Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease, characterized by pain and stiffness of the joints,
inflammatory arthritis and extra-articular involvement. This
systematic autoimmune disorder commonly induces the accumulation of
immune cells, including T cells, B cells and macrophages, in the
inflamed joints, which may lead to synovial hyperplasia, as well as
cartilage and bone erosion (1). T
cells have an important role in the pathogenesis of RA, which is
therefore considered a typical T-cell-mediated disease, which is
arbitrated in particular by CD4+ T helper (Th) cells.
Regulatory T cells (Tregs) are another subtype of T cells, which
exert a suppressive effect and are considered to have a protective
role against the autoimmune response (2).
Treg cells were initially identified as
CD4+CD25high T cells, and the most recent
accurately characterized Treg population is
CD4+CD25high Forkhead box
(Fox)P3+. Studies have indicated that the majority of
the Foxp3+Tregs are however within the
CD4+CD25highCD127− cell
population; the latter population is therefore usually used for
determining the Treg cell count and function (3). Since their identification, a number of
studies have investigated the number and function of Tregs in RA
patients. Tregs have been suggested to have a protective role in
mouse models of arthritis (4,5).
However, although a number of studies have come to the same
conclusion that Tregs were enriched in RA synovial fluid, reports
on Treg proportions in the peripheral blood (PB) of patients with
RA have provided conflicting results. The majority of studies
indicated that the percentage of circulating Tregs in RA was
reduced compared with that in healthy individuals, while others
have reported increased or similar cell percentages in RA patients
compared with those in normal controls or patients with
osteoarthritis (OA) (6–9).
Although Tregs are thought to exert their
suppressive effect via secretion of the inhibitory cytokines
interleukin (IL)-10 and transforming growth factor-β, and cell-cell
contact via cytotoxic T-lymphocyte-associated protein 4 and the
membrane glycoprotein lymphocyte-activation gene 3, the exact
mechanism of Treg function has remained to be fully elucidated
(10,11). IL-35 is a recently discovered
cytokine of the IL-12 family, which also includes Epstein-Barr
virus induced gene 3 (EBI3) and p35. IL-35 was initially reported
to be secreted by Tregs (12) and
has recently been revealed to be produced by other cell types,
including regulatory B cells and activated B cells (13,14).
IL-35 not only has an important role in promoting the suppressive
function of Treg cells (12), but
also induces the generation of Tregs that produce IL-35 (iTr35
cells); these induced iTr35 cells in turn produce more IL-35
(15). Several studies have assessed
the role of IL-35 in autoimmune diseases; in a mouse model,
Niedbala et al (16) revealed
that recombinant IL-35 effectively attenuated collagen-induced
arthritis, and a subsequent study by Kochetkova et al
(17) reported similar results.
Several clinical studies indicated that serum IL-35 levels were
significantly lower in patients with RA; furthermore, treatment
with IL-35 suppressed inflammatory cytokine levels and enhanced the
regulatory function of Tregs (18,19).
However, few studies have analyzed the number and function of Tregs
in patients with RA.
In the present study, the IL-35 concentration and
Treg frequency in patients with RA was analyzed, and the
association between IL-35, Tregs and indicators of RA activity was
further explored. This preliminary study provides a basis for
understanding the role IL-35 of in RA and may serve as a reference
for further investigation to develop novel diagnostic tools or
treatments for RA.
Materials and methods
Patients and clinical data
Peripheral blood was obtained from 37 patients with
active-phase RA (PA-AP), 18 patients with chronic-phase RA (RA-CP)
and 20 healthy controls (HC). HC subjects were recruited from local
staff volunteers. All of the patients with RA fulfilled the
American College of Rheumatology criteria for RA (20). The following clinical parameters were
acquired from the patients' medical records: Erythrocyte
sedimentation rate (ESR), C-reactive protein (CRP) levels,
rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP)
antibody. The 28-joint disease activity score (DAS28) was used to
determine disease activity (21).
All patients were free of infectious diseases, cancer,
cardiovascular disease and any other inflammatory diseases. The
characteristics of the patients with RA and the HC are presented in
Table I. The final protocol for the
use of patient samples was approved by the local Institutional
Review Board of Yantai Yuhuangding Hospital (Yantai, China). All
patients and controls voluntarily joined the present study and
provided their written informed consent.
 | Table I.Clinical parameters of RA patients
and HC. |
Table I.
Clinical parameters of RA patients
and HC.
| Parameter | HC (n=20) | RA-CP (n=18) | RA-AP (n=37) |
|---|
| Gender |
|
Male | 10 | 5 | 11 |
|
Female | 10 | 13 | 26 |
| Age (years) |
| Median
range | 43 (26–59) | 53
(15–76) | 54 (18–77) |
| CRP (mg/dl) | – | 4.00±0.43 |
31.94±4.96a |
| ESR (mm/h) | – | 23.94±2.64 |
60.76±4.09a |
| CCP (IU/ml) | – | 65.51±29.14 | 114.5±13.14 |
| RF (IU/ml) | – | 318.1±119.7 | 461.5±138.0 |
| DAS28 | – | 2.20±0.07 |
5.67±0.14a |
Treg detection
For analysis of Tregs, 5 µl peridinin chlorophyll
cyanine 5.5-conjugated anti-human CD3 (cat no. 340949), 5 µl
phycoerythrin-conjugated anti-human CD25 (cat no. 341009), 5 µl
fluorescein isothiocyanate-conjugated anti-human CD4 (cat no.
340133) and 5 µl Alexa Fluor 647-conjugated anti-human CD127 (cat.
no. 558598; all antibodies obtained from BD Biosciences, Franklin
Lakes, NJ, USA) were mixed with 100 µl fresh
EDTA-K2-anti-coagulated whole blood, followed by incubation at room
temperature in the dark for 30 min. Equal volumes of corresponding
mouse immunoglobulin isotypes: Alexa Fluor 647-conjugated IgG1
isotype (BD Biosciences; cat no. 565571) and
phycoerythrin-conjugated IgG1 isotype (BD Biosciences; cat no.
555749) were used as controls. Following incubation, red blood
cells were lysed with lysis solution (BD Biosciences; cat. no.
349202) for 10 min at room temperature and washed with PBS,
followed by dilution with 0.5 ml PBS for analysis by flow
cytometry. The analysis was performed using a BD FACS Canto II flow
cytometer (BD Biosciences). At least 50,000 events were collected
for each specimen and the results were analyzed using Diva 7.0
software (BD Biosciences).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
PB mononuclear cells (PBMC) were isolated through
Ficoll-Hypaque density gradient centrifugation from PB of patients
with RA and the HC. Total mRNA was isolated using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) by
the one-step extraction method. RNA concentrations and purity were
determined by reading their absorbance at 260 nm. Complementary DNA
was prepared using the RevertAid First Strand cDNA Synthesis Kit
(Thermo Fisher Scientific, inc.; cat. no. K1622) according to the
manufacturer's instructions. Real-time PCR amplification was
performed using SYBR® Selected Master mix (Applied
Biosystems; Thermo Fisher Scientific). The sequences of specific
primer pairs (Invitrogen; Thermo Fisher Scientific Inc.) were as
follows: p35 forward, 5′-AGGAATGTTCCCATGCCTTCA-3′ and reverse,
5′-CCAATGGTAAACAGGCCTCCAC-3′; EBI3 forward,
5′-TCCCAGAGATCTTCTCACTGAAGTA-3′ and reverse,
5′-GCACAGCCCTGAGGATGAA-3′; GAPDH forward,
5′-AACGGATTTGGTCGTATTGGG-3′ and reverse,
5′-CCTGGAAGATGGTGATGGGAT-3′. The thermocycling steps were as
follows: 95°C for 10 min, followed by 39 cycles of 95°C for 30 sec,
60°C for 1 min and 65°C for 30 sec. Each sample was analyzed in
triplicate. The relative mRNA expression was calculated using the
2−ΔΔCq method (22). All
samples were normalized to GAPDH, which was used as a control.
Measurement of serum IL-35 levels by
ELISA
Serum IL-35 levels of samples were determined using
IL-35 ELISA kits (Cusabio Biotech, Wuhan, China cat no. CSB-E13126
h) according to the manufacturer's protocols. All samples were
measured in triplicate and the mean value was calculated for
statistical analysis. IL-35 levels were calculated based on a
standard curve.
Statistical analysis
Statistical significance was evaluated with data
from at least three independent experiments. Statistical
comparisons between two groups were performed using an unpaired
t-test, while multigroup comparisons were performed by one-way
analysis of variance followed by a Student-Newman-Keuls post-hoc
test (Prism 6.0 software; GraphPad Inc., La Jolla, CA, USA).
Correlation analysis was performed by determining Pearson's
correlation coefficient. Values are expressed as the mean ±
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
Low frequency of
CD4+CD25+/highCD127−/low Tregs in
PB of patients with RA
Overall, 55 patients with RA were included in the
present study. Their clinical characteristics, including gender,
age, CRP, ESR, CCP, RF and DAS28, are presented in Table I. The level of CRP, the ESR and the
DAS28 of RA-AP patients were significantly higher than those in
RA-CP patients (P<0.05). However, there was no significant
difference in sex, age, RF and CCP levels between the RA-AP and
RA-CP groups. Furthermore, CD4+T cells and
CD4+CD25highCD127− Tregs in
patients with RA and in HC were analyzed. In Fig. 1A, CD3+CD4+
cells (gate P3) indicate Th cells and
CD25highCD127− cells (gate P4) indicate
Tregs. The percentage of CD25highCD127− Tregs
in patients with RA (RA-AP or RA-CP) was revealed to be
significantly lower than that in HC (Fig. 1B). However, no significant difference
in the percentage of Tregs between the RA-AP and RA-CP groups was
identified. The quantified results regarding the T-cell subsets
detected are presented in Table
II.
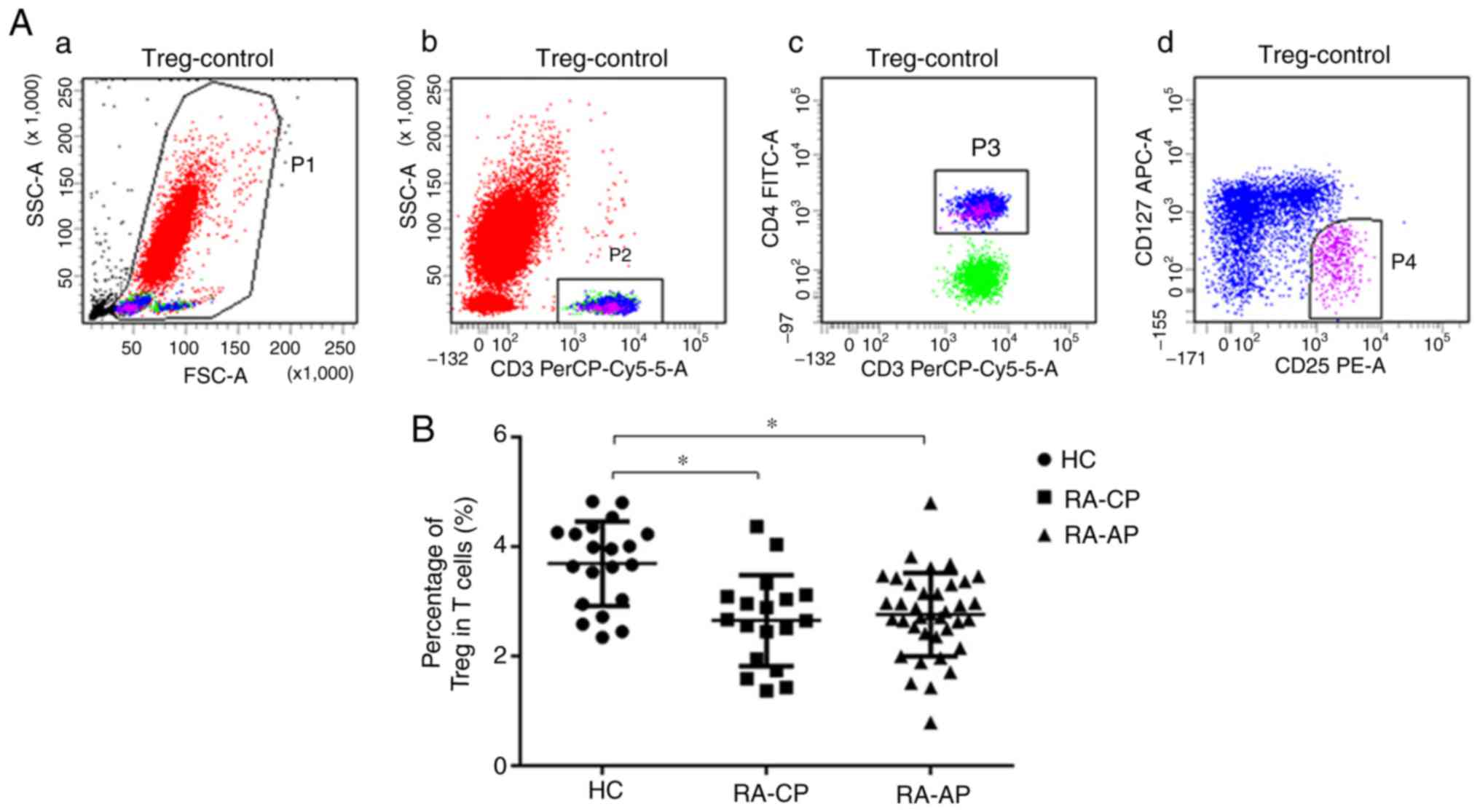 | Figure 1.Percentage of
CD4+CD25highCD127− Tregs in
peripheral blood of RA patients and HC. (A) Representative dot
plots of Treg analysis by flow cytometry. (a) Gate P1 (red)
contains all of the nucleated cells in the peripheral blood; (b)
gate P2 (green) contains the CD3+ T cells in the P1
gate; (c) gate P3 (blue) contains CD3+CD4+T
helper cells in the P2 gate; (d) gate P4 (purple) includes the
CD3+CD4+CD25highCD127−
regulatory T cells in the P3 gate. (B) Quantified Treg frequency in
the PA-AP, RA-CP and HC groups. *P<0.05. RA-AP, rheumatoid
arthritis in active phase; RA-CP, RA in chronic phase; HC, healthy
controls; Treg, T-regulatory cell; PerCP, peridinin chlorophyll;
FSC, forward scatter, SSC, side scatter; Cy, cyanine; FITC,
fluorescein isothiocyanate; APC, allophycocyanine. |
 | Table II.Percentage of Th cells and Tregs in
RA patients and HC. |
Table II.
Percentage of Th cells and Tregs in
RA patients and HC.
| Cell type | HC (n=20) | RA-CP (n=18) | RA-AP (n=37) | P-value |
|---|
| Th (% of total T
cells) | 53.05±1.67 | 58.54±2.61 | 60.22±1.87 | a0.0159; b0.0712; c0.6458 |
| Treg (% of total T
cells) | 3.55±0.18 | 2.69±0.25 | 2.90±0.14 | a0.0083; b0.0072; c0.4701 |
| Treg/Th | 0.067±0.003 | 0.046±0.004 | 0.048±0.002 | a<0.0001; b<0.0001; c0.5912 |
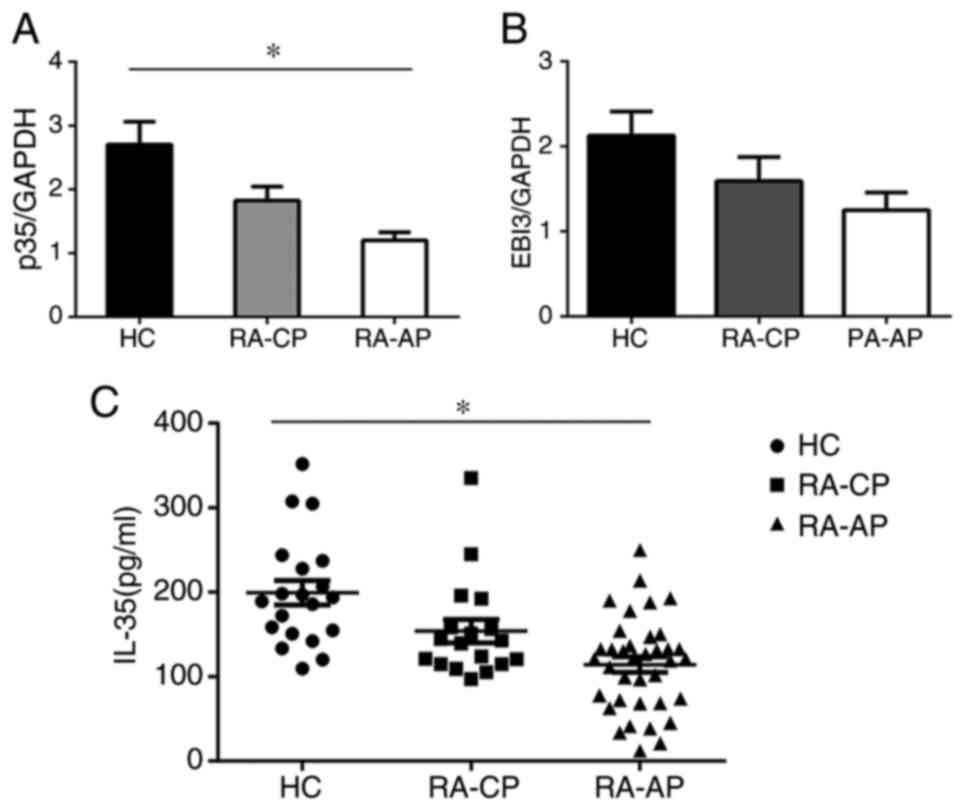
IL-35 is decreased in patients with
RA
As IL-35 has an important role in the generation and
function of Tregs, which themselves produce IL-35, the levels of
IL-35 in patients with RA were assessed (Fig. 2). The serum IL-35 levels in the RA-AP
patient group (114.2±9.097 pg/ml) were significantly lower than
those in the RA-CP group (153.8±13.83 pg/ml) and the control group
(199.3±14.45 pg/ml). The serum IL-35 levels in the RA-CP patient
group were also significantly lower than those in the control
group, which suggests a potential link between IL-35 and disease
progression (Fig. 2C). Furthermore,
PBMCs were isolated from RA patients and IL-35 mRNA levels were
determined with RT-qPCR. Consistent with the above results, the
data revealed that mRNA levels of p35, a subunit of IL-35, were
significantly decreased in RA-AP patients compared with those in
the RA-CP or control group, and also the p35 levels in the PA-CP
group were lower than those in the control group (Fig. 2A). However, no significant difference
in EBI3 mRNA expression was detected (Fig. 2B), which may be due to EBI3 also
being a component of other cytokines. These results suggested that
IL-35 has an important role in the development of RA.
Correlation between IL-35 and disease
activity in patients with RA
The association between IL-35 and disease activity
in RA-AP patients was investigated, and it was revealed that serum
IL-35 levels were not significantly correlated with the levels of
CRP (r=−0.1762, P=0.6929; Fig. 3A),
RF (r=−0.0293, P=0.8693; Fig. 3C) or
anti-CCP antibodies (r=−0.0822, P=0.6545; Fig. 3D) in patients with RA. However, serum
IL-35 levels were negatively correlated with the ESR (r=−0.4247,
P<0.01; Fig. 3B) and DAS28
(r=−0.4909, P<0.01; Fig. 3E) in
patients with RA. The DAS28 is an important indicator of disease
activity, and therefore, these results further suggested that IL-35
may prevent the progression of RA.
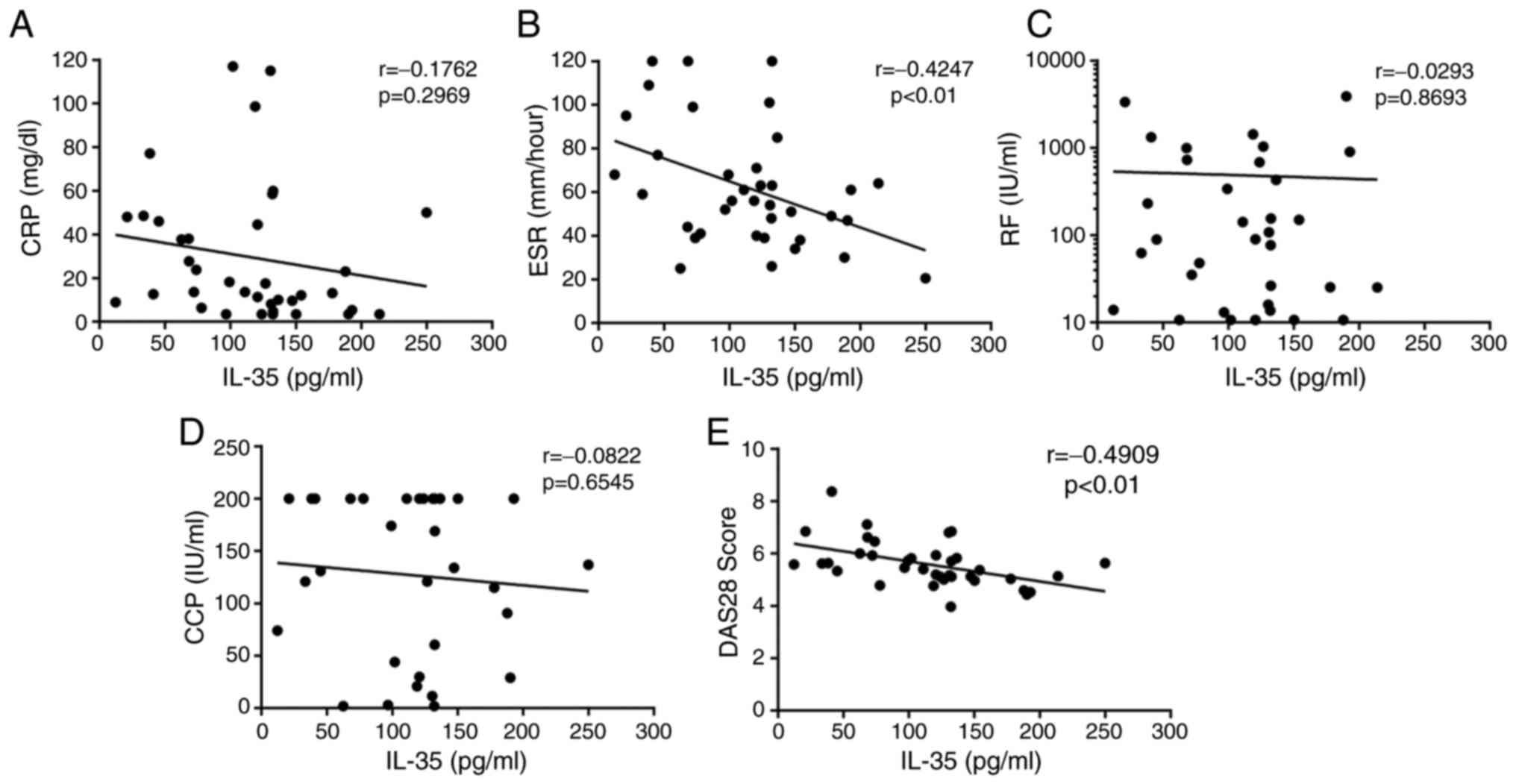 | Figure 3.Correlations between IL-35 levels and
(A) CRP, (B) ESR, (C) RF, (D) anti-CCP antibody and (E) DAS28 score
in patients with rheumatoid arthritis. Pearson's correlation
coefficient was determined in the correlation analysis. Significant
negative correlations were observed between the serum IL-35
concentration and the ESR (r=−0.4247; P<0.01), as well as the
DAS28 (r=−0.4909; P<0.01). IL, interleukin; ESR, erythrocyte
sedimentation rate; CRP, C-reactive protein; CCP, cyclic
citrullinated peptide; RF, rheumatoid factor; DAS28, 28-joint
disease activity score. |
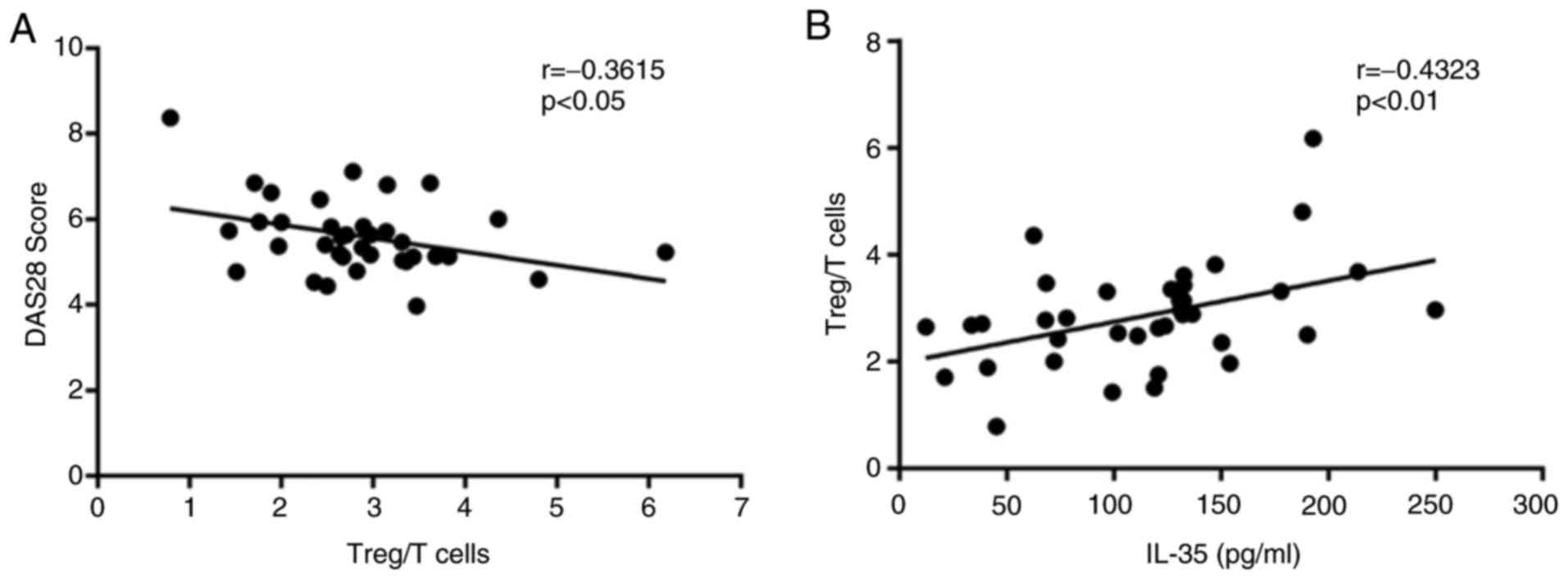
Correlation between IL-35 and
percentage of Tregs in patients with RA
The correlation between the percentage of Tregs and
the DAS28 in RA-AP patients was investigated. The correlation
analysis indicated that the percentage of Tregs was negatively
correlated with the DAS28 (r=−0.3615, P<0.05; Fig. 4A), which confirmed the role of Tregs
in the progression of RA. In addition, the percentages of Tregs
were identified to be significantly positively associated with the
serum IL-35 concentration (r=0.4323, P<0.01; Fig. 4B), which was in accordance with the
above. These results suggested that IL-35 may have an
immunosuppressive role by enhancing the Treg population in patients
with RA.
Discussion
IL-35 is a newly described anti-inflammatory
cytokine involved in various autoimmune diseases, which is produced
primarily by Tregs and in turn induces the generation of Tregs
(12,15). In the present study, the levels of
IL-35 and the percentage of Tregs in RA patients were investigated,
and the possible link between IL-35, Tregs and disease activity in
RA was analyzed. The results indicated that the IL-35 concentration
and the percentage of Tregs in patients with RA was significantly
lower than that in HC. In addition, the IL-35 concentration and the
percentage of Tregs were negatively correlated with the DAS28,
which suggested that IL-35 may have a role in the inflammatory
processes of RA development.
Several studies have investigated the frequency of
Tregs in RA; however, they provided conflicting results, including
a decreased, similar or increased Treg percentage compared with
that in HC (7–9). Of note, the establishment of CD127 as
an additional surface marker of Tregs has promoted the consistent
identification of Tregs as the
CD4+CD25highCD127− phenotype
(23). To date, only few studies
have provided data on
CD4+CD25highCD127− Tregs in
patients with RA; Kawashiri et al (24) reported that the frequency of Tregs
was lower in RA-AP and similar in RA-CP patients compared with that
in controls, and Moradi et al (25) indicated that the mean Treg frequency
was comparable between RA and OA patients. In the present study, it
was revealed that
CD4+CD25highCD127− Tregs in RA-AP
and RA-CP patients were obviously lower than those in HC. The
results indicated that there was no significant difference in the
percentage of Tregs between the RA-AP and RA-CP groups, which may
be due to the limitation of small sample size; however, these
results are consistent with those of previous studies (24,25). In
addition, it was also revealed that the Treg frequency was
negatively correlated with the DAS28, which suggested a role of
Tregs in the development of RA. Administration of Tregs has been
indicated to be a promising treatment for autoimmune diseases
including RA, and previous studies have reported that induced
pluripotent stem cell-derived Tregs suppress arthritis development
(26,27).
IL-35 is primarily involved in the function of Treg
effector cells, and therefore, it is important in the study of
autoimmune disease (28). Mice
deficient of IL-35 produce B cells which cannot recover from T
cell-mediated experimental autoimmune encephalomyelitis (14). However, another study has indicated
that IL-35 gene transfer enhanced the severity of collagen-induced
arthritis (29). In a clinical study
on systemic lupus erythematosus, a decreased IL-35 concentration
was detected and a negative correlation with disease severity was
observed (30). In multiple
sclerosis patients, the serum levels of IL-35 were not different
from those in HC (31). In RA, the
serum levels of IL-35 were significantly lower than in HC and were
negatively correlated with RF, the percentage of neutrophils and
articular erosion (18). In the
present study, it was also revealed that the serum levels of IL-35
in RA-AP and RA-CP patients was significantly decreased compared
with that in HC. It was also indicated that serum IL-35 in the
RA-AP patient group was significantly decreased compared with that
in the RA-CP group. These results are consistent with those of
other studies (18,19). Furthermore, the correlation between
the IL-35 concentration and disease activity indicators was
analyzed, and the results suggested that serum IL-35 levels were
not significantly correlated with CRP, RF or anti-CCP antibodies
but negatively correlated with the ESR and DAS28 in patients with
RA, which suggests that the levels of IL-35 may reflect RA disease
activity. A previous study indicated that recombinant human IL-35
enhanced natural Treg function in vitro and suppressed Treg
proliferation and inflammatory cytokines in patients with RA, which
suggested that IL-35 is involved in the Treg-mediated suppression
of autoimmunity in RA (19). Other
studies indicated that IL-35 caused an upregulaion of the
expression of Foxp3 and resulted in a significant increase in the
proportions of
CD4+CD25+Foxp3+Tregs in
apolipoprotein E−/− mice (32), and administration of IL-35
significantly increased the number of Tregs (33). Consistent with this, the present
study revealed that the percentage of Tregs in PB samples was
significantly positively correlated with the serum levels of IL-35
in patients with RA. However, the precise regulatory mechanism of
IL-35 expression in patients with RA and the molecular pathways
involved require to be further elucidated.
In conclusion, the present study revealed decreased
serum IL-35 levels and a decreased Treg percentage in patients with
RA when compared with those in HC. Further analysis demonstrated
that the IL-35 concentration is negatively correlated with the ESR
and DAS28, and that the percentage of Tregs is significantly
positively correlated with IL-35 levels in patients with RA. This
suggested a possible protective role of IL-35 and Tregs regarding
the development of RA. Current treatment strategies for RA mainly
aim to control inflammation, and in the future, IL-35 and Tregs may
provide multiple therapeutic targets.
Acknowledgements
Not applicable.
Funding
The current study was supported by The National
Natural Science Foundation of China (grant no. 81202069) and the
Natural Scientific Research Foundation of Hunan Province (grant no.
2018JJ3365).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Xia Zhang and Xiaolu Zhang: Performed the
experiments and wrote the paper; Lili Zhuang and Guili Zhang:
Collection and assembly of data; Cangcang Xu and Tao Li: Data
analysis and manuscript revision. Xia Zhang and Ying Liu:
Conception and design, financial support and final approval of
manuscript.
Ethical approval and consent to
participate
The final protocol for the use of patient samples
was approved by the local Institutional Review Board of Yantai
Yuhuangding Hospital (Yantai, China).
Patient consent for publication
Written informed consent was obtained from all
patients prior to enrolment.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wing K and Sakaguchi S: Regulatory T cells
exert checks and balances on self tolerance and autoimmunity. Nat
Immunol. 11:7–13. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Guo H, Lu L, Zahorchak AF,
Wiseman RW, Raimondi G, Cooper DK, Ezzelarab MB and Thomson AW:
Sequential monitoring and stability of ex vivo expanded autologous
and nonautologous regulatory T cells following infusion in nonhuman
primates. Am J Transplant. 15:1253–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgan ME, Flierman R, van Duivenvoorde
LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR and Toes
RE: Effective treatment of collagen induced arthritis by adoptive
transfer of CD25+ regulatory T cells. Arthritis Rheum.
52:2212–2221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen LT, Jacobs J, Mathis D and Benoist
C: Where FoxP3-dependent regulatory T cells impinge on the
development of inflammatory arthritis. Arthritis Rheum. 56:509–520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alunno A, Manetti M, Caterbi S,
Ibba-Manneschi L, Bistoni O, Bartoloni E, Valentini V, Terenzi R
and Gerli R: Altered immunoregulation in rheumatoid arthritis: The
role of regulatory T cells and proinflammatory Th17 cells and
therapeutic implications. Mediators Inflamm. 2015:7517932015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiao Z, Wang W, Jia R, Li J, You H, Chen L
and Wang Y: Accumulation of FoxP3 expressing CD4+CD25+ T cells with
distinct chemokine receptors in synovial fluid of patients with
active rheumatoid arthritis. Scand J Rheumatol. 36:428–433. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Möttönen M, Heikkinen J, Mustonen L,
Isomäki P, Luukkainen R and Lassila O: CD4+ CD25+ T cells with the
phenotypic and functional characteristics of regulatory T cells are
enriched in the synovial fluid of patients with rheumatoid
arthritis. Clin Exp Immunol. 140:360–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han GM, O'Neil-Andersen NJ, Zurier RB and
Lawrence DA: CD4+CD25high T cell numbers are enriched in the
peripheral blood of patients with rheumatoid arthritis. Cell
Immunol. 253:92–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sansom DM and Walker LS: The role of CD28
and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell
biology. Immunol Rev. 212:131–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamura T, Fujio K, Sumitomo S and
Yamamoto K: Roles of LAG3 and EGR2 in regulatory T cells. Ann Rheum
Dis. 2 Suppl 71:i96–i100. 2012. View Article : Google Scholar
|
|
12
|
Collison LW, Workman CJ, Kuo TT, Boyd K,
Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS and Vignali DA:
The inhibitory cytokine IL-35 contributes to regulatory T-cell
function. Nature. 450:566–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang RX, Yu CR, Dambuza IM, Mahdi RM,
Dolinska MB, Sergeev YV, Wingfield PT, Kim SH and Egwuagu CE:
Interleukin-35 induces regulatory B cells that suppress autoimmune
disease. Nat Med. 20:633–641. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen P, Roch T, Lampropoulou V, O'Connor
RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C,
et al: IL-35-producing B cells are critical regulators of immunity
during autoimmune and infectious diseases. Nature. 507:366–370.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collison LW, Chaturvedi V, Henderson AL,
Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ,
Brown SA, et al: IL-35-mediated induction of a potent regulatory T
cell population. Nat Immunol. 11:1093–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niedbala W, Wei XQ, Cai B, Hueber AJ,
Leung BP, McInnes IB and Liew FY: IL-35 is a novel cytokine with
therapeutic effects against collagen-induced arthritis through the
expansion of regulatory T cells and suppression of Th17 cells. Eur
J Immunol. 37:3021–3029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kochetkova I, Golden S, Holderness K,
Callis G and Pascual DW: IL-35 stimulation of CD39+ regulatory T
cells confers protection against collagen II-induced arthritis via
the production of IL-10. J Immunol. 184:7144–7153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ning X, Jian Z and Wang W: Low serum
levels of interleukin 35 in patients with rheumatoid arthritis.
Tohoku J Exp Med. 237:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakano S, Morimoto S, Suzuki S, Tsushima
H, Yamanaka K, Sekigawa I and Takasaki Y: Immunoregulatory role of
IL-35 in T cells of patients with rheumatoid arthritis.
Rheumatology (Oxford). 54:1498–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH and Luthra
HS: The american rheumatism association 1987 revised criteria for
the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prevoo ML, van'tHof MA, Kuper HH, van
Leewen MA, van de Putte LB and van Riel PL: Modified disease
activity scores that include twenty-eight-joint counts. Development
and validation in a prospective longitudinal study of patients with
rheumatoid arthritis. Arthritis Rheum. 38:44–48. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hartigan-O'Connor DJ, Poon C, Sinclair E
and McCune JM: Human CD4+ regulatory T cells express lower levels
of the IL-7 receptor α chain (CD127), allowing consistent
identification and sorting of live cells. J Immunol Methods.
319:41–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawashiri SY, Kawakami A, Okada A, Koga T,
Tamai M, Yamasaki S, Nakamura H, Origuchi T, Ida H and Eguchi K:
CD4+CD25(high)CD127(low/-) Treg cell frequency from peripheral
blood correlates with disease activity in patients with rheumatoid
arthritis. J Rheumatol. 38:2517–2521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moradi B, Schnatzer P, Hagmann S, Rosshirt
N, Gotterbarm T, Kretzer JP, Thomsen M, Lorenz HM, Zeifang F and
Tretter T: CD4+CD25+/highCD127low/- regulatory T cells are enriched
in rheumatoid arthritis and osteoarthritis joints-analysis of
frequency and phenotype in synovial membrane, synovial fluid and
peripheral blood. Arthritis Res Ther. 16:R972014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haque R, Lei F, Xiong X, Bian Y, Zhao B,
Wu Y and Song J: Programming of regulatory T cells from pluripotent
stem cells and prevention of autoimmunity. J Immunol.
189:1228–1236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wright GP, Notley CA, Xue SA, Bendle GM,
Holler A, Schumacher TN, Ehrenstein MR and Stauss HJ: Adoptive
therapy with redirected primary regulatory T cells results in
antigen-specific suppression of arthritis. Proc Natl Acad Sci USA.
106:19078–19083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pope RM and Shahrara S: Possible roles of
IL-12-family cytokines in rheumatoid arthritis. Nat Rev Rheumatol.
9:252–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thiolat A, Denys A, Petit M, Biton J,
Lemeiter D, Herve R, Lutomski D, Boissier MC and Bessis N:
Interleukin-35 gene therapy exacerbates experimental rheumatoid
arthritis in mice. Cytokine. 69:87–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ouyang H, Shi YB, Liu ZC, Wang Z, Feng S,
Kong SM and Lu Y: Decreased interleukin 35 and CD4+EBI3+ T cells in
patients with active systemic lupus erythematosus. Am J Med Sci.
348:156–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jafarzadeh A, Jamali M, Mahdavi R,
Ebrahimi HA, Hajghani H, Khosravimashizi A, Nemati M, Najafipour H,
Sheikhi A, Mohammadi MM and Daneshvar H: Circulating levels of
interleukin-35 in patients with multiple sclerosis: Evaluation of
the influences of FOXP3 gene polymorphism and treatment program. J
Mol Neurosci. 55:891–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tao L, Zhu J, Chen Y, Wang Q, Pan Y, Yu Q,
Zhou B and Zhu H: IL-35 improves Treg-mediated immune suppression
in atherosclerotic mice. Exp Ther Med. 12:2469–2476. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zongyi Y, Funian Z, Hao L, Xin W, Ying C,
Jialin Z, Yongfeng L and Baifeng L: Interleukin-35 mitigates the
function of murine transplanted islet cells via regulation of
Treg/Th17 ratio. PLoS One. 12:e01896172017. View Article : Google Scholar : PubMed/NCBI
|