Introduction
Nasopharyngeal carcinoma (NPC) is a highly invasive
and metastatic head and neck cancer (1). NPC is associated with many factors,
including Epstein-Barr virus infection, genetic and environmental
factors (2). Although radiotherapy
is effective for early-stage tumors, patients with advanced NPC
succumb to skull base and neck lymph node invasion and metastasis
(3). It is reported that the median
survival of patients with metastatic or advanced NPC is only 5–11
months (4). The poor prognosis is
mainly due to high recurrence and metastasis rates (5). Consequently, there is an urgent
requirement to explore new therapies against NPC.
Phosphoinositide 3-kinase (PI3K)/protein kinase B
(AKT)/mammalian target of rapamycin (mTOR) signaling is commonly
associated with tumorigenesis (6).
During the development of tumors, PI3K pathway deregulation
commonly occurs via the inactivation of the tumor suppressor
phosphatase and tensin homolog (7),
and mTOR is activated and associated with cell growth,
proliferation, differentiation and apoptosis (8). Thus, the PI3K/AKT/mTOR pathway provides
a promising target for cancer therapy. NVP-BEZ235 is an imidazo
[4,5-c]quinoline derivative and suppresses the activities of PI3K
and mTOR (9). Previous reports have
demonstrated that NVP-BEZ235 exerts an anti-cancer function in
breast cancer (10), ovarian cancer
(11) and prostate cancer (12). Although NVP-BEZ235 has been used in
patients with advanced NPC for phase I/II clinical trials, the
outcomes of these trials have been unsatisfactory (13).
Rapamycin, as an mTOR signaling inhibitor, reduces
cell proliferation in NPC (1). The
main aim of the present study was to investigate the role of the
combination of NVP-BEZ235 and rapamycin on NPC cell viability,
cellular apoptosis and PI3K/AKT/mTOR signaling. The results
indicated that both NVP-BEZ235 and rapamycin caused morphological
changes, inhibited cell viability, induced cellular apoptosis and
affected the activation of the PI3K/AKT/mTOR pathway. The
combination of NVP-BEZ235 and rapamycin significantly improved the
effects of the drug therapy. Therefore, the present study
identified potential novel anti-NPC drugs with more efficiency and
less toxicity.
Materials and methods
Cell culture
The NPC cell line SUNE1 was obtained from Wuxi
Innovate Biomedical Technology Co., Ltd. (Wuxi, China). SUNE1 cells
were incubated with Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C.
SUNE1 cells were seeded into a 24-well plate at the density of 80%,
and then were incubated with 100 nM NVP-BEZ235 (cat. no. A506167)
(14), 100 nM rapamycin (cat. no.
A606203; both Sangon Biotech Co., Ltd., Shanghai, China) (15), or 100 nM NVP-BEZ235 and 100 nM
Rapamycin for 48 h at 37°C. The control group was administered the
same amount of dimethyl sulphoxide (DMSO; Sangon Biotech Co.,
Ltd.). Cell morphology following treatment was observed with an
inverted phase contrast microscope (Olympus Corporation, Tokyo,
Japan; magnification, ×100).
Cellular apoptosis detected by
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling (TUNEL) assay and flow cytometry
SUNE1 cells from the four groups were fixed with 4%
paraformldehyde at room temperature for 5 min, washed with PBS and
incubated with 50 mL TUNEL detection solution (2 ml terminal
deoxynucleotidyl-transferase enzyme and digoxigenin-dUTP reaction
buffer) at 37°C for 1 h. Following washing with PBS, SUNE1 cells
were stained with DAPI (1 mg/ml) in PBS for 10 min at room
temperature. A total of 5 non-overlapping fields were captured with
a fluorescence inverted microscope (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA; magnification, ×100).
For the flow cytometry assay, SUNE1 cells from the
four groups were washed with PBS twice, and incubated with 400 ml
1X binding buffer and 5 ml fluorescein isothiocyanate-labeled
Annexin-V from the Annexin V-FITC/PI kit (cat. no. APOAF-20TST;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 15 min in the
dark at room temperature. Then, SUNE1 cells were incubated with 10
ml propidium iodide for 5 min in the dark at room temperature.
Cellular apoptosis was detected with fluorescence-activated cell
sorting (FACS) using FCSExpress version 3.0 (De Novo Software,
Glendale, CA, USA) in a flow cytometer (BD FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA).
Cell viability examined by MTT assay
and flow cytometry
SUNE1 cells in the exponential growth phase were
collected and seeded in a 96-well plate at 5×103
cells/well in 100 ml culture medium. Cells were incubated with
NVP-BEZ235 and/or rapamycin at final concentrations of 100 nM for
48 h at 37°C, in the groups detailed above. Following incubation,
the medium was removed and 100 ml fresh medium containing 10% MTT
(5 mg/ml) was added into each well. Following 4 h of incubation at
37°C, DMSO (200 ml) was added to cells for 10 min and the
absorbance was measured at 570 nm using a multifunctional
microplate reader (Thermo Fisher Scientific, Inc.). To calculate
the relative cell viability, 6-well replication was used. Data were
analyzed in three independent samples.
SUNE1 cells from the four groups were digested with
trypsin and fixed with 70% ethanol overnight at 4°C. The following
morning, SUNE1 cells were washed with PBS and subsequently stained
with propidium iodide (50 mg/ml) and RNase A (100 mg/ml; cat. no.
B300068; Sangon Biotech Co., Ltd.) for 1 h in the dark. The cell
cycle was analyzed FACS using FCSExpress software in BD FACSCalibur
flow cytometer.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SUNE1 cells using
TRIzon Reagent (Beijing ComWin Biotech, Co., Ltd., Beijing, China).
The quality and quantity of RNA was examined with a NanoDrop-2000
ultramicrospectrophotometer (NanoDrop; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) at wavelengths of 260 and 280 nm.
Subsequently, 1 mg RNA was reverse transcribed into cDNA using the
SuperRT cDNA Synthesis kit (Beijing ComWin Biotech, Co., Ltd.)
according to the manufacturer's protocol, and qPCR was carried out
with SYBR Green qPCR SuperMix (Invitrogen; Thermo Fisher
Scientific, Inc.) in CFX96™ Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc.). The thermocycling conditions
were as follows: 95°C for 5 min, followed by 40 cycles of 95°C for
30 sec and 60°C for 30 sec, and a final extension at 72°C for 5
min. The relative expression of genes was normalized to GAPDH using
the 2−ΔΔCq method (16).
The primers for RT-qPCR were as follows: GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′; PI3K forward,
5′-GCCCAGGCTTACTACAGAC-3′ and reverse, 5′-AAGTAGGGAGGCATCTCG-3′;
AKT forward, 5′-CTCATTCCAGACCCACGAC-3′ and reverse,
5′-ACAGCCCGAAGTCCGTTA-3′; and mTOR forward,
5′-CTGGGACTCAAATGTGTGCAGT-3′ and reverse,
5′-GAACAATAGGGTGAATGATCCGGG-3′.
Western blotting
Proteins were extracted using
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and centrifuged at 12,000 × g
at 4°C for 20 min. The concentration of protein was detected with a
bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Subsequently, 30–50 mg
protein was subjected to SDS-PAGE on a 10% gel and transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% non-fat milk in Tris-buffered
saline containing 0.05% Tween-20 (TBST) at room temperature for 1
h, the membranes were probed with the following mouse monoclonal
antibodies: Anti-PI3K (ab86714; 1:1,000), anti-AKT (ab175354;
1:1,000), anti-phosphorylated (p)-AKT (ab105731; 1:500), anti-mTOR
(ab87540; 1:1,000), anti-GAPDH (AB8245; 1:1,000) and rabbit
monoclonal anti-p-mTOR (ab109268; 1:1,000; all Abcam, Cambridge,
MA, USA) at 4°C overnight. The membranes were incubated with the
rabbit anti-mouse IgG (ab6728; 1:10,000) or goat anti-rabbit IgG
(ab205718; 1:10,000) horseradish peroxidase-labeled secondary
antibodies (both Abcam) at room temperature for 1 h. Following
washing with TBST, the blot was examined with
electrochemiluminescence solution (Pierce; Thermo Fisher
Scientific, Inc.). The relative expression was normalized to GAPDH
using Quantity One software version 4.2 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
Statistical analysis was performed with SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). All experiments were carried out at
least three times. Data are expressed as the mean ± standard
deviation, and differences were analyzed using one-way analysis of
variance with Tukey's post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Rapamycin and NVP-BEZ235 promote
cellular apoptosis
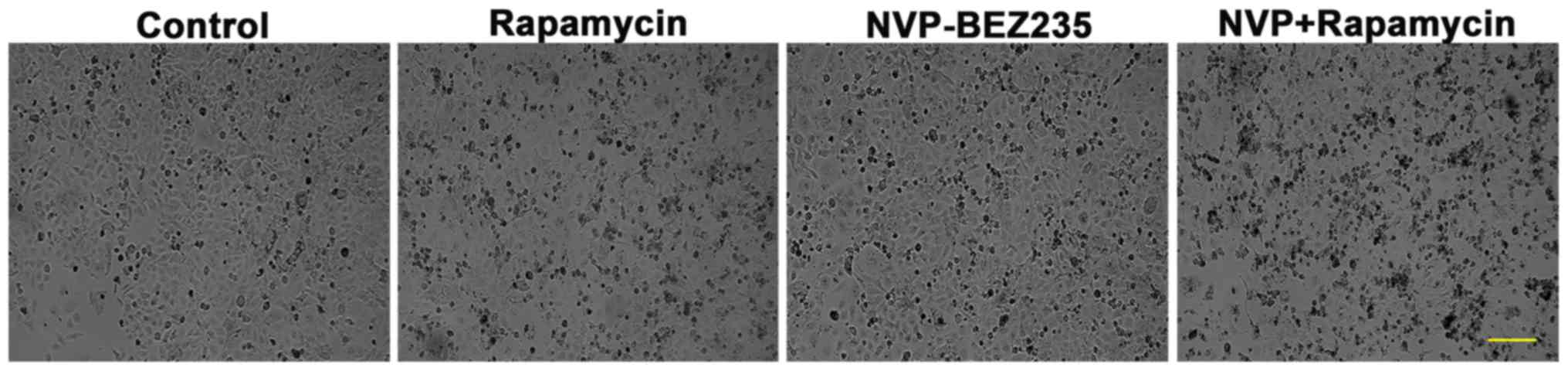
As presented in Fig.
1, cells in the control group possessed clear cell contours,
boundaries and round cellular nuclei, with good cell growth status.
However, once SUNE1 cells were incubated with NVP-BEZ235 or
rapamycin, they exhibited shrinkage and cell size reduction.
Furthermore, more marked morphological changes were observed in
NVP-BEZ235+rapamycin induced cells. These data suggested that the
above drugs inhibited the growth of SUNE1 cells.
To further examine the effect of NVP-BEZ235 and
rapamycin on SUNE1 cellular apoptosis, TUNEL and flow cytometry
assays were performed. As presented in Fig. 2A, very few apoptotic cells were
observed in the control group. However, compared with the control
group, the number of TUNEL-positive cells in the NVP-BEZ235 or
rapamycin groups was markedly increased, and the number of
apoptotic cells in NVP-BEZ235+rapamycin was the greatest. To
further validate the results, flow cytometry was performed. As
presented in Fig. 2B and C, the
trend in the number of early apoptotic cells (the fourth quadrant)
and the late apoptotic cells (the second quadrant) was as follows:
NVP-BEZ235+rapamycin group > rapamycin group > NVP-BEZ235
group > control group. These data were in accordance with the
TUNEL assay results. These results suggested that NVP-BEZ235 and
rapamycin induced SUNE1 cellular apoptosis, and the combination
enhanced this promoting effect.
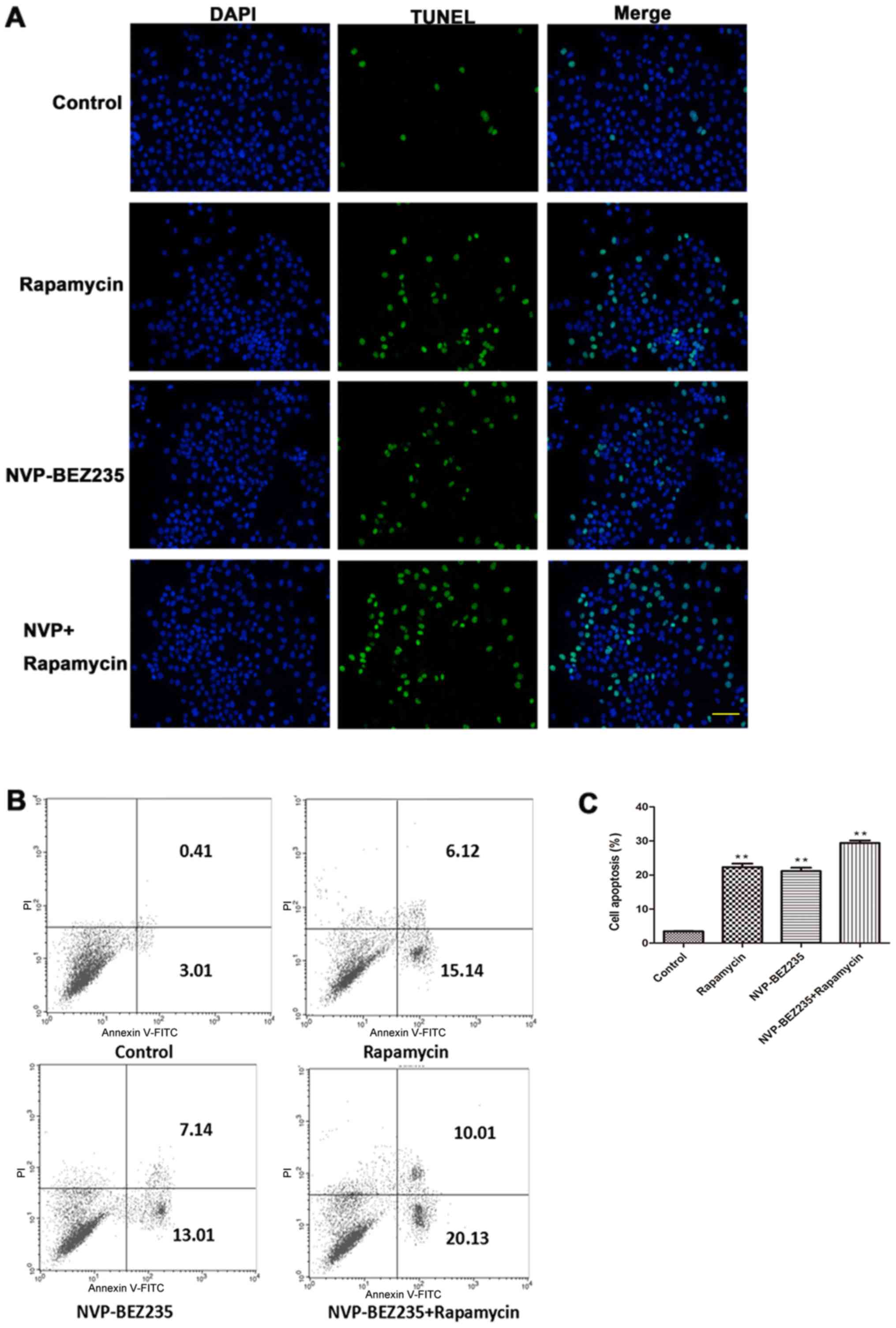 | Figure 2.NVP-BEZ235 and rapamycin promoted
cellular apoptosis. SUNE1 cells were cultured with 100 nM
NVP-BEZ235, 100 nM rapamycin, or 100 nM NVP-BEZ235 and 100 nM
rapamycin. The control group was administered the same amount of
vehicle. Following 48 h, cells were collected for further
experiments. (A) NVP-BEZ235 and rapamycin induced cellular
apoptosis, as detected by TUNEL. TUNEL-positive cells were marked
green, and nuclei were stained with DAPI in blue. Scale bar, 100
µm. (B) Cellular apoptosis was detected via flow cytometry. The
fourth and second quadrants indicated the early apoptotic cells and
the late apoptotic cells, respectively. (C) The percentage of
apoptotic cells from different groups is presented. Few apoptotic
cells were observed in the control group. The addition of
NVP-BEZ235 and rapamycin increased the number of apoptotic cells,
and NVP-BEZ235+rapamycin enhanced the effects. **P<0.01 vs.
control. TUNEL, terminal deoxynucleotidyl-transferase-mediated dUTP
nick end labeling; NVP, NVP-BEZ235. |
Rapamycin and NVP-BEZ235 suppress cell
viability
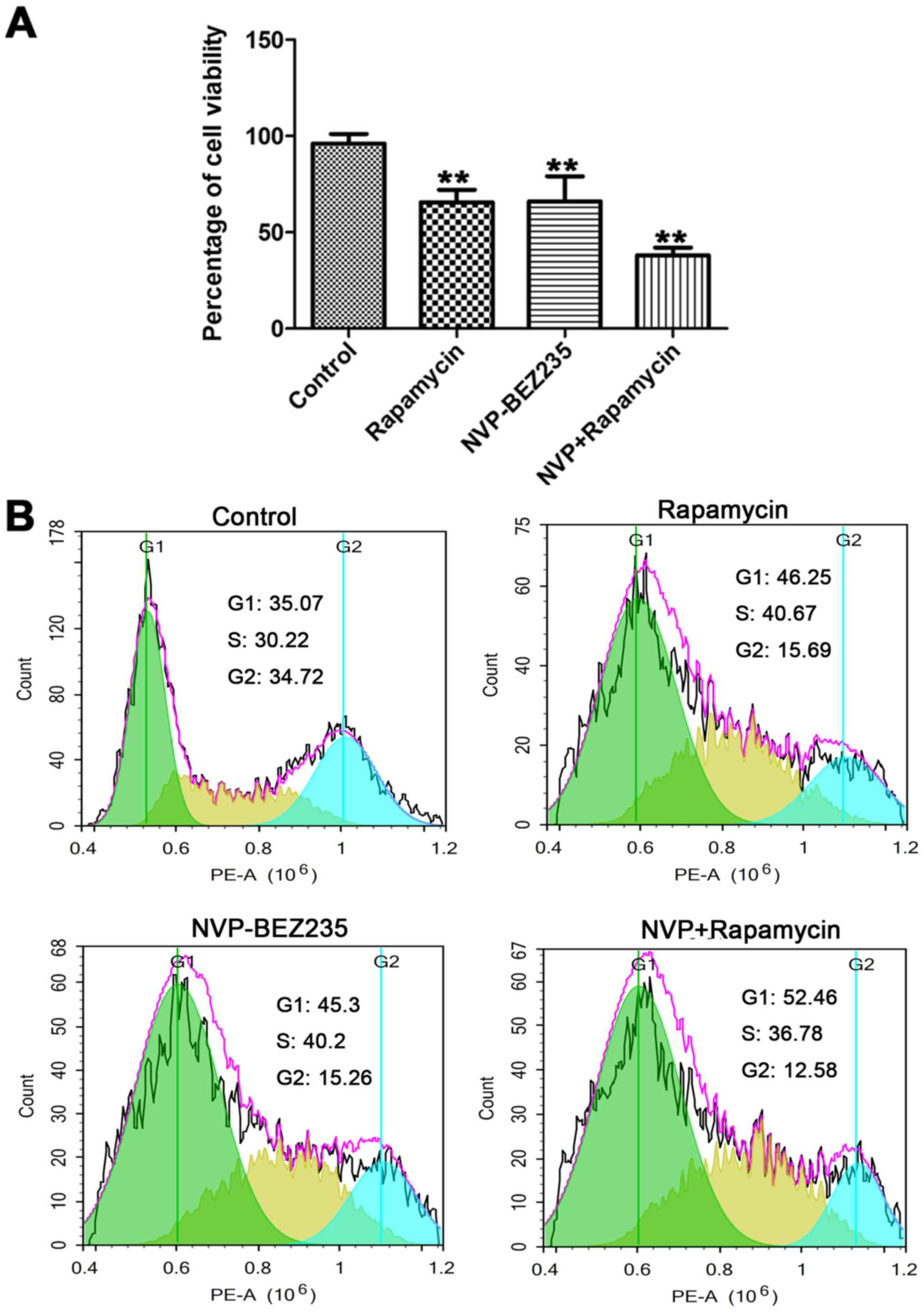
To further explore the role of rapamycin and
NVP-BEZ235 on cell viability, MTT and cell cycle distribution
assays were performed following the different drugs treatments
(Fig. 3). As presented in Fig. 3A, compared with the control group,
the cell viability in the NVP-BEZ235 group (66.76±2.16%) and the
rapamycin group (63.92±2.52%) was significantly decreased compared
with the control group (P<0.01). In addition,
NVP-BEZ235+rapamycin cell survival (38.15±1.94%) was markedly
decreased compared with NVP-BEZ235 or rapamycin alone. In Fig. 3B, compared with the control group,
the addition of NVP-BEZ235 or rapamycin markedly enhanced the
percentage of cells at the G1 phase. The percentage of cells at G1
phage was greatest in the NVP-BEZ235+rapamycin group. These data
indicated that NVP-BEZ235 and rapamycin arrested the cell cycle at
the G1 phase, and that the drug combination heightened the
intensity of the effect.
Rapamycin and NVP-BEZ235 suppress the
activation of PI3K/AKT/mTOR pathway
To explore the mechanism by which NVP-BEZ235 and
rapamycin affect cellular apoptosis and viability, the expression
of PI3K, AKT and mTOR were evaluated (Fig. 4). As presented in Fig. 4A-C, compared with the control group,
NVP-BEZ235 significantly suppressed the mRNA levels of PI3K, AKT
and mTOR (P<0.01); rapamycin only significantly decreased the
expression of mTOR (P<0.01), but had no significant effect on
the mRNA levels of PI3K and AKT (P>0.05). The combination of
NVP-BEZ235 and rapamycin exerted more marked inhibition on the mRNA
levels of PI3K, AKT and mTOR compared with the control group
(P<0.01). Similarly, the protein levels of PI3K, AKT, p-AKT,
mTOR and p-mTOR were detected following the different drugs
treatments. As presented in Fig.
4D-K, it was demonstrated that rapamycin suppressed the protein
level of p-AKT, mTOR, p-mTOR, p-AKT/AKT, and p-mTOR/mTOR compared
with the control group (P<0.01), but had no significant effect
on the expression of AKT and PI3K (P>0.05). However, NVP-BEZ235
alone significantly repressed the protein levels of PI3K, AKT,
p-AKT, mTOR, p-mTOR, p-AKT/AKT and p-mTOR/mTOR compared with the
control group (P<0.01). NVP-BEZ235+rapamycin induced more marked
inhibition of PI3K, AKT, p-AKT, mTOR, p-mTOR, p-AKT/AKT and
p-mTOR/mTOR. Together, these findings suggested that rapamycin and
NVP-BEZ235 inhibited the activation of the PI3K/AKT/mTOR pathway,
and the combination of the two drugs promoted the inhibition.
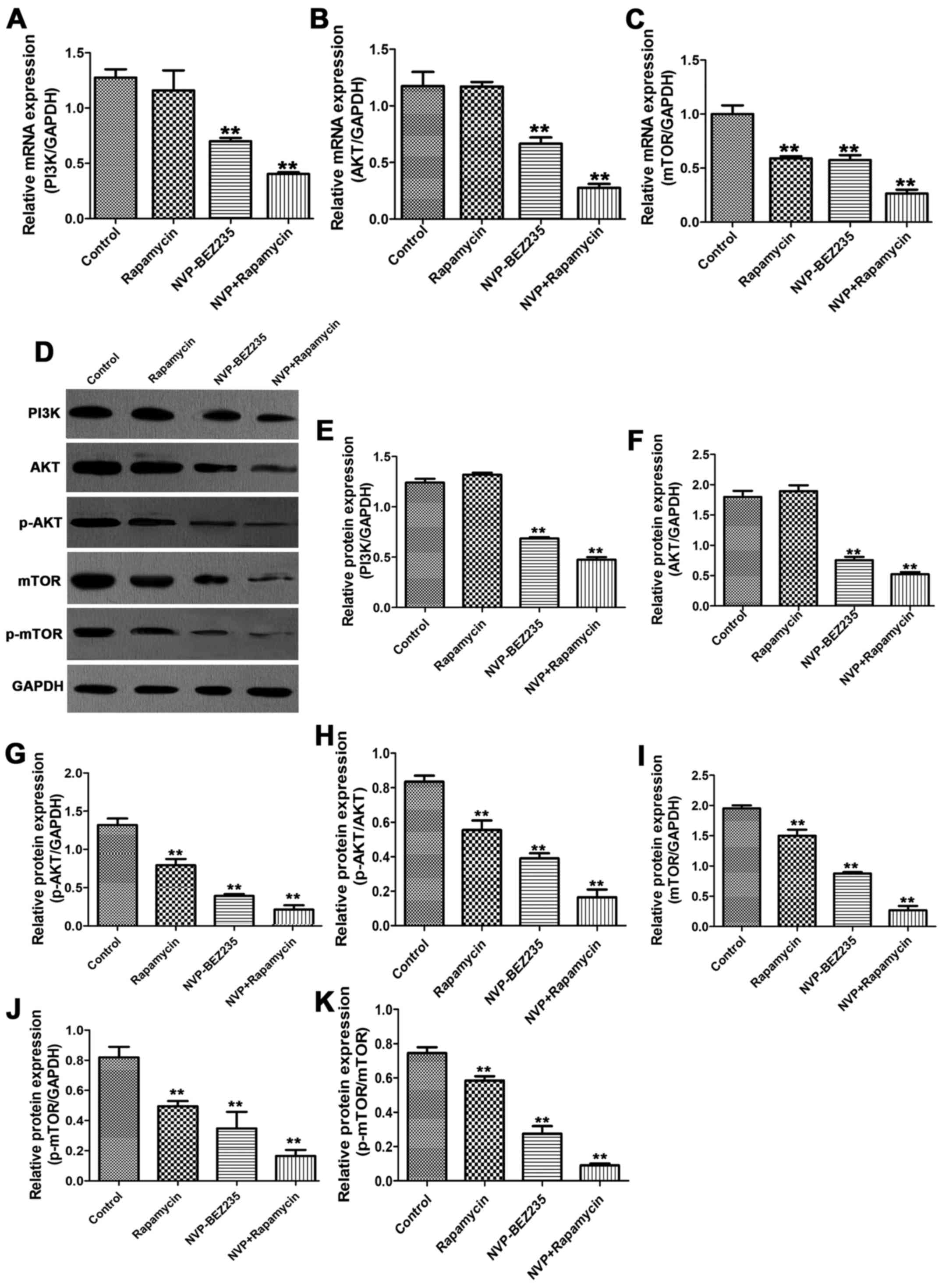 | Figure 4.NVP-BEZ235 and rapamycin repressed the
PI3K/AKT/mTOR pathway. SUNE1 cells were treated with 100 nM
NVP-BEZ235, 100 nM rapamycin, or 100 nM NVP-BEZ235 and 100 nM
rapamycin for 48 h. The control group was administered the same
amount of vehicle. Total RNA from the four groups was isolated for
reverse transcription-quantitative polymerase chain reaction. The
mRNA levels of (A) PI3K, (B) AKT and (C) mTOR were normalized to
the internal control GAPDH. (D) The protein levels of PI3K, AKT,
p-AKT, mTOR and p-mTOR were detected by western blotting. (E-K) The
protein density of PI3K, AKT, p-AKT, mTOR and p-mTOR was calculated
relative to the internal control GAPDH, and the p-AKT/AKT and
p-mTOR/mTOR ratios were calculated. **P<0.01 vs. control. PI3K,
phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian
target of rapamycin; p, phosphorylated; NVP, NVP-BEZ235. |
Discussion
Despite the progress that has been made in
researching NPC, the molecular mechanism underlying NPC remains
elusive. A previous study demonstrated that NVP-BEZ235
significantly inhibited cell proliferation in NPC via inhibition of
the PI3K/AKT/mTOR pathway (17).
However, the outcomes for NVP-BEZ235 in phase I/II clinical trials
in advanced patients have been unsatisfactory (13). In the present study, the role of the
combination of NVP-BEZ235 and rapamycin on NPC cellular apoptosis,
cell viability, and PI3K/AKT/mTOR signaling was investigated, which
might provide a novel drug for NPC therapy. The findings suggested
that the combination of NVP-BEZ235 and rapamycin significantly
induced cellular apoptosis, blocked cell viability and inhibited
the activation of PI3K/AKT/mTOR pathway.
The PI3K/AKT/mTOR signaling pathway is associated
with various biological events, including proliferation and
apoptosis (18), and provides a good
target for cancer treatment (19).
NVP-BEZ235 is a synthetic low molecular mass compound and blocks
PI3K catalytic activity by competing at its ATP-binding site
(10), and represses the catalytic
activity of mTOR (20). It has been
revealed that AKT acts as a key regulator in PI3K/AKT/mTOR
signaling, regulates cell proliferation by monitoring the
expression of the cell cycle proteins c-myc and cyclin D1, and
mediates cell survival by regulating a cascade of pro-apoptotic and
anti-apoptotic proteins (21).
However, the application of NVP-BEZ235 significantly suppresses the
activation of AKT, S6 ribosomal protein and 4EBP1 in breast cancer
(19). In the present study, it was
demonstrated that the administration of NVP-BEZ235 significantly
promoted cellular apoptosis, repressed cell viability, and
modulated the expression of PI3K, AKT, p-AKT, mTOR, p-mTOR,
p-AKT/AKT and p-mTOR/mTOR in SUNE1 cells. These data revealed that
NVP-BEZ235 regulated NPC development via regulation of the
PI3K/AKT/mTOR signaling pathway, which was in accordance with a
previous study (9).
mTOR is a serine/threonine kinase which is
associated with the PI3K/AKT/mTOR pathway (22). A number of studies have indicated
that mTOR regulates the synthesis of key proteins for cell growth
and proliferation (23–25). Rapamycin, as an inhibitor of mTOR,
significantly inhibits NPC cell proliferation in vitro and
the formation of tumors in vivo (26). In the present study, treatment with
rapamycin promoted cellular apoptosis, suppressed cell viability
and blocked the expression of p-AKT, mTOR, p-mTOR, p-AKT/AKT and
p-mTOR/mTOR, but had no effect on the mRNA and protein levels of
PI3K and AKT. These data indicated that rapamycin participated in
the inhibition of NPC development by repressing the expression of
mTOR, which supported previous findings (1).
Although previous studies demonstrated that
NVP-BEZ235 and rapamycin functioned as therapies for NPC (2,17), the
outcome of the single drugs was unsatisfactory. The aim of the
present study was to identify a more effective drug for NPC
therapy, thus the effect of NVP-BEZ235+rapamycin was detected on
SUNE1 cellular apoptosis and viability. In the present study, it
was demonstrated that the combination of NVP-BEZ235 and rapamycin
more markedly promoted cellular apoptosis and repressed cell
viability than either single drug treatment (NVP-BEZ235 or
rapamycin alone). Furthermore, the administration of
NVP-BEZ235+rapamycin reduced the expression of PI3K, AKT, p-AKT,
p-mTOR, p-mTOR, p-AKT/AKT and p-mTOR/mTOR. Thus, it was concluded
that the combination of NVP-BEZ235 and rapamycin modulated cell
viability and apoptosis via the PI3K/AKT/mTOR pathway in SUNE1
cells; and that the outcome with the two drugs was more positive
than with either single drug. These results provided insight for
exploring novel drug therapies for NPC.
However, there were certain limitations in the
present study. First, the effect of NVP-BEZ235+rapamycin was
evaluated in SUNE1 cells with a lack of in vivo animal
experiments. Therefore, authors may perform further studies on the
effect of NPV-BEZ235+rapamycin on animals in vivo, which may
provide important findings for a future clinical trial. Secondly,
although it was demonstrated that NVP-BEZ235+rapamycin modulated
cell viability and apoptosis via the PI3K/AKT/mTOR pathway, the
molecular mechanism by which NPV-BEZ235+rapamycin regulated the
PI3K/AKT/mTOR pathway remains elusive. Further investigations are
required to elucidate this mechanism.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jiangxi Science
and Technology Department Supoort Project (grant no.
20151BBG70233).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HLu and Y-YY analyzed the data and were major
contributors in writing the manuscript. H-MC cultured the cells,
performed the cell apoptosis and cell cycle distribution detection
via flow cytometry, and prepared Figs.
1, 2 and 3. WW and YL performed the RNA and protein
extraction for reverse transcription-quantitative polymerase chain
reaction and western blot analyses, respectively, and prepared
Fig. 4. HLi directed the study and
revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
AKT
|
protein kinase B
|
|
mTOR
|
mammalian target of rapamycin
|
|
FACS
|
fluorescence-activated cell
sorting
|
References
|
1
|
Yang C, Zhang Y, Zhang Y, Zhang Z, Peng J,
Li Z, Han L, You Q, Chen X, Rao X, et al: Downregulation of cancer
stem cell properties via mTOR signaling pathway inhibition by
rapamycin in nasopharyngeal carcinoma. Int J Oncol. 47:909–917.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shueng PW, Shen BJ, Wu LJ, Liao LJ, Hsiao
CH, Lin YC, Cheng PW, Lo WC, Jen YM and Hsieh CH: Concurrent
image-guided intensity modulated radiotherapy and chemotherapy
following neoadjuvant chemotherapy for locally advanced
nasopharyngeal carcinoma. Radiat Oncol. 6:952011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee AW, Ng WT, Chan YH, Sze H, Chan C and
Lam TH: The battle against nasopharyngeal cancer. Radiother Oncol.
104:272–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stoker SD, van Diessen JN, de Boer JP,
Karakullukcu B, Leemans CR and Tan IB: Current treatment options
for local residual nasopharyngeal carcinoma. Curr Treat Options
Oncol. 14:475–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YH, Wei MF, Wang CW, Lee HW, Pan SL,
Gao M, Kuo SH, Cheng AL and Teng CM: Dual phosphoinositide
3-kinase/mammalian target of rapamycin inhibitor is an effective
radiosensitizer for colorectal cancer. Cancer Lett. 357:582–590.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li JR, Cheng CL, Yang CR, Ou YC, Wu MJ and
Ko JL: Dual inhibitor of phosphoinositide 3-kinase/mammalian target
of rapamycin NVP-BEZ235 effectively inhibits cisplatin-resistant
urothelial cancer cell growth through autophagic flux. Toxicol
Lett. 220:267–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong SW, Shin JS, Moon JH, Kim YS, Lee J,
Choi EK, Ha SH, Lee DH, Chung HN, Kim JE, et al: NVP-BEZ235, a dual
PI3K/mTOR inhibitor, induces cell death through alternate routes in
prostate cancer cells depending on the PTEN genotype. Apoptosis.
19:895–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maira SM, Stauffer F, Brueggen J, Furet P,
Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker
K, et al: Identification and characterization of NVP-BEZ235, a new
orally available dual phosphatidylinositol 3-kinase/mammalian
target of rapamycin inhibitor with potent in vivo antitumor
activity. Mol Cancer Ther. 7:1851–1863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muranen T, Selfors LM, Worster DT,
Iwanicki MP, Song L, Morales FC, Gao S, Mills GB and Brugge JS:
Inhibition of PI3K/mTOR leads to adaptive resistance in
matrix-attached cancer cells. Cancer Cell. 21:227–239. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dubrovska A, Kim S, Salamone RJ, Walker
JR, Maira SM, García-Echeverría C, Schultz PG and Reddy VA: The
role of PTEN/Akt/PI3K signaling in the maintenance and viability of
prostate cancer stem-like cell populations. Proc Natl Acad Sci USA.
106:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian XJ, Li YT, Yu Y, Yang F, Deng R, Ji
J, Jiao L, Li X, Wu RY, Chen WD, et al: Inhibition of DNA
methyltransferase as a novel therapeutic strategy to overcome
acquired resistance to dual PI3K/mTOR inhibitors. Oncotarget.
6:5134–5146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishikawa C, Senba M and Mori N: Effects of
NVP-BEZ235, a dual phosphatidylinositol 3-kinase/mammalian target
of rapamycin inhibitor, on HTLV-1-infected T-cell lines. Oncol
Lett. 15:5311–5317. 2018.PubMed/NCBI
|
|
15
|
Wang D, Gao L, Liu X, Yuan C and Wang G:
Improved antitumor effect of ionizing radiation in combination with
rapamycin for treating nasopharyngeal carcinoma. Oncol Lett.
14:1105–1108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma BB, Lui VW, Hui CW, Lau CP, Wong CH,
Hui EP, Ng MH, Cheng SH, Tsao SW, Tsang CM, et al: Preclinical
evaluation of the mTOR-PI3K inhibitor BEZ235 in nasopharyngeal
cancer models. Cancer Lett. 343:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serra V, Markman B, Scaltriti M, Eichhorn
PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S,
et al: NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K
signaling and inhibits the growth of cancer cells with activating
PI3K mutations. Cancer Res. 68:8022–8030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guertin DA, Stevens DM, Thoreen CC, Burds
AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ and Sabatini DM:
Ablation in mice of the mTORC components raptor, rictor, or mLST8
reveals that mTORC2 is required for signaling to Akt-FOXO and
PKCalpha, but not S6K1. Dev Cell. 11:859–871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ocana A, Vera-Badillo F, Al-Mubarak M,
Templeton AJ, Corrales-Sanchez V, Diez-Gonzalez L, Cuenca-Lopez MD,
Seruga B, Pandiella A and Amir E: Activation of the PI3K/mTOR/AKT
pathway and survival in solid tumors: Systematic review and
meta-analysis. PLoS One. 9:e952192014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benjamin D, Colombi M, Moroni C and Hall
MN: Rapamycin passes the torch: A new generation of mTOR
inhibitors. Nat Rev Drug Discov. 10:868–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghayad SE, Bieche I, Vendrell JA, Keime C,
Lidereau R, Dumontet C and Cohen PA: mTOR inhibition reverses
acquired endocrine therapy resistance of breast cancer cells at the
cell proliferation and gene-expression levels. Cancer Sci.
99:1992–2003. 2008.PubMed/NCBI
|
|
24
|
Ma Y, Vassetzky Y and Dokudovskaya S:
mTORC1 pathway in DNA damage response. Biochim Biophys Acta.
1865:1293–1311. 2018. View Article : Google Scholar
|
|
25
|
Balaji S, Ahmed M, Lorence E, Yan F, Nomie
K and Wang M: NF-kappaB signaling and its relevance to the
treatment of mantle cell lymphoma. J Hematol Oncol. 11:832018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang C, Peng J, Jiang W, Zhang Y, Chen X,
Wu X, Zhu Y, Zhang H, Chen J, Wang J, et al: mTOR activation in
immature cells of primary nasopharyngeal carcinoma and anti-tumor
effect of rapamycin in vitro and in vivo. Cancer Lett. 341:186–194.
2013. View Article : Google Scholar : PubMed/NCBI
|