Introduction
Metabolic syndrome is a clustering of medical
conditions that increases the risk of type 2 diabetes mellitus and
cardiovascular disease (1). In the
United States, the prevalence of the metabolic syndrome is >20%
and increases with aging (2). With
changes in people's diet structure and increased incidence of
obesity, the incidence of metabolic syndrome is predicted to be
significantly increased in the near future (3). Patients with metabolic syndrome usually
present atherogenic dyslipidemia, visceral adiposity, insulin
resistance, elevated blood pressure, endothelial dysfunction,
genetic susceptibility and chronic stress. Among these, insulin
resistance is the major cause of the development of type 2 diabetes
mellitus in these patients (4),
while chronic inflammation, which is characterized by abnormal
production of certain cytokines, such as interleukin-1 (IL-1) and
tumor necrosis factor α (TNF-α), is closely correlated with the
development of insulin resistance (5). Therefore, improving chronic
inflammation will benefit the recovery of metabolic syndrome.
Exercise therapy is a treatment strategy to improve
certain medical conditions through physical activity. Exercise
therapy has displayed promising therapeutic effects in the
treatment of various human diseases, including stroke (6), chronic fatigue syndrome (7) and chronic low back pain (8). In addition, exercise has also been used
as an anti-inflammatory therapy in the treatment of certain
diseases, such as rheumatic diseases (9), indicating its potential in improving
insulin resistance in patients with metabolic syndrome. A recent
study has revealed that diet structure management and exercise
therapy can improve certain aspects of metabolic syndrome,
including the endothelial function (10). However, the effects of exercise
therapy on chronic inflammation and insulin resistance in patients
with metabolic syndrome have not been well studied to date.
In the present study, patients with metabolic
syndrome were treated with swimming intervention, and the effects
of this therapy on insulin resistance and key inflammatory factors
were investigated. Furthermore, the effects of swimming
intervention on key insulin signal transduction pathways were
explored.
Patients and methods
Patients
A total of 100 patients with metabolic syndrome
admitted between January 2015 and January 2017 at the University
Hospital of Chuzhou University (Chuzhou, China) were selected. All
patients were diagnosed according to the criteria established by a
previous study (11). Patients who
met three of the following four criteria were diagnosed with
metabolic syndrome: i) Overweight and/or obese, with a body mass
index of ≥25; ii) had a fasting plasma glucose of ≥6.1 mmol/l (110
mg/dl) and/or 2-h plasma glucose of ≥7.8 mmol/l (140 mg/dl), and/or
had been diagnosed with diabetes and treated; iii) had
systolic/diastolic blood pressure of ≥140/90 mmHg, and/or had been
diagnosed with hypertension and treated; and iv) had a fasting
triglyceride level of ≥1.7 mmol/l (150 mg/dl), and/or a
high-density lipoprotein cholesterol level of <0.9 mmol/l (35
mg/dl) for males and <1.0 mmol/l (39 mg/dl) for females. The
enrolled patients included 44 males and 56 females, and their age
ranged between 22 and 76 years, with a mean age of 46±19.2 years.
In addition, 100 normal healthy individuals were also selected to
serve as the control group, including 49 males and 51 females with
an age ranging between 25 and 73 years, and a mean age of 43±17.8
years. No significant differences in terms of the age and sex were
detected between the patient and control groups. The homeostatic
model assessment of β-cell function and insulin resistance
(HOMA-IR) in serum was calculated using fasting insulin (FINS) and
fasting blood glucose (FBG) with the following formula:
HOMA-IR=FINS/(22.5×10−FBG) (12). HOMA-IR in normal adult is generally
<2.7. A higher HOMA-IR indicates that a patient possesses a
metabolic disorder.
Patients were randomly divided into five groups,
including groups A-E (n=20 per group). Patients in group A were
treated with conventional drug treatment using metformin (250 mg
per time, twice per day) and insulin sensitizers
(thiazolidinediones; 20 mg, once per day). Besides conventional
treatment, patients in group B-E were also subjected to swimming
intervention for 15, 30, 45 and 60 min each time, respectively,
four times a week for 3 months. This study was approved by the
Ethics Committee of Chuzhou University, and all patients signed
informed consent forms prior to participation.
Serum and tissue collection and
processing
Fasting blood (10 ml) was extracted from each
participant in the morning of the day before and at 3 months after
swimming intervention. Blood samples were kept at room temperature
for 1 h, followed by centrifugation at 1,200 × g for 10 min at room
temperature to collect the serum. Serum samples were stored at
−80°C prior to use. Patients were asked to rest for 3 h, and muscle
biopsies (150–200 mg) were obtained from the vastus lateralis
muscle under local anesthesia using a modified Bergstrom needle
following treatment.
Measurement of serum levels of high
sensitivity C-reactive protein (hs-CRP), TNF-a, IL-1 and IL-8
The serum levels of IL-1 (cat. no. DLB50), hs-CRP
(cat. no. DCRP00; both R&D Systems, Inc., Minneapolis, MN,
USA), TNF-α (cat. no. RPN5967; GE Healthcare, Chicago, IL, USA) and
IL-8 (cat. no. D8000C; R&D Systems, Inc.) were measured by
sandwich enzyme-linked immunosorbent assay (ELISA) using
corresponding ELISA kit. Serum samples were diluted to 1:10 in
dilution buffer and then the 100-µl mixture was transferred to the
ELISA plate (GE Healthcare). Next, the assay was performed
according to the manufacturer's protocol.
Western blot analysis
A radioimmunoprecipitation assay solution (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to extract
total protein from the muscle tissues obtained from three patients
each from the healthy control, group A and group E (13). A plasma membrane protein extraction
kit (cat. no. ab65400; Abcam, Cambridge, MA, USA) was used to
extract the plasma membrane protein, and protein samples were
quantified by a bicinchoninic acid assay. Next, 20 µg protein from
each sample was subjected to 10% SDS-PAGE to separate proteins with
different molecular weights, followed by transfer to a
polyvinylidene difluoride membrane. Membranes were then blocked
with 5% skimmed milk at room temperature for 2 h. Subsequent to
washing using TBST membrane (0.3% Tween), The membranes was with
TBS with 0.3% Tween and then incubated overnight at 4°C with the
corresponding primary antibodies, including rabbit anti-insulin
receptor substrate-1 (IRS1; 1:2,000; cat. no. ab52167), anti-p-IRS1
(phospho-Y896; 1:2,000; cat. no. ab4873), anti-glucose transporter
type 4 (GLUT4; 1:2,000; cat. no. ab33780), anti-p-protein kinase B
(Akt; 1:2,000; cat. no. ab18206), anti-Akt (1:2,000; cat. no.
ab126811) and rabbit anti-β-actin (1:1,000; cat. no. ab8226; all
purchased from Abcam) antibodies. Subsequent to washing, membranes
were further incubated with anti-rabbit horseradish
peroxidase-conjugated IgG secondary antibody (1:1,000; cat. no.
MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room
temperature for 4 h. Following further washing, the enhanced
chemiluminescence method (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was applied to detect the signals. Image J software was
used to normalize the relative expression level of each protein to
that of the endogenous control β-actin.
Statistical analysis
IBM SPSS software (version 19.0; IBM Corp., Armonk,
NY, USA) was used for statistical analysis of the data. Normal
distribution data were expressed as the mean ± standard deviation.
A Student's t-test was used for comparisons between two groups of
normally distributed data, while analysis of variance and least
significant difference tests were performed for comparisons among
multiple groups. P<0.05 was considered to denote a statistically
significant difference.
Results
Comparison of insulin resistance
between patients with metabolic syndrome and the control group
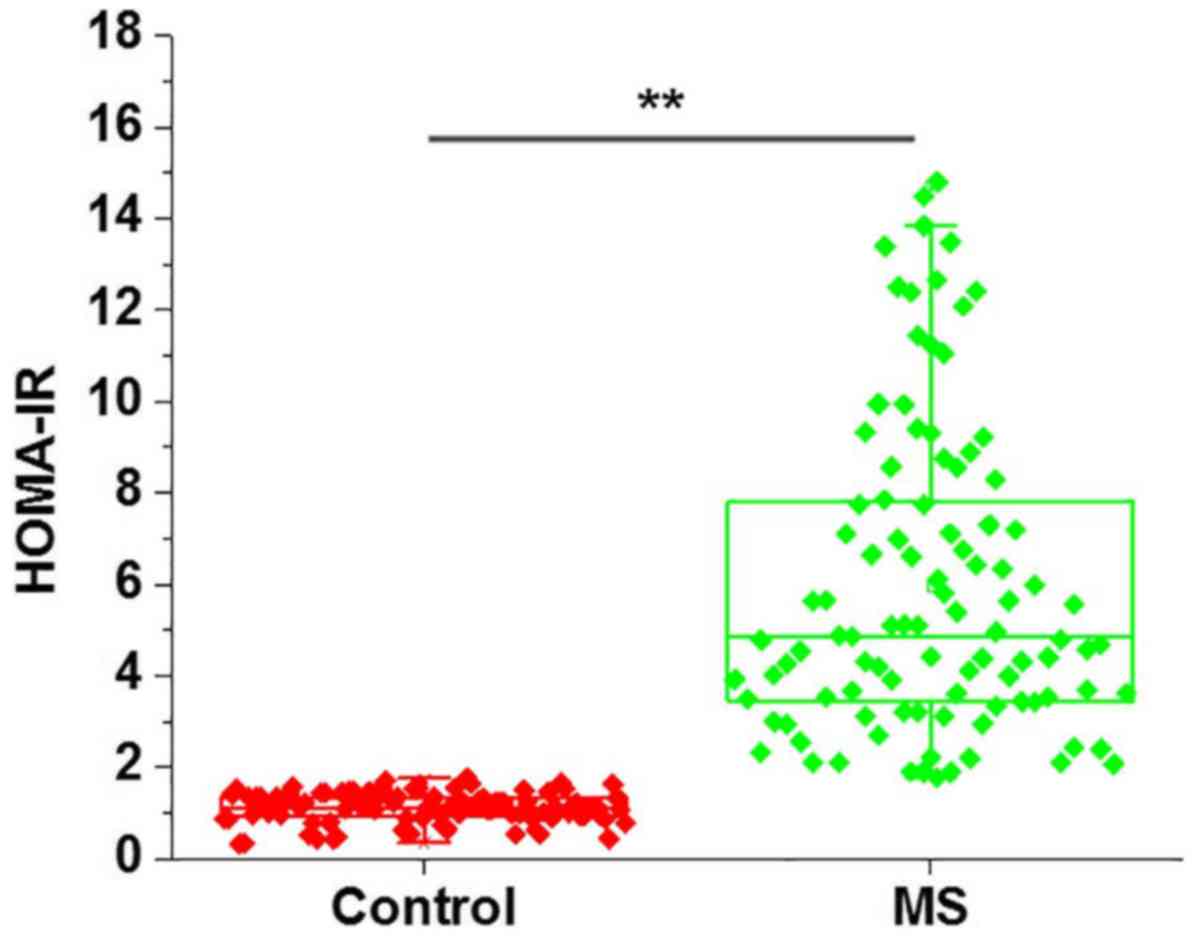
As shown in Fig. 1,
HOMA-IR score was significantly higher in patients with metabolic
syndrome in comparison with that in the control group (P<0.01),
indicating the existence of insulin resistance in metabolic
syndrome patients.
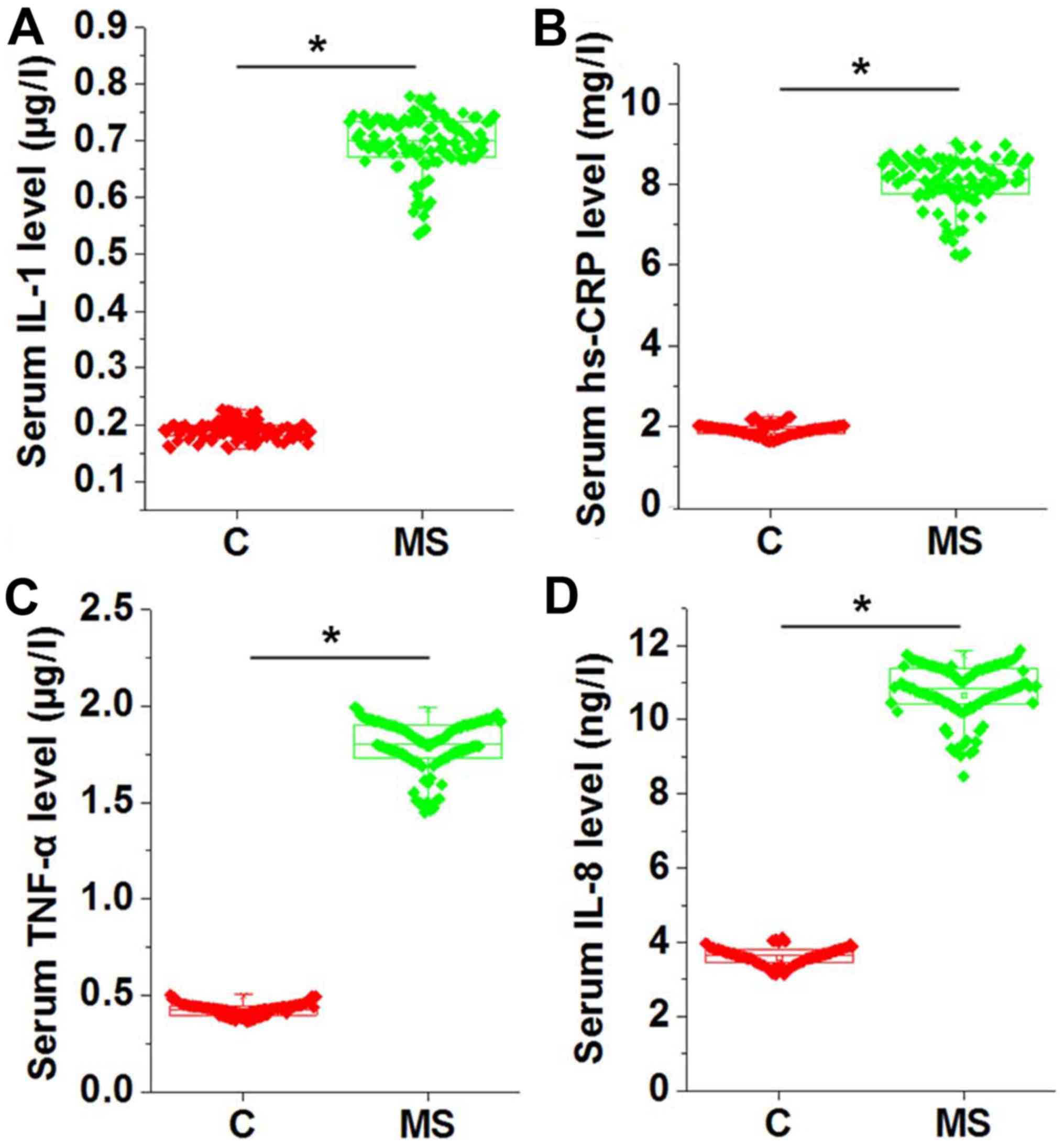
Comparison of serum levels of IL-1,
hs-CRP, TNF-α and IL-8 between patients with metabolic syndrome and
the control group
Chronic inflammation is common in patients with
metabolic syndrome. Therefore, the levels of several serum
inflammatory factors, including IL-1, hs-CRP, TNF-α and IL-8, were
measured and compared between the two groups. As shown in Fig. 2, the serum levels of IL-1 (Fig. 2A), hs-CRP (Fig. 2B), TNF-α (Fig. 2C) and IL-8 (Fig. 2D) were significantly higher in
patients with metabolic syndrome as compared with those in the
normal controls (P<0.05), indicating the presence of
inflammatory response in those patients.
Effects of swimming intervention on
HOMA-IR and serum IL-1, hs-CRP, TNF-α and IL-8 levels in patients
with metabolic syndrome
The 100 patients were randomly divided into groups
A-E, and no statistically significant differences were identified
in HOMA-IR score and the serum levels of IL-1, hs-CRP, TNF-α and
IL-8 among the five groups prior to swimming intervention.
Following swimming intervention for 3 months, the decrease in
HOMA-IR increased with the increase in the swimming time per
session; however, no significant differences were detected among
groups A-D (Fig. 3A). As compared
with group A, which did not receive swimming intervention, the
decrease in HOMA-IR was significantly higher in group E, which was
subjected to 60 min of swimming intervention at each session
(P<0.05; Fig. 3A). Similarly,
compared with group A, the decrease in the serum levels of IL-1
(Fig. 3B), hs-CRP (Fig. 3C), TNF-α (Fig. 3D) and IL-8 (Fig. 3E) were also higher in groups B-E.
Decreases in serum levels of IL-1 (Fig.
3B), hs-CRP (Fig. 3C), TNF-α
(Fig. 3D) and IL-8 (Fig. 3E) increased continually in groups
B-E.
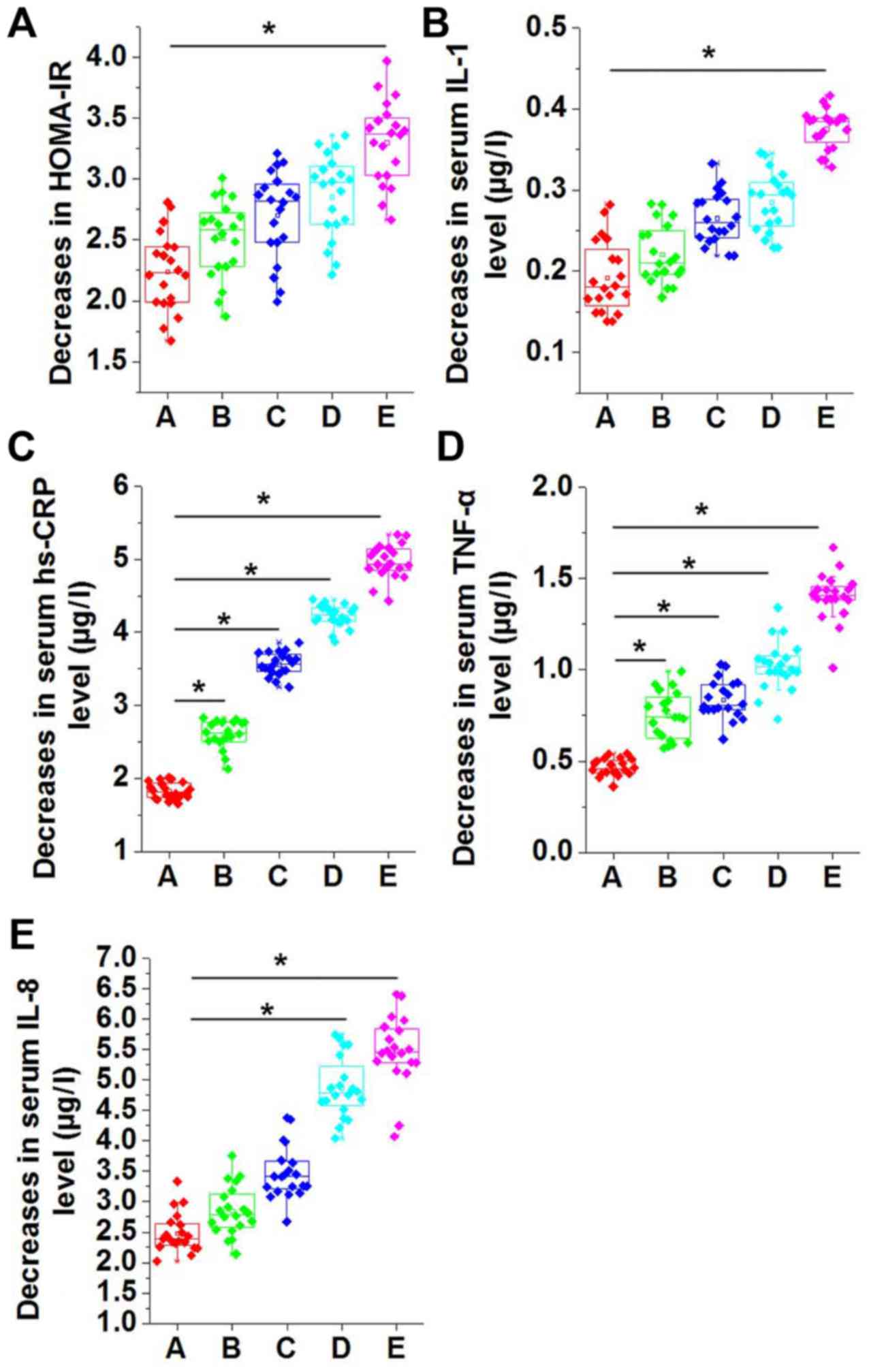 | Figure 3.Comparison of decreases in (A)
HOMA-IR, and serum levels of (B) IL-1, (C) hs-CRP, (D) TNF-α and
(E) IL-8 in different groups of patients with MS. The decrease in
HOMA-IR score increased along with the increase in swimming time
per session. No significant differences in HOMA-IR were detected
among groups A-D, while the score significantly increased in group
E compared with group A. Decreases in serum levels of IL-1, hs-CRP,
TNF-α and IL-8 were significantly higher in groups B-E compared
with group A. *P<0.05. HOMA-IR, homeostatic model assessment of
β-cell function and insulin resistance; IL, interleukin; hs-CRP,
high sensitivity C-reactive protein; TNF-α, tumor necrosis factor
α; group A, metabolic syndrome with no swimming intervention;
groups B-E, swimming intervention for 15, 30, 45 and 60 min per
session, respectively. |
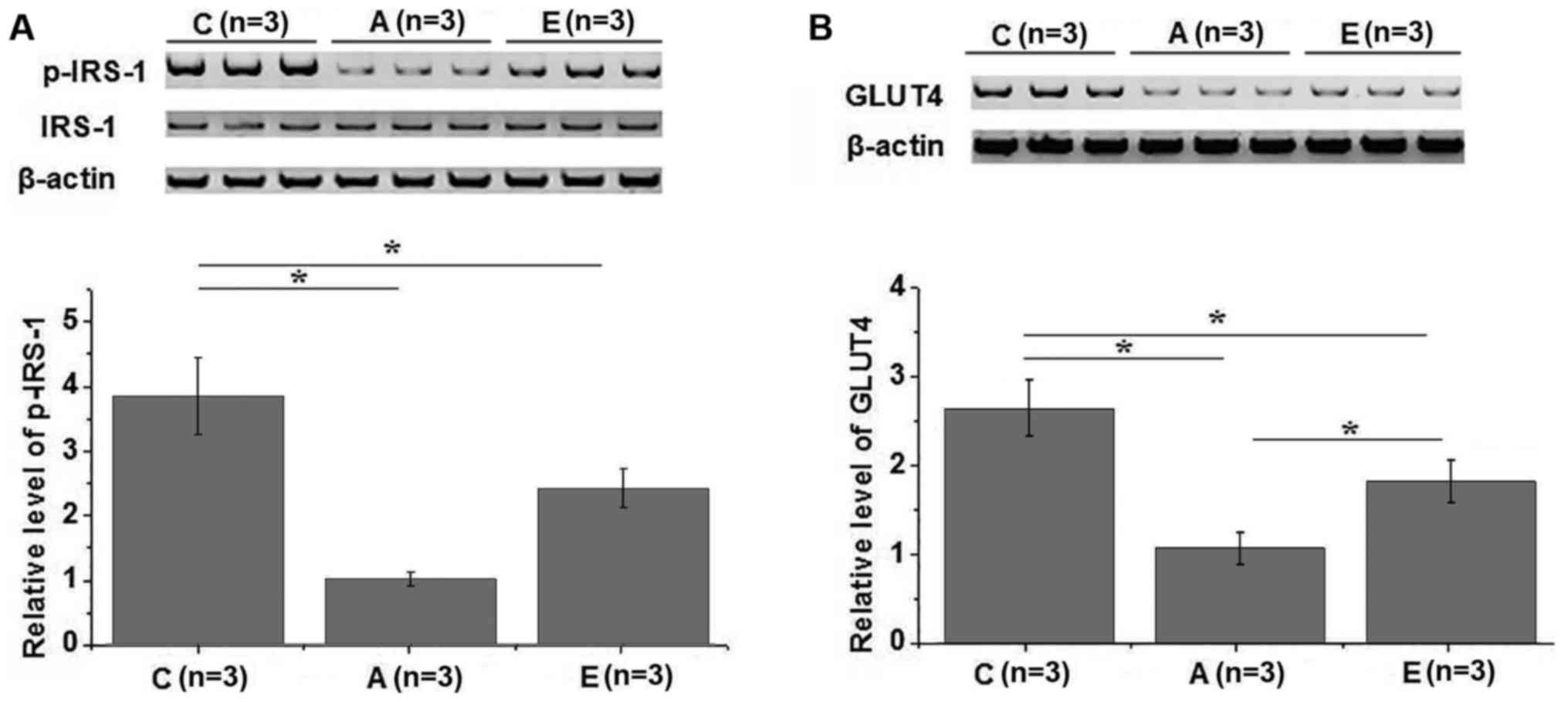
Effects of swimming intervention on
IRS-1 phosphorylation and GLUT4 expression
Western blot analysis was performed to investigate
the effects of swimming intervention on IRS-1 and GLUT4. In total,
3 healthy controls, 3 group A patients and 3 group E patients were
included in this experiment. Compared with the healthy controls, no
significant changes in the expression level of IRS-1 were observed
in groups A and E (Fig. 4A).
However, the phosphorylation level of IRS-1 was significantly lower
in groups A and E in comparison with that in the healthy control
group (P<0.05). In addition, compared with group A, the
phosphorylation level of IRS-1 was significantly higher in group E
(P<0.05; Fig. 4A). Similarly, the
expression level of GLUT4 in the plasma membrane was significantly
lower in groups A and E as compared with that in the control group
(P<0.05), while it was significantly higher in group E compared
with group A (Fig. 4B). IRS-1 and
GLUT4 serve pivotal roles in insulin signal transduction (14,15);
therefore, the aforementioned data suggest that swimming
intervention may promote IRS-1 phosphorylation and GLUT4 expression
to improve insulin resistance.
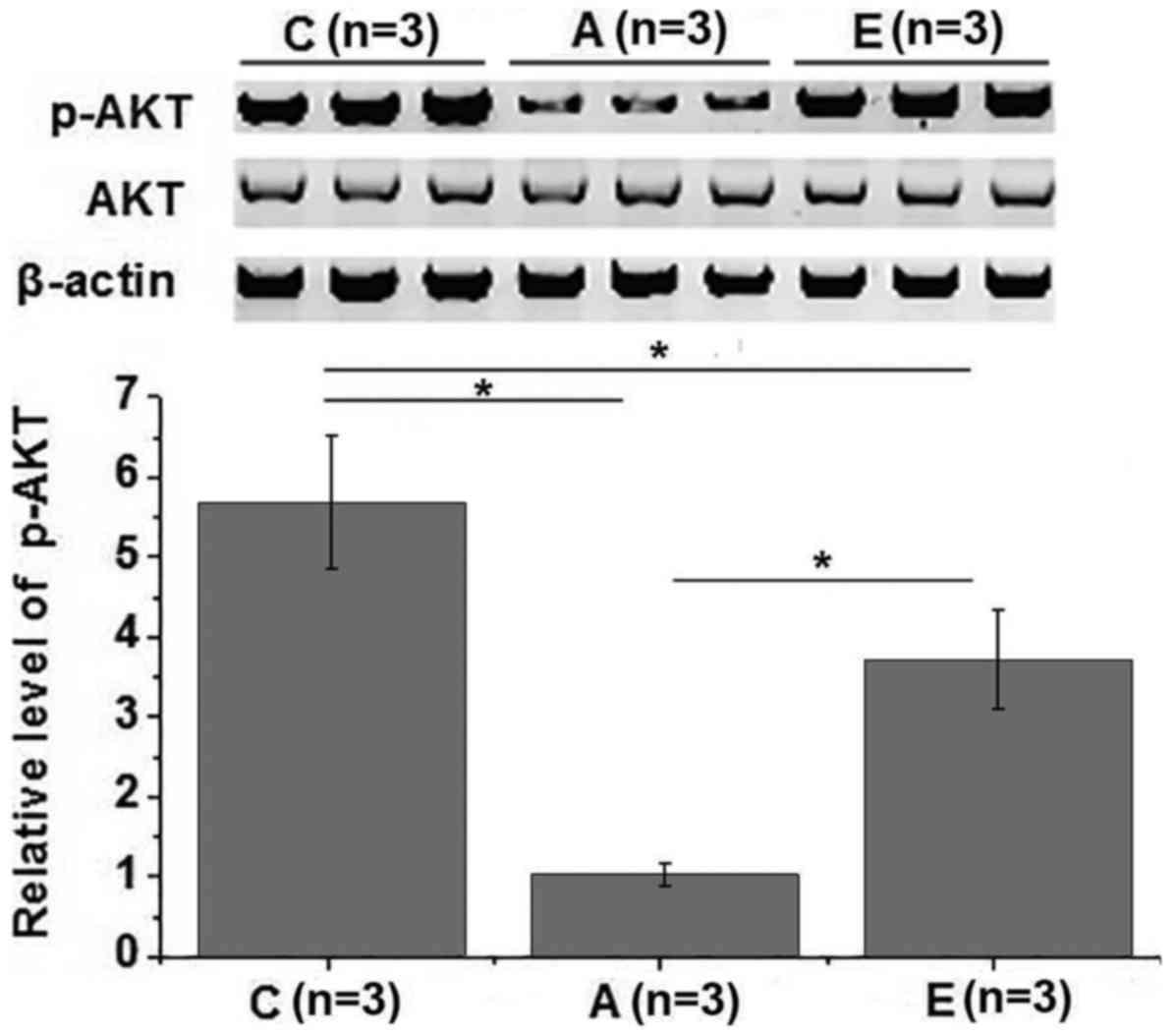
Effects of swimming intervention on
phosphoinositide 3-kinase (PI3K)/Akt pathway
The PI3K/Akt signaling pathway is also involved in
insulin signal transduction (16).
Therefore, the effects of swimming intervention on PI3K/Akt pathway
were also investigated in the present study. No significant
differences in the expression level of Akt were observed among
different groups. However, the phosphorylation level of Akt was
significantly lower in groups A and E as compared with that in the
control group (P<0.05). The phosphorylation level of Akt was
also significantly higher in group E in comparison with that in
group A (P<0.05; Fig. 5). These
data suggest that swimming intervention is able to improve insulin
resistance by activating the PI3K/Akt signaling pathway.
Discussion
In recent years, the prevalence of metabolic
syndrome has markedly increased in both developed and developing
countries (17). Insulin resistance,
as a common medical condition in metabolic syndrome, is closely
correlated with the development of type 2 diabetes mellitus
(1). Although the cutoff score of
HOMA-IR separating metabolic syndrome patients varies in different
regions, increased HOMA-IR scores compared with healthy people
usually indicate aggregated pathological conditions of metabolic
syndrome (18). Consistent with
previous studies, in the present study, HOMA-IR scores were
significantly higher in metabolic syndrome patients in comparison
with those in normal control individuals, indicating the existence
of insulin resistance in these patients. Chronic inflammation has
been proven to be a major cause of the development of insulin
resistance (5). A recent study has
demonstrated that, when insulin resistance occurs, the levels of
inflammation-associated factors, including osteopontin, monocyte
chemoattractant protein 1, fractalkine, TNF-α and IL-6, will be
significantly increased in the human body, leading to inflammatory
reactions (19). Similar results
were reported in the current study, which demonstrated that the
serum levels of the pro-inflammatory factors IL-1, hs-CRP, TNF-α
and IL-8 were significantly increased in patients with metabolic
syndrome compared with the normal control individuals.
Exercise therapy aims to improve certain medical
conditions through the application of physical activity. Numerous
studies have reported that different types of exercise therapies,
such as cycling, walking and swimming, can relieve swelling, pain
and inflammation caused by injuries and different types of chronic
diseases (20). In a study of
patients with major depression, Knapen et al (21) have demonstrated that exercise
therapy, as a valuable complementary treatment to the traditional
therapies, was able to significantly reduce the risk of
depression-induce medical conditions, including metabolic syndrome,
type 2 diabetes and cardiovascular diseases. Furthermore, the
authors reported that exercise therapy also improved the body
image, which in turn improved the quality of life of patients. In
another study, Almeida et al (22) reported that swimming intervention for
5 weeks was sufficient to reduce increased expression levels of
brain-derived neurotrophic factor and nerve growth factor induced
by nerve injury without significantly affecting glial-derived
neurotrophic factor. Swimming intervention also inhibited
phosphorylation of phospholipase Cγ1, and reversed microglia
hyperactivity and astrocytes in the dorsal horn following nerve
injury, thus improving neuropathic pain (22). Recent studies have also indicated
that the exercise habits of individuals are closely associated with
insulin resistance in the body, and a well-designed exercise
therapy plan can effectively improve insulin resistance and inhibit
the development of its complications (23). Furthermore, different types of
exercise therapies can also regulate the expression of
inflammation-associated factors though different pathways,
including epigenetic modifications, which in turn inhibits
inflammatory responses (24). In the
present study, patients with metabolic syndrome were treated with
swimming intervention for 3 months at a frequency of four times per
week. Compared with patients who did not receive swimming
intervention, the HOMA-IR score and serum levels of key
pro-inflammatory factors IL-1, hs-CRP, TNF-α and IL-8 were
significantly reduced in patients treated with swimming
intervention. The therapeutic effects of swimming intervention were
increased with the increase in the intensity of exercise. These
data suggest that swimming intervention is able to improve insulin
resistance and inhibit inflammatory reactions in patients with
metabolic syndrome.
IRS-1 serves a pivotal role in insulin signal
transduction, and the polymorphisms of IRS-1 expression are closely
correlated with insulin resistance (25). GLUT-4 is an insulin-regulated glucose
transporter that promotes the transportation of circulating glucose
into muscle and fat cells to be processed, which in turn reduces
the level of glucose in the blood (26). Translocation of GLUT-4 to the plasma
membrane is critical for the transduction of insulin signaling. In
the present study, the phosphorylation level of IRS-1 and
expression level of GLUT-4 were significantly reduced in muscle
tissues of metabolic syndrome patients, while swimming intervention
promoted IRS-1 phosphorylation and GLUT-4 translocation to plasma
membrane. Furthermore, the PI3K/Akt pathway has important functions
in insulin signal transduction (27). In the current study, the
phosphorylation level of Akt was significantly lower in metabolic
syndrome patients as compared with that in the normal controls,
while swimming intervention increased the phosphorylation level of
IRS-1. These data suggest that swimming intervention activated
IRS-1 and PI3K/Akt pathway, and promoted GLUT-4 translocation to
plasma membrane, thus improving the metabolic syndrome.
Only HOMA-IR scoring was used to reflect the degree
of insulin resistance due to the limited resources, which is a
limitation of the present study. Our future study will detect more
indexes, including the blood glucose level and glycosylated
hemoglobin, to further verify the conclusions of the current study.
Besides PI3K/Akt pathway, the insulin signaling transduction is
also affected by other pathways, such as the Ras/ERK signaling
pathway (28). Our further studies
will also focus on the effects of swimming intervention on those
pathways.
In conclusion, swimming intervention reduced the
HOMA-IR score and serum levels of IL-1, hs-CRP, TNF-α and IL-8. In
addition, it promoted IRS-1 and Akt phosphorylation, and GLUT4
translocation, therefore improving the metabolic syndrome. However,
the present study is limited by the small sample size, and future
studies with bigger sample size are required to confirm the
conclusions of the current study.
Acknowledgements
Not applicable.
Funding
The study was financially supported by grants from
the Key Project of Natural Science of Anhui Provincial Education
Department (grant no. KJ2017A427), the School Grade Project of
Chuzhou University (grant no. 2014sk09), and the 2018 Anhui College
Excellent Youth Backbone Personnel Domestic Interview Research and
Study Project (grant no. gxgnfx2018048).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JT and LG designed the experiments, and read and
approved the manuscript. JT performed experiments and collected the
data. LG analyzed and interpreted data, and wrote the
manuscript.
Ethics approval and consent to
participate
The current study was approved by the University
Hospital of Chuzhou University. All participants signed informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
O'neill S and O'driscoll L: Metabolic
syndrome: A closer look at the growing epidemic and its associated
pathologies. Obes Rev. 16:1–12. 2015. View Article : Google Scholar
|
|
2
|
Ford ES, Giles WH and Dietz WH: Prevalence
of the metabolic syndrome among US adults: Findings from the third
National Health and Nutrition Examination Survey. JAMA.
287:356–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ussar S, Griffin NW, Bezy O, Fujisaka S,
Vienberg S, Softic S, Deng L, Bry L, Gordon JI and Kahn CR:
Interactions between gut microbiota, host genetics and diet
modulate the predisposition to obesity and metabolic syndrome. Cell
Metab. 22:516–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaur J: A comprehensive review on
metabolic syndrome. Cardiol Res Pract. 2014:9431622014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu H, Barnes GT, Yang Q, Yang D, Chou CJ,
Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H: Chronic
inflammation in fat plays a crucial role in the development of
obesity-related insulin resistance. J Clin Invest. 112:1821–1830.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwakkel G, van Peppen R, Wagenaar RC, Wood
Dauphinee S, Richards C, Ashburn A, Miller K, Lincoln N, Partridge
C, Wellwood I and Langhorne P: Effects of augmented exercise
therapy time after stroke. Stroke. 35:2529–2539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larun L, Brurberg KG, Odgaard-Jensen J and
Price JR: Exercise therapy for chronic fatigue syndrome. Cochrane
Database Syst Rev. 10:CD0032002016.
|
|
8
|
Hayden JA, Cartwright JL, Riley RD and
vanTulder MW; the Chronic Low Back Pain IPD Meta-Analysis Group, .
Exercise therapy for chronic low back pain: Protocol for an
individual participant data meta-analysis. Syst Rev. 1:642012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benatti FB and Pedersen BK: Exercise as an
anti-inflammatory therapy for rheumatic diseases-myokine
regulation. Nat Rev Rheumatol. 11:86–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuzawa Y, Sugiyama S, Sugamura K,
Sumida H, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H,
Nakayama N, et al: Successful diet and exercise therapy as
evaluated on self-assessment score significantly improves
endothelial function in metabolic syndrome patients. Circ J.
77:2807–2815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma CM, Yin FZ, Liu XL, Wang R, Lou DH and
Lu Q: How to simplify the diagnostic criteria of metabolic syndrome
in adolescents. Pediatr Neonatol. 58:178–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentration in man. Diabetologia. 28:412–419.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morino K, Petersen KF, Dufour S, Befroy D,
Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al:
Reduced mitochondrial density and increased IRS-1 serine
phosphorylation in muscle of insulin-resistant offspring of type 2
diabetic parents. J Clin Invest. 115:3587–3593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klip A, Sun Y, Chiu TT and Foley KP:
Signal transduction meets vesicle traffic: The software and
hardware of GLUT4 translocation. Am J Physiol Cell Physiol.
306:C879–C886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun XJ, Miralpeix M, Myers MG Jr, Glasheen
EM, Backer JM, Kahn CR and White MF: Expression and function of
IRS-1 in insulin signal transmission. J Biol Chem. 267:22662–22672.
1992.PubMed/NCBI
|
|
16
|
Yao H and Han X and Han X: The
cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling
pathway. Am J Cardiovasc Drugs. 14:433–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonomini F, Rodella LF and Rezzani R:
Metabolic syndrome, aging and involvement of oxidative stress.
Aging Dis. 6:109–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gayoso-Diz P, Otero-González A,
Rodriguez-Alvarez MX, Gude F, García F, De Francisco A and Quintela
AG: Insulin resistance (HOMA-IR) cut-off values and the metabolic
syndrome in a general adult population: Effect of gender and age:
EPIRCE cross-sectional study. BMC Endocr Disord. 13:472013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daniele G, Guardado Mendoza R, Winnier D,
Fiorentino TV, Pengou Z, Cornell J, Andreozzi F, Jenkinson C,
Cersosimo E, Federici M, et al: The inflammatory status score
including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and
adiponectin underlies whole-body insulin resistance and
hyperglycemia in type 2 diabetes mellitus. Acta Diabetol.
51:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moore G, Durstine JL and Painter P:
American College of Sports Medicine: ACSM's Exercise Management for
Persons with Chronic Diseases and Disabilities, 4E(M). Human
Kinetics. 2016.
|
|
21
|
Knapen J, Vancampfort D, Moriën Y and
Marchal Y: Exercise therapy improves both mental and physical
health in patients with major depression. Disabil Rehabil.
37:1490–1495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Almeida C, DeMaman A, Kusuda R, Cadetti F,
Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR and Lucas
G: Exercise therapy normalizes BDNF upregulation and glial
hyperactivity in a mouse model of neuropathic pain. Pain.
156:504–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fedewa MV, Gist NH, Evans EMa and Dishman
RK: Exercise and insulin resistance in youth: A meta-analysis.
Pediatrics. 133:e163–e174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horsburgh S, Robson-Ansley P, Adams R and
Smith C: Exercise and inflammation-related epigenetic
modifications: Focus on DNA methylation. Exerc Immunol Rev.
21:26–41. 2015.PubMed/NCBI
|
|
25
|
Giandalia A, Pappalardo MA, Russo GT,
Romeo EL, Alibrandi A, Di Bari F, Vita R, Cucinotta D and Benvenga
S: Influence of peroxisome proliferator-activated receptor
(PPAR)-(gamma) exon 2 (Pro12Ala) and exon 6 (His447His) and
Gly972Arg insulin receptor substrate (IRS)-1 polymorphisms on
insulin resistance (IR) and beta cell function in southern
mediterranean women with polycystic ovary syndrome (PCOS). J Clin
Transl Endocrinol. 13:1–8. 2017.
|
|
26
|
Sano H, Peck GR, Blachon S and Lienhard
GE: A potential link between insulin signaling and GLUT4
translocation: Association of Rab10-GTP with the exocyst subunit
Exoc6/6b. Biochem Biophys Res Commun. 465:601–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y,
Liang D, Zhang R, Zhang S, Wang H and Cao F: Apelin stimulates
glucose uptake through the PI3K/Akt pathway and improves insulin
resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 353:305–313.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gomes AP and Blenis JA: Nexus for cellular
homeostasis: The interplay between metabolic and signal
transduction pathways. Curr Opin Biotechnol. 34:110–117. 2015.
View Article : Google Scholar : PubMed/NCBI
|