Introduction
As a chronic airway disease, asthma is characterized
by airway inflammation, remodeling and hyper-responsiveness
(1). The disorder affects over 300
million people worldwide and can be induced by environmental
antigens that increase the secreting activity of lymphocytes and
eosinophils (2). Significant
heterogeneities exist among asthma patients, which makes the
clinical management of asthma challenging currently (3,4).
Therefore, a comprehensive understanding of the mechanism driving
the onset of asthma is imperative for the development of novel
treatment strategies.
In recent years, it is well recognized that ‘airway
remodeling’ is a relatively more chronic feature of asthma
(1). Airway remodeling represents
structural abnormities in bronchial walls and is characterized by
airway smooth muscle (ASM) hypertrophy and hyperplasia (1). The induced secreting activity of
lymphocytes and eosinophils during asthma initiates inflammatory
responses and stimulates the accumulation of extracellular matrix
in ASM cells (ASMCs) (1,5). The secretion of extracellular matrix in
ASMCs has been inferred to be the major driving force of epithelial
dysplasia and denudation, and mucus gland hyperplasia associated
with asthma (6,7). Other previous studies have also
confirmed the increase of ASMC mass following exposure to
inflammatory factors (8), implying
the complex interplay among inflammation, ASMCs and asthma attacks.
Therefore, attenuating hypertrophy and hyperplasia of ASMCs has
been proposed to be a promising method for inhibiting airway
remodeling and managing asthma (1,9).
Transforming growth factor-β1 (TGF-β1) protein is a
crucial regulator for the establishment of body structure and
tissue differentiation by influencing cell proliferation,
differentiation and migration (10).
This agent has been widely employed as an inducer for an in
vitro hyperplasia model of ASMCs (11–13).
TGF-β1 exerts its function in cell hyperplasia via multiple
mechanisms, including Smad-dependent and non-Smad-dependent manners
(10). In the study by Meng et
al (14), the authors
demonstrated that the disruption of Smad4 influences the signaling
transduction of TGF-β1/Smad3, which attenuates inflammation and
fibrosis in the kidney. As reported by Chen and Khalil (11), the phosphorylation of
mitogen-activated protein kinases (MAPKs) by TGF-β1 increases the
proliferation of ASMCs. Furthermore, interactions among TGF-β1,
Smad and MAPK signaling have also been verified by different
studies (10,11). Taken together, it is reasonable to
verify the possibility of managing asthma by interrupting the
interactions among TGF-β1, Smad and MAPK signaling.
Uncaria rhynchophylla, also known as ‘Gou
Teng’, is a traditional Chinese herb that has been used in the
treatment of cardiovascular and brain disorders for centuries
(15–17). The major pharmacologically active
components of Uncaria rhynchophylla include rhynchophylline
(Rhy), isorhynchophylline, hirsutine and corynantheine (15,18),
among which Rhy has displayed the potential to inhibit the
proliferation of ASMCs (19,20). Given the fact that Uncaria
rhynchophylla is capable of attenuating asthma as a Chinese
medicine formula (21), it is
hypothesized that Rhy may serve a key role in the anti-asthma
effect of Uncaria rhynchophylla. To verify this hypothesis,
asthma symptoms were induced in mice using ovalbumin (OVA), and the
treatment potential of Rhy was assessed in the present study.
Furthermore, the inhibiting effect of Rhy on ASMC hyperplasia
induced by TGF-β1 was detected. By focusing on TGF-β1, Smad and
MAPK pathways, the current study also attempted to uncover the
mechanism associated with the anti-asthma effect of Rhy. The data
revealed that Rhy effectively attenuated the symptoms of asthma and
inhibited the proliferation of ASMCs by blocking TGF-β1-induced
activation of Smad and MAPK signaling.
Materials and methods
Chemicals and agents
OVA (cat. no. A5503) and MTT (cat. no. M-2128) were
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Enzyme-linked immunosorbent assay (ELISA) kits for the detection of
murine immunoglobulin E (IgE; cat. no. EK2751), interleukin 4
(IL-4; cat. no. EK2041/2), IL-5 (cat. no. EK2051) and IL-13 (cat.
no. EK2131/2) were purchased from MultiSciences Biotech Co., Ltd.
(Hangzhou, China). TGF-β1 (cat. no. RPA124Mu01) was obtained from
USCN Business Co., Ltd. (Wuhan, China). Rhy (cat. no. R102720) and
the TGF-β1 inhibitor SB431542 (cat. no. S125924) were obtained from
Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
Radioimmunoprecipitation assay lysis buffer (cat. no. P0013B) and a
protein concentration determination kit using the bicinchoninic
acid (BCA) method (cat. no. P0009) were purchased from Beyotime
Institute of Biotechnology (Shanghai, China).
Antibodies
An antibody against TGF-β1 (cat. no. BA0290) was
purchased from Boster Biological Technology, Ltd. (Wuhan, China).
Antibodies against Smad2 (cat. no. 5339), phosphorylated (p)-Smad2
(Ser465/467; cat. no. 3108), Smad3 (cat. no. 9523), p-Smad3
(Ser423/425; cat. no. 9520), extracellular signal-regulated kinase
(ERK; cat. no. 4695) and p-ERK (Thr202/Tyr204; cat. no. 4370) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Antibodies against Smad4 (cat. no. D120124), Smad7 (cat. no.
D160746), p38 (cat. no. D151619) and p-p38 (Thr180/Tyr182; cat. no.
D151619) were obtained from Sangon Biotech Co., Ltd. (Shanghai,
China). Antibodies against proliferating cell nuclear antigen
(PCNA), α-smooth muscle actin (α-SMA) and calponin were purchased
from ProteinTech Group, Inc. (Chicago, IL, USA). Horseradish
peroxidase (HRP)-conjugated goat-anti rabbit (cat. no. A0208), goat
anti-mouse (cat. no. A0216) IgG secondary and Cy3-labeled secondary
antibodies (cat. no. A0516) were obtained from Beyotime Institute
of Biotechnology. Antibody against internal reference protein
β-actin were purchased from Bioss (Beijing, China).
Allergic asthma induction and Rhy
treatment
All the animal assays were performed following the
Institutional Animal Ethics Committee and Animal Care Guidelines
for the Care and Use of No.1 People's Hospital (Jining, China). A
total of 24 female BALB/c mice (8-week-old) were purchased from
Changsheng Biotechnology Co., Ltd. (Liaoning, China) and housed in
cages at room temperature (20–25°C) with a constant humidity
(55±5%) and with food and water available ad libitum. The 24
mice were randomly divided into four groups (6 mice in each group),
including the blank (untreated mice), sham, asthma and Rhy groups,
and were raised for 42 days under the same conditions. To induce
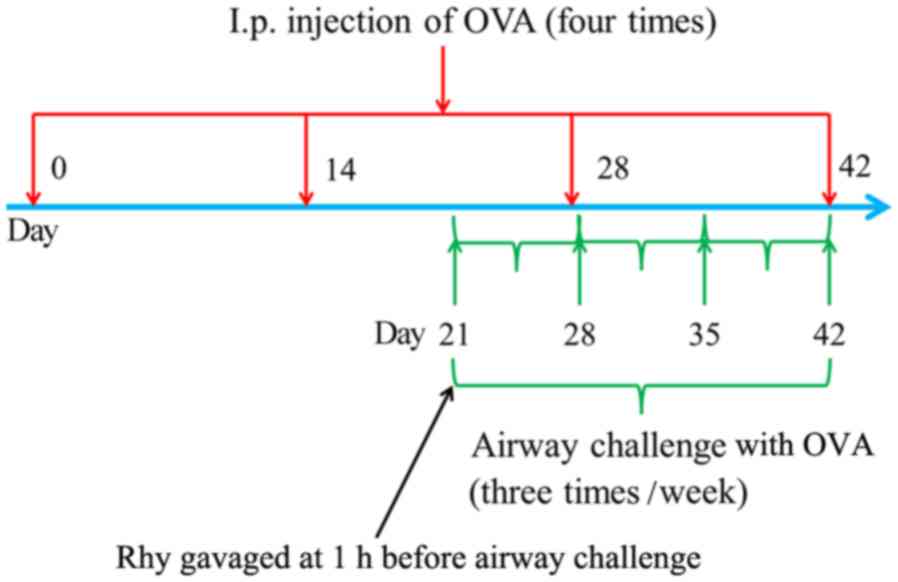
allergic asthma, mice in the asthma and Rhy groups were
intraperitoneally injected with 20 µg OVA (22) for four times, on days 0, 14, 28 and
42 of the model induction, respectively (Fig. 1). Between days 21 and 42 of the
induction, mice were subjected to airway challenges with OVA (1%,
w/v) using an atomizer for 30 min three times per week (Fig. 1). Mice in the sham group underwent
the same procedure as that conducted in the asthma and Rhy groups,
but with PBS replacing OVA. In the Rhy treatment group, mice were
gavaged with 40 mg/kg Rhy, as previously described (23,24), at
1 h before airway challenge with OVA between days 21 and 42 of the
induction (Fig. 1). At 24 h after
the last challenge, blood samples and bronchoalveolar lavage fluid
(BALF) of mice were collected. Subsequently, mice were sacrificed
by pentobarbital sodium overdose (200 mg/kg). Following perfusion
of the left ventricle using normal saline, lungs were collected,
fixed in 10% neutral buffered formalin and stored at −70°C for
subsequent assays.
Hematoxylin and eosin (H&E)
staining
H&E staining of BALF samples was conducted
following previously published protocols (25). Briefly, ~1 ml BALF was recovered
through centrifugation at 1,367 × g for 15 min at 4°C, and cellular
component was obtained from the centrifugal sediment. Next, 0.1 ml
sediment was smeared onto a slide and placed into Bouin solution
(4% formaldehyde) for perfusion fixation and dehydrated using
different concentrations of alcohol. Subsequent to vitrifying in
dimethylbenzene, the slide was embedded in paraffin, sectioned and
stained with H&E. Images were captured using a microscope
(BX53; Olympus Corporation, Tokyo, Japan) at magnification, ×200
and the average number of eosinophils per 500 cells was calculated
for each group.
ELISA for determination of IgE, IL-4,
IL-5 and IL-13 levels
The production of IgE in the serum of mice, and the
production of IL-4, IL-5, and IL-13 in BALF samples were detected
using the corresponding ELISA kits, according to the manufacturer's
protocol.
Western blot analysis
Cells or tissues were initially incubated with
radioimmunoprecpitation assay lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology) supplemented with 1%
phenylmethane sulfonyl fluoride and placed on ice for 5 min. Next,
the mixture was centrifuged at 10,005 × g for 4 min, and total
protein was collected from the supernatant. Protein concentration
was then determined using the BCA method. In total, 20 µg protein
in a 20 µl solution was subjected to 5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis at 80 V for 2.5 h and
then transferred onto polyvinylidene difluoride membranes at 80 V
for 1–2 h. Following rinsing with Tris-buffered saline/Tween-20
(TBST) for 5 min, the membranes were blocked with skimmed milk
solution (5%, m/v) for 1 h at room temperature. The membranes were
then incubated at 4°C overnight with primary antibodies against
TGF1β (1:300), p-Smad2 (1:1,000), Smad2 (1:1,000), p-Smad3
(1:1,000), Smad3 (1:1,000), Smad4 (1:500), Smad7 (1:500), p-ERK1/2
(1:2,000), ERK1/2 (1:1,000), p-p38 (1:1,000), p38 (1:500), and
β-actin (1:500). After washing with TBST for four times,
HRP-conjugated IgG secondary antibodies (1:5,000) were added onto
the membranes and incubated for 45 min at 37°C. Blots were then
developed by incubating the membranes with Beyo ECL Plus reagent
(Beyotime Institute of Biotechnology) for 5 min, and the relative
expression levels of proteins were analyzed using the
Gel-Pro-Analyzer software (Media Cybernetics, Inc., USA).
Isolation of ASMCs
ASMCs were isolated from the healthy female BALB/c
8-week-old mice (which were purchased and housed under the
aforementioned conditions) following previously published
procedures (26). Briefly, 10 mice
were sacrificed using 50 mg/kg pentobarbital sodium, and bronchus
tissues were collected. Following washing twice using PBS, the
tissues were cut into small sections, and incubated with 0.1%
collagenase and 0.1% trypsin in 15-ml tubes at 37°C for 20 min.
Cultures were then filtered with a 150-µm strainer and centrifuged
at 309 × g for 7 min in Dulbecco's modified Eagle's medium (DMEM).
Subsequent to incubation with 0.1% collagenase and 0.1% trypsin and
further centrifugation at 309 × g for 7 min at 37°C, the
supernatants were discarded, and precipitates were cultured in DMEM
at 37°C in an atmosphere containing 5% CO2 and 95% air.
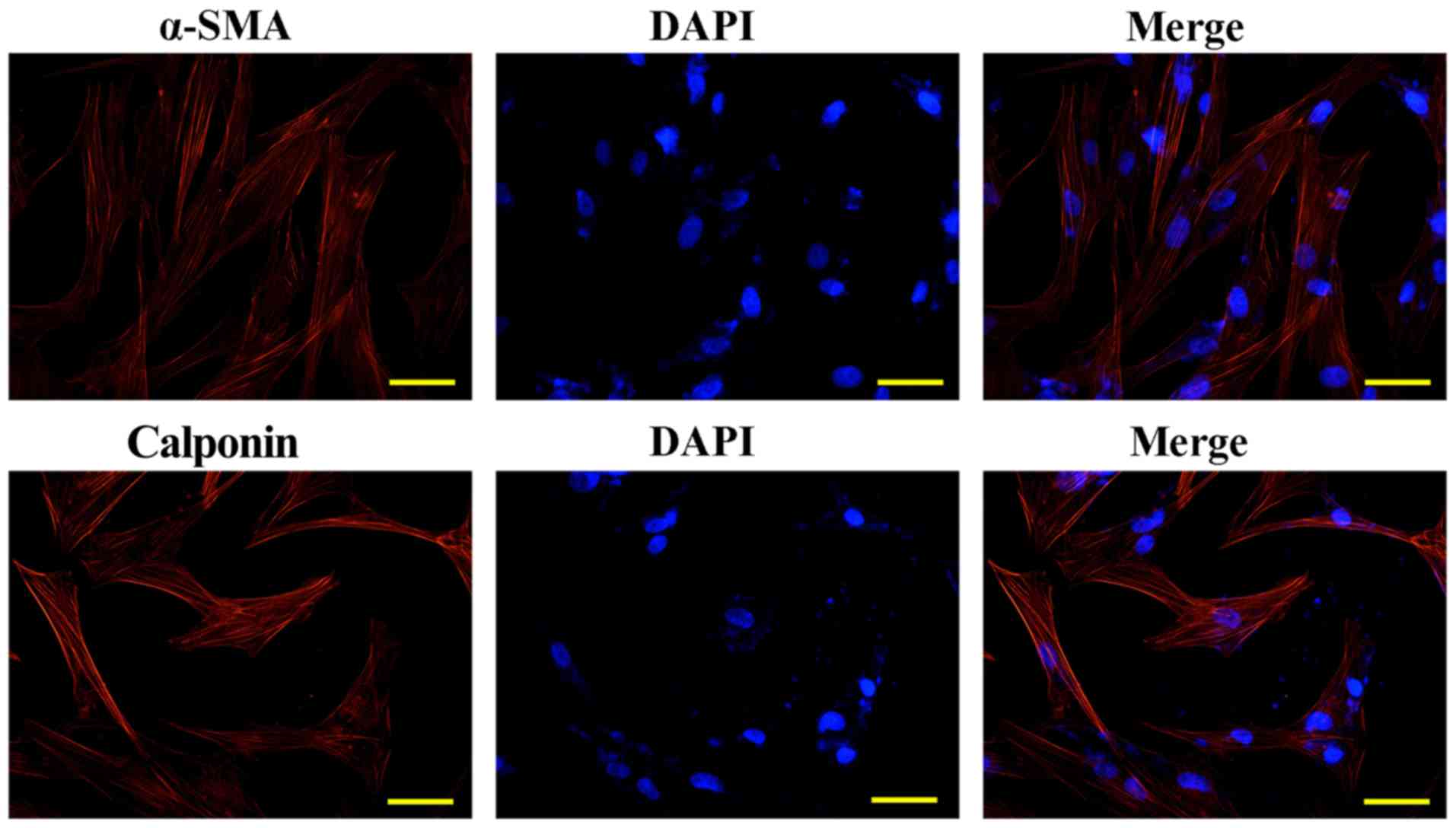
ASMCs were identified by immunofluorescence detection of calponin
and α-SMA (Fig. 2), and cells of
passages 3–5 were employed for subsequent assays.
For in vitro assays, cells were divided into
four groups as follows: Blank group, which contained ASMCs; TGF-β1
group, in which ASMCs were initially cultured in 0.2% BSA/DMEM
serum-free medium to arrest cell growth and then incubated with 5
ng/ml TGF-β1 for 24 h (11); Rhy
group, in which ASMCs were initially cultured in 0.2% BSA/DMEM
serum-free medium, and then incubated with 5 ng/ml TGF-β1 and 10 µM
Rhy for 24 h; SB431542 group, in which ASMCs were initially
cultured in 0.2% BSA/DMEM serum-free medium, and then incubated
with 5 ng/ml TGF-β1 and 10 µM SB431542 for 24 h (27). Upon completion of the culture, cells
were collected for subsequent assays.
MTT assay
The cell viability of ASMCs was detected by an MTT
assay. Briefly, the culture medium of ASMCs was replaced by DMEM
supplemented with 0.5 mg/ml MTT, and cells were cultured for
another 4 h at 37°C. Next, supernatants were aspirated, and 200 µl
dimethyl sulfoxide was added into each well of a 96-well plate
(4×103/well). Cell viability was represented by the
optical density value at 570 nm, as detected using a microplate
reader (ELX-800; BioTek Instruments, Inc., Winooski, VT, USA).
Immunofluorescence analysis
Cells were seeded in 14-well chambers
(4×103/well) and allowed to grow into a monolayer. Next,
cells were fixed with 4% paraformaldehyde for 15 min and
permeabilized with 0.1% Triton X-100 for 30 min. Subsequent to
incubation with 10% goat serum for 15 min at room temperature,
cells were incubated with primary antibodies against PCNA (1:50),
α-SMA (1:50) and calponin (1:50) at 4°C overnight. Following three
washings using PBS, Cy3-labeled secondary antibody (1:200) was
added and incubated for 1 h at room temperature in the dark. After
washing with PBS, cells were stained with
4′,6-diamino-2-phenylindole for 5 min. Images were captured with a
fluorescent microscope (BX53; Olympus Corporation) at
magnification, ×400.
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance and post-hoc multiple comparisons were
performed using a general linear model. Duncan's test was used for
post-hoc multiple comparisons in order to control type I error. A
statistically significant difference was considered when the
two-tailed P-value was <0.05. All the statistical analyses and
graph plotting were conducted using GraphPad Prism version 6.0
(GraphPad Software, Inc., San Diego, CA, USA).
Results
Rhy attenuates the recruitment of
inflammatory cells in BALF induced by OVA
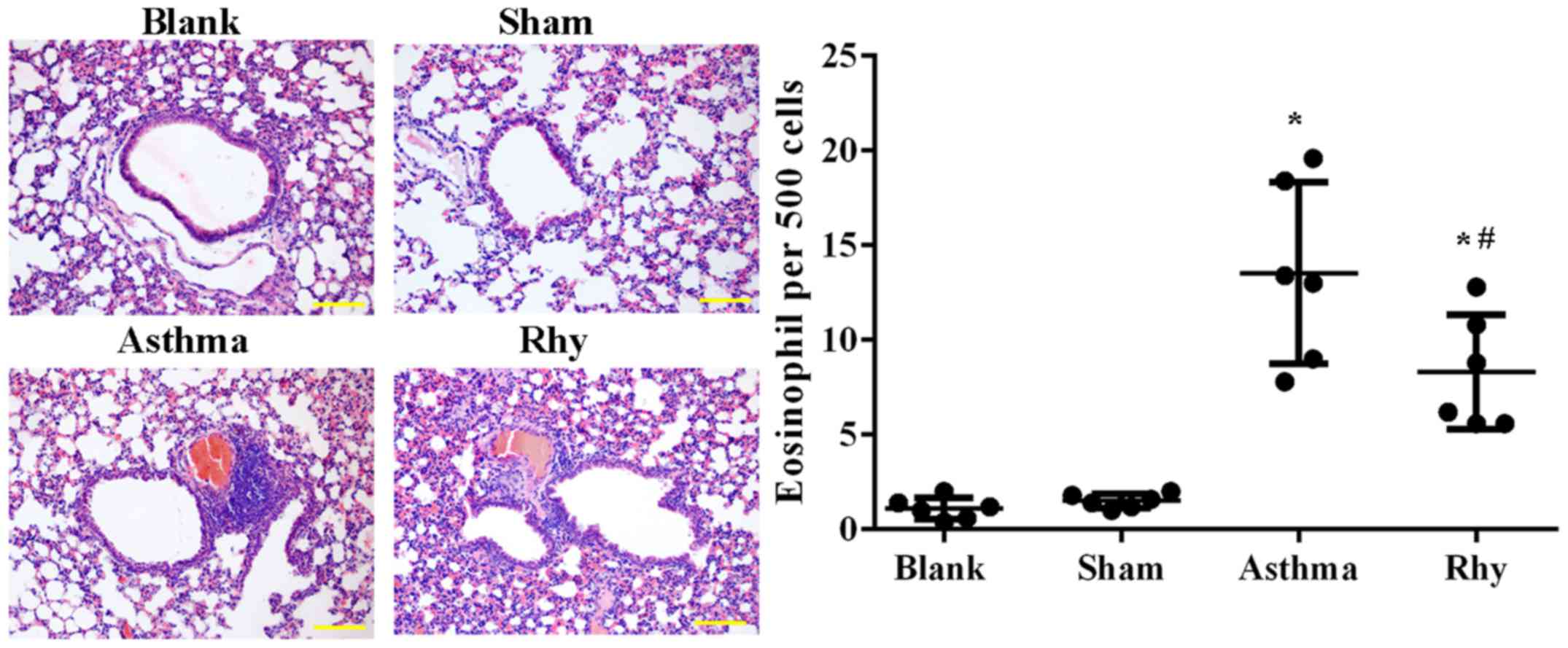
The induction of the asthma model was first
evaluated by H&E staining. As shown in Fig. 3, a significantly greater number of
eosinophils was recorded in mice in the asthma group as compared
with those in the blank and sham groups (P<0.05). The results
confirmed the establishment of the allergic asthma model.
Similarly, asthma mice treated with Rhy had a significantly lower
number of eosinophils (Fig. 3)
compared with that in the asthma group (Fig. 3), evidently indicating the control of
inflammatory cell recruitment by Rhy treatment.
Rhy suppresses the production of IgE
and pro-inflammatory cytokines induced by OVA
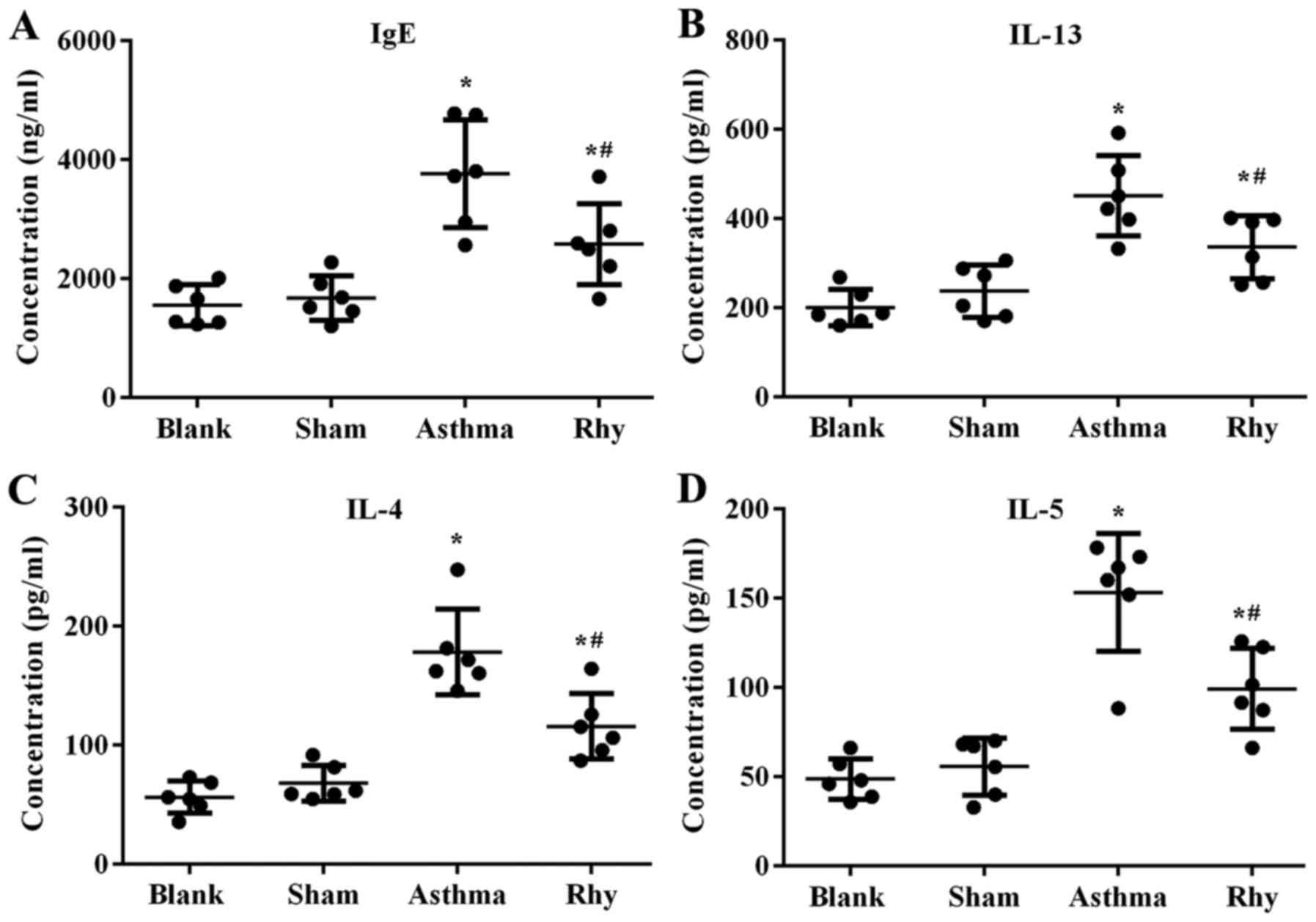
Concomitant with the increase in eosinophil number,
the levels of IgE in the serum and pro-inflammatory cytokines
IL-13, IL-4 and IL-5 in BALF were found to be induced by OVA
administration (Fig. 4),
representing the initiation of OVA-induced allergic and
inflammatory responses in the lungs. By contrast, mice treated with
Rhy displayed lower levels of IgE and pro-inflammatory cytokines
compared with those in the asthma group (P<0.05; Fig. 4). The results revealed the
anti-inflammation effect of Rhy during the onset of asthma.
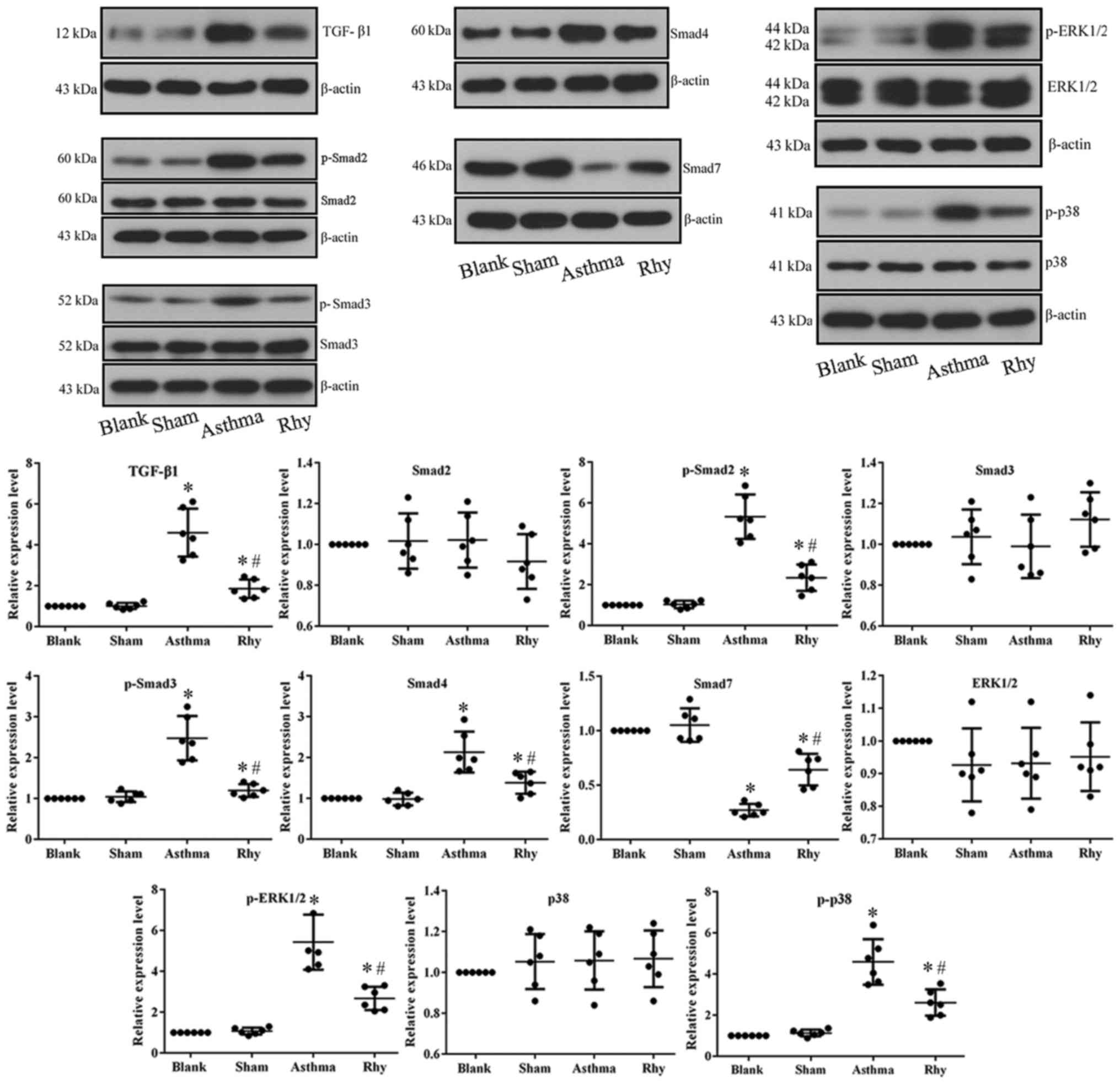
Rhy inhibits the TGF-β1-induced
activation of Smad and MAPK signaling in vivo
To uncover the mechanism driving the anti-asthma
effect of Rhy, the activation of TGF-β1-mediated Smad and MAPK
signaling in lung tissues was detected. The data demonstrated that
OVA administration in the asthma group significantly induced the
expression of TGF-β1, which further initiated Smad signaling by
markedly increasing the expression of Smad4, and the
phosphorylation of Smad2 and Smad3, while significantly decreasing
the expression of Smad7 (Fig. 5).
The mechanism is important for the fibrosis process associated with
asthma (14). Furthermore, the
overproduction of TGF-β1 in the asthma group induced the activation
of MAPK pathway (i.e., the increased the levels of p-ERK1/2 and
p-p38; Fig. 5), which is known to
promote hyperplasia of ASMCs in the airway and exacerbate asthma
symptoms (10,11). However, treatment with Rhy reversed
the expression patterns of all aforementioned indicators in lung
tissues (Fig. 5), inhibiting the
pro-fibrosis and pro-hyperplasia signaling transduction induced by
OVA.
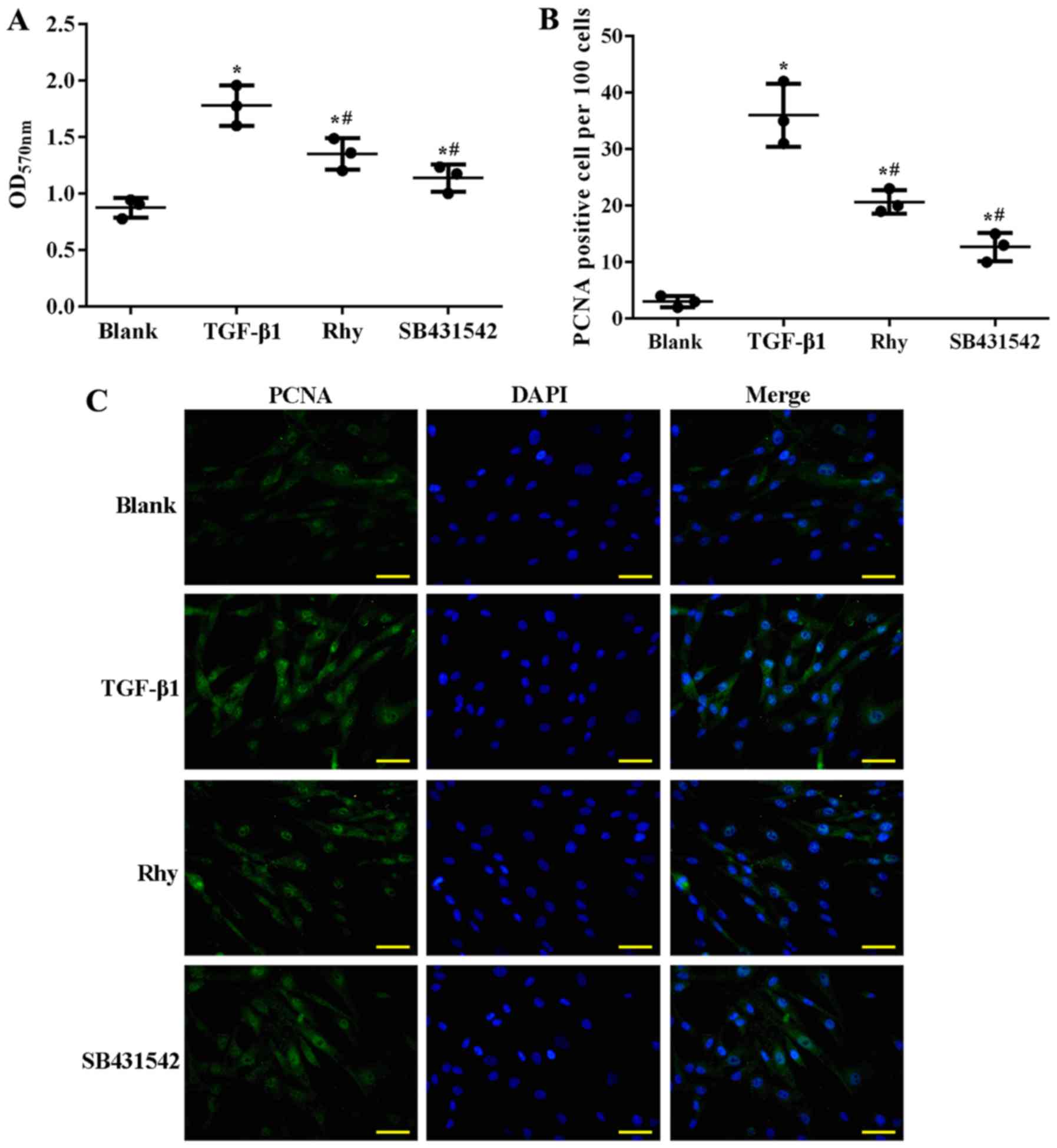
Rhy inhibits ASMC proliferation by
blocking TGF-β1-mediated Smad and MAPK signaling in vitro
To verify whether Rhy exerted its anti-asthma effect
by suppressing hyperplasia of ASMCs, treatment with Rhy or TGF-β1
inhibitor SB431542 in TGF-β1-treated ASMCs was performed in the
current study, and the effect of the administrations on the
proliferation of ASMCs and on Smad and MAPK signaling was assessed.
Similar to the effect of OVA on mice, TGF-β1 induced proliferation
of ASMCs. As detected by MTT assay, significantly higher cell
viability (Fig. 6A) and higher
production of PCNA (Fig. 6B and C)
were observed in the TGF-β1 group as compared with the blank group
(P<0.05). However, when ASMCs were co-incubated with TGF-β1 and
Rhy, or TGF-β1 and SB431542, the cell viability and production of
PCNA were significantly inhibited (P<0.05; Fig. 6A-C), evidently inferring that Rhy
inhibited the proliferation of ASMCs in a parallel pattern to that
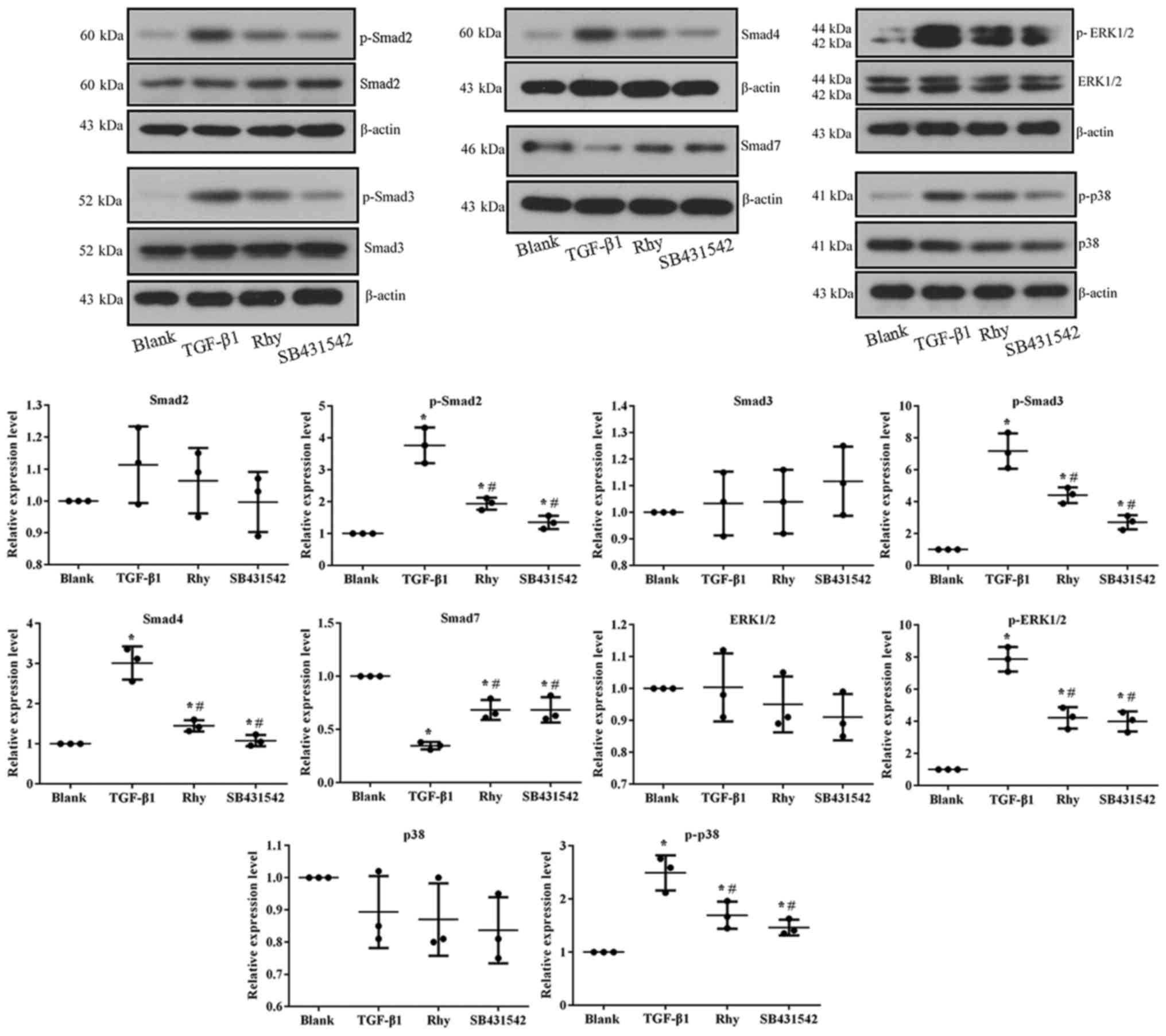
of TGF-β1 inhibitor. At the molecular level, TGF-β1 treatment
induced the activation of Smad and MAPK pathways, while treatment
with Rhy or SB431542 reversed the expression levels of factors
involved in these pathways (Fig. 7),
similar to the observations in the mouse model.
Discussion
Hyperplasia of ASMCs has been reported to induce a
variety of pathological symptoms, including atherosclerosis,
hypertension and asthma (28,29).
Among the factors causing ASMC hyperplasia, TGF-β1 is effective in
inducing ASMC proliferation and has been employed as a method to
establish asthma in vitro models (11–13).
Therefore, targeting TGF-β1 has been conceived to be a promising
strategy for the treatment of asthma. Rhy, one of the major
pharmacologically active components of Uncaria
rhynchophylla, was selected in the current study for the
management of asthma in vivo and in vitro. The
findings of the current study demonstrated that Rhy was able to
attenuate inflammatory and allergic symptoms in vivo, and to
inhibit hyperplasia of ASMCs in vitro by blocking the
TGF-β1-mediated Smad and MAPK signaling.
Uncaria rhynchophylla is a herb that is
widely used in traditional Chinese medicine against hypertension,
light headedness, dizziness, convulsion and numbness (20). In the study by Sun et al
(21), the authors concluded that
total alkaloids extracted from Uncaria rhynchophylla
exhibited an anti-asthma effect in cavy asthma models. However, few
studies have followed on these results and conducted a more
comprehensive assessment to reveal the pharmacological components
involved in the treatment of asthma with this herb. The active
components purified from Uncaria rhynchophylla include Rhy,
isorhynchophylline, hirsutine and corynantheine, among which Rhy
has been reported to inhibit the proliferation of ASMCs (19,20).
Therefore, it was hypothesized in the current study that the
anti-asthma effect of Uncaria rhynchophylla total alkaloids
may depend on the function of Rhy. Based on the results of in
vivo assays, it was revealed that the administration of Rhy
attenuated the recruitment of inflammatory cells and suppressed the
production of IgE, as well as pro-inflammatory cytokines, in asthma
mice, representing the effective treatment of Rhy against asthma.
Furthermore, incubating TGF-β1-treated ASMCs with Rhy inhibited the
proliferation of these cells. Taken together, the results evidently
indicated that Rhy was able to attenuate the progression of asthma
by inhibiting hyperplasia of ASMCs.
To explore the mechanism driving the effect of Rhy
on ASMCs, the activities of TGF-β1-mediated Smad and MAPK pathways
were detected. Rhy treatment inhibited the activation of Smad
pathway by inducing the expression of Smad7, and markedly
suppressing the expression levels of Smad4, p-Smad2 and p-Smad3.
The effect was comparable to that observed upon exposure to the
TGF-β1 specific inhibitor SB431542, indicating a TGF-β1
inhibition-dependent pattern of Rhy in treating asthma. Two sources
for TGF-β1 recruitment exist in airways, including inflammatory
cells and residential airway cells (30,31), and
this recruitment in turn leads to increased production of collagen
I and fibronectin (32). Thus, it is
concluded that TGF-β1 serves a determining role in the progression
of chronic asthma with repeated episode of injury and inflammation
(32). In addition, a previous study
demonstrated that overproduction of TGF-β1 enhances proliferation
of ASMCs via phosphorylation of MAPKs (11). Activation of MAPK pathway is required
for TGF-β1-mediated initiation of Smad signaling, which is central
to inflammation and fibrosis processes (14). In addition, co-expression of Smad2
and Smad4 enhances the activation of p38 (11). Interactions between TGF-β1, Smads and
MAPKs constitute a positive loop in promoting the proliferation of
ASMCs, and further induce pathological symptoms associated with
asthma. Therefore, Rhy would interrupt signaling transduction
between Smads and MAPKs, and attenuate impairments induced by
TGF-β1 overproduction during asthma attacks.
However, shortcomings also exist in the current
experimental design. The endotoxin contamination of Rhy cannot be
excluded and only a preliminary conclusion that Rhy was able to
attenuate allergic bronchial asthma could be provided in the
present study. In order to fully explore the medicinal value of Rhy
and other active components of Uncaria rhynchophylla total
alkaloids, further comprehensive investigation is required in the
future.
In conclusion, the current study demonstrated the
anti-asthma effect of Rhy, which depended on the inhibition of
TGF-β1-mediated Smad and MAPK signaling. The findings suggested the
clinical application potential of Rhy for managing asthma and other
disorders resulting from smooth muscle cell hyperplasia.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of Shandong Province (no. 2016ZRA08005),
the Traditional Chinese Medicine Science and Technology Development
Project of Shandong Province (no. 2015-443), the Traditional
Chinese Medicine Science and Technology Development Project of
Jining City (no. ZYY2015018), and the Scientific Research Project
of Jining Medical College (no. JY2015KJ027).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MW and HL collected data and wrote the manuscript.
YZ and CL also performed data collection and analyzed the data. GZ
designed the experiment, approved the submission and was
responsible for the project.
Ethics approval and consent to
participate
All the animal assays were performed following the
Institutional Animal Ethics Committee and Animal Care Guidelines
for the Care and Use of the No. 1 People's Hospital (Jining,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ammit AJ, Hastie AT, Edsall LC, Hoffman
RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S
and Panettieri RA Jr: Sphingosine 1-phosphate modulates human
airway smooth muscle cell functions that promote inflammation and
airway remodeling in asthma. FASEB J. 15:1212–1214. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bousquet J, Mantzouranis E, Cruz AA,
Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, Brightling CE, Burney
P, Bush A, Busse WW, et al: Uniform definition of asthma severity,
control, and exacerbations: Document presented for the world health
organization consultation on severe asthma. J Allergy Clin Immunol.
126:926–938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung KF, Wenzel SE, Brozek JL, Bush A,
Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et
al: International ERS/ATS guidelines on definition, evaluation and
treatment of severe asthma. Eur Respir J. 43:343–373. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wenzel SE: Asthma phenotypes: The
evolution from clinical to molecular approaches. Nat Med.
18:716–725. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pei QM, Jiang P, Yang M, Qian XJ, Liu JB,
Zheng H, Zhao LH and Kim SH: Upregulation of a disintegrin and
metalloproteinase-33 by VEGF in human airway smooth muscle cells:
Implications for asthma. Cell Cycle. 15:2819–2826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung KF: Airway smooth muscle cells:
Contributing to and regulating airway mucosal inflammation? Eur
Respir J. 15:961–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Que CL, Maksym G and Macklem PT:
Deciphering the homeokinetic code of airway smooth muscle. Am J
Respir Crit Care. 161:S161–S163. 2000. View Article : Google Scholar
|
|
8
|
Dunnill MS, Massarella GR and Anderson JA:
A comparison of the quantitative anatomy of the bronchi in normal
subjects, in status asthmaticus, in chronic bronchitis, and in
emphysema. Thorax. 24:176–179. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao SS, Mu Q, Zeng Y, Cai PC, Liu F, Yang
J, Xia Y, Zhang Q, Song LJ, Zhou LL, et al: Calpain-activated
mTORC2/Akt pathway mediates airway smooth muscle remodelling in
asthma. Clin Exp Allergy. 47:176–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen G and Khalil N: TGF-beta1 increases
proliferation of airway smooth muscle cells by phosphorylation of
map kinases. Respir Res. 7:22006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Ge Q, Black JL, Deng L, Burgess JK
and Oliver BG: Differential regulation of extracellular matrix and
soluble fibulin-1 levels by TGF-β1 in airway smooth muscle cells.
PLoS one. 8:e655442013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie S, Sukkar MB, Issa R, Oltmanns U,
Nicholson AG and Chung KF: Regulation of TGF-beta1-induced
connective tissue growth factor expression in airway smooth muscle
cells. Am J Physiol Lung Cell Mol Physiol. 288:L68–L76. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng XM, Huang XR, Xiao J, Chung AC, Qin
W, Chen HY and Lan HY: Disruption of Smad4 impairs TGF-β/Smad3 and
Smad7 transcriptional regulation during renal inflammation and
fibrosis in vivo and in vitro. Kidney Int. 81:266–279. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi JS, Yu JX, Chen XP and Xu RX:
Pharmacological actions of Uncaria alkaloids, rhynchophylline and
isorhynchophylline. Acta Pharmacol Sin. 24:97–101. 2003.PubMed/NCBI
|
|
16
|
Zhou J and Zhou S: Antihypertensive and
neuroprotective activities of rhynchophylline: The role of
rhynchophylline in neurotransmission and ion channel activity. J
Ethnopharmacol. 132:15–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao W, Wang Y, Lv X, Yu X, Li X, Li H,
Wang Y, Lu D, Qi R and Wang H: Rhynchophylline prevents cardiac
dysfunction and improves survival in lipopolysaccharide-challenged
mice via suppressing macrophage I-κBα phosphorylation. Int
Immunopharmacol. 14:243–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu J, Gong T, Ma B, Zhang L, Kano Y and
Yuan D: Comparative study of fourteen alkaloids from Uncaria
rhynchophylla hooks and leaves using HPLC-diode array
detection-atmospheric pressure chemical ionization/MS method. Chem
Pharm Bull (Tokyo). 60:23–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YL: Effect of rhynchophylline and
isorhynchophylline on the proliferation of artery smooth muscle
cells induced by angiotensin II (Translated). Zhong Guo Yao Li Xue
Tong Bao. 24:62008.(In Chinese).
|
|
20
|
Kamat PK, Rai S, Swarnkar S, Shukla R, Ali
S, Najmi AK and Nath C: Okadaic acid-induced tau phosphorylation in
rat brain: Role of NMDA receptor. Neuroscience. 238:97–113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun AS, Huang XZ, Liu WG, Zhang XD and Ke
MM: The anti-asthma effect of rhynchophylla total alkaloids
(translated). Gui Zhou Yi Yao. 2:1983.(In Chinese).
|
|
22
|
Shin IS, Shin NR, Jeon CM, Kwon OK, Sohn
KY, Lee TS, Kim JW, Ahn KS and Oh SR: EC-18, a synthetic
monoacetyldiglyceride (1-palmitoyl-2-linoleoyl-3-acetylglycerol),
attenuates the asthmatic response in an aluminum
hydroxide/ovalbumin-induced model of asthma. Int Immunopharmacol.
18:116–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Liu W, Peng Q, Jiang M, Luo C, Guo
Y, Liu Y, Fang M and Mo Z: Effect of rhynchophylline on conditioned
place preference on expression of NR2B in methamphetamine-dependent
mice. Biochem Biophys Res Commun. 452:695–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu AK, Hung KW, Huang H, Gu S, Shen Y,
Cheng EY, Ip FC, Huang X, Fu WY and Ip NY: Blockade of EphA4
signaling ameliorates hippocampal synaptic dysfunctions in mouse
models of Alzheimer's disease. Proc Natl Acad Sci USA.
111:9959–9964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong YH, Shi Q, Han N, Zhang L, Zhang YY,
Gao TX, Chen C and Li YL: Structural modulation of gut microbiota
in rats with allergic bronchial asthma treated with recuperating
lung decoction. Biomed Environ Sci. 29:574–583. 2016.PubMed/NCBI
|
|
26
|
Deng H, Dokshin GA, Lei J, Goldsmith AM,
Bitar KN, Fingar DC, Hershenson MB and Bentley JK: Inhibition of
glycogen synthase kinase-3beta is sufficient for airway smooth
muscle hypertrophy. J Biol Chem. 283:10198–10207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schuliga M, Harris T, Xia Y, Wang Z, Zhang
X, Lee P and Stewart A: FGF-2 modulates human airway smooth muscle
contractile protein expression and cell stiffness. Eur Respiratory
Soc. 42:P5662013.
|
|
28
|
Ranganna K, Yatsu FM, Hayes BE, Milton SG
and Jayakumar A: Butyrate inhibits proliferation-induced
proliferating cell nuclear antigen expression (PCNA) in rat
vascular smooth muscle cells. Mol Cell Biochem. 205:149–161. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Natarajan R and Nadler JL: Lipoxygenases
and lipid signaling in vascular cells in diabetes. Front Biosci.
8:s783–s795. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vignola AM, Chiappara G, Chanez P,
Merendino AM, Pace E, Spatafora M, Bousquet J and Bonsignore G:
Growth factors in asthma. Monaldi Arch Chest Dis. 52:159–169.
1997.PubMed/NCBI
|
|
31
|
Duvernelle C, Freund V and Frossard N:
Transforming growth factor-beta and its role in asthma. Pulm
Pharmacol Ther. 16:181–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Coutts A, Chen G, Stephens N, Hirst S,
Douglas D, Eichholtz T and Khalil N: Release of biologically active
TGF-beta from airway smooth muscle cells induces autocrine
synthesis of collagen. Am J Physiol Lung Cell Mol Physiol.
280:L999–L1008. 2001. View Article : Google Scholar : PubMed/NCBI
|