Introduction
Ankylosing spondylitis is a type of arthritis
characterized by the long-term inflammation of the joints of the
spine (1). This disease mainly
affects spine joints, while other joints, including hips or
shoulders, may also be affected in certain cases (1). Stiffness of the affected joints becomes
worse with the development of ankylosing spondylitis, leading to
impaired back mobility and reduced life quality (2,3).
Ankylosing spondylitis mainly affects individuals aged 20–30 years
and the early diagnosis rate is low (4). Although human leukocyte antigen (HLA)
B27 subtypes (B*2701-2759) and bacteria (e.g. Enterobacter)
have been proved to be correlated with the progression of
ankylosing spondylitis, their diagnostic value for this disease is
low (4). Therefore, identification
of novel effective and reliable diagnostic markers is urgently
required to improve the survival of patients with ankylosing
spondylitis.
Long non-coding (lnc)RNAs are a class of RNA
transcripts comprising >200 nucleotides with no protein coding
capacity but the ability to post-transcriptionally regulate the
expression of certain genes (5).
lncRNAs have important functions in normal physiological processes
and pathological changes in the human body (6,7). The
role of the lncRNA maternally expressed gene 3 (MEG3) as a tumor
suppressor has been extensively characterized in different types of
cancer (8).
A recent study proved that MEG3 may promote the
development of osteoporosis (9),
which has mechanistic similarities with ankylosing spondylitis
(10), while its possible role in
ankylosing spondylitis has remained elusive. Therefore, the present
study investigated the involvement of MEG3 in ankylosing
spondylitis, revealing that lncRNA MEG3 was downregulated in
ankylosing spondylitis and is associated with disease activity,
disease duration, hospitalization time and re-hospitalization rate
within 2 years after discharge. The present study provided
references for the diagnosis and prognosis of ankylosing
spondylitis.
Materials and methods
Subjects
A total of 172 patients with ankylosing spondylitis
who presented at Yongchuan Hospital of Chongqing Medical University
(Chongqing, China) between January 2013 and January 2015 were
included in the present study. All patients were diagnosed
according to the New York criteria established in 1984 (11). Those patients included 98 males and
74 females, and their age ranged from 10 to 45 years, with a mean
age of 25.6±5.4 years. All patients were diagnosed and treated for
the first time. Disease activity was evaluated based on the
Ankylosing Spondylitis Disease Activity Score (ASDAS) (12), which was interpreted as follows:
<1.3, inactive disease; 1.3–2.1, moderate disease activity;
2.1–3.5, high disease activity; and >3.5 very high disease
activity. Patients transferred to other hospitals during treatment
were not included. All patients were followed up for 5 years and
re-hospitalization within 2 years after discharge was recorded. At
the same time, 98 control subjects with normal physiological
conditions were also included to serve as a healthy control group.
The control group included 55 males and 43 females, and the age
ranged from 14 to 42 years, with a mean age of 26.1±6.3 years. No
significant differences in age and sex were identified between the
patient and the control group.
Specimen collection
Blood (~15 ml) was obtained from the elbow vein of
each of the patients and healthy control subjects on the day of
admission. Blood was kept at room temperature for 1.5 h and then
centrifuged at 800 × g for 20 min at room temperature to collect
the serum. Open sacroiliac joint biopsies were collected from 42
patients and 36 healthy controls. All specimens were stored in
liquid nitrogen prior to analysis. Open sacroiliac joint biopsies
were performed on healthy controls to detect potential lesions, but
those potential lesions were not found and no medical conditions
were observed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Biopsies were ground in liquid nitrogen, followed by
addition of TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) to extract total RNA. Serum samples were
directly mixed with TRIzol reagent to extract total RNA. Total RNA
samples were then used as template to synthesize complementary
(c)DNA through RT using SuperScript IV Reverse Transcriptase
(Thermo Fisher Scientific, Inc.). The reaction conditions were as
follows: 25°C for 5 min, 55°C for 20 min and 80°C for 20 min. The
PCR reaction system was prepared using the SYBR™ Green
PCR Master mix (cat. no. LS4309155; Applied Biosystems™;
Thermo Fisher Scientific, Inc.) and cDNA samples. The sequences of
the primers used for PCR were as follows: Human lncRNA-MEG3
forward, 5′-CTGCCCATCTACACCTCACG-3′ and reverse,
5′-CTCTCCGCCGTCTGCGCTAGGGGCT-3′; human β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
PCR analyses were performed on the CFX384 Touch™
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using the following thermocycling conditions:
95°C for 45 sec, followed by 40 cycles of 95°C for 10 sec and 52°C
for 35 sec according to manufacturer's protocol. The quantification
of mRNA expression was performed using the 2−ΔΔCq method
(13). The expression levels of
lncRNA-MEG3 relative to those of the endogenous control β-actin
were determined.
Statistical analysis
SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was
used for all statistical analyses. Receiver operating
characteristics (ROC) curve analysis was performed using default
parameters. Comparisons of measurement data expressed as the mean ±
standard deviation between two groups were performed by using the
unpaired t-test. Count data were compared by Chi-squared test.
Correlation analyses were performed by a Pearson correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of expression levels of
lncRNA-MEG3 in serum and open sacroiliac joint biopsies between
ankylosing spondylitis patients and healthy controls
RT-qPCR was performed to detect the expression of
lncRNA-MEG3 in serum and open sacroiliac joint biopsies collected
from ankylosing spondylitis patients and healthy controls. As
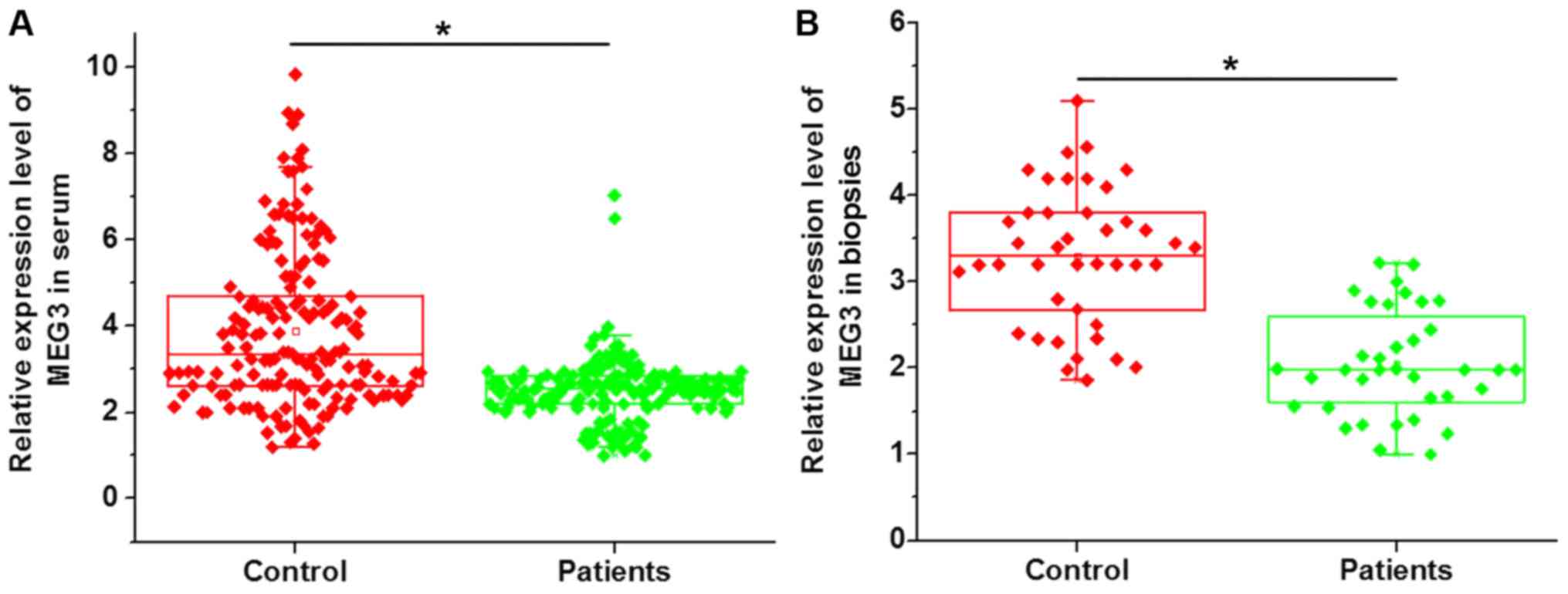
presented in Fig. 1, a significantly
downregulated MEG3 expression in the serum (Fig. 1A) and open sacroiliac joint biopsies
(Fig. 1B) of ankylosing spondylitis
patients compared with that in healthy controls was identified.
These results suggest that downregulation of lncRNA-MEG3 expression
is likely to be involved in the pathogenesis of ankylosing
spondylitis.
Diagnostic value of lncRNA-MEG3
expression in serum and open sacroiliac joint biopsies for
ankylosing spondylitis
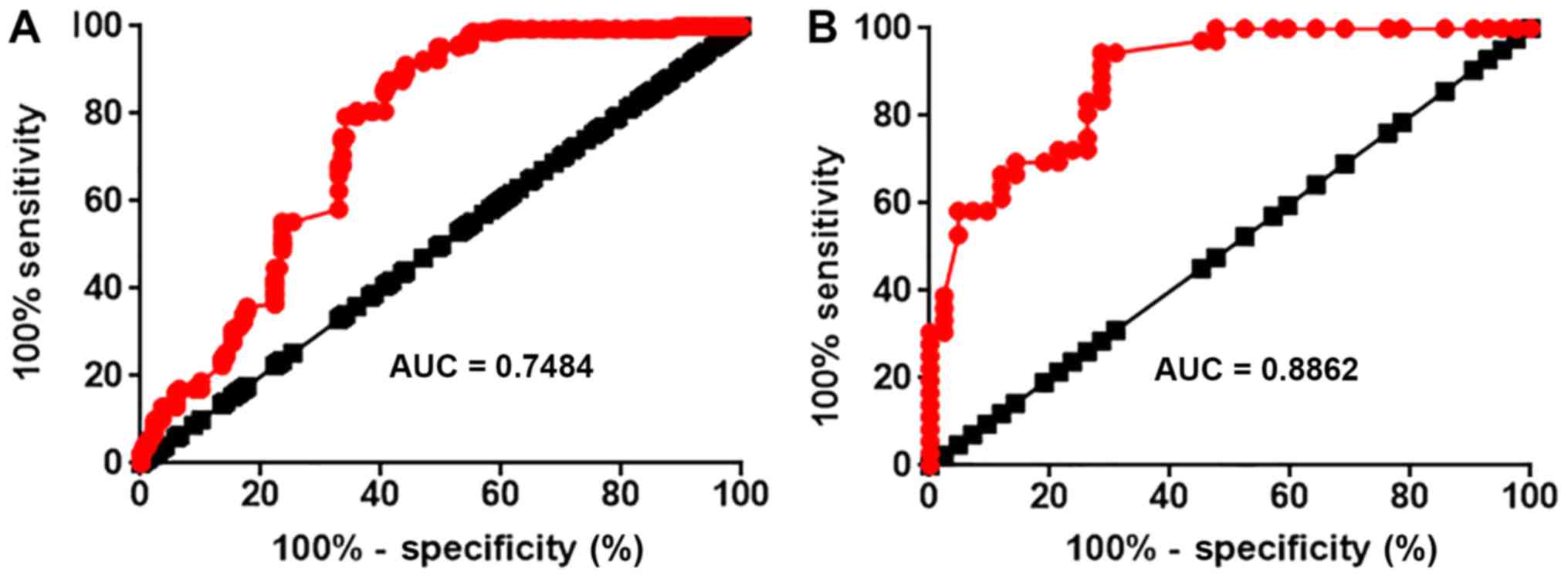
ROC curve analysis was performed to evaluate the
diagnostic values of lncRNA-MEG3 expression in serum and open
sacroiliac joint biopsies for ankylosing spondylitis. The ROC curve
for serum lncRNA-MEG3 is presented in Fig. 2A. The area under the curve (AUC) of
the ROC for the use of serum lncRNA-MEG3 in the diagnosis of
ankylosing spondylitis was 0.7484 with a 95% confidence interval
(CI) of 0.6950–0.8017 (P<0.0001). The ROC curve for lncRNA-MEG3
in open sacroiliac joint biopsies is presented in Fig. 2B. The AUC of the ROC for the use of
lncRNA-MEG3 expression in pen sacroiliac joint biopsies in the
diagnosis of ankylosing spondylitis was 0.8862 with a 95% CI of
0.8163–0.9562 (P<0.0001).
Correlation between serum circulating
MEG3 levels with clinicopathological characteristics of ankylosing
spondylitis patients
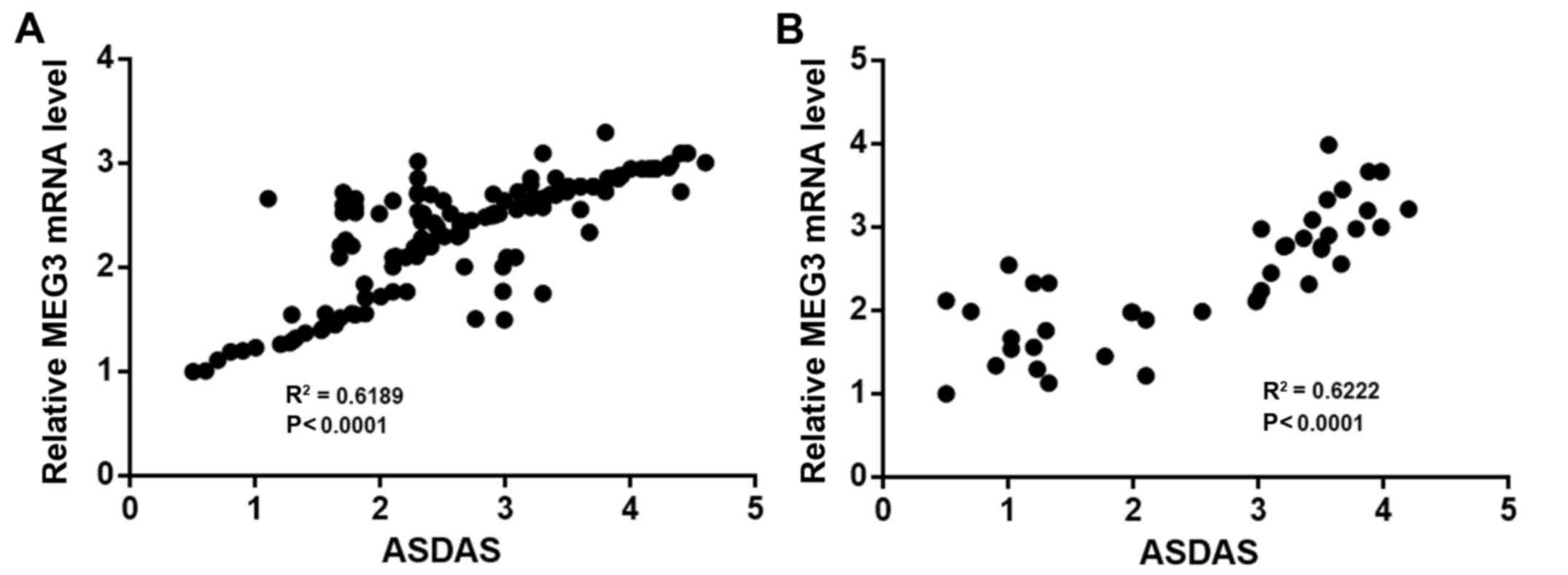
A linear correlation analysis was performed with
MEG3 expression as ‘X’ values and ASDAS scores as ‘Y’ values. The
results suggested that the levels of MEG3 in the serum
(R2=0.6198, P<0.0001; Fig.
3A) and open sacroiliac joint biopsies (R2=0.6222,
P<0.0001; Fig. 3B) were
significantly positively correlated with ASDAS. According to the
medium expression levels of MEG3, patients were divided into
high-level and low-level groups (n=86). The differences between
these high-level and low-level groups regarding the general
clinicopathological data were further analyzed by using the
chi-square test. As presented in Tables
I and II, respectively, the
serum circulating MEG3 levels and the expression levels of MEG3 in
open sacroiliac joint biopsies were not significantly correlated
with the patients' sex, age and lifestyle habits, including smoking
and drinking. However, significant differences in the disease
duration and ASDAS were identified between the high- and low-level
MEG3 expression groups.
 | Table I.Association of circulating MEG3 levels
with clinicopathological data of ankylosing spondylitis
patients. |
Table I.
Association of circulating MEG3 levels
with clinicopathological data of ankylosing spondylitis
patients.
| Variables | Cases (n) | High-expression group
[n (%)] | Low-expression group
[n (%)] | χ2 | P-value |
|---|
| Sex |
|
|
| 0.095 | 0.76 |
| Male | 98 | 48 (49.0) | 50 (51.0) |
|
|
|
Female | 74 | 38 (51.4) | 36 (48.6) |
|
|
| Age (years) |
|
|
| 0.374 | 0.54 |
| ≥25 | 80 | 38 (47.5) | 42 (52.5) |
|
|
|
<25 | 92 | 48 (52.2) | 44 (47.8) |
|
|
| Disease duration
(years) |
|
|
| 18.241 | <0.0001 |
| ≥5 | 84 | 28 (33.3) | 56 (66.7) |
|
|
|
<5 | 88 | 58 (65.9) | 30 (34.1) |
|
|
| ASDAS |
|
|
| 19.066 | <0.001 |
|
<1.3 | 32 | 17 (53.1) | 15 (46.9) |
|
|
|
1.3–2.1 | 46 | 22 (47.8) | 24 (52.2) |
|
|
|
2.1–3.5 | 44 | 24 (54.5) | 20 (45.5) |
|
|
|
>3.5 | 50 | 24 (48.0) | 26 (52.0) |
|
|
| Smoking |
|
|
| 0.212 | 0.65 |
| Yes | 77 | 37 (48.1) | 40 (51.9) |
|
|
| No | 95 | 49 (51.6) | 46 (48.4) |
|
|
| Drinking |
|
|
| 1.147 | 0.28 |
| Yes | 93 | 43 (46.2) | 50 (53.8) |
|
|
| No | 79 | 43 (54.4) | 36 (45.6) |
|
|
 | Table II.Correlation between expression levels
of MEG3 in open sacroiliac joint biopsies with clinicopathological
data of ankylosing spondylitis patients. |
Table II.
Correlation between expression levels
of MEG3 in open sacroiliac joint biopsies with clinicopathological
data of ankylosing spondylitis patients.
| Variables | Cases (n) | High-expression group
[n (%)] | Low-expression
group [n (%)] | χ2 | P-value |
|---|
| Sex |
|
|
| 1.71 | 0.79 |
|
Male | 28 | 12 (42.9) | 16 (57.1) |
|
|
|
Female | 14 | 9
(64.3) | 5
(35.7) |
|
|
| Age (years) |
|
|
| 0.47 | 0.49 |
|
≥25 | 30 | 14 (46.7) | 16 (53.3) |
|
|
|
<25 | 12 | 7
(58.3) | 5
(41.7) |
|
|
| Disease duration
(years) |
|
|
| 6.11 | 0.01 |
| ≥5 | 20 | 6
(30.0) | 14 (70.0) |
|
|
|
<5 | 22 | 15 (68.2) | 7
(31.8) |
|
|
| ASDAS |
|
|
| 6.27 | 0.01 |
|
<1.3 | 10 | 7
(70.0) | 3
(30.0) |
|
|
|
1.3–2.1 | 8 | 6
(75.0) | 2
(25.0) |
|
|
|
2.1–3.5 | 12 | 4
(33.3) | 8
(66.7) |
|
|
|
>3.5 | 12 | 4
(33.3) | 8
(66.7) |
|
|
| Smoking |
|
|
| 0.87 | 0.35 |
|
Yes | 23 | 10 (43.5) | 13 (56.5) |
|
|
| No | 19 | 11 (57.9) | 8
(42.1) |
|
|
| Drinking |
|
|
| 1.56 | 0.21 |
|
Yes | 24 | 10 (41.7) | 14 (58.3) |
|
|
| No | 18 | 11 (61.1) | 7
(38.9) |
|
|
Effects of serum levels of circulating
MEG3 on hospitalization time and re-hospitalization rate
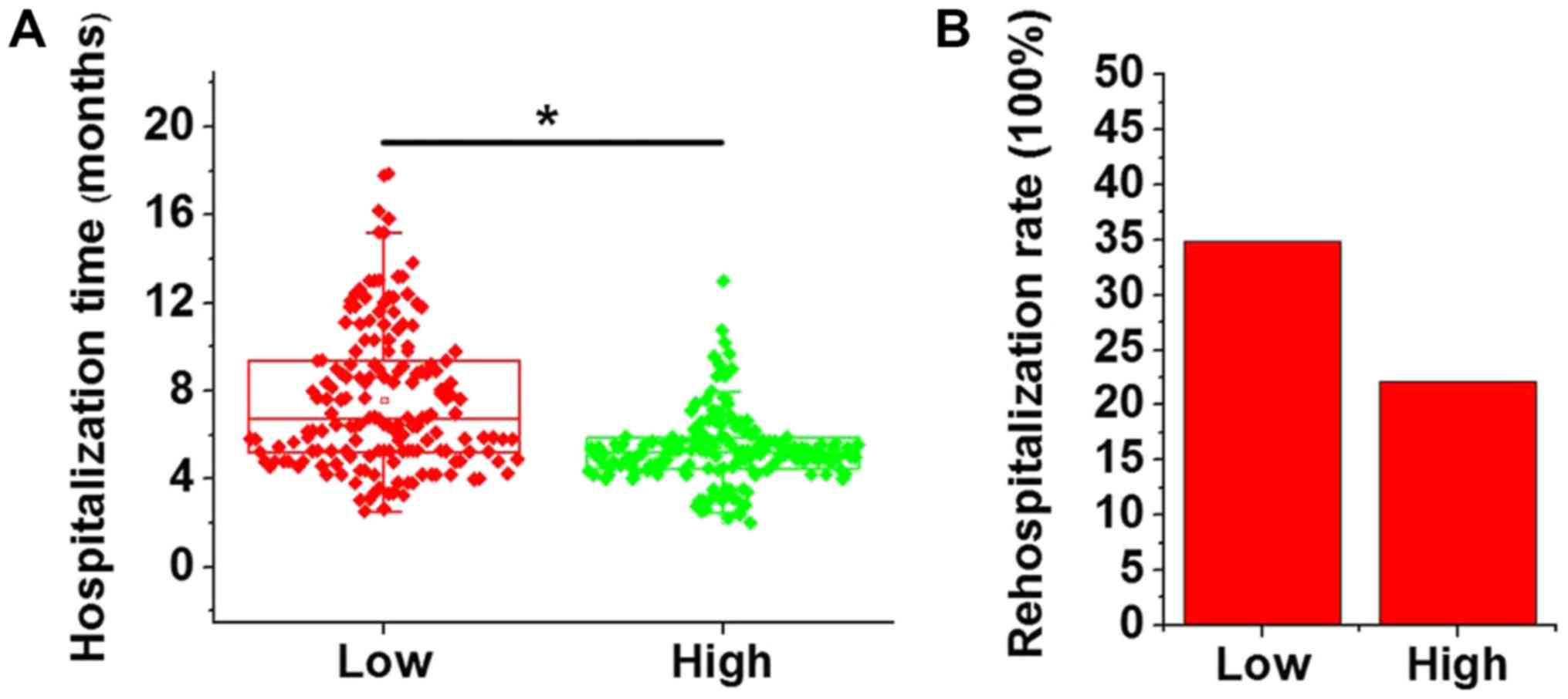
According to the median expression levels of MEG3,
patients were divided into high-level and low-level groups (n=86),
and the hospitalization time and re-hospitalization rate were
compared between the two groups. As presented in Fig. 4A, the hospitalization time in the
low-level group was significantly longer than that in the
high-level group (P<0.05). In addition, the re-hospitalization
rate in the low-expression group (30 out of 86, 34.9%) within 2
years after discharge was also higher than that in the high-level
group (19 out of 86, 22.1%; Fig.
4B). However, the difference in the re-hospitalization rate
between the two groups was not significant (P=0.06).
Discussion
The onset, development and progression of ankylosing
spondylitis are complex processes with multiple internal and
external factors involved (1). It
has been reported that HLA-B27 subtypes and bacteria are likely to
be involved in the pathogenesis of ankylosing spondylitis (4). Furthermore, the development of
ankylosing spondylitis is usually accompanied with changes in
expression patterns of a large set of genes, including miRNAs and
lncRNAs, indicating the involvement of lncRNAs in this disease
(14,15). However, studies on the detailed
expression patterns of those lncRNAs, as well as their prognostic
and diagnostic values for this disease, remain insufficient. In a
recent study, Li et al (16)
characterized the expression pattern of lncRNA-AK001085 in the
blood of patients with ankylosing spondylitis and revealed that
lncRNA-AK001085 was frequently downregulated in ankylosing
spondylitis patients compared with that in healthy controls. lncRNA
MEG3 has been proved to be overexpressed in patients with
osteoporosis (9), which has similar
mechanisms to those of ankylosing spondylitis (10). In the present study, a significantly
downregulated MEG3 expression in the serum and open sacroiliac
joint biopsies of ankylosing spondylitis patients compared with
that in healthy controls was identified, indicating that
downregulation of MEG3 is likely to be involved in ankylosing
spondylitis. The opposite expression pattern of MEG3 in ankylosing
spondylitis and osteoporosis may be explained by the inverse
pathological processes between those two diseases (17). In the present study, marked overlaps
in the levels of MEG3 between patients and healthy controls were
observed, which indicates that differences between individuals may
prevail.
The development of diseases is always accompanied
with changes of certain substances (e.g. circulating lncRNAs) in
the blood (18). Therefore, the
detection of the changes of those substances may provide references
for the diagnosis of specific diseases (18). In the present study, ROC curve
analysis indicated that serum circulating MEG3 may be used to
effectively distinguish patients with ankylosing spondylitis from
healthy individuals. It is known that the expression of certain
lncRNAs may be affected by tobacco consumption and alcohol abuse
(19). In addition, a genome-wide
alteration of lncRNA expression was identified with aging (20). In the present study, serum
circulating MEG3 levels were not significantly associated with age,
sex or the patients' smoking and drinking habits, but were
significantly associated with the disease duration and disease
activity. These results suggest that serum circulating MEG3 may
serve as a reliable and effective diagnostic biomarker for
ankylosing spondylitis. Our future studies will work on the
optimization of specificity, sensitivity and other parameters.
Although various serum lncRNAs have a promising diagnostic value
for multiple human diseases (21),
the serum levels of circulating lncRNAs may fail to fully reflect
the expression pattern in lesion tissues. In the present study, it
was observed that 3 patients with serum levels of MEG3 above the
median value had expression levels of MEG3 in open sacroiliac joint
biopsies below the median values. In another 6 patients, the serum
levels of MEG3 below the median and expression levels of MEG3 in
open sacroiliac joint biopsies above the median were observed. In
addition, the ROC curve analysis indicated that the expression
levels of MEG3 in open sacroiliac joint biopsies have a higher
diagnostic value for ankylosing spondylitis compared with that of
the serum circulating levels of MEG3. However, open sacroiliac
joint biopsy as an invasive technique is unbearable for all
patients (only 42 out of 172 patients were willing to receive it),
particularly teenagers. Furthermore, the patients' health condition
and availability of financial resources should be fully considered
when selecting the diagnostic techniques. In the present study, the
serum levels of circulating MEG3 were also identified to be
significantly correlated with the ASDAS scores, which reflect the
activity of ankylosing spondylitis, indicating the possible role of
MEG3 as a marker for determining disease activity. In addition, a
lower expression level of MEG3 was identified to be associated with
a longer hospitalization time and a higher re-hospitalization rate
within 2 years after discharge. These results suggest that serum
circulating MEG3 may be used to guide the treatment and prognosis
of ankylosing spondylitis.
The present study did not identify the downstream
targets of MEG3 in ankylosing spondylitis. It has been reported
that angiogenesis (22) and
inflammation (23) have pivotal
roles in the pathogenesis of ankylosing spondylitis. A previous
study indicated that MEG3 knockdown aggravates retinal vessel
dysfunction by promoting inflammatory responses (24). In addition, an inverse association
between MEG3 and VEGF has been observed in osteoarthritis (25). Therefore, a reduction in MEG3 may
participate in ankylosing spondylitis through inducing inflammation
and angiogenesis. Besides the ASDAS, other important indicators,
including the erythrocyte sedimentation rate, C-reactive protein
and procalcitonin should also be analyzed. In the present study
those indicators were not detected due to the limited resources,
but this will be addressed in future studies.
In conclusion, the present study revealed that MEG3
is downregulated in ankylosing spondylitis patients compared with
that in healthy controls. Serum circulating MEG3 and MEG3
expression in sacroiliac joint biopsies are biomarkers for
ankylosing spondylitis. The expression levels of MEG3 were not
associated with the patients' age, sex and smoking/drinking habits,
but were associated with the duration of disease and closely
correlated with the disease activity. Furthermore, patients with
lower expression levels of MEG3 had a longer hospitalization time
and a higher re-hospitalization rate within 2 years after
discharge. It may therefore be concluded that lncRNA MEG3 is
downregulated in ankylosing spondylitis and it may hold predictive
value regarding disease activity, disease duration, hospitalization
time and re-hospitalization rate within 2 years after
discharge.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and ZL designed experiments. WL, LH and CZ
performed experiments. WL, LH and ZL analyzed data. ZL interpreted
data and drafted the manuscript. All authors read and approved the
manuscript.
Ethical approval and consent to
participate
The present study was approved by the ethics
committee of Yongchuan Hospital of Chongqing Medical University
(Chongqing, China) and all patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atouf O, Benbouazza K, Brick C, Saoud B,
Benseffaj N, Amine B, Hajjaj-Hassouni N and Essakalli M:
Distribution of HLA class I and II genes in ankylosing spondylitis
patients from Morocco. Pathol Biol (Paris). 60:e80–e83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilgil E, Kaçar C, Tuncer T and Bütün B:
The association of syndesmophytes with vertebral bone mineral
density in patients with ankylosing spondylitis. J Rheumatol.
32:292–294. 2005.PubMed/NCBI
|
|
4
|
Braun J, Brandt J, Listing J, Zink A,
Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider
M, et al: Treatment of active ankylosing spondylitis with
infliximab: A randomised controlled multicentre trial. Lancet.
359:1187–1193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: A tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Li Y and Zhang Y, Ma L, Lin L,
Meng J, Jiang L, Wang L, Zhou P and Zhang Y: LncRNA MEG3 inhibited
osteogenic differentiation of bone marrow mesenchymal stem cells
from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed
Pharmacother. 89:1178–1186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klingberg E, Nurkkala M, Carlsten H and
Forsblad-d'Elia H: Biomarkers of bone metabolism in ankylosing
spondylitis in relation to osteoproliferation and osteoporosis. J
Rheumatol. 41:1349–1356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984.
|
|
12
|
Lukas C, Landewe R, Sieper J, Dougados M,
Davis J, Braun J, van der Linden S and van der Heijde D; Assessment
of SpondyloArthritis international Society, . Development of an
ASAS-endorsed disease activity score (ASDAS) in patients with
ankylosing spondylitis. Ann Rheum Dis. 68:18–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Wang C, Jia Z, Tong W, Liu D, He
C, Huang X and Xu W: Differentially expressed mRNAs, lncRNAs, and
miRNAs with associated co-expression and ceRNA networks in
ankylosing spondylitis. Oncotarget. 8:113543–113557.
2017.PubMed/NCBI
|
|
15
|
Xie Z, Li J, Wang P, Li Y, Wu X, Wang S,
Su H, Deng W, Liu Z, Cen S, et al: Differential expression profiles
of long noncoding RNA and mRNA of osteogenically differentiated
mesenchymal stem cells in ankylosing spondylitis. J Rheumatol.
43:1523–1531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Chai W, Zhang G, Ni M, Chen J, Dong
J, Zhou Y, Hao L, Bai Y and Wang Y: Down-Regulation of
lncRNA-AK001085 and its influences on the diagnosis of ankylosing
spondylitis. Med Sci Monit. 23:11–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyakoshi N, Itoi E, Murai H, Wakabayashi
I, Ito H and Minato T: Inverse relation between osteoporosis and
spondylosis in postmenopausal women as evaluated by bone mineral
density and semiquantitative scoring of spinal degeneration. Spine
(Phila Pa 1976). 28:492–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Yin C, Dang Y, Ye F and Zhang G:
Identification of the long non-coding RNA H19 in plasma as a novel
biomarker for diagnosis of gastric cancer. Sci Rep. 5:115162015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soares do Amaral N, Cruz E, Melo N, de
Melo Maia B and Malagoli Rocha R: Noncoding RNA profiles in
tobacco-and alcohol-associated diseases. Genes (Basel). 8(pii):
E62016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grammatikakis I, Panda AC, Abdelmohsen K
and Gorospe M: Long noncoding RNAs (lncRNAs) and the molecular
hallmarks of aging. Aging (Albany NY). 6:992–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J,
Wei M, Xu C, Wu C, Zhang Z, et al: Long non-coding RNA metastasis
associated in lung adenocarcinoma transcript 1 derived miniRNA as a
novel plasma-based biomarker for diagnosing prostate cancer. Euro J
Cancer. 49:2949–2959. 2013. View Article : Google Scholar
|
|
22
|
Goldberger C, Dulak J, Duftner C,
Weidinger F, Falkenbach A and Schirmer M: Vascular endothelial
growth factor (VEGF) in ankylosing spondylitis-a pilot study. Wien
Med Wochenschr. 152:223–225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sveaas SH, Berg IJ, Provan SA, Semb AG,
Olsen IC, Ueland T, Aukrust P, Vøllestad N, Hagen KB, Kvien TK and
Dagfinrud H: Circulating levels of inflammatory cytokines and
cytokine receptors in patients with ankylosing spondylitis: A
cross-sectional comparative study. Scand J Rheumatol. 44:118–124.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu GZ, Tian W, Fu HT, Li CP and Liu B:
Long noncoding RNA-MEG3 is involved in diabetes mellitus-related
microvascular dysfunction. Biochem Biophys Res Commun. 471:135–141.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su W, Xie W, Shang Q and Su B: The long
noncoding RNA MEG3 is downregulated and inversely associated with
VEGF levels in osteoarthritis. Biomed Res Int. 2015:3568932015.
View Article : Google Scholar : PubMed/NCBI
|