Introduction
The ductus arteriosus (DA) is an important component
of the fetal cardiovascular system. In the normal fetal
circulation, the DA diverts ~78% of the right ventricle output or
46% of the combined cardiac output away from the non-ventilated
lungs and joins the descending aorta to supply the lower body
(1,2). Furthermore, as an important pathway
between pulmonary and systemic circulations, the DA has a vital
role in modulating the flow distribution between the left and right
side of the heart to support the development of important organs,
even in the case of congenital heart disease (CHD) (3,4). The
hemodynamic status of the DA is a major factor that is being
considered in the prenatal diagnosis of CHD and decision-making
regarding its management (5).
Spectral Doppler sonography is widely used in the
clinic due to its reliability in providing quantitative data for
the comprehensively antenatal evaluation of fetal DA. In general,
two sonographic views are independently used to obtain the flow
waveforms of fetal DA, namely the traditional longitudinal ductal
arch (LDA) view or the new introduced three vessel and trachea
(3VT) view. However, it remains controversial which of the two
sonography views is superior in the evaluation of fetal DA, and
differences in the values of velocity parameters measured using
these two views have been noted by numerous specialists in fetal
cardiology (6–10). To address these limitations in
knowledge, a detailed study was performed to confirm whether
sonography assessment from the LDA and 3VT views indeed provides
different Doppler parameters of the fetal DA. Furthermore, the
present study aimed to determine the exact differences and their
potential correlation with the gestational age (GA).
Patients and methods
Study population
In the present study, fetuses referred to the Center
for Medical Ultrasound of the Affiliated Hospital of Nanjing
Medical University (Suzhou, China) for routine screening for fetal
CHD from May 2017 to June 2017 were enrolled. The Ethics Committee
of the Affiliated Suzhou Municipal Hospital of Nanjing Medical
University approved this study, and all pregnant subjects provided
their written informed consent.
The fetal GA was determined based on the time-point
of the last menstruation of the mother. If this information was not
available, the fetal GA was estimated according to the combination
of abdominal circumference, femur length and biparietal diameter
obtained by sonography. The criteria for inclusion were as follows:
i) Absence of congenital malformations and intrauterine growth
retardation revealed by routine ultrasound screening in obstetrics,
ii) normal anatomy, diameter and flow supply of the fetal DA, iii)
normal fetal heart rate (120–160 bpm) and rhythm, iv) low risk for
aneuploidy suggested by cell-free fetal DNA testing, v) absence of
maternal conditions potentially affecting fetal hemodynamics.
Ultrasound equipment
Fetal echocardiography was performed according to
the American Society of Echocardiography guidelines (11), using a Voluson E8 ultrasound system
(GE Healthcare Ultrasound, Milwaukee, WI, USA) coupled with a 3.5
MHz curved-array transducer.
Standardized technique
A standard protocol on waveform recording and
measurement of parameters for detecting fetal DA was used for each
fetus. First, routine obstetric ultrasound scanning was performed
to exclude the presence of congenital malformations, fetal
intrauterine growth retardation and abnormal placental function.
Subsequently, the normal anatomy, diameter and flow supply of the
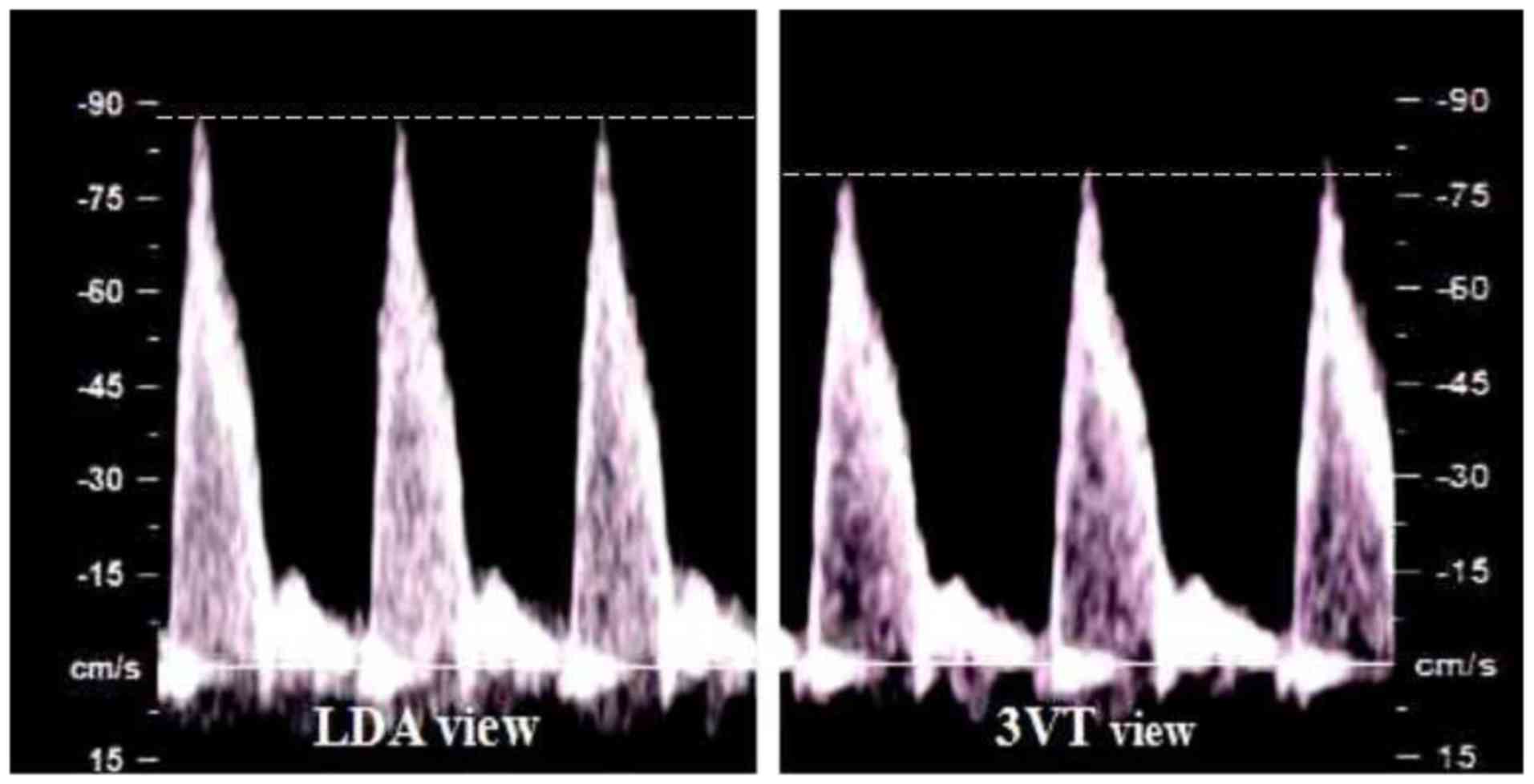
fetal DA was confirmed from the LDA and 3VT views. In particular,
the 3VT view was obtained at the level of the fetal upper
mediastinum by moving the transducer cranially to maintain a
transverse position from the four-chamber view, as described by
Yagel et al (12). The
characteristic V-shape to the left of the fetal trachea in 3VT view
represented the convergence of the aortic isthmus and DA, and the
LDA view was obtained by slightly tilting the transducer from the
right ventricular outflow tract (RVOT) view until the RVOT, major
pulmonary artery trunk and DA were aligned in the shape of a
‘hockey stick-like’ line (13). The
flow waveform of fetal DA in the LDA and the 3VT view was then
separately obtained by using a pulsed wave, ensuring that the fetus
had no obvious breathing movement during the Doppler sampling, and
the sampling point was placed at the middle point between the
origin of the left pulmonary artery and the beginning of the
descending aorta.
To standardize the reproducibility and accuracy of
the measurements, machine settings included the following: i) A
high frame rate with increased contrast and high resolution, ii) a
high-pass wall motion filter (50 Hz) and low-energy output levels
(<50 mW/cm2), iii) absence of Doppler aliasing and
iv) an angle of insonation as close to 0° as possible (<15°). In
a continuous attempt to minimize the measurement and observation
biases, flow waveform obtainment and tracing were evaluated by the
same experienced fetal echocardiography specialist. Furthermore, to
minimize intra-observer variations, the average of three repeated
measurements under the same conditions taken as the final value.
The peak systolic velocity (PSV), end-diastolic velocity (EDV),
time-averaged maximum velocity (TAMXV) and velocity-time integral
(VTI) in the two views were recorded for post-processing (Fig. 1). The pulsatility index (PI) and
resistance index (RI) were calculated according to the following
formulas: PI=(PSV-EDV)/TAMXV and RI=(PSV-EDV)/PSV (14).
Statistical analysis
Statistical analysis was performed with SPSS version
19 for Windows (IBM Corp., Armonk, NY, USA). The entirety of the
data was checked for normality of distribution using the
Kolmogorov-Smirnov test and for homogeneity of variance with
Levene's test. Normally distributed continuous variables were
expressed as the mean ± standard deviation or range. The
significance of differences in PSV, EDV, TAMXV, VTI, PI and RI of
the DA between the LDA and 3VT views was assessed using unpaired
Student's t-tests. Bland-Altman plot analysis was used for
illustrating differences in the impedance indices PI and RI.
Correlations between differences in all indices and the GA were
evaluated by calculating Pearson's linear coefficient of
correlation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Basline characteristics
During the study period, satisfactory flow waveforms
of the fetal DA were successfully obtained in the LDA and 3VT
sonographic planes for 52 normal singleton fetuses (23 males and 29
females). The demographic features are presented in Table I. The mean maternal age at the
time-point of the sonography scan was 31.5 years (range, 23–37
years), and the mean GA was 31.7 weeks (range, 21–39 weeks). The
mean fetal heart rate was 141 bpm (range, 121–157 bpm). The mean
fetal biparietal diameter, abdominal circumference and femur length
were 81 mm (range, 50–95 mm), 275 mm (range, 160–350 mm) and 61 mm
(range, 35–76 mm), respectively. Cell-free fetal DNA testing of all
subjects revealed low risks for trisomies 21, 18 and 13.
 | Table I.Demographic data for all subjects. |
Table I.
Demographic data for all subjects.
| Item | Value |
|---|
| Maternal age
(years) | 31.5 (23–37) |
| Gestational age
(weeks) | 31.7 (21–39) |
| Fetal heart rate
(bpm) | 141 (112–157) |
| Fetal biometry
(mm) |
|
| BPD | 81 (50–95) |
| AC | 275 (160–350) |
| FL | 61 (35–76) |
| Cell-free fetal DNA
testing (z-score) |
|
| Trisomy
21 | 0.23
(−0.07–0.42) |
| Trisomy
18 | −0.59
(−1.19–0.27) |
| Trisomy
13 | −0.24
(−0.89–0.43) |
Differences of Doppler parameters
The parameter values were continuous variables and
normally distributed according to the homogeneity of variance
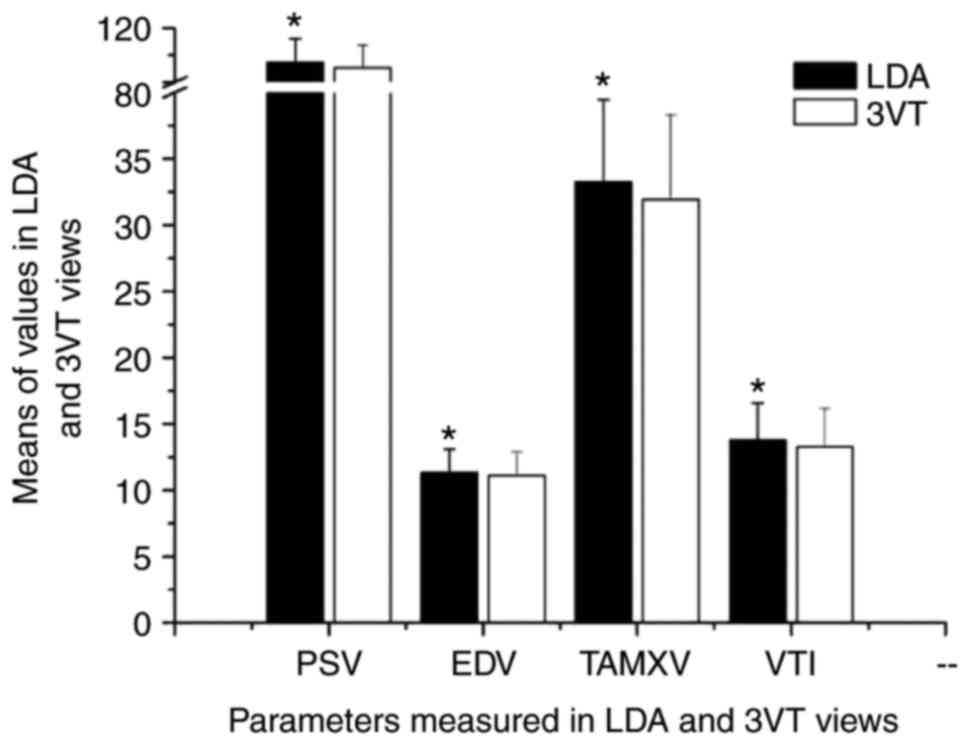
analysis. The PSV, EDV, TAMXV and VTI measured in the LDA view were
significantly increased compared with those measured in the 3VT
view (P<0.05; Table II and
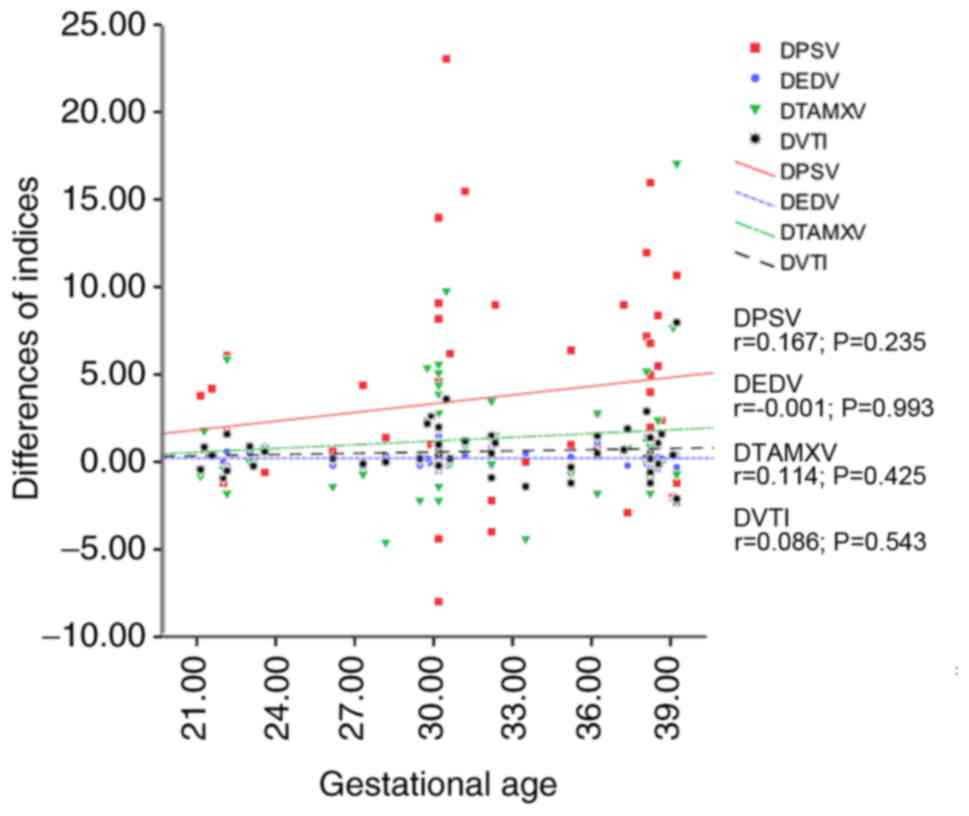
Fig. 2). Furthermore, no significant
correlation was identified between these parameters and fetal GA,
which means that the differences in velocity parameters measured in
the LDA and 3VT views were independent of the GA (P>0.05;
Fig. 3). However, no significant
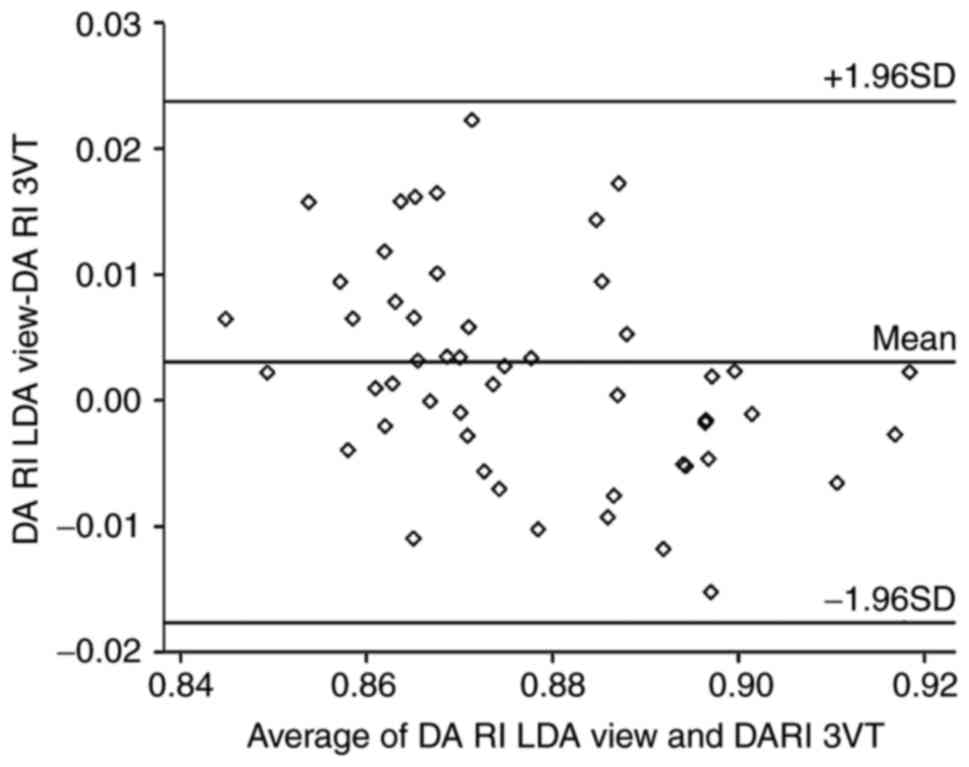
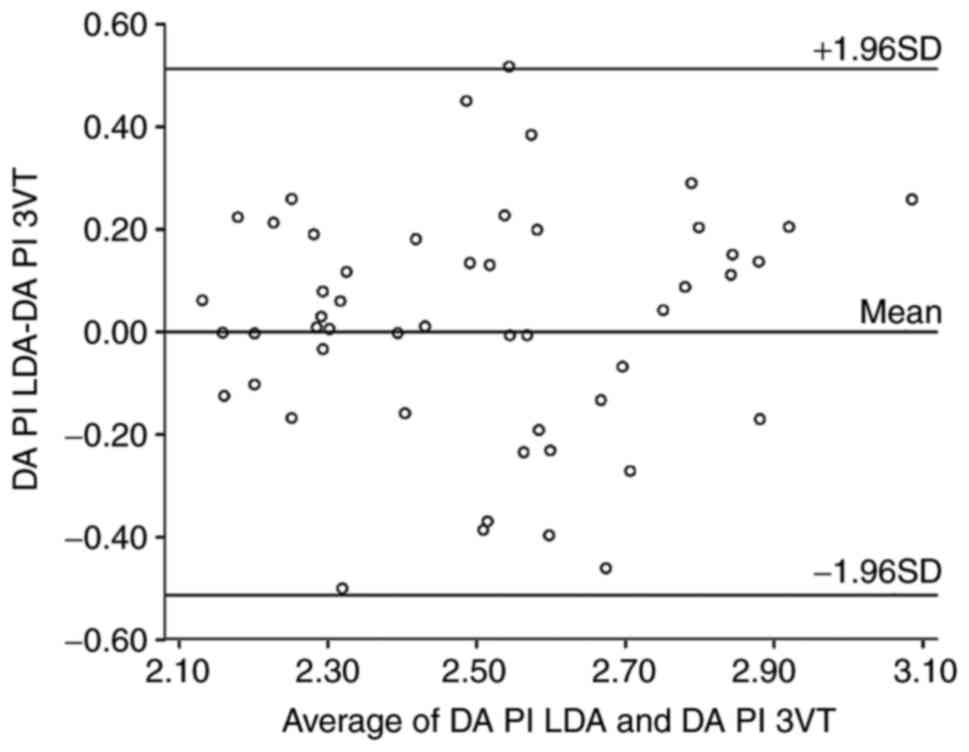
differences in the impedance indices PI and RI were identified
between the LDA view and 3VT view (P>0.05; Table I). The Bland-Altman analysis
demonstrated the agreement of RI and PI measured in two different
views (Figs. 4 and 5).
 | Table II.Doppler parameters of fetal ductus
arteriosus measured at the 3VT and LDA views (n=52). |
Table II.
Doppler parameters of fetal ductus
arteriosus measured at the 3VT and LDA views (n=52).
|
| LDA | 3VT |
|
|---|
|
|
|
|
|
|---|
| Parameter | Mean ± SD | Range | Mean ± SD | Range | P-value |
|---|
| PSV | 94.49±17.59 | 62.80–155.00 | 90.66±16.98 | 59.00–143.00 | <0.01 |
| EDV | 11.33±1.77 | 7.80–14.10 | 11.11±1.80 | 7.60–14.10 | <0.01 |
| TAMXV | 33.27±6.20 | 22.30–53.30 | 31.93±6.38 | 19.10–48.10 | 0.01 |
| VTI | 13.79±2.76 | 8.27–22.10 | 13.24±2.92 | 7.75–21.30 | 0.02 |
| PI | 2.50±0.27 | 2.07–3.21 | 2.50±0.27 | 2.07–3.08 | 0.99 |
| RI | 0.88±0.17 | 0.85–0.92 | 0.88±0.21 | 0.82–0.92 | 0.10 |
Discussion
Quantitative analysis of the fetal DA hemodynamics
has an important role in the comprehensive antenatal evaluation of
the fetal cardiovascular function, particularly in those fetuses
with DA-dependent CHD. The accuracy and reliability of sonographic
parameters are affected by numerous factors, including the
sonographic view used for data measurement or calculation. The
present study revealed differences in the parameter values obtained
from the LDA and 3VT views, while the two have been widely used in
previous relevant studies. Specifically, the present study revealed
a slight increase in the PSV, EDV, TAMXV, and VTI obtained in the
LDA view compared with those obtained in the 3VT view, and that
those increases are not correlated with the GA. However, no
significant differences in PI and RI were observed between the two
sonographic planes. The possible explanations for this phenomenon
are further discussed below.
The angle between fetal DA and the ultrasound beam
is the key factor affecting the differences in Doppler measurements
between the LDA and 3VT views. The LDA view has a greater
probability than the 3VT view to form a smaller angle or even a
parallel alignment of the central axis of the bloodstream flowing
through the DA and the ultrasound beam. First, it is well-known
that the fetal DA and aortic arch are not at the same horizontal
level of the fetal body (15,16); in
fact, the DA is situated slightly lower than the aortic arch, which
can be verified by the earlier emergence of DA compared with the
transverse arch when sliding the transducer cranially from the
four-chamber plane towards the fetal upper mediastinum (17). The central axis of the lumen of the
fetal DA, where the blood flow is fastest, is not included in the
3VT view where the classic ‘V-like’ sign is formed by the pulmonary
artery and the aortic arch, and the DA seen from the 3VT view is
actually the upper part of the fetal DA lumen. Furthermore,
physiologically, the angle between the DA and the descending aorta
(DAO, 98.4±12.5°) is significantly larger than that between the
aortic arch and the DAO (88.5±5.5°) (18–20).
Since the 3VT view is obtained in the horizontal direction of the
fetal body, the angle between the DA and DAO is relatively large,
thereby reducing the probability of forming the smallest angle
between the DA and the ultrasound beam compared with that including
the aortic arch. However, in the LDA view, the sagittal section of
the DA arch was clearly visualized in a ‘hockey stick’ shape
(13). The transducer may be slid in
any direction to obtain the smallest angle between the DA flow and
ultrasound beam regardless of the spatial association with the
aortic arch and the DAO. Therefore, compared to the 3VT view, the
LDA view allows for achieving a smaller angle between the DA and
the ultrasound beam. Therefore, higher velocity parameters of fetal
DA may be measured in the 3VT view. However, there were no
differences in the PI and RI calculated in the two views, which was
consistent with the well-known theory that the impedance indices
are independent of the sampling angle of the target structure and
ultrasound beam.
The present study revealed that the velocity
parameters of the fetal DA measured in the LDA view are
significantly higher than those obtained in the 3VT view, and the
differences are independent of fetal GA. This means that in the
second half of gestation, the LDA view is better than the 3VT view
in the assessment of the flow restriction or the tendency of
premature closure of fetal DA. In addition, the present study also
demonstrated that the impedance indices PI and RI of the flow
within the fetal DA are similar between the LDA and 3VT views. In
other words, it is not necessary to obtain the LDA view, as is
performed for the measurement of velocity parameters, if only the
impedance indices of the fetal DA are required.
The primary limitations of the present study were as
follows: First, the relative spatial associations among the fetal
DA, aortic arch and DAO may vary with advancing gestation, which
was not taken into consideration in detail. Of note, most obstetric
ultrasound screening examinations are performed during the second
half of gestation. Furthermore, the present study did not include
any fetuses with premature restriction, closure of the DA or
arteriosus aneurysm. In those cases, it is required to slightly
adjust the transducer to identify the smallest angle between the DA
and ultrasound beam to obtain the optimal waveform, which were not
usually in the standard LDA or 3VT views.
In conclusion, the LDA view has a greater
probability than the 3VT view to obtain higher values of velocity
parameters of the fetal DA, including PSV, EDV, TMAXV and VTI, and
the differences are independent of the fetal GA. However, no
significant variations were observed in the impedance indices PI
and RI between these two sonographic planes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Suzhou Basic
Research in Medical and Health Application grant (grant no.
SYSD2016109).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG conceived of the study, collected the data,
acquire funding and wrote the manuscript. JZ, ZW and SL collected
the data. XY analyzed the data and wrote and edited the manuscript.
ZG, XY, JZ, ZW and SL designed the study. XD acquired funding and
performed the experiments.
Ethics approval and consent to
participate
The Ethics Committee of the Affiliated Suzhou
Municipal Hospital of Nanjing Medical University approved this
study. All pregnant subjects provided their written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gournay V: The ductus arteriosus:
Physiology, regulation, and functional and congenital anomalies.
Arch Cardiovasc Dis. 104:578–585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mielke G and Benda N: Cardiac output and
central distribution of blood flow in the human fetus. Circulation.
103:1662–1668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rudolph AM: Congenital cardiovascular
malformations and the fetal circulation. Arch Dis Child Fetal
Neonatal Ed. 95:F132–E136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han W, Xie M, Cheng TO, Wang Y, Zhang L,
Hu Y, Cao H, Hong L, Yang Y, Sun Z and Yu L: The vital role the
ductus arteriosus plays in the fetal diagnosis of congenital heart
disease: Evaluation by fetal echocardiography in combination with
an innocative caediovascular cast techonology. Int J Cardiol.
202:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weichert J, Hartge DR and Axt-Fliedner R:
The fetal ductus arteriosus and its abnormalities-a review.
Congenit Heart Dis. 5:398–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Howley L, Wood C, Patel SS, Zaretsky MV,
Crombleholme T and Cuneo B: Flow patterns in the ductus arteriosus
during open fetal myelomeningocele repair. Prenat Diagn.
35:564–570. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mari G, Deter RL and Uerpairojkit B: Flow
velocity waveforms of the ductus arteriosus in appropriate and
small-for-gestational-age fetuses. J Clin Ultrasound. 24:185–196.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopes LM, Carrilho MC, Francisco RP, Lopes
MA, Krebs VL and Zugaib M: Fetal ductus arteriosus constriction and
closure: analysis of the causes and perinatal outcome related to 45
consecutive cases. J Matern Fetal Neonatal Med. 29:638–645. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mielke G and Benda N: Blood flow velocity
waveforms of the fetal pulmonary artery and the ductus arteriosus:
Reference ranges from 13 weeks to term. Ultrasound Obstet Gynecol.
15:213–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi JY, Park JD, Jung MJ and Kim HS:
Doppler flow velocity waveforms of human fetal ductus arteriosus
and branch pulmonary artery. J Korean Med Sci. 12:409–415. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rychik J, Ayres N, Cuneo B, Gotteiner N,
Hornberger L, Spevak PJ and Van Der Veld M: American Society of
Echocardiography guidelines and standards for performance of the
fetal echocardiogram. J Am Soc Echocardiogr. 17:803–810. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yagel S, Arbel R, Anteby EY, Raveh D and
Achiron R: The three vessels and trachea view (3VT) in fetal
cardiac scanning. Ultrasound Obstet Gynecol. 20:340–345. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MY and Won HS: Technique of fetal
echocardiography. Obstet Gynecol Sci. 56:217–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Del Río M, Martínez JM, Figueras F,
Bennasar M, Palacio M, Gómez O, Coll O, Puerto B and Cararach V:
Doppler assessment of fetal aortic isthmus blood flow in two
different sonographic planes during the second half of gestation.
Ultrasound Obstet Gynecol. 26:170–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satomi G: Guidelines for fetal
echocardiography. Pediatr Int. 57:1–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brezinka C, DeRuiter M, Slomp J, den
Hollander N, Wladimiroff JW and Gittenberger-de Groot AC:
Anatomical and sonographic correlation of the fetal ductus
arteriosus in first and second trimester pregnancy. Ultrasound Med
Biol. 20:219–224. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jackson CM, Sandor GG, Lim K, Duncan WJ
and Potts JE: Diagnosis of fetal ductus arteriosus aneurysm:
Importance of the three-vessel view. Ultrasound Obstet Gynecol.
26:57–62. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Espinoza J, Gotsch F, Kusanovic JP,
Gonçalves LF, Lee W, Hassan S, Mittal P, Schoen ML and Romero R:
Changes in fetal cardiac genometry with gestation: Implications for
3- and 4-dimensional fetal echocardiography. J Ultrasound Med.
26:437–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arya B, Bhat A, Vernon M, Conwell J and
Lewin M: Utility of novel fetal echocardiographic morphometric of
the aortic arch in the diagnosis of neonatal coarctation of the
aorta. Prenat Diagn. 36:127–134. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mielke G and Benda N: Reference ranges for
two-dimensional echocaridographic examination of the fetal ductus
arterious. Ultrasound Obstet Gynecol. 15:219–225. 2000. View Article : Google Scholar : PubMed/NCBI
|