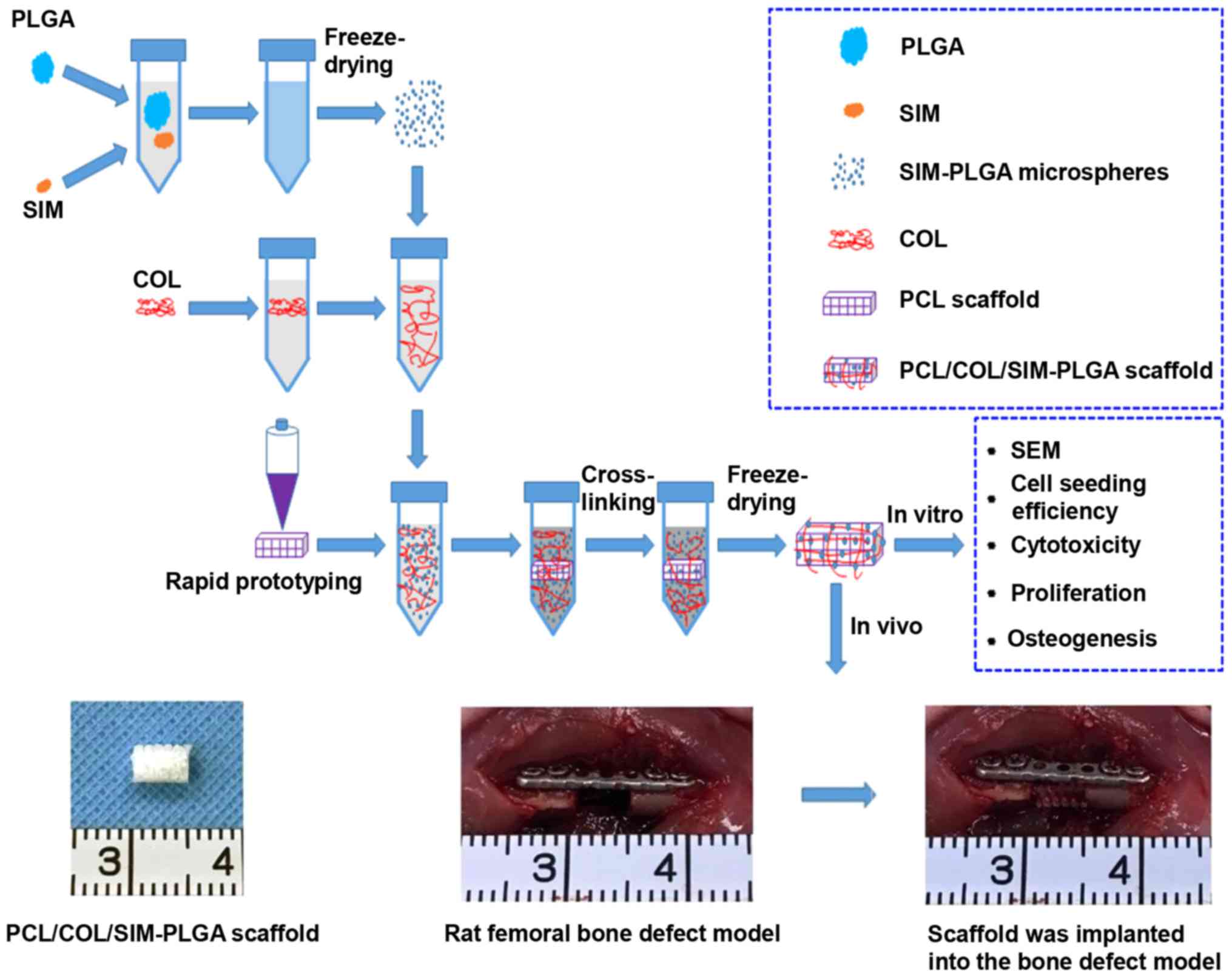
Introduction
Bone defects arise following surgery for bone
tumors, traumatic injury or congenital disorders, and often require
surgical intervention using effective bone grafts. Ideal scaffolds
for bone tissue engineering usually require strong mechanical
properties as well as good biocompatibility (1), new bone-synchronizing degradation
abilities (2,3), adjustable pore structure and
osteogenesis-promoting capabilities (4). To date, the most frequently used bone
substitute materials include metal, ceramics and natural or
synthetic polymers (5). However,
metallic scaffolds may weaken bone growth due to stress shielding
as a result of excessive rigid fixation, while ceramic is
associated with brittleness and poor degradability (6–8). Due to
these issues with the current bone repair materials available,
further investigation is required in order to identify novel bone
tissue engineering scaffolds.
Poly(ε-caprolactone) (PCL) has drawn much attention
in the field of bone tissue engineering due to its excellent
biocompatibility, biodegradability, mechanical properties and
workability (9). However, the major
limitations of PCL are that it lacks a cell recognition site and is
hydrophobic, thus, it can induce in vivo fibrous
encapsulation (10). Therefore, a
number of previous studies have been performed to investigate
methods of enhancing the cell affinity of PCL, such as through
surface coating with collagen (11–13).
An ideal bone tissue engineering scaffold should be
as similar as possible to the natural bone, which is a type of
mineralized collagen composite with hierarchical structure; thus,
many previous studies have focused on using biomimetic strategies
in order to fabricate scaffolds (5,14,15). As
a completely computer aided process, three-dimensional (3D)
printing technology, also known as rapid prototyping (RP), provides
a new method to accurately design and produce scaffolds with high
porosity and connectable pore networks (8,16). In
order to build a scaffold that matches the function and structure
of natural bone, a hierarchical composite scaffold is constructed
by combining the RP technology with the bionic functional
manufacturing strategy, as applied in the present study.
Simvastatin (SIM), which is currently used
clinically to lower blood cholesterol and low-density lipoprotein,
has been hypothesized to promote osteoblast proliferation and
differentiation, inhibit osteoclast activity and support immune
cells such as macrophages; thus, it has been proposed to enhance
bone repair (17–21). A controlled drug release system is
able to provide a sustained stimulus for bone regeneration. For
this reason, the controlled release of SIM was applied in the
present study with poly(lactic-co-glycolic acid) (PLGA)
microspheres. Owing to its good biocompatibility and drug-loading
properties, PLGA has been approved by the US Food and Drug
Administration for application in pharmaceuticals, medical
materials and tissue engineering (22). Furthermore, microspheres based on
PLGA are of stable degradability and are easy to fabricate since
the size and distribution of particles are controllable; they are
therefore becoming increasingly favorable as drug carriers
(23–26).
In the present study, a PCL macro-porous framework
construct was first generated using RP technology, which had
favorable mechanical properties to support nascent bone tissue in
growth. Then, collagen (COL) incorporating SIM-loaded PLGA
microspheres was coated on to the PCL framework using the
evacuation method to formulate bone-like microporous networks and
to provide a sustained osteoinduction stimulus. The in vitro
osteogenic effect of the scaffolds was evaluated using bone
marrow-derived mesenchymal stem cells (BMSCs), and their in
vivo osteogenic potential was investigated using a rat femur
defect model.
Materials and methods
Materials
PCL was purchased from Shenzhen Esun Industrial Co.,
Ltd. (Shenzhen, China). COL was purchased from Sichuan Mingrang
Bio-Tech Co., Ltd. (Sichuan, China).
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
crystalline (EDC), SIM and PLGA were purchased from Sigma Aldrich
(Merck KGaA, Darmstadt, Germany). A Cell Counting Kit-8 (CCK-8) was
obtained from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). Polyvinyl alcohol (PVA) and dichloromethane were purchased
from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China).
Generation of SIM-loaded PLGA
(SIM-PLGA) microspheres
SIM-PLGA microspheres were prepared by utilizing the
single emulsion solvent evaporation method, as previously described
(27). A total of 460 mg PLGA and 23
mg SIM were dissolved in 6 ml dichloromethane. The mixture was
ultrasonically shaken for 30 sec and left standing at room
temperature for 30 min. It was then slowly dropped into aqueous
solution containing 3% PVA, and stirred for 6 h until it fully
solidified into microspheres. Then the microspheres were rinsed
three times with deionized water, centrifuged at 300 × g and 4°C
for 10 min, pre-frozen at −80°C for 2 h and restored following
lyophilization for 24 h.
Encapsulation efficiency
The content of SIM in the microspheres was
determined using an ultraviolet (UV) spectrophotometer (Beckman
Coulter, Inc., Brea, CA, USA) (26,28–30). UV
scanning from 100 to 1,000 nm was used to identify the absorption
peak of SIM. It was identified that the absorbance of SIM solution
was greatly affected by its concentration at 250 nm. The standard
absorption curve of SIM was calculated according to the absorbance
of samples with known SIM concentrations at 250 nm. The SIM content
in the solution was calculated according to the standard absorption
curve. Briefly, 1 mg SIM-PLGA microspheres was dissolved in 5 ml
acetonitrile and sonicated at 40,000 Hz for 40 sec at room
temperature. The UV absorbance of the sample was examined and the
concentration of SIM was determined using the standard curve.
Encapsulation efficiency of the SIM-PLGA microspheres was
calculated according to the following formula: Encapsulation
efficiency (%)=(amount of encapsulated SIM)/(amount of total SIM)
×100%.
Release properties
A total of 1 mg of PLGA microspheres was incubated
with 5 ml PBS and placed in a vibrating screen with constant
stirring (60 rpm) at 37°C. After 1, 3, 7, 14, 21 and 28 days, 2.5
ml of the supernatant was removed and an equal volume of fresh PBS
was added. The content of SIM in the solution was quantified using
a UV spectrophotometer as aforementioned.
PCL scaffold generation and
functionalization
PCL scaffolds were generated with a fused deposition
modeling 3D printer (Glarun Technology Co., Ltd., Nanjing, China)
as previously described (5).
Briefly, a 3D porous CAD model was established and modified in stl
format using Magics software 2.1 (Materialise, Leuven, Belgium),
processed in the 0-60-120° pattern with a filament width of 500 µm,
and the final vertical and lateral pore sizes of 1,000 and 250 µm,
respectively. The PCL scaffold was then produced according to the
stl module. Firstly, the raw PCL material was heated to 120°C, so
that PCL melted to a half flow state. Using the computer controls,
according to the shape of the STL file module, PCL with a diameter
of 500 µm was squeezed from the nozzle along the X- and Y-axis,
cooled to room temperature and allowed to solidify. Then, the
nozzle was raised to a specific height along the Z-axis and the
process was repeated. The new half flow PCL was squeezed from the
nozzle and tightly bound to the lower layer after cooling and
solidifying.
Generation of the PCL/COL/SIM-PLGA
scaffold
For surface activation, the PCL scaffolds were first
alkaline-treated with 5 M NaOH solution at 37°C for 24 h, and then
subsequently immersed in COL solution (1 wt% in 0.1 M acetic acid),
vacuumed for 30 min, frozen at −80°C and freeze-dried at −50°C for
48 h in order to obtain the preliminary COL-PCL composite. The
scaffolds were then cross-linked with 5 mM EDC solution for 24 h,
thoroughly washed with 5 wt% glycine solution and distilled water
three times, and freeze-dried at −50°C for 24 h. To incorporate the
SIM-PLGA into the PCL/COL scaffolds, 7 mg of microspheres was added
to 10 ml COL solution, thoroughly mixed by manual agitation and
freeze-dried as aforementioned.
Morphology and porosity
observations
The morphologies of the scaffold and the
microspheres were observed by field-emission scanning electron
microscopy (SEM; Hitachi S-4800; Hitachi, Ltd., Tokyo, Japan) at a
beam energy of 10 and 3 keV, respectively. To evaluate the PCL/COL
scaffold, the molded samples were cut into 5×2.5×2.5 mm pieces,
which were then gold-coated prior to observation. The porosity of
the scaffolds was measured using an ethanol infiltration method.
The sample was slowly immersed into a known volume (V1)
of absolute alcohol solution (Aladdin Bio-Chem Technology Co.,
Ltd.) inside a measuring cylinder. After 1 h, the total volume of
alcohol and sample was regarded as V2. Following careful
removal of the sample, the residual volume of alcohol solution was
measured as V3. The results were determined from the
following formula: Porosity
(%)=(V1-V3)/(V2-V3)
×100%.
In vitro cell seeding, proliferation,
viability and cytotoxicity evaluations
BMSCs were obtained from newborn Sprague-Dawley rats
as described in a previous study with ethical approval from the
Animal Care and Use Committee of Shanghai Ninth People's Hospital
(Shanghai, China) (31). The
protocol conformed to the National Institutes of Health Guidelines
concerning the Care and Use of Laboratory Animals (32). The cell culture medium consisted of
89% Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). Passages 2–3 of BMSCs w ere used for the subsequent
experiments.
For evaluation of cell-seeding efficiency,
5×105 cells in 100 µl D10 medium were added into the
constructs (PCL, PCL/COL and PCL/COL/SIM-PLGA; n=5 each group). The
cell number was counted as W1. Following incubation for
4 h, the seeded constructs were removed and washed with PBS. Cells
that had adhered were digested with trypsin and were counted as
W2; thus, the cell seeding efficiency was calculated
according to the following formula: Cell seeding
efficiency=W2/W1.
To evaluate cytotoxicity, 1×103 BMSCs
were seeded onto a 96-well plate and incubated for 4 h in D10
medium, then the medium was replaced with fresh medium, fresh D10
medium with 5% DMSO, or the extract fluid of PCL, PCL/COL, or
PCL/COL/SIM-PLGA, which was prepared by immersing the constructs in
D10 medium for 72 h (n=5). CCK-8 assays were then performed to
assess the cytotoxicity 1 day later at a wavelength of 450 nm.
To evaluate cell proliferation, 3×104
cells in 20 µl D10 medium were seeded into the PCL, PCL/COL,
PCL/COL/SIM-PLGA, or decalcified bone matrix (DBM; cut to 5×2.5×2.5
mm) in 24-well plates and incubated in 1 ml of D10 medium for 1 h.
The procedure was then repeated on the other side of the
constructs. At 1, 3 and 7 days after seeding, cell proliferation on
the different constructs was assessed by CCK-8 assay. DBM was
prepared as described in a previous study (33).
Alkaline phosphatase (ALP) and
Alizarin red staining
Since it is difficult to extract the mRNA of cells
directly cultured on scaffolds, and the blank scaffold would have
very poor osteoinduction since this property is primarily due to
the encapsulated drug, cells were cultured on the well plate and
scaffolds were placed on the Transwell membrane above the cells
(34). BMSCs (1×104) of
passage 3 were seeded in 24-well plates. After 6 h, 1 ml of
osteogenic induction medium (50 mg/ml ascorbic acid, 10 mM
β-glycerophosphate and 10 nM dexamethasone) was added. Then, the
0.8-µm-pore inserts and the different scaffolds were added to the
wells of the culture plates. On day 7 following osteogenic
induction, the cells were fixed in 4% paraformaldehyde for 20 min
at room temperature and stained in ALP staining solution (Beyotime
Institute of Biotechnology, Haimen, China) at 37°C for 10 min in
the dark. ALP activity after 7 and 14 days of culture was
quantified using the QuantiChrom™ Alkaline Phosphatase Assay kit
(BioAssay Systems; Thermo Fisher Scientific, Inc.), and the total
protein content was assessed with a BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). The mineralized matrix nodules formed by
BMSCs were evaluated using Alizarin red staining. On day 21, the
cells were washed three times with PBS, fixed in 4%
paraformaldehyde at room temperature for 30 min and incubated with
Alizarin red solution (Beyotime Institute of Biotechnology) for 10
min at room temperature. To quantify the levels of mineralization,
the mineralized matrix nodules were dissolved in 10% (w/v)
cetylpyridinium chloride (Aladdin Bio-Chem Technology Co., Ltd.)
and the absorbance was measured at 450 nm using a microplate
reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression of genes associated with osteogenesis
was assessed by RT-qPCR, as previously described (35). After 7 and 14 days of culture, total
RNA was prepared using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) in order to determine the mRNA expression of ALP
and COL type 1 (COL 1), which are associated with osteogenesis.
cDNA was obtained by reverse transcription at 37°C for 1 h using
BeyoRT™ II First Strand cDNA Synthesis kit with gDNA Eraser
(Beyotime Institute of Biotechnology) from 1 µg RNA and stored at
−20°C. qPCR was conducted using SYBR GreenER qPCR SuperMix reagents
(Invitrogen; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the PCR: Initial
denaturation at 95°C for 10 min; 30 cycles of 95°C for 15 sec, and
60°C for 1 min; and a final dissociation cycle at 95°C for 15 sec,
60°C for 1 min and 95°C for 15 sec. The following 5′-3′ primer
sequences were used: ALP forward, CGTGGAAACCTGATGTATGCT and
reverse, ACTCCTATGACTTCTGCGTCTG; COL 1 forward,
GAAAGAGAAAGACCCCAGTTAC and reverse, ATACCATCTCCCAGGAACAT; β-actin
forward, CCTCTATGCCAACACAGT and reverse, AGCCACCAATCCACACAG; BSP
forward, GATAGTTCGGAGGAGGAGGG and reverse, CTAACTCCAACTTTCCAGCGT;
OPN forward CCTGGACCTCATCAGCATTT and reverse TTGGAGCAAGGAGAACCC;
RUNX2 forward GCACCCAGCCCATAATAGA and reverse
GACGGTTATGGTCAAGGTGAA; OCN forward GGAGGGCAGTAAGGTGGTGAA and
reverse GAAGCCAATGTGGTCCGCTA. β-actin forward CACGAAACTACCTTCAACTCC
and reverse CATACTCCTGCTTGCTGATC. The 2−ΔΔCq method was
used to calculate the relative expression of each target gene
against the β-actin (36).
In vivo study of segmental defect
repair on rat femurs
The following animal experiments were performed with
ethical approval from the Animal Care and Use Committee of Shanghai
Ninth People's Hospital (Shanghai, China), and conformed to the
National Institutes of Health Guidelines concerning the Care and
Use of Laboratory Animals (37).
A diagram of the experimental procedure is presented
in Fig. 1. A total of 12 male
Sprague-Dawley rats aged 6–8 weeks (~200 g) were purchased from the
Shanghai Jiao Tong University Research Center of Laboratory Animal
(Shanghai, China). The animals were housed and acclimatized at the
experimental animal laboratory of Shanghai Ninth People's Hospital
(Shanghai, China) under controlled temperature (25°C) and humidity
(55%) with a 12-h light/dark cycle, with free access to food and
water. The rats were randomly divided into three groups: i) PCL
(bone defect + blank PCL scaffold); ii) PCL/COL (bone defect + PCL
scaffold coated with COL); and iii) PCL/COL/SIM-PLGA (bone defect +
PCL scaffold coated with COL and SIM-loaded PLGA microspheres; n=4
each group). Rats were anesthetized via intraperitoneal injection
of 1% pentobarbital sodium (Invitrogen; Thermo Fisher Scientific,
Inc.; 40 mg/kg). Under a standard surgical procedure, the femur was
fully exposed and holes were made on both femoral ends with a micro
bone drill. Then, a titanium plate (15×2.8 mm) was fixed along the
femur using tapping screws (1 mm in diameter, 6 mm in length).
Then, a 5 mm-long segmental defect was made in the middle position
corresponding to the plate with the bone drill, which was then
cleaned with 0.9% saline and the PCL, PCL/COL and PCL/COL/SIM-PLGA
scaffolds were transferred to the defect area respectively.
X-ray examination was performed at 4 and 12 weeks
after surgery. At 12 weeks post-surgery, the animals were
sacrificed and the femurs of rats were fixed in 4% paraformaldehyde
at room temperature for a week. The specimens were then detected by
micro-computed tomography (micro-CT; SCANCO µ-80 Micro-CT; SCANCO
Medical AG, Brüttisellen, Switzerland). 3D reconstruction and
calculation of the new bone tissue was subsequently performed with
the built-in micro-CT software. The regions of interest were
restricted to the cylindrical part covering the defect, whereas
regions with CT values of 1,300–3,500 were determined as bone
tissue.
Histological assay
Following micro-CT testing, the specimens were
decalcified with 10% EDTA, dehydrated, paraffin embedded, sectioned
into 5-µm-thick slices for hematoxylin and eosin (HE; 30 min at
room temperature) and Masson trichrome (20 min at room temperature)
staining, and examined by light microscopy.
For immunohistochemistry, the sections were blocked
with 5% bovine serum albumin (Invitrogen; Thermo Fisher Scientific,
Inc.) for 30 min at room temperature and then incubated with the
anti-osteocalcin (OCN; Abcam, Cambridge, UK; cat. no. ab198228;
1:200) primary antibody overnight at room temperature. The sections
were then incubated with the horseradish peroxidase-conjugated goat
anti-rabbit IgG (H+L) secondary antibody (Beyotime Institute of
Biotechnology; cat. no. A0208) at a dilution of 1:200 for 1 h at
room temperature, visualized with 3,3-diaminobenzidine solution,
and counter-stained with hematoxylin (Beyotime Institute of
Biotechnology) for 1 h at room temperature. Image Pro Plus software
6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used to
analyze the results.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The results were evaluated by one-way analysis of
variance with Tukey's post-hoc using SPSS 15.0 software for Windows
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Surface characterization
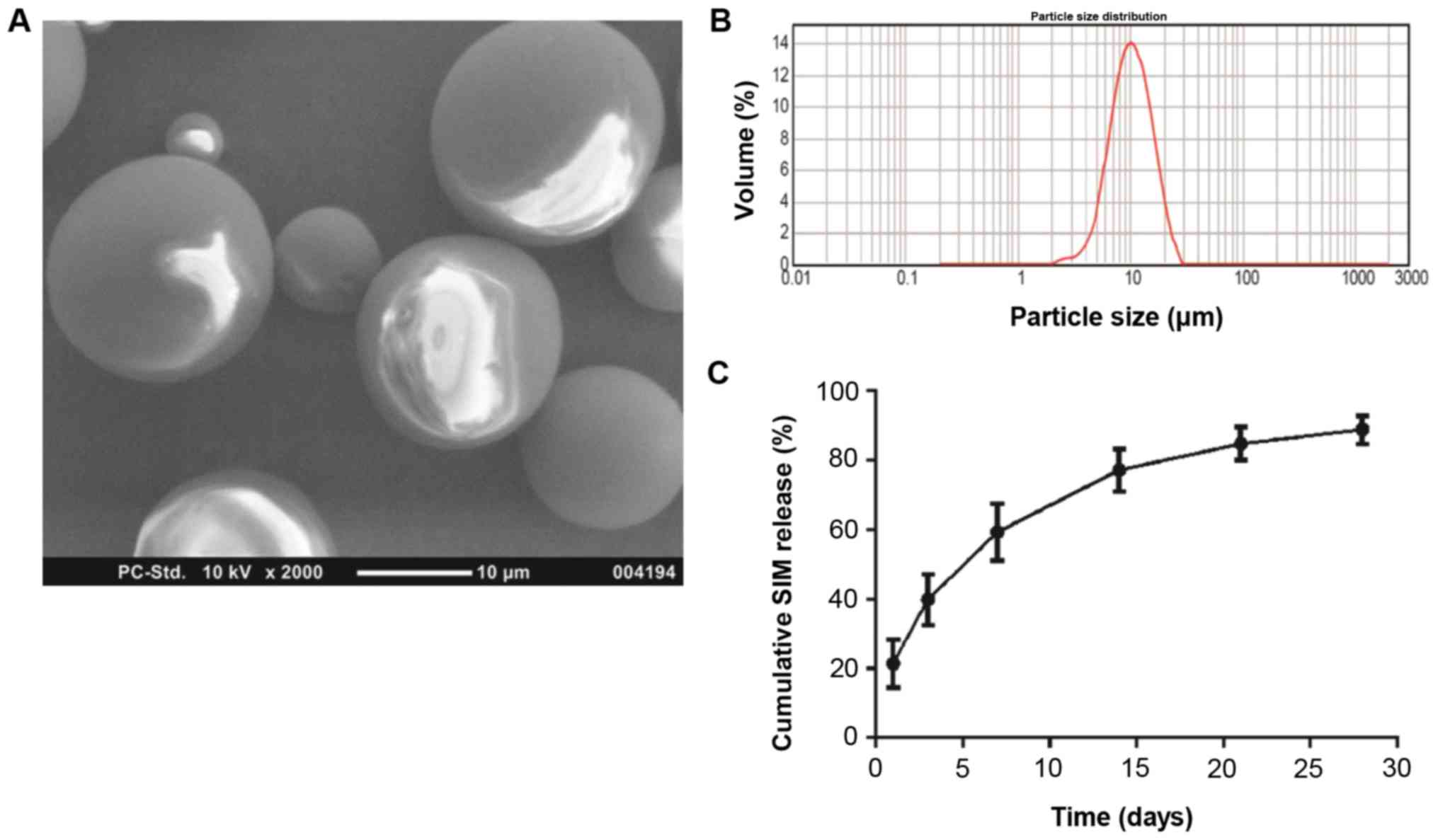
As demonstrated in the SEM images, the SIM-PLGA
microspheres assumed a configuration with a smooth, glossy surface
and regular spherical shape (Fig.
2A), indicating that the experimental conditions for the
manufacture of microspheres were stable and suitable (31,38,39). The
particle diameter (Fig. 2B) ranged
from 2–20 µm (mean, 12.11 µm). The narrow particle size
distribution suggested that the drug release from scaffolds would
be relatively stable (40,41). In addition, the encapsulation
efficiency of SIM inside the PLGA microspheres reached ~86.3%. An
initial outbreak release was detected in the first 3 days. As
indicated in the in vitro release diagram, ~40% of the
primarily integrated SIM was leaked from the PLGA microspheres
within the first 3 days, which was followed by sustained release
over 27 days (Fig. 2C). At the end
of the release assay, almost 90% of the SIM-loaded microspheres
were released (Fig. 2C), and the
cumulative drug release achieved ~86.9%.
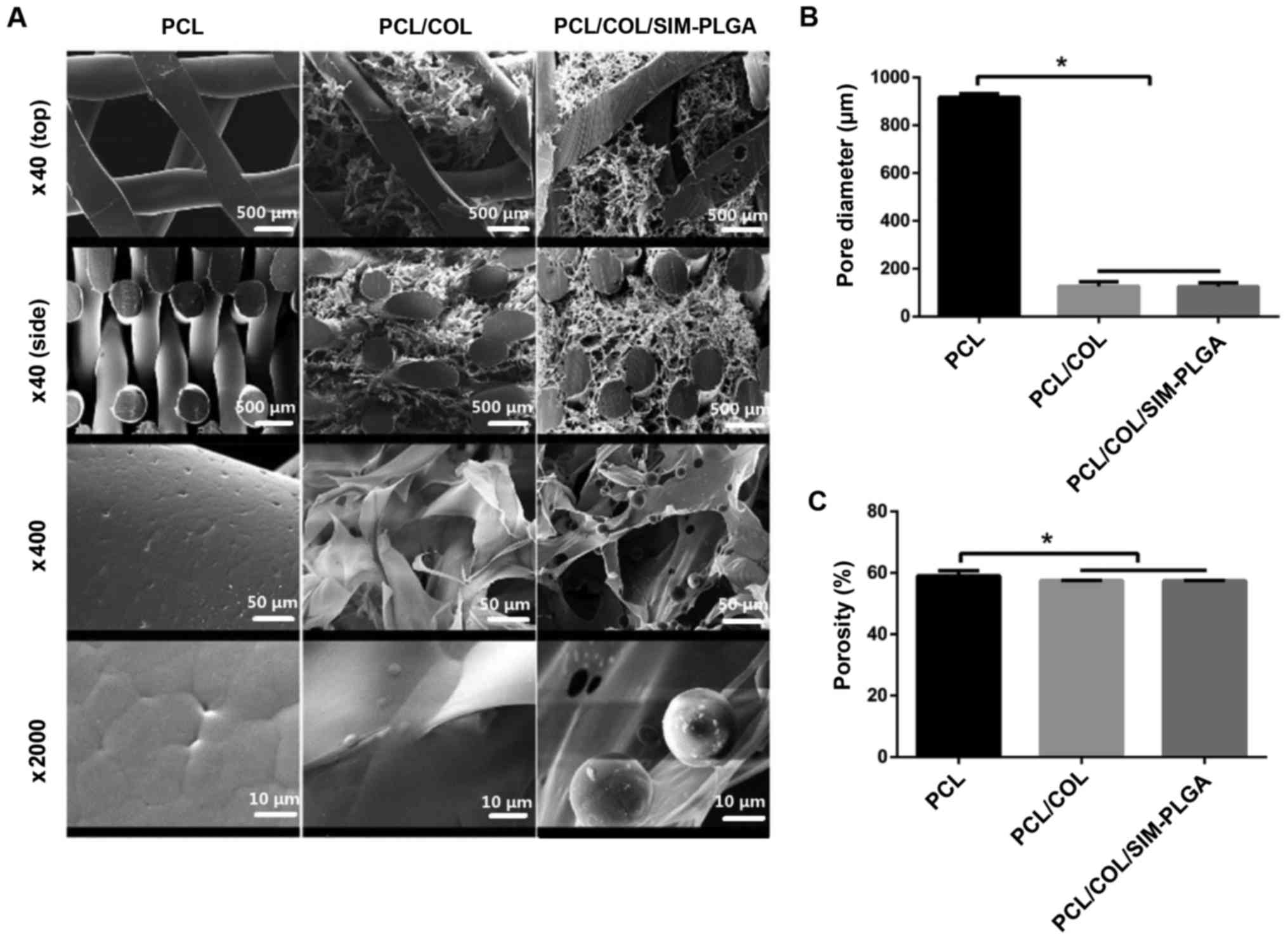
In the present study, the PCL scaffold alone
exhibited a vertical aperture at 917±14.8 µm. Porous COL with good
biological activity was well compounded onto the PCL scaffold by
vacuuming and freeze-drying, forming a thin coating layer. COL
displayed porosity with homogeneous pore sizes, filling in the
horizontal pores in a reticular pattern and covering the trabecula
of the PCL scaffold. Therefore, the PCL/COL composite possessed a
two-level pore structure, consisting of macro-porous PCL (~1,000
µm) and microporous COL (~100 µm). Following further incorporation,
PLGA microspheres were evenly distributed in the COL network
(Fig. 3A). The pore size of PCL/COL
was 126.7±18.42 µm, whereas that of PCL/COL/SIM-PLGA was
125.7±15.82 µm (Fig. 3B). In
addition, the porosity of the three constructs was 59.05±1.7,
57.5±0.1 and 57.4±0.1%, respectively (Fig. 3C). These results revealed that a
reduced but hierarchical structure was formed following COL
modification, while the incorporation of SIM-PLGA had no
significant effect on porosity.
In vitro cellular evaluation
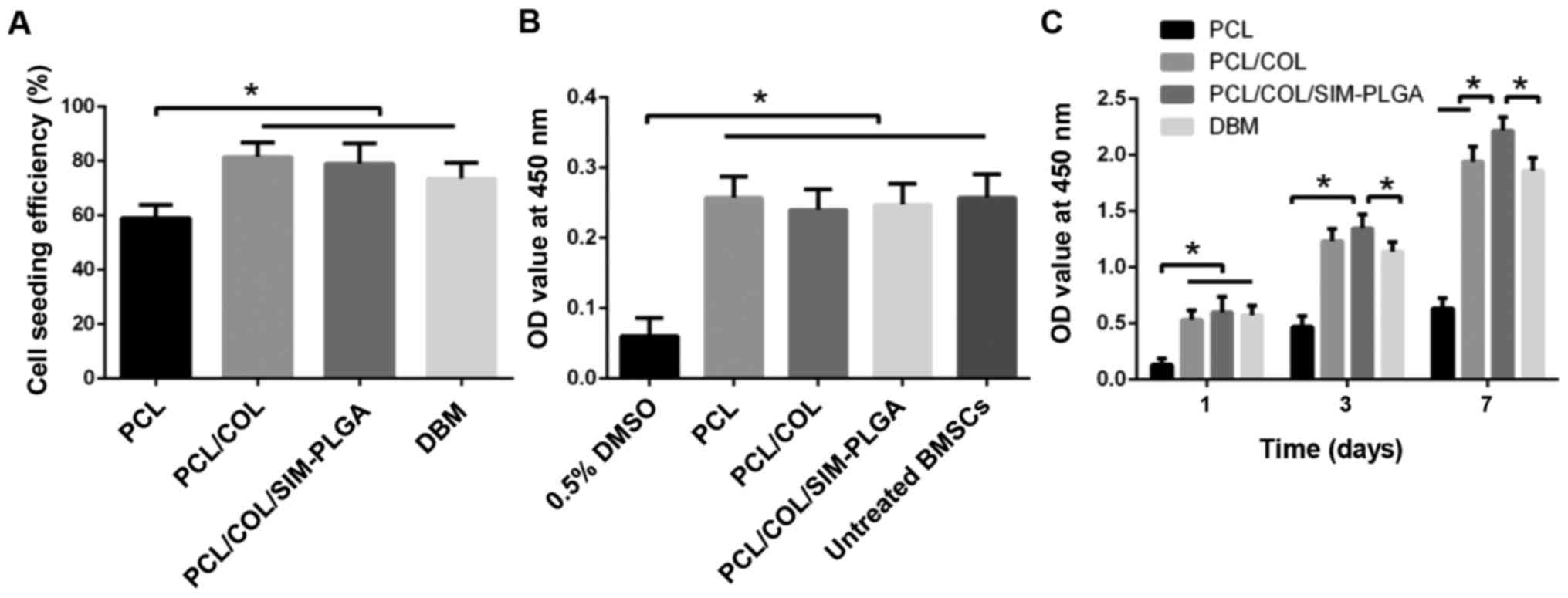
Cell proliferation and viability
BMSCs were directly seeded on the scaffold. The
cell-seeding efficiency was revealed to be 54±2.2% for PCL,
88.6±2.0% for PCL/COL and 89±1.6% for PCL/COL/SIM-PLGA. PCL/COL and
PCL/COL/SIM-PLGA exhibited a significantly higher efficiency (1.64-
and 1.65-fold increase, respectively) when compared with PCL alone
(Fig. 4A). The improvement in cell
seeding may be attributable to the greater contact area with the
COL network and more adhesion sites from the COL matrix, while PLGA
microspheres may have exerted little influence. The extracted fluid
test demonstrated that there was no significant cytotoxicity in the
scaffolds when compared with the untreated BMSC group, indicating
that no toxic substances were identified during the COL
cross-linking process, and thus demonstrating its biosafety
(Fig. 4B). Furthermore, the greatest
levels of proliferation were produced by the SIM-PLGA group, which
may be caused by the SIM released from the scaffold (Fig. 4C).
Osteogenic potency of BMSCs
To assess the osteogenic potency of BMSCs treated
with the different constructs, the present study performed Alizarin
red and ALP staining. Cells were cultured on the well plate and
scaffolds were placed on a Transwell membrane above the cells.
Typical osteogenic differentiation was observed in all three
groups. However, the SIM/PLGA group exhibited higher ALP activity
and more mineralized nodules (Fig.
5A). Despite the increase in ALP expression noted in all three
groups following 14 days of differentiation when compared with that
observed after 7 days (Fig. 5B), the
SIM-PLGA group exhibited significantly higher expression levels
when compared with the other three groups at 7 and 14 days.
Furthermore, quantitative analysis of Alizarin red staining
indicated that the OD value of the SIM/PLGA group was the highest
among the four groups, suggesting that the mineral deposition and
the osteogenic differentiation of BMSCs improved. By contrast, the
DBM group exhibited similar osteogenesis to the PCL group, as
determined by the quantitative results of Alizarin red staining
(Fig. 5C).
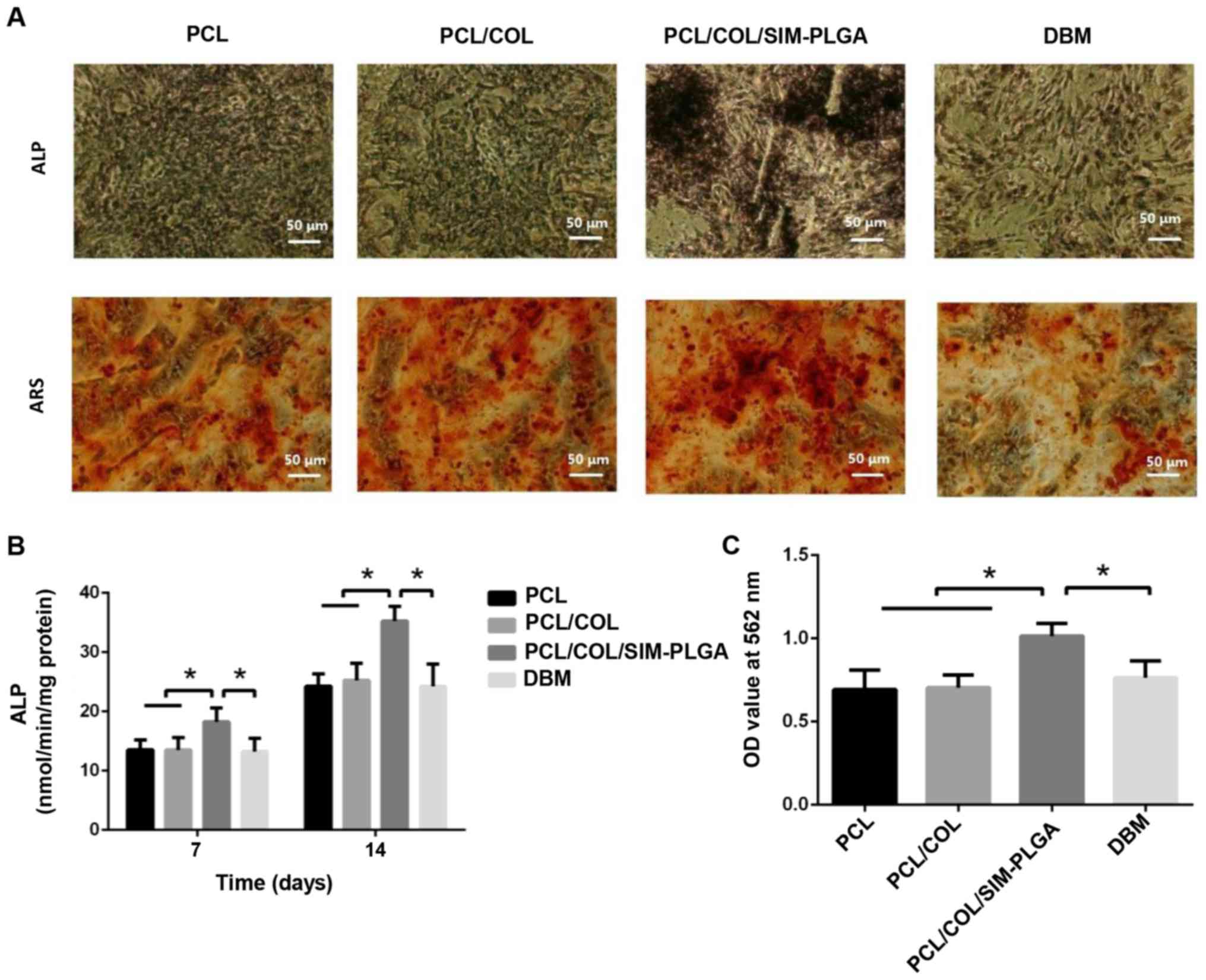 | Figure 5.Early and late stages of BMSC
osteogenic differentiation, as determined by ALP staining and ARS,
respectively. (A) Representative images of ALP staining after
14-day culture and ARS after 21-day culture. (B) ALP activity
results. (C) Summary of ARS results. The PCL/COL/SIM-PLGA construct
significantly promoted the osteogenic differentiation of BMSCs in
both the early and late stages. *P<0.05, as indicated. PLGA,
poly(lactic-co-glycolic acid); SIM, simvastatin; COL, collagen;
PCL, poly(ε-caprolactone); BMSC, bone marrow-derived mesenchymal
stem cells; ALP, alkaline phosphatase; ARS, Alizarin red staining;
OD, optical density. |
Osteogenic gene expression
RT-qPCR was performed to examine the mRNA levels of
ALP, bone sialoprotein, COL 1, runt-related transcription factor 2,
osteopontin and OCN in the BMSCs of the different groups. As
presented in Fig. 6, the SIM-PLGA
group exhibited significantly higher expression levels of all
osteogenic genes when compared with the other groups at days 7 and
14.
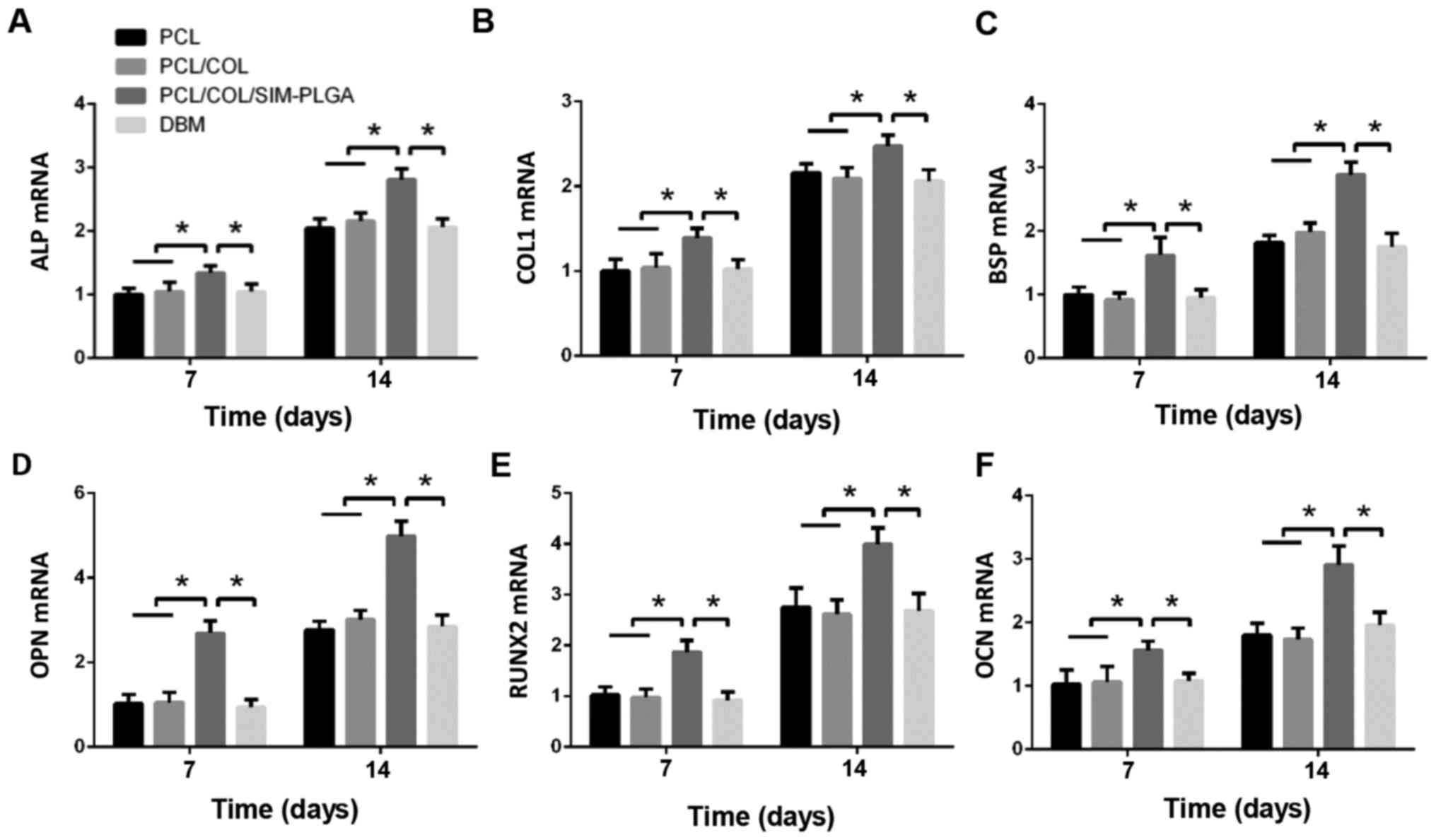 | Figure 6.Gene expression analyses of (A) ALP,
(B) COL 1, (C) BSP, (D) OPN, (E) RUNX2 and (F) OCN in the different
groups after 7 and 14 days of culture. The results are presented as
relative ratios to the PCL group at day 7. The expression of
osteogenic genes was greatly enhanced by the PCL/COL/SIM-PLGA
scaffold. *P<0.05, as indicated. PLGA, poly(lactic-co-glycolic
acid); SIM, simvastatin; COL, collagen; PCL, poly(ε-caprolactone);
ALP, alkaline phosphatase; COL 1, collagen type 1; BSP, bone
sialoprotein; RUNX2, runt-related transcription factor 2; OPN,
osteopontin; OCN, osteocalcin. |
In vivo study of rat femur defects
X-ray and micro-CT examination
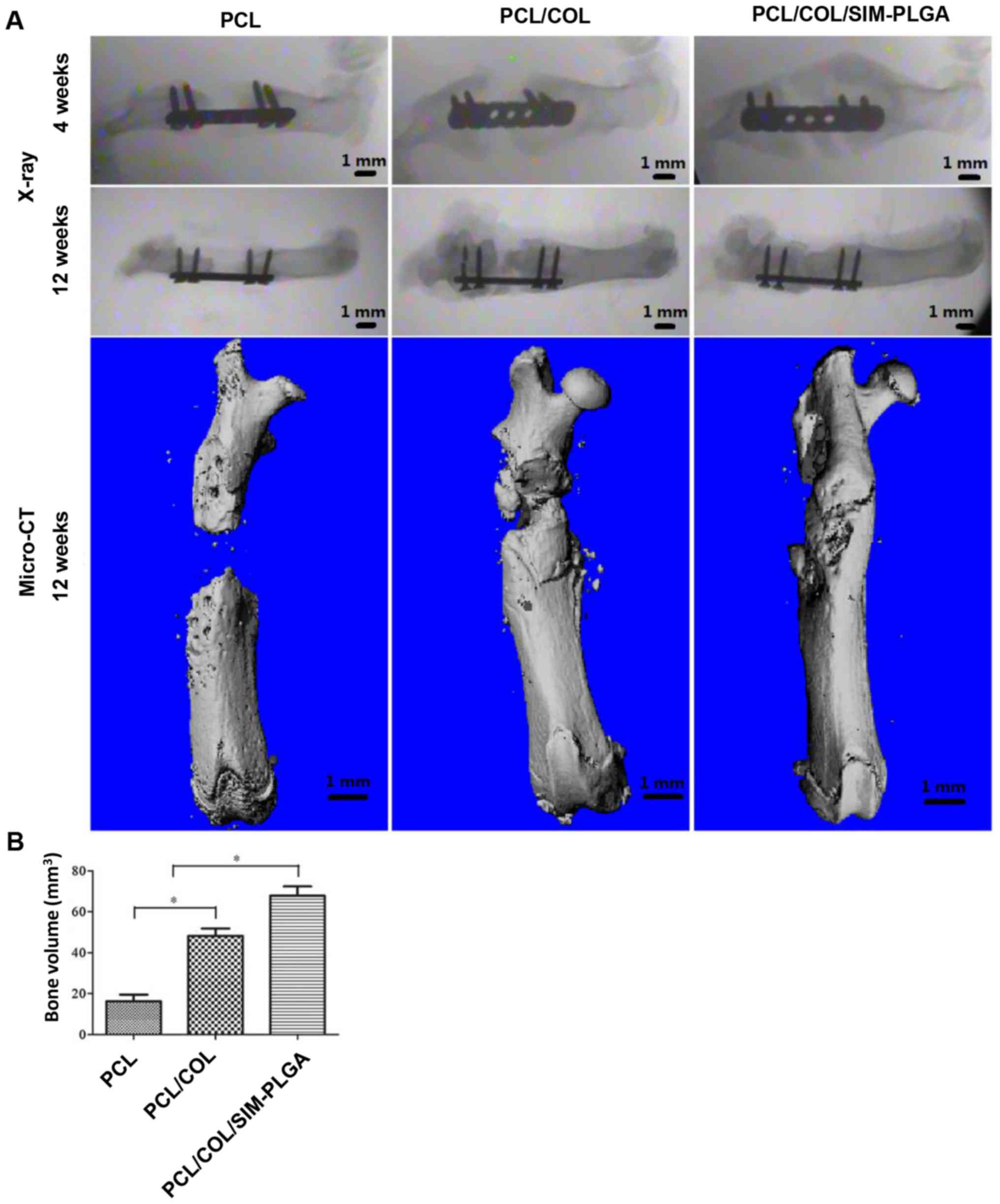
As indicated in Fig.
7A, at 4 weeks post-surgery, the X-ray images revealed that the
PCL group exhibited only a small amount of bone tissue growth, with
no significant increase in bone deposition at 12 weeks and no bone
fracture healing. On the other hand, significant levels of bone
tissue growth were observed at 4 weeks in the PCL/COL and
PCL/COL/SIM-PLGA groups. Notably, complete defect repair with
continuous cortical bone was achieved in the PCL/COL/SIM-PLGA group
at 12 weeks post-implantation. The results of micro-CT demonstrated
that there was scattered bone tissue near the defect edges as
opposed to obvious newly formed bone in the PCL group. As shown in
the 3D reconstructed images, new bone formation reconnected the
fractured ends, with the newly formed cortical bone enveloping the
scaffolds. Both the PCL/COL and PCL/COL/SIM-PLGA attained improved
results compared with the PCL group, and the incorporation of
SIM-PLGA further enhanced the osteogenic ability of the PCL/COL
scaffolds. Furthermore, the new bone volume calculated according to
the 3D reconstructed images indicated that the PCL/COL/SIM-PLGA
group exhibited the best bone regeneration among the three groups
(Fig. 7B).
Histological qualitative and
quantitative analysis
The HE staining results revealed that the defect
area was filled with loose fibrous connective tissue, which was
stained a light red, and was wrapped around the PCL trabecula
(Fig. 8A). The results of Masson
trichrome staining were in accordance with that of HE, as the
fibrous tissue, stained a light blue, surrounded the scaffolds
forming a fibrous interface at the scaffold-bone juncture. In the
PCL/COL group, only a marginal amount of fibrous tissue formed, and
the defect was filled mostly with newly formed bone tissue; both
mature (dark blue) and immature (light blue) bone tissue was
observed. In the PCL/COL/SIM-PLGA composite scaffold group, the
bone tissue in the bone defect was further improved, and was
primarily composed of mature bone tissue stained a dark blue. The
new bone tissue directly integrated with the scaffold material by
connecting with the original bone tissue at the fracture end, which
was consistent with the results of micro-CT. Thus, the
PCL/COL/SIM-PLGA construct served well in osteogenesis. In
addition, quantitative analysis of new bone area also indicated
that the PCL/COL exhibited an improved osteoinduction ability
compared with the blank PLC scaffold and the PCL/COL/SIM-PLGA
scaffold exhibited the highest new bone area among the three
groups.
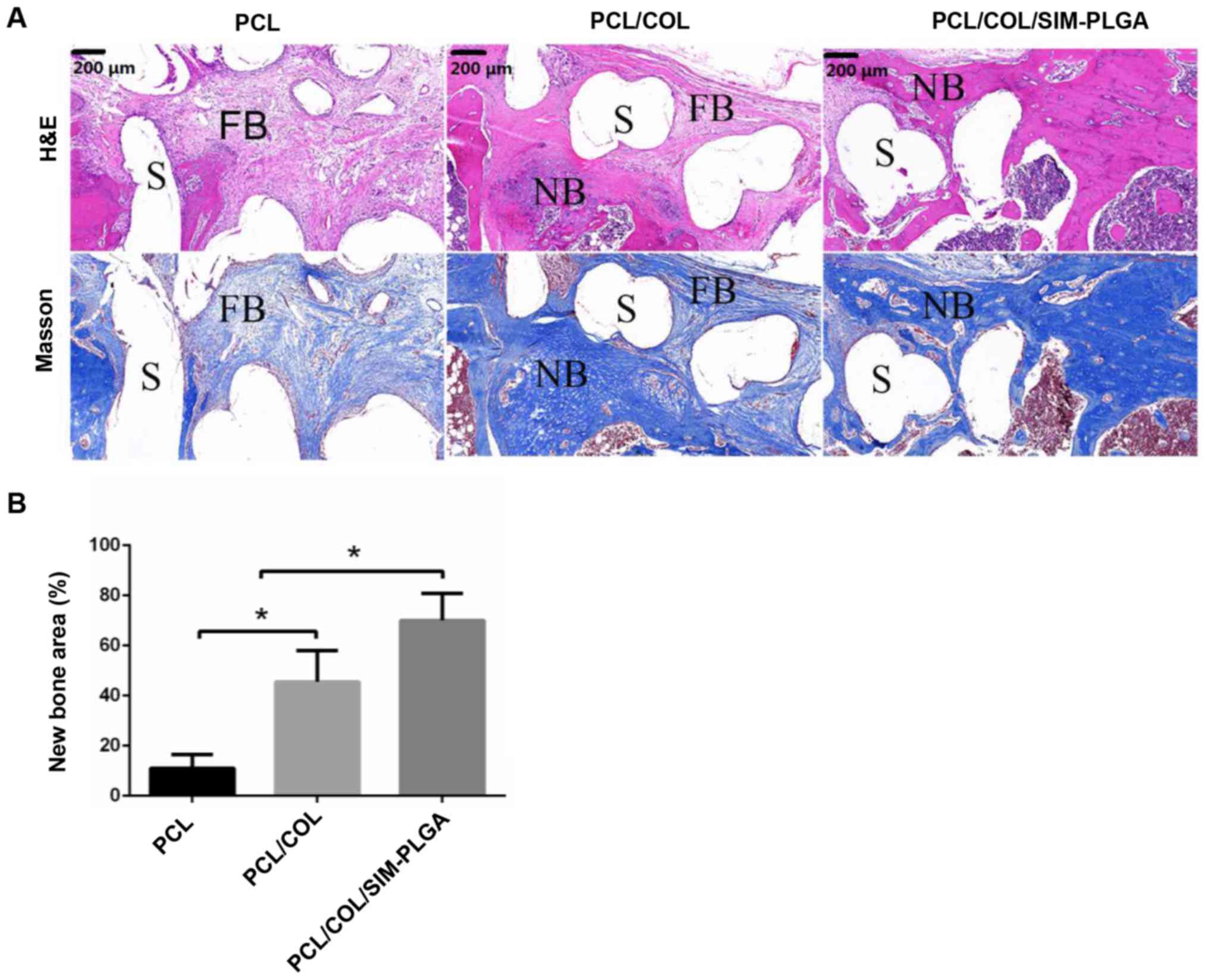 | Figure 8.Analysis of bone regeneration of the
femur defect by using HE and Masson staining. (A) HE and Masson
stained tissue sections of bone regeneration in the defect areas,
implanted with PCL, PCL/COL or PCL/COL/SIM-PLGA constructs, at 3
months after surgery. (B) The SIM-loaded scaffold demonstrated the
most robust osteogenic activity, and the majority of the defect
area in the SIM-loaded scaffold group was filled with eosin-stained
newly formed bone tissue. *P<0.05, as indicated. S, scaffolds;
NB, newly formed bone; FB, fibrosis; PLGA, poly(lactic-co-glycolic
acid); SIM, simvastatin; COL, collagen; PCL, poly(ε-caprolactone);
HE, hematoxylin and eosin. |
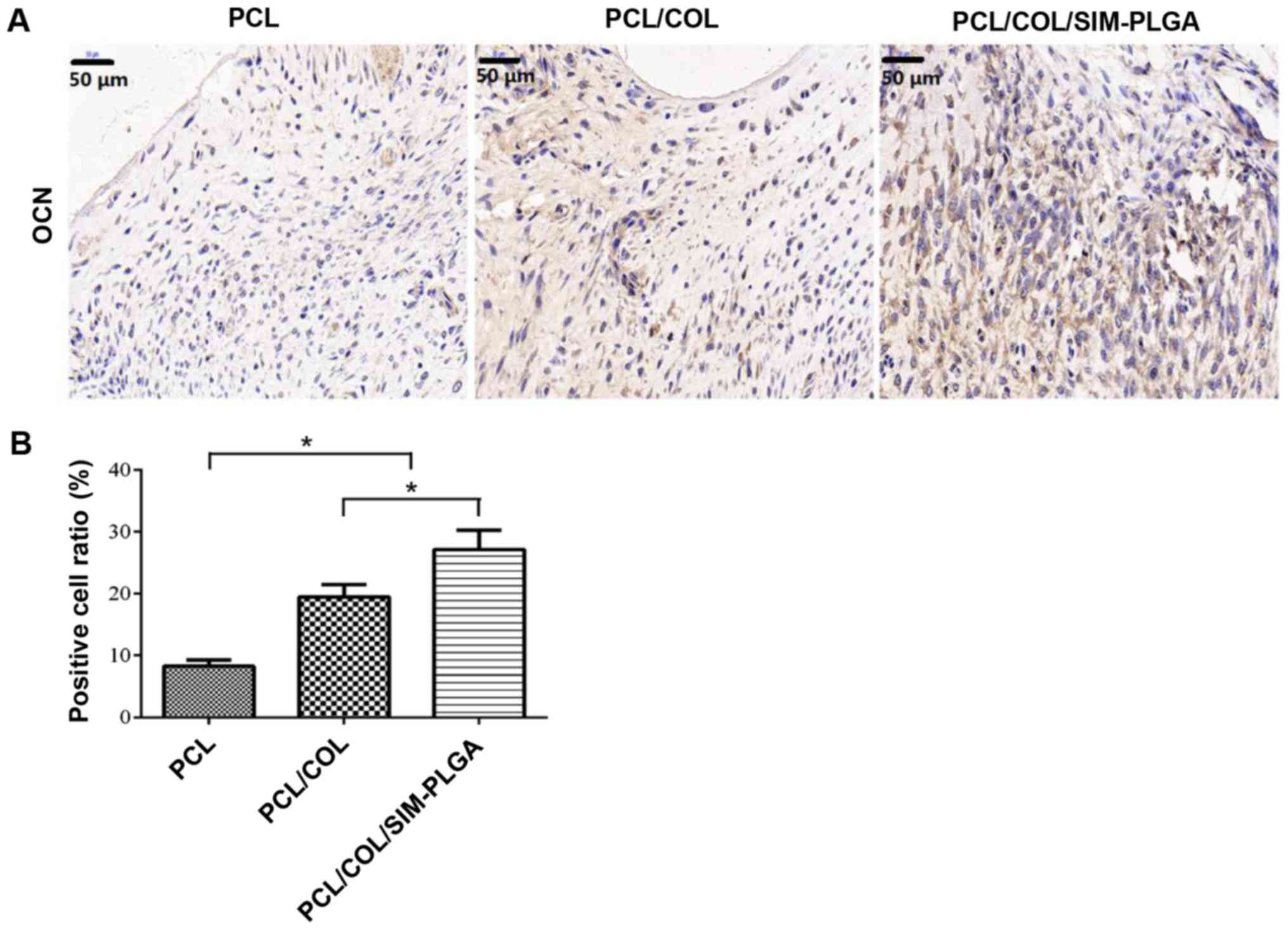
Immunohistochemical staining of
OCN
The immunohistochemical staining results (Fig. 9) demonstrated that the
PCL/COL/SIM-PLGA group exhibited significantly higher OCN
expression compared with the PCL/COL group, as determined by the
number of OCN-positive areas (indicated by brown staining). In
addition, the level of OCN expression in the PCL/COL and
PCL/COL/SIM-PLGA groups was significantly increased compared with
the PCL group, which suggested that the composited PCL possessed
improved osteogenic ability, with collagen facilitating the PCL to
form bone tissue and SIM-PLGA promoting further osteogenesis.
Discussion
In order to accelerate bone reconstruction, an
optimal bone implant for clinical practice requires both a porous
structure and optimal biocompatibility. The present study employed
hybrid 3D PCL scaffolds consisting of PCL, COL and SIM/PLGA, and
the results revealed two key points. Firstly, the low degradation
rate of the PCL scaffold with an interconnected porous structure
provided good mechanical strength and allowed for the delivery of
nutrients through the scaffolds. COL modification produced
scaffolds with macro/micro hierarchical structures where cells
attached and proliferated well among the COL RGD (Arg-Gly-Asp
motif) domains and integrin receptors (13). Secondly, the scaffolds conferred high
absorbability to the SIM-PLGA microspheres, enhancing osteogenesis
and the osteointegration of biomimetic PCL/COL scaffolds.
In our previous study, PCL frames that were
incorporated with a COL scaffold significantly increased the
surface area and bulk modulus, making best use of the advantages,
and bypassing the disadvantages, of using scaffold materials
(5). In the present study, in order
to further improve the biological functionalities of the composite,
SIM-PLGA was subsequently incorporated into the COL scaffold in
order to achieve controlled SIM discharge. To elucidate the impact
of SIM-PLGA incorporation, the pore size, release diagram,
mechanical structure of the scaffolds, cell activity and in
vivo bone restoration effect were measured.
As indicated in previous research, SIM enhances bone
formation by reducing apoptosis and increasing osteogenesis in MSCs
(21), as well as inhibiting bone
resorption by suppressing the proliferation and differentiation of
osteoclasts (42). In the present
study, it was also observed that SIM could augment the
proliferative and differentiative capabilities of BMSCs, indicating
its potential as a bone regenerative molecule. This was also
confirmed by the superior osteogenesis elicited by the
incorporation of SIM-PLGA in femur bone defect repair. However, it
remains difficult to systemically manipulate the uptake of SIM due
to its poor drug absorptivity. On the other hand, sufficient doses
via local injection may cause inflammation (15). To improve the efficacy of SIM and
circumvent its side effects, the present study selected PLGA
microspheres as a drug vehicle due to their agreeable
biocompatibility, low toxicity and controllable rate of degradation
(24,25,43).
Sustained release of SIM plays an essential role in bone
regeneration, as a release time of 2–3 weeks is required for growth
factors to fulfill ideal bone repair (44). As demonstrated in the release
diagram, the release of SIM from the PLGA microspheres lasted
>27 days, indicating that PLGA microspheres may serve as
appropriate carriers for growth factors.
Porosity is an important determinant of optimal
regenerative materials (45).
Osteochondral formation usually occurs in pores of a small size;
and non-chondral osteogenesis is usually present in pores of a
larger size (46,47). Based on previous work (5), the present study fabricated scaffolds
with a pore size of 1,000 µm through 3D printing. To simulate the
anatomic constitution of human cancellous bone, COL was combined
with the PCL scaffold via dry-freezing, thereby acquiring a
hierarchical porous structure with a saturated void space (48). Within the COL network, the PLGA
microspheres exhibited homogeneous distribution with the pore size
slightly diminished.
Since bones function as load-bearing parts, the
materials for bone implants must retain strong mechanical
properties in order to secure all of the osteogenic cells with
sufficient space for tissue regeneration. Due to its good
mechanical strength and low rate of degradation, PCL is widely
considered to be an appropriate candidate for osteogenic scaffolds.
Previous studies have revealed that the compressive strength and
modulus of PCL, with a hierarchical pore structure, is similar to
that of human cancellous bone (1,49) and
could be utilized for bone tissue engineering (1,4), as
demonstrated by the present study.
In conclusion, the results of the present study
provide the basis for a new strategy for bone tissue regeneration,
which incorporates polyesters and biomolecules, forming a
hierarchical structure containing a PCL scaffold of a macro-scale
filled with micro-sized COL. With the sustained release of SIM, the
present study obtained superior bone formation with sufficient
mechanical strength.
Acknowledgements
The authors would like to thank Dr Jinbing Wang and
Dr Yu Li (Shanghai Key Laboratory of Tissue Engineering, Department
of Plastic and Reconstructive Surgery, Shanghai Ninth People's
Hospital, School of Medicine, Shanghai Jiao Tong University,
Shanghai, China) for their help in the preparation of the
scaffolds.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The final version of the manuscript has been read
and approved by all authors, and each author believes that the
manuscript represents honest work. ZZZ and HZZ collaborated to
design the study. ZZZ was responsible for experiments. ZZZ and ZYZ
analyzed the data. All authors collaborated to interpret results
and prepare the manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Shanghai Ninth People's
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weisgerber DW, Erning K, Flanagan CL,
Hollister SJ and Harley BAC: Evaluation of multi-scale mineralized
collagen-polycaprolactone composites for bone tissue engineering. J
Mech Behav Biomed Mater. 61:318–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kasuya A, Sobajima S and Kinoshita M: In
vivo degradation and new bone formation of calcium phosphate
cement-gelatin powder composite related to macroporosity after in
situ gelatin degradation. J Orthop Res. 30:1103–1111. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Wang L, Zhang W, Zhang M and Luo
ZP: Synchronization of calcium sulphate cement degradation and new
bone formation is improved by external mechanical regulation. J
Orthop Res. 33:685–691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li L, Zuo Y, Zou Q, Yang B, Lin L, Li J
and Li Y: Hierarchical structure and mechanical improvement of an
n-HA/GCO-PU composite scaffold for bone regeneration. ACS Appl
Mater Interfaces. 7:22618–22629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Wu D, Zhang Z, Li J, Shen Y, Wang
Z, Li Y, Zhang ZY and Sun J: Biomimetically ornamented rapid
prototyping fabrication of an apatite-collagen-polycaprolactone
composite construct with nano-micro-macro hierarchical structure
for large bone defect treatment. ACS Appl Mater Interfaces.
7:26244–26256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pilia M, Guda T and Appleford M:
Development of composite scaffolds for load-bearing segmental bone
defects. Biomed Res Int. 2013:4582532013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inzana JA, Olvera D, Fuller SM, Kelly JP,
Graeve OA, Schwarz EM, Kates SL and Awad HA: 3D printing of
composite calcium phosphate and collagen scaffolds for bone
regeneration. Biomaterials. 35:4026–4034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papadimitropoulos A, Riboldi SA,
Tonnarelli B, Piccinini E, Woodruff MA, Hutmacher DW and Martin I:
A collagen network phase improves cell seeding of open-pore
structure scaffolds under perfusion. J Tissue Eng Regen Med.
7:183–191. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zein I, Hutmacher DW, Tan KC and Teoh SH:
Fused deposition modeling of novel scaffold architectures for
tissue engineering applications. Biomaterials. 23:1169–1185. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siddiqui N, Asawa S, Birru B, Baadhe R and
Rao S: PCL-based composite scaffold matrices for tissue engineering
applications. Mol Biotechnol. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim W, Jang CH and Kim G: Optimally
designed collagen/polycaprolactone biocomposites supplemented with
controlled release of HA/TCP/rhBMP-2 and HA/TCP/PRP for hard tissue
regeneration. Mater Sci Eng C Mater Biol Appl. 78:763–772. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui Z, Lin L, Si J, Luo Y, Wang Q, Lin Y,
Wang X and Chen W: Fabrication and characterization of chitosan/OGP
coated porous poly(epsilon-caprolactone) scaffold for bone tissue
engineering. J Biomater Sci Polym Ed. 28:826–845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiran S, Nune KC and Misra RD: The
significance of grafting collagen on polycaprolactone composite
scaffolds: Processing-structure-functional property relationship. J
Biomed Mater Res A. 103:2919–2931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pek YS, Gao S, Arshad MS, Leck KJ and Ying
JY: Porous collagen-apatite nanocomposite foams as bone
regeneration scaffolds. Biomaterials. 29:4300–4305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie C, Lu X, Han L, Xu J, Wang Z, Jiang L,
Wang K, Zhang H, Ren F and Tang Y: Biomimetic mineralized
hierarchical graphene oxide/chitosan scaffolds with adsorbability
for immobilization of nanoparticles for biomedical applications.
ACS Appl Mater Interfaces. 8:1707–1717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Zhao Q and Wang M: Cryogenic 3D
printing for producing hierarchical porous and rhBMP-2-loaded
Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering.
Biofabrication. 9:0250312017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan Q, Xiao LQ, Tan L, Sun W, Wu T, Chen
LW, Mei Y and Shi B: Controlled release of simvastatin-loaded
thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue
regeneration: In vitro and in vivo characteristics. J Biomed Mater
Res A. 103:3580–3589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papadimitriou K, Karkavelas G, Vouros I,
Kessopoulou E and Konstantinidis A: Effects of local application of
simvastatin on bone regeneration in femoral bone defects in rabbit.
J Craniomaxillofac Surgy. 43:232–237. 2015. View Article : Google Scholar
|
|
19
|
Montero J, Manzano G and Albaladejo A: The
role of topical simvastatin on bone regeneration: A systematic
review. J Clin Exp Dent. 6:e286–e290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanigo T, Takaoka R and Tabata Y:
Sustained release of water-insoluble simvastatin from biodegradable
hydrogel augments bone regeneration. J Control Release.
143:201–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JB: The use of simvastatin in bone
regeneration. Med Oral Patol Oral Cir Bucal. 14:e485–e488.
2009.PubMed/NCBI
|
|
22
|
Mir M, Ahmed N and Rehman AU: Recent
applications of PLGA based nanostructures in drug delivery.
Colloids Surf B Biointerfaces. 159:217–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Liao H, Bao C, Xiao Y and Wang Q:
Preparation and evaluations of mangiferin-loaded PLGA scaffolds for
alveolar bone repair treatment under the diabetic condition. AAPS
Pharm Sci Tech. 18:529–538. 2017. View Article : Google Scholar
|
|
24
|
Hu X, Zhang J, Tang X, Li M, Ma S, Liu C,
Gao Y, Zhang Y, Liu Y, Yu F, et al: An accelerated release method
of risperidone loaded PLGA microspheres with good IVIVC. Curr Drug
Deliv. 15:87–96. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang J, Chen Z, Li Y, Li L and Zhang G:
Rifapentine-linezolid-loaded PLGA microspheres for interventional
therapy of cavitary pulmonary tuberculosis: Preparation and in
vitro characterization. Drug Des Devel Ther. 11:585–592. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y and Zhang Z and Zhang Z: Porous
chitosan/nano-hydroxyapatite composite scaffolds incorporating
simvastatin-loaded PLGA microspheres for bone repair. Cells Tissues
Organs. 205:20–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernández-Sánchez L, Bravo-Osuna I, Lax P,
Arranz-Romera A, Maneu V, Esteban-Pérez S, Pinilla I,
Puebla-González MDM, Herrero-Vanrell R and Cuenca N: Controlled
delivery of tauroursodeoxycholic acid from biodegradable
microspheres slows retinal degeneration and vision loss in P23H
rats. PLoS One. 12:e01779982017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeong C, Kim SE, Shim KS, Kim HJ, Song MH,
Park K and Song HR: Exploring the in vivo anti-inflammatory actions
of simvastatin-loaded porous microspheres on inflamed tenocytes in
a collagenase-induced animal model of achilles tendinitis. Int J
Mol Sci. 19:E8202018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao F, Zhang J, Wang J, Du B, Huang X,
Pang L and Zhou Z: Silk fibroin-coated PLGA dimpled microspheres
for retarded release of simvastatin. Colloids Surf B Biointerfaces.
158:112–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu WL, Sun TW, Qi C, Zhao HK, Ding ZY,
Zhang ZW, Sun BB, Shen J, Chen F, Zhu YJ, et al: Enhanced
osteogenesis and angiogenesis by mesoporous hydroxyapatite
microspheres-derived simvastatin sustained release system for
superior bone regeneration. Sci Rep. 7:441292017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y and Zhang ZZ: Sustained curcumin
release from PLGA microspheres improves bone formation under
diabetic conditions by inhibiting the reactive oxygen species
production. Drug Des Devel Ther. 12:1453–1466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Chang Q, Xu L, Li G, Yang G, Ding
X, Wang X, Cui D and Jiang X: Graphene oxide-copper
nanocomposite-coated porous CaP scaffold for vascularized bone
regeneration via activation of Hif-1α. Adv Healthc Mater.
5:1299–1309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie H, Wang Z, Zhang L, Lei Q, Zhao A,
Wang H, Li Q, Cao Y, Zhang Jie W and Chen Z: Extracellular
vesicle-functionalized decalcified bone matrix scaffolds with
enhanced pro-angiogenic and pro-bone regeneration activities. Sci
Rep. 7:456222017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen X, Zhang Y, Gu Y, Xu Y, Liu Y, Li B
and Chen L: Sequential and sustained release of SDF-1 and BMP-2
from silk fibroin-nanohydroxyapatite scaffold for the enhancement
of bone regeneration. Biomaterials. 106:205–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Gao X and Wang J: Human
adipose-derived mesenchymal stem cell-conditioned media suppresses
inflammatory bone loss in a lipopolysaccharide-induced murine
model. Exp Ther Med. 15:1839–1846. 2018.PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
National Research Council Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
The National Academies Collection: Reports funded by National
Institutes of Health, . In: Guide for the Care and Use of
Laboratory Animals. th (ed). National Academies Press (US) National
Academy of Sciences; Washington (DC): 2011
|
|
38
|
Sun X, Wang J, Wang Y and Zhang Q:
Collagen-based porous scaffolds containing PLGA microspheres for
controlled kartogenin release in cartilage tissue engineering.
Artif Cells Nanomed Biotechnol. 1–10. 2017. View Article : Google Scholar
|
|
39
|
Fahimipour F, Rasoulianboroujeni M,
Dashtimoghadam E, Khoshroo K, Tahriri M, Bastami F, Lobner D and
Tayebi L: 3D printed TCP-based scaffold incorporating VEGF-loaded
PLGA microspheres for craniofacial tissue engineering. Dent Mater.
33:1205–1216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ramazani F, Chen W, van Nostrum CF, Storm
G, Kiessling F, Lammers T, Hennink WE and Kok RJ: Strategies for
encapsulation of small hydrophilic and amphiphilic drugs in PLGA
microspheres: State-of-the-art and challenges. Int J Pharm.
499:358–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giteau A, Venier-Julienne MC,
Aubert-Pouëssel A and Benoit JP: How to achieve sustained and
complete protein release from PLGA-based microparticles? Int J
Pharm. 350:14–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oryan A, Kamali A and Moshiri A: Potential
mechanisms and applications of statins on osteogenesis: Current
modalities, conflicts and future directions. J Control Release.
215:12–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Du L, Yang S, Li W, Li H, Feng S, Zeng R,
Yu B, Xiao L, Nie HY and Tu M: Scaffold composed of porous
vancomycin-loaded poly(lactide-co-glycolide) microspheres: A
controlled-release drug delivery system with shape-memory effect.
Mater Sci Eng C Mater Biol Appl. 78:1172–1178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guelcher SA, Brown KV, Li B, Guda T, Lee
BH and Wenke JC: Dual-purpose bone grafts improve healing and
reduce infection. J Orthop Trauma. 25:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H, Yang L, Zhang E, Zhang R, Cai D,
Zhu S, Ran J, Bunpetch V, Cai Y, Heng BC, et al: Biomimetic tendon
extracellular matrix composite gradient scaffold enhances
ligament-to-bone junction reconstruction. Acta Biomater.
56:129–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J,
Noh I, Mikos AG and Zhang S: Selective laser sintering scaffold
with hierarchical architecture and gradient composition for
osteochondral repair in rabbits. Biomaterials. 137:37–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Amini AR, Wallace JS and Nukavarapu SP:
Short-term and long-term effects of orthopedic biodegradable
implants. J Long Term Eff Med Implants. 21:93–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nezu T and Winnik FM: Interaction of
water-soluble collagen with poly(acrylic acid). Biomaterials.
21:415–419. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hollister SJ: Porous scaffold design for
tissue engineering. Nat Mater. 4:518–524. 2005. View Article : Google Scholar : PubMed/NCBI
|