Introduction
Anesthetic agents are used to overcome
intraoperative and postoperative pain for adult and pediatric
surgeries (1). Among them,
isoflurane is the typical inhalation anesthetic primarily used in
general anesthesia and bupivacaine is an amide, and used as a local
anesthetic (2,3). However, a number of previous studies
have suggested that anesthetics, including isoflurane and
bupivacaine, may unavoidably induce neuron injury, particularly in
older populations, even at normal clinical doses (3,4).
Isoflurane was demonstrated to induce neuroapoptosis and inhibit
protein kinase B (AKT) activity in the hippocampus of neonatal rats
(4). Bupivacaine treatment resulted
in significant levels of apoptosis in human proximal tubular HK-2
cells (5,6).
Apoptosis is the process of programmed cell death,
accompanied by nuclear fragmentation, cell shrinkage, chromatin
condensation, membrane blebbing and the formation of apoptotic
bodies (7). Neuron apoptosis is the
primary cause of neurotoxicity (8),
and the mechanisms of anesthesia-induced apoptosis have not been
well studied. Increased expression levels of the proapoptotic
protein B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax)
results in mitochondrial membrane breakage and activation of
caspases, which results in cell apoptosis (8). Previously, a number of studies
suggested that neuronal apoptosis made mice and human susceptible
to a series of acute and chronic diseases. including Alzheimer's
disease (AD), amyotrophic lateral sclerosis (ALS) and Parkinson's
disease (PD) (9). Raynaud and
Marcilhac (10) suggested that
progressively decreasing numbers of neurons accounted for the
irreversible pathogenesis of AD in adult brain tissue. Increased
numbers of apoptotic neuronal cells and expression of caspase 3 and
Bax were identified in the PD nigra compared with age-matched
controls (11). PD was originally
attributed to neuronal loss within the substantia nigra pars
compacta and a concomitant loss of dopamine. Therefore, there may
be a close association between neuronal apoptosis and human
disease.
An important factor known to regulate cell apoptosis
is hypoxia-inducible factor 1 (HIF-1). The HIF-1α subunit of HIF-1
is the master regulator of the cellular response to hypoxia
(12). HIF-1 contains an
oxygen-regulated α subunit and a constitutive HIF-1β subunit, which
regulates the transcription of a number of genes involved in cell
proliferation, angiogenesis and glycolysis (13). Pyruvate kinase M2 (PKM2), as a HIF-1
target gene, transcribes an important metabolic enzyme that is also
a regulatory protein of HIF-1 activity (13).
Previous studies have identified that the
phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway serves a
major role in regulating the homeostasis between cell survival and
apoptosis (14–16). AKT-associated serine-threonine
kinases regulate cell survival and serve an important role in the
pathogenesis of degenerative diseases and in cancer (15). Increasing evidence has suggested that
a number of chemical drugs activate apoptosis in cells by
inhibiting the PI3K/AKT pathway (16). However, it remains unknown whether
dexmedetomidine (Dex) affects the PI3K/AKT pathway during apoptosis
in neuronal cells, particularly in hippocampal neuronal HT22
cells.
Dex, as a typical α2 adrenergic receptor agonist,
attenuates isoflurane- and bupivacaine-induced neuronal apoptosis
(6,17). However, the exact mechanisms
underlying its anti-apoptotic activity are largely unknown. In the
present study, it was identified that the Dex protected hippocampal
neuronal HT22 cells from isoflurane or bupivacaine-induced
apoptosis primarily through suppressing HIF-α/PKM2 and activating
the PI3K-AKT pathway.
Materials and methods
Cells and cell culture
The mouse HT22 cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml streptomycin and 100 U/ml penicillin (Beijing
Xias Biotechnology Co., Ltd., Beijing, China), and maintained at
37°C with 5% CO2 in a humidified atmosphere. All
experiments were performed on logarithmically growing cells.
In order to conduct the following assays, cells were
seeded into plates and divided into seven groups: the HT22 cell
control group; the Isoflurane (1.12 mM) group; the Isoflurane (1.12
mM) + Dex (200 µM) group; the Isoflurane (1.12 mM) + IGF-1 (100 nM)
group; the Bupivacaine (900 µM) group; the Bupivacaine (900 µM) +
Dex (200 µM) group; and the Bupivacaine (900 µM) + IGF-1 (100 nM)
group.
Reagents
Primary antibodies against PKM2 (cat. no.
sc-365684), HIF-α (cat. no. sc-13515), cleaved caspase-3 (cat. no.
sc-271759), AKT (cat. no. sc-8312), phosphorylated (p)-AKT (cat.
no. sc-271964), Bax (cat. no. sc-20067), Bcl-2 (cat. no. sc-23960)
and cytochrome c (cat. no. sc-13156) were purchased from Santa Cruz
Biotechnology (Dallas, TX, USA). Trypsin was purchased from Beijing
Solarbio Science & Technology Co., Ltd., (Beijing, China). A
Cell Counting Kit-8 (CCK-8) was obtained from Beyotime Institute of
Biotechnology (Haimen, China). An annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) double staining
apoptosis kit was purchased from Nanjing KeyGen Biotech Co., Ltd.,
(Nanjing, China). Dex was purchased from sigma (Sigma Aldrich;
Merck KGaA, Darmstadt, Germany; cat. no. SML0956). IGF 1 was
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
Senescence-associated β-galactosidase
(SA β-gal) staining
Hippocampal neuronal HT22 cells exposed to
sub-cytotoxic oxidative stress undergo stress-induced premature
senescence, which may be identified using SA β-gal staining
(18). HT22 cells were exposed to
100 mM tert Buty1 hydroperoxide (t-BHP) for 2 h at 37°C.
The SA β-gal staining kit (Beyotime Institute of
Biotechnology) was used to detect senescent cells according to the
manufacturer's protocol. Briefly, cells were seeded into 96-well
culture plates (Corning Incorporated, Corning, NY, USA) at a
density of 5×103 cells/well and cultured for 24 h. Then,
the cells were washed twice with PBS and fixed in 1 ml 3%
formaldehyde at room temperature for 15 min. Following fixation,
cells were washed with PBS three times and stained with 1 ml
freshly prepared cell staining working solution (containing 10 µl
SA β-gal staining solution A, 10 µl SA β-gal staining solution B,
930 µl SA β-gal staining solution C and 50 µl X-Gal solution;
obtained from Beyotime Institute of Biotechnology) at 37°C for 12 h
in the dark. Finally, a light microscope (magnification, ×200;
Olympus Corporation, Tokyo, Japan) was used to identify the
blue-stained cells.
Cell proliferation assay
Cells were seeded into 96-well culture plates
(Corning Incorporated) at a density of 5×103 cells/well
and cultured for 24 h. Then, the cells were divided into seven
groups and cultured for 24 h at 37°C. Then, 10 µl/well CCK-8
solution was added into the cell culture supernatant at 37°C for 2
h. Thereafter, the optical density was measured at 450 nm
wavelength using a microplate reader (Sigma-Aldrich; Merck
KGaA).
Observation of morphological
changes
HT22 cells were seeded into 24-well culture plates
(Corning Incorporated) at a density of 2×104 cells/well
and divided into seven groups. Then, cellular morphology was
observed using a phase contrast microscope (magnification, ×200;
Leica Microsystems GmbH, Wetzlar, Germany).
Flow cytometry analysis of annexin
V-FITC/PI double staining
The annexin V-FITC/PI double staining assay was
performed using quantitative flow cytometry. Cells were harvested
at 37°C for 24 h. Subsequently, harvested cells were using 0.25%
trypsin, centrifuged at 352 × g for 5 min at 4°C and then washed
twice with PBS. The supernatant was discarded, and the pellet was
resuspended with 300 µl binding buffer (Hepes 10 mM, NaCl 140 mM,
CaCl2 2.5 mM, pH 7.4 in tri-distilled water). Cells were incubated
at 37°C with 5 µl FITC-conjugated Annexin V for 15 min and then
incubated with 10 µl PI for 15 min at room temperature in the dark.
The samples were analyzed by a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). The fluorescent scatter plots indicate
three cell populations: Live (Annexin
V-FITC−/PI−), necrotic (Annexin
V-FITC+/PI+), and apoptotic (Annexin
V-FITC+/PI−). Quadrant analysis (BD FACSuite™
v 1.0; BD Biosciences) was performed on the gated fluorescent
scatter plot to examine the percentage of live, necrotic and
apoptotic cell populations.
Western blot analysis
HT22 cells were divided into seven groups and
cultured for 24 h at 37°C. Then, cells were harvested; washed twice
with PBS and then lysed in lysis buffer (50 mM HEPES pH 7.4, 1%
Triton X-100, 2 mM sodium orthovanadate, 100 mM sodium fluoride, 1
mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 µg/ml aprotinin and 10 µg/ml
leupeptin) at 4°C for 60 min. Lysates were centrifuged at 15,000 ×
g for 10 min at 4°C, and the supernatants were used for western
blot analysis. Protein content of the supernatants was determined
using a Bio-Rad protein assay reagent (Bio Rad Laboratories, Inc.,
Hercules, CA, USA). Equal amounts (50 µg) of the total protein were
separated by 10–11% gel electrophoresis and electrophoretically
transferred to nitrocellulose membranes (NC membranes, 0.45 µm;
Thermo Fisher Scientific, Inc.) at 4°C for 2 h. The NC membranes
were then blocked with 5% skimmed milk at room temperature for 1 h.
Proteins were detected with the primary antibodies (all 1:1,000)
against PKM2, HIF-α, cleaved caspase-3, AKT, p-AKT, Bax, Bcl-2 and
cytochrome c overnight at 4°C, followed by a horseradish peroxidase
(HRP)-conjugated secondary antibody (cat. no. sc-2789; 1:5,000;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature.
Protein bands were visualized by using enhanced chemiluminescence
(BeyoECL Plus; Beyotime Institute of Biotechnology) as the HRP
substrate. Band density of the specific protein was analyzed with
Quantity one image software (Image Lab™ software version 5.1;
Bio-Rad Laboratories, Inc.).
Statistical analysis
Data obtained by at least three independent
experiments were expressed as mean ± standard deviation. Comparison
of multiple groups was performed by one-way analysis of variance
followed by Dunnett's test using GraphPad Prism v7 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Dex inhibits anesthetic-induced
apoptotic body formation and attenuates the proliferation
inhibition of HT22 cells
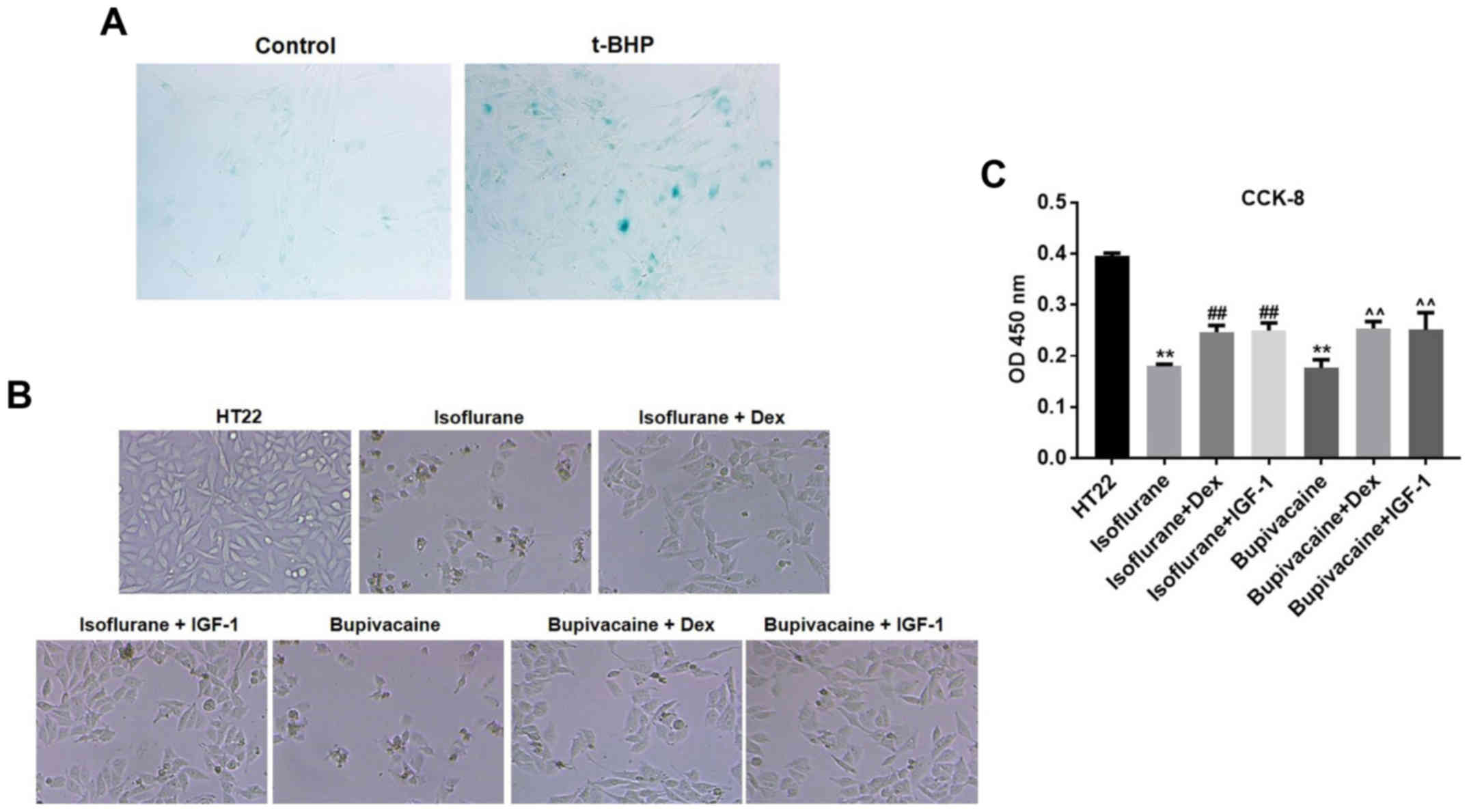
HT22 cell senescence was first induced by t-BHP,
which was then confirmed by the β-gal staining (Fig. 1A). Next, to determine the features of
HT22 cell growth, the morphological changes were observed by phase
contrast microscopy. Marked membrane blebbing, cell shrinkage and
formation of apoptotic bodies were observed following treatment
with isoflurane and bupivacaine. However, the changes were less
significant in the presence of Dex or insulin-like growth factor 1
(IGF-1), the PI3K/AKT agonist (Fig.
1B). To additionally evaluate the potential effect of Dex on
cell proliferation, a CCK-8 assay was conducted. As demonstrated in
Fig. 1B, the growth of HT22 cells
was markedly inhibited by isoflurane or bupivacaine compared with
the control group (P<0.01). However, Dex significantly decreased
the isoflurane or bupivacaine-mediated proliferation inhibition of
HT22 cells (Fig. 1C).
Treatment with Dex rescues HT22 cells
from anesthetic-induced apoptosis
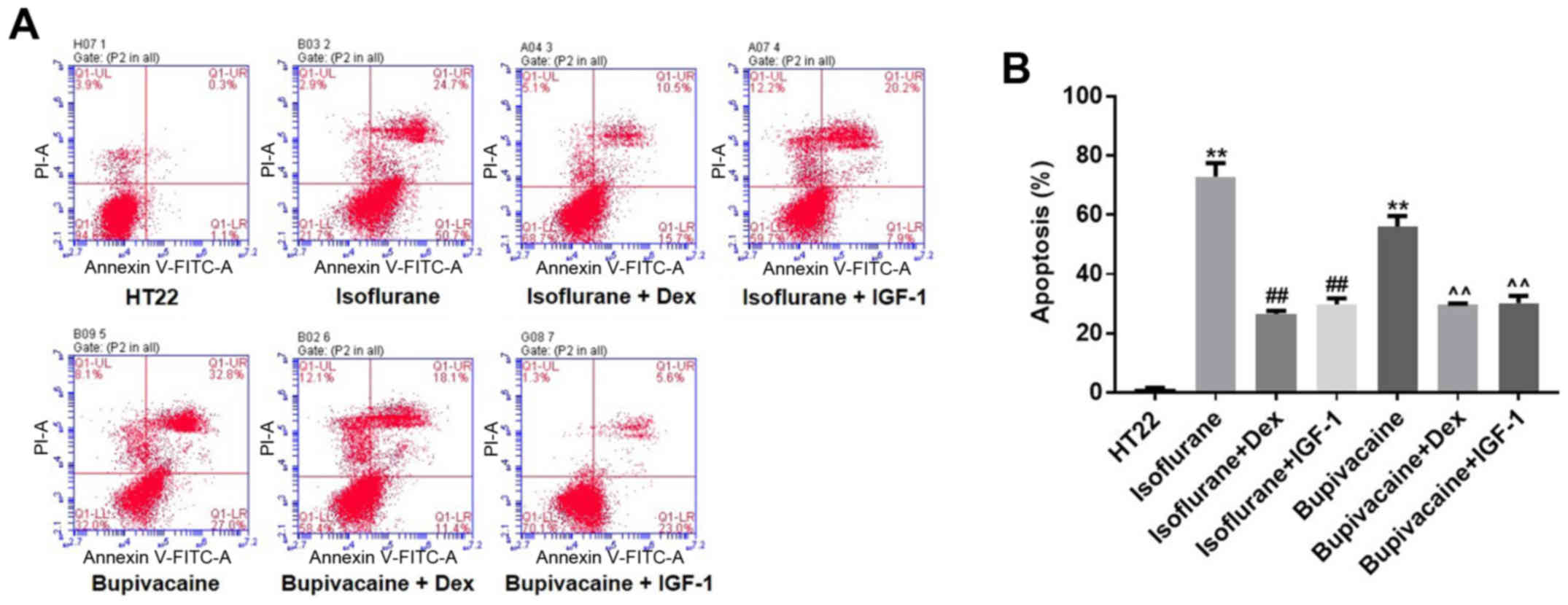
Annexin V and PI double staining was used to examine
HT22 cell apoptosis. As indicated in Fig. 2A, isoflurane (1.12 mM) treatment for
24 h resulted in 75.4% apoptosis compared with the vehicle (1.4%).
Similarly, exposing HT22 cells to 900 µM bupivacaine for 24 h
resulted in 59.8% apoptosis. However, Dex treatment decreased
isoflurane- or bupivacaine-induced cell apoptosis to 26.2 and
29.5%, respectively. Similarly, the percentage of isoflurane or
bupivacaine-induced apoptotic cells decreased to 28.1 and 28.6%,
respectively, following incubation with IGF-1 (Fig. 2B). In conclusion, it was confirmed
that treatment with Dex protected HT22 cells from
anesthetic-induced apoptosis.
Anesthetics induce apoptosis via
activating the HIF-α/PKM2 pathway and inhibiting the PI3K-AKT
pathway in HT22 cells, which is reversed by Dex
Isoflurane or bupivacaine significantly decreased
the protein expression of p-PI3K and p-AKT; while treatment with
Dex or IGF-1 increased their expression levels. Concomitantly, Dex
or IGF-1 treatment resulted in equivalent inhibitory effects on the
isoflurane- or bupivacaine-induced expression of these proteins
(Fig. 3A). Data from the western
blot analysis indicated the same effects (Fig. 3B-D).
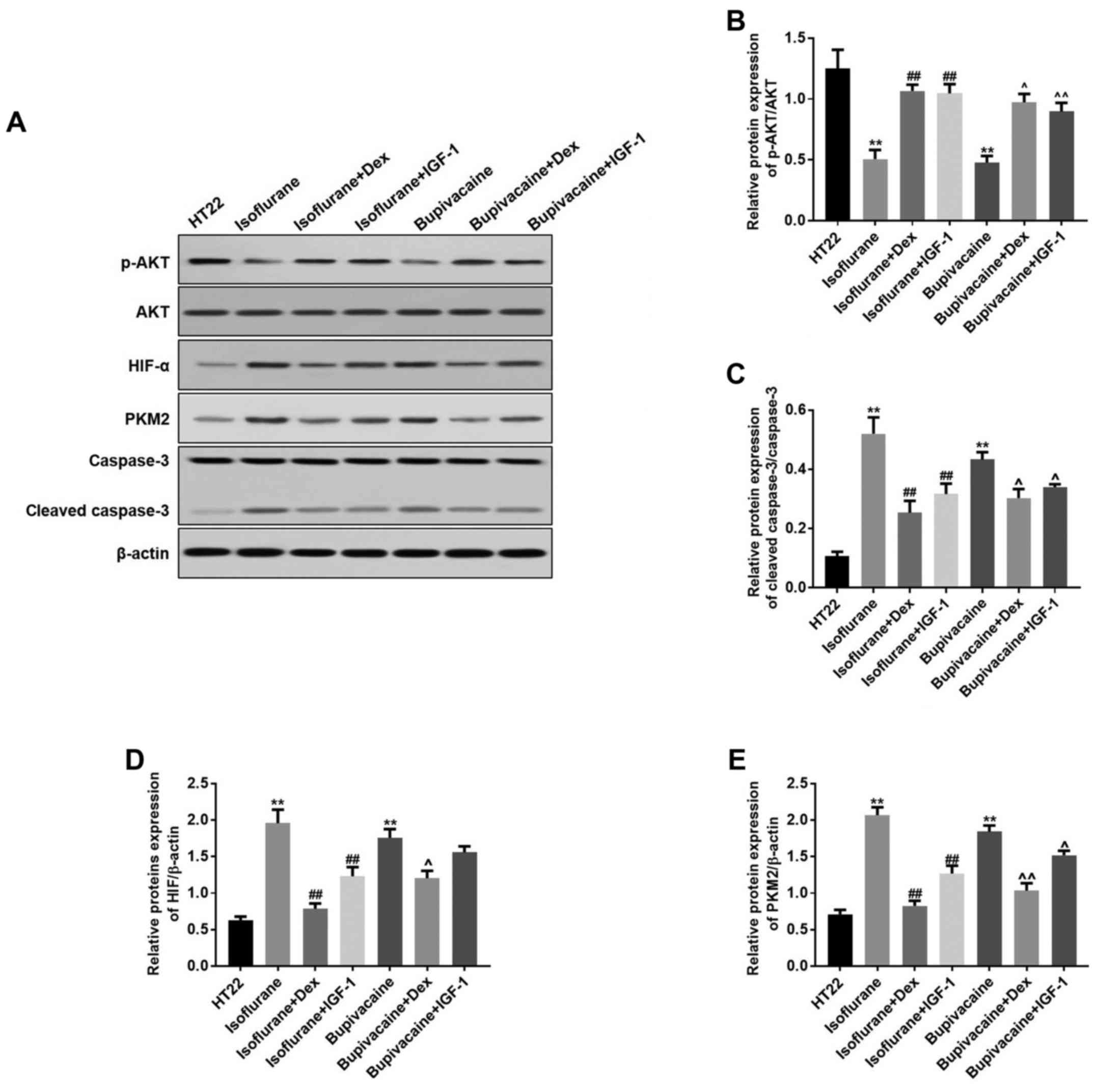 | Figure 3.Dex regulates the proteins expression
levels of HIF-α, PKM2, p-AKT and cleaved caspase-3 in vitro.
(A) HIF-α, PKM2, p-AKT and cleaved caspase-3 levels were detected
by western blot analysis. β-actin was used as a loading control.
(B) The quantitative analysis of the ratio of p-Akt to Akt. (C)
Cleaved caspase-3, (D) HIF-α and (E) PKM2 levels were analyzed by
one-way analysis of variance and Dunnett's post-hoc test. n=3; data
are presented as mean ± standard deviation. **P<0.01 vs. HT22;
##P<0.01 vs. Isoflurane; ^P<0.05 and
^^P<0.01 vs. Bupivacaine. HIF-α, hypoxia-inducible
factor α; PKM2, pyruvate kinase M2; p, phosphorylated; AKT, protein
kinase B; IGF-1, insulin-like growth factor 1; Dex,
dexmedetomidine. |
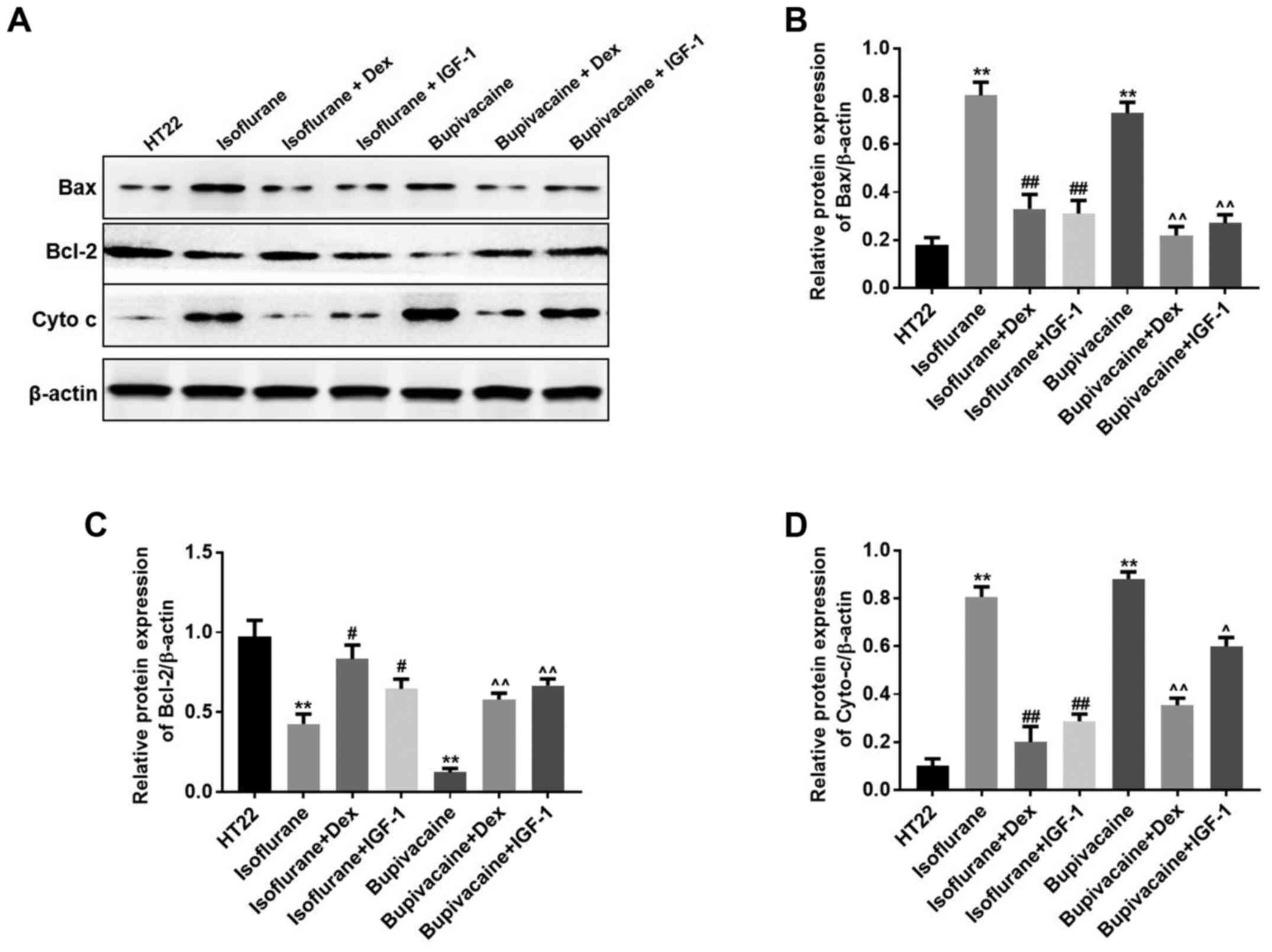
Anesthetics-induced apoptosis may
occur through a mitochondria-dependent intrinsic apoptosis
pathway
Western blot analysis was performed to additionally
examine the mechanism of anesthetics-induced cell death. It has
been established that the release of cytochrome c into the
cytoplasm and an increase of the Bax:Bcl-2 ratio leads to apoptosis
(4). Bcl-2 is a potent inhibitor of
apoptotic cell death, while Bax accelerates apoptosis by
contributing to the permeabilization of the outer mitochondrial
membrane (4). In the present study,
when considering the intrinsic apoptosis pathway, the translocation
of the anti-apoptotic protein Bcl-2 to the mitochondria was
markedly decreased, while the pro-apoptotic translocation of Bax to
the mitochondria and the release of cytochrome c into cytoplasm
were increased in the anesthetics-treated groups. However,
treatment with Dex or IGF-1 inhibited these effects in HT22 cells
(Fig. 4A). The results indicated
that the intrinsic apoptosis pathway was upregulated by anesthetics
in the hippocampal neuronal HT22 cells. Data from the western blot
analysis indicated similar results (Fig.
4B-D).
Discussion
To assess the protective mechanism of Dex on the
anesthetic-induced damage in the elderly population, an aging model
with t-BHP-stimulated HT22 cells was designed in the present study.
Exposure of HT22 cells to 100 mM tert-Butyl hydroperoxide (t-BHP)
for 2 h decreased their proliferative life span and increased the
proportion of cells positive for the SA β-gal activity (18). The results demonstrated that the
apoptosis of HT22 cells induced by anesthetic treatment was caused
by HIF-α/PKM2 activation and PI3K-AKT pathway downregulation. Dex
simultaneously inhibited the HIF-α/PKM2 activation and PI3K-AKT
downregulation in anesthetic-treated HT22 cells, thereby
attenuating the intrinsic apoptosis pathway. These data provided
novel insights into the process of anesthetic injury in HT22 cells
and the protective potential of Dex on anesthetic-treated HT22
cells. Consistent with previous data, isoflurane or bupivacaine
treatment increased the expression of cleaved caspase-3 and
promoted the expression of HIF-α and PKM2.
There are a number of factors affecting neuronal
cells apoptosis. Oxidative stress in neuronal cells leads to
apoptosis through the release of cytochrome c from the mitochondria
and caspase-3 activation (19). In
addition, tumor necrosis factor-α released by neuronal cells is
associated with glutamate-induced neuronal cell death (20). Lauritzen et al (21) identified that rat mature cerebellar
granule cells die through apoptosis when cultured in a medium
containing physiological concentrations of K+. A number
of studies have suggested that anesthetics induce neurodegeneration
in multiple brain regions (22,23).
However, the present study specially focused on hippocampal
neuronal HT22 cells, as previous studies had demonstrated that
isoflurane induced a severe hippocampal lesion in neonatal rats,
accompanied by an abnormal response to contextual fear conditioning
(22).
Anesthetics including isoflurane and bupivacaine are
the most common clinical drugs used during surgical procedures and
are generally safe (23,24). Isoflurane has been demonstrated to be
neuroprotective and neurotoxic (25–28).
Short-time exposure of isoflurane provides neuroprotection via the
moderate activation of inositol triphosphate (IP3) receptors and
activation of AKT-mediated neuroprotection. However, long-time
exposure of isoflurane induces neurotoxicity via overactivation of
IP3 receptors and activates excessive Ca2+ release from
the endoplasmic reticulum (25–28).
Previously, increasing data have indicated that anesthetics are
neurotoxic even at normal clinical doses (29,30). The
primary neurotoxic effect is mediated through the activation of
apoptotic death (22). Previous
studies have suggested that isoflurane induced neurocognitive
impairment and neuroapoptosis in neonatal rats (22). It has also been demonstrated that
isoflurane induced neuroapoptosis throughout the cortex, thalamus
and hippocampus regions, accompanying increased caspase-3 levels
(22). Bupivacaine, as a local
anesthetic, has been demonstrated to induce neural dysfunction and
cell apoptosis in vitro (31). Bupivacaine led to the inhibition of
mitochondrial respiratory complexes, decreased mitochondrial
membrane potential and overproduction of reactive oxygen species
(ROS), with cytochrome c liberation and activation of the
caspase-3-dependent apoptosis pathway (32,33).
Dex is usually used as an antianxiety treatment,
sedative and analgesic. Dex may relieve stress and maintain the
stable function of the cardiovascular system (34). During the anesthesia recovery phase,
Dex maintained patients in a continuous calm state with good
respiratory function (35). Dex is
an α2-adrenergic agonist, and exhibited neuroprotective effects
against ischemic cerebral injury through activating the
α2-adrenergic receptors and binding at imidazoline 1 and 2
receptors (4). Dex attenuated
isoflurane-induced injury in the developing brain, providing
neurocognitive protection (4). Dex
attenuated bupivacaine-induced cytotoxicity in the mouse
neuroblastoma N2 cell line, primarily by decreasing the release of
ROS and the expression of caspase-3, and ultimately inhibiting
apoptosis in N2 cells (17).
Consistent with the aforementioned results, the present study
identified that Dex protected the hippocampal neuronal HT22 cells
against isoflurane-and bupivacaine-induced apoptosis. However, a
previous study suggested that Dex itself induced neuroapoptosis
in vivo and in vitro, respectively, and although Dex
exhibited neuroprotective effect at clinical doses, the high
cumulative doses and concentrations induced neuroapoptosis
(36).
Apoptosis is activated by a series of caspases,
namely the cysteine aspartic-specific proteases. The apoptotic
pathway is activated by intracellular and extracellular signals.
There are two different pathways leading to apoptosis: The
intrinsic (mitochondrial) and extrinsic (death receptor) pathways
(37). The intrinsic pathway is
associated with changes in the permeability transition of the outer
mitochondrial membrane, and this permeability is primarily
controlled by the Bcl-2 family of proteins, including Bax, through
regulating the formation of apoptotic protein-conducting pores in
the mitochondrial membrane (37).
Then, permeabilization allows the release of intermembrane proteins
cytochrome c, which functions to activate caspase-9, thereby
promoting the expression of caspase-3, activating apoptosis
(37). Cleaved caspase-3 expression
is a marker of apoptotic cell death (37). In the present study, western blot
analysis data demonstrated that isoflurane and bupivacaine
increased the expression of cleaved caspase-3, Bax, Bcl-2 and
cytochrome c in the hippocampal neuronal HT22 cells, while Dex
inhibited this increase. This suggests that the intrinsic apoptosis
pathway was upregulated by treatment with anesthetics in the
hippocampal neuronal HT22 cells.
In the present study, isoflurane or bupivacaine
significantly decreased the protein expression levels of p-PI3K and
p-AKT, while treatment with Dex or IGF-1 increased the expression
of p-PI3K and p-AKT. This indicates that the neuroprotective
effects of Dex may be mediated by activating the PI3K/AKT pathway.
The results of the present study are consistent with the study of
Li et al (4), which suggested
that Dex treatment induced neuroprotective effects against
isoflurane-induced neuroapoptosis in the hippocampus of neonatal
rats by preserving PI3K/AKT pathway activity. HIF-1α and PKM2 are
associated with glucose metabolism and mitochondrial respiratory
chain (38). In the present study,
Dex protected hippocampal neuronal HT22 cells from isoflurane- or
bupivacaine-induced apoptosis primarily through suppressing the
HIF-α/PKM2 axis. Thereby, the anti-apoptosis effect of Dex may be
associated with the regulation of the HIF-α/PKM2 pathway. However,
the detailed interactions how Dex preserves PI3K/AKT activity and
suppresses the HIF-α/PKM2 pathway remain unclear and require
additional investigation.
In conclusion, the present study suggested that Dex
treatment protected against anesthetic-induced intrinsic apoptosis
in vitro, indicating that it exhibits anti-apoptotic
qualities. It was demonstrated that the neuroprotective effect of
Dex against anesthetic-induced cell apoptosis occurred primarily
through preserving PI3K/AKT activity and suppressing the HIF-α/PKM2
pathway. These data provide not only novel insight into the complex
associations between anesthetics and cell death, but also the
possibility for the treatment of anesthetic-induced injury using
Dex.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical and
Health Science and Technology Plan of Zhejiang Province
(2014kyb082, 2018KY390).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FB, XK and JW contributed to the experimental
design, tissue collection, the execution of experiments and were
the major contributors in developing the first draft of the
manuscript. QX performed staining assay, and analyzed and
interpreted the data. FB reviewed and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kehlet H and Dahl JB: Anaesthesia,
surgery, and challenges in postoperative recovery. Lancet.
362:1921–1928. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie Z, Culley DJ, Dong Y, Zhang G, Zhang
B, Moir RD, Frosch MP, Crosby G and Tanzi RE: The common inhalation
anesthetic isoflurane induces caspase activation and increases
amyloid beta-protein level in vivo. Ann Neurol. 64:618–627. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malik O, Kaye AD, Kaye A, Belani K and
Urman RD: Emerging roles of liposomal bupivacaine in anesthesia
practice. J Anaesthesiol Clin Pharmacol. 33:151–156.
2017.PubMed/NCBI
|
|
4
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by preserving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Zhang QG, Lai LY, Wen XJ, Zheng T,
Cheung CW, Zhou SQ and Xu SY: Neuroprotective effect of ginkgolide
B on bupivacaine-induced apoptosis in SH-SY5Y cells. Oxid Med Cell
Longev. 2013:1598642013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng J, Drobish JK, Liang G, Wu Z, Liu C,
Joseph DJ, Abdou H, Eckenhoff MF and Wei H: Anesthetic
preconditioning inhibits isoflurane-mediated apoptosis in the
developing rat brain. Anesth Analg. 119:939–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malikova J, Zdarilova A and Hlobilkova A:
Effects of sanguinarine and chelerythrine on the cell cycle and
apoptosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
150:5–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun J, Chen XL, Zheng JY, Zhou JW and Ma
ZL: Astragaloside IV protects new born rats from anesthesia-induced
apoptosis in the developing brain. Exp Ther Med. 12:1829–1835.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Becker EB and Bonni A: Cell cycle
regulation of neuronal apoptosis in development and disease. Prog
Neurobiol. 72:1–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raynaud F and Marcilhac A: Implication of
calpain in neuronal apoptosis. A possible regulation of Alzheimer's
disease. FEBS J. 273:3437–3443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tatton NA: Increased caspase 3 and Bax
immunoreactivity accompany nuclear GAPDH translocation and neuronal
apoptosis in Parkinson's disease. Exp Neurol. 166:29–43. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koh MY, Darnay BG and Powis G:
Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and
ubiquitinates hypoxia-inducible factor 1alpha, leading to its
oxygen-independent degradation. Mol Cell Biol. 28:7081–7095. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Wit RH, Mujić-Delić A, van Senten JR,
Fraile-Ramos A, Siderius M and Smit MJ: Human cytomegalovirus
encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in
glioblastoma cells. Oncotarget. 7:67966–67985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tüfek A, Kaya S, Tokgöz O, Firat U,
Evliyaoğlu O, Çelik F and Karaman H: The protective effect of
dexmedetomidine on bupivacaine-induced sciatic nerve inflammation
is mediated by mast cells. Clin Invest Med. 36:E95–E102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao C, Chen X, Zhu Y, Zeng Y and Jin J: A
senescence model induced by tertbutyl-hydroperoxide in rats. J
Fujian Medical Univ. 37:19–22. 2003.
|
|
19
|
Annunziato L, Amoroso S, Pannaccione A,
Cataldi M, Pignataro G, D'Alessio A, Sirabella R, Secondo A, Sibaud
L and Di Renzo GF: Apoptosis induced in neuronal cells by oxidative
stress: Role played by caspases and intracellular calcium ions.
Toxicol Lett. 139:125–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kogo J, Takeba Y, Kumai T, Kitaoka Y,
Matsumoto N, Ueno S and Kobayashi S: Involvement of TNF-alpha in
glutamate-induced apoptosis in a differentiated neuronal cell line.
Brain Res. 1122:201–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lauritzen I, Zanzouri M, Honoré E, Duprat
F, Ehrengruber MU, Lazdunski M and Patel AJ: K+-dependent
cerebellar granule neuron apoptosis. Role of task leak K+ channels.
J Biol Chem. 278:32068–32076. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanders RD, Xu J, Shu Y, Januszewski A,
Halder S, Fidalgo A, Sun P, Hossain M, Ma D and Maze M:
Dexmedetomidine attenuates isoflurane-induced neurocognitive
impairment in neonatal rats. Anesthesiology. 110:1077–1085. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Auroy Y, Benhamou D, Bargues L, Ecoffey C,
Falissard B, Mercier FJ, Bouaziz H and Samii K: Major complications
of regional anesthesia in France: The SOS Regional Anesthesia
Hotline Service. Anesthesiology. 97:1274–1280. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park CJ, Park SA, Yoon TG, Lee SJ, Yum KW
and Kim HJ: Bupivacaine induces apoptosis via ROS in the Schwann
cell line. J Dent Res. 84:852–857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang G, Wang Q, Li Y, Kang B, Eckenhoff
MF, Eckenhoff RG and Wei H: A presenilin-1 mutation renders neurons
vulnerable to isoflurane toxicity. Anesth Analg. 106:492–500. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao X, Yang Z, Liang G, Wu Z, Peng Y,
Joseph DJ, Inan S and Wei H: Dual effects of isoflurane on
proliferation, differentiation, and survival in human
neuroprogenitor cells. Anesthesiology. 118:537–549. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang H, Liang G, Hawkins BJ, Madesh M,
Pierwola A and Wei H: Inhalational anesthetics induce cell damage
by disruption of intracellular calcium homeostasis with different
potencies. Anesthesiology. 109:243–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Liang G, Chen Q, Joseph DJ, Meng
Q, Eckenhoff RG, Eckenhoff MF and Wei H: Anesthetic-induced
neurodegeneration mediated via inositol 1,4,5-trisphosphate
receptors. J Pharmacol Exp Ther. 333:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Griffiths JD, Le NV, Grant S, Bjorksten A,
Hebbard P and Royse C: Symptomatic local anaesthetic toxicity and
plasma ropivacaine concentrations after transversus abdominis plane
block for Caesarean section. Br J Anaesth. 110:996–1000. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gibbs NM and Rodoreda P: Primary
anaesthetic deaths in Western Australia from 1985–2008: Causation
and preventability. Anaesth Intensive Care. 41:302–310.
2013.PubMed/NCBI
|
|
31
|
Perez-Castro R, Patel S, Garavito-Aguilar
ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ and Xu F:
Cytotoxicity of local anesthetics in human neuronal cells. Anesth
Analg. 108:997–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arai Y, Kondo T, Tanabe K, Zhao QL, Li FJ,
Ogawa R, Li M and Kasuya M: Enhancement of hyperthermia-induced
apoptosis by local anesthetics on human histiocytic lymphoma U937
cells. J Biol Chem. 277:18986–18993. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cela O, Piccoli C, Scrima R, Quarato G,
Marolla A, Cinnella G, Dambrosio M and Capitanio N: Bupivacaine
uncouples the mitochondrial oxidative phosphorylation, inhibits
respiratory chain complexes I and III and enhances ROS production:
Results of a study on cell cultures. Mitochondrion. 10:487–496.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng K, Wu SR, Ji FH and Li J:
Premedication with dexmedetomidine in pediatric patients: A
systematic review and meta-analysis. Clinics (Sao Paulo).
69:777–786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia ZQ, Chen SQ, Yao X, Xie CB, Wen SH and
Liu KX: Clinical benefits of dexmedetomidine versus propofol in
adult intensive care unit patients: A meta-analysis of randomized
clinical trials. J Surg Res. 185:833–843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu JR, Yuki K, Baek C, Han XH and Soriano
SG: Dexmedetomidine-induced neuroapoptosis is dependent on its
cumulative dose. Anesth Analg. 123:1008–1017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KH, Seo HS, Choi HS, Choi I, Shin YC
and Ko SG: Induction of apoptotic cell death by ursolic acid
through mitochondrial death pathway and extrinsic death receptor
pathway in MDA-MB-231 cells. Arch Pharm Res. 34:1363–1372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen G, Feng W, Zhang S, Bian K, Yang Y,
Fang C, Chen M, Yang J and Zou X: Metformin inhibits gastric cancer
via the inhibition of HIF1α/PKM2 signaling. Am J Cancer Res.
5:1423–1434. 2015.PubMed/NCBI
|