Introduction
Osteoarthritis (OA) is a degenerative joint disease
mainly affecting the elderly (1).
With the growth of aging population, incidence of OA shows an
increasing trend. Most elderly people show symptoms of systemic
multi-articular OA, and approximately 60% of OA elderly patients
need to be treated (2). At present,
the pathogenesis of OA has not yet been elucidated, and clinically
there is no effective means for diagnosing early OA. Magnetic
resonance imaging (MRI) and X-ray are common methods for diagnosing
OA, but it has certain limitations. Joints are often affected even
when the X-ray results are normal. MRI has a higher resolution in
the diagnosis of early OA, however, the application of MRI is
limited by the high cost (3,4). Although there are many clinical methods
for treating OA, the treatment cycle is long and the effect is
often not ideal. With the development of OA, joints are gradually
destroyed, and internal structure of the joint undergoes
pathological changes and disorders. In clinical practice, it often
manifests as joint pain, swelling, morning stiffness, and poor
joint stability. In severe cases, joint deformity and function may
also occur (5,6). Therefore, early diagnosis and treatment
of OA is particularly important. With the development of molecular
biology, the application of biological markers in the diagnosis of
OA have attracted increasing attention. C-terminal telopeptides of
collagen type II (CTX-II) is a biomarker that can reflect the
pathological changes and metabolism of joint tissue. It can be
detected in urine, blood, and synovial fluid (7). YKL-40 is a member of the 18 glycosyl
hydrolase family and is widely distributed in synoviocytes and
chondrocytes (8). Studies have shown
that CTX-II and YKL-40 are closely related to the pathological
changes of articular cartilage and can reflect the degree of
inflammation of OA (9). Glucosamine
is an important component of cartilage tissue. Glucosamine
supplementation can reduce the destruction of cartilage tissue and
cells. Diacerein can induce cartilage production and has
anti-inflammatory, analgesic and antipyretic effects. Both
glucosamine and diacerein supplementation can relieve joint pain
and improve joint activity, thereby delaying the course of OA
(10). Clinically, WOMAC is a
scoring system specially designed for hip and knee arthritis, which
can assess the severity of arthritis and its therapeutic effect
according to the related symptoms and signs of patients (11). Previous studies on CTX-II and YKL-40
mainly focused on the development of OA articular cartilage tissue.
There are few studies on the diagnostic value of serum CTX-II and
YKL-40 in patients with OA. This study examined the expression of
CTX-II and YKL-40 in the serum of patients with early OA and
explored the role of CTX-II and YKL-40 in the diagnosis of early
OA, assessment of disease status and evaluation of therapeutic
effect.
Materials and methods
General information
Diagnostic experiment of 90 patients with OA
diagnosed and treated in The First Affiliated Hospital, Guangzhou
Medical University (Guangzhou, China) from March 2015 to January
2018 were selected as the study group. The study group included 38
males and 52 females, and age ranged from 49 to 78 years, with an
average age of 58.8±6.7 years. Kellgren Lawrence (K-L) (12) classification: 30 cases of grade I, 23
cases of grade II, 19 cases of grade III and 18 cases of grade IV.
Inclusion criteria: i) Patients met OA diagnostic criteria
established by the American College of Rheumatology (ACR; Atlanta,
GA, USA) (11); ii) K-L (13) grade <1, imaging shows no
osteophyte hyperplasia and joint space is normal; and iii) knee
pain and soreness last for at least 4 months. Exclusion criteria:
i) Patients who have previously received knee joint treatment; ii)
patients with fever or skin lesions at the site of the disease;
iii) patients with severe hepatorenal and hematopoietic disorders;
iv) patients with other bony diseases such as gout and bone cancer;
v) individuals with a history of mental illness or having a family
history of mental illness; and vi) age ≤39 years or age ≥85 years.
At the same time, 50 healthy elderly individuals were selected as
the control group. The control group included 23 males and 27
females, and age ranged from 43 to 75 years, with a mean age of
59.2±5.1 years. The study was approved by the Ethics Committee of
The First Affiliated Hospital, Guangzhou Medical University, and
all participants signed an informed consent.
Grouping and treatment
The study group was divided into three subgroups
including group A [29 cases, oral intake of 500 mg glucosamine
sulfate (14), batch no. H20090305;
Hubei Aipu Bio-Engineering Co., Ltd., Wuhan, China], group B [29
cases, oral intake of 50 mg diacerein (15), batch no. J20100150; Kunming Jida
Pharmaceutical Co., Ltd., Kunming, China] and group C (32 cases,
oral intake of 500 mg glucosamine sulfate and 50 mg diacerein).
Treatment was performed twice a day. Patients' adverse reactions
and toxic side effects during the treatment were recorded.
Observation of curative effect
Western Ontario and McMaster Universities
Osteoarthritis Index (WOMAC) was used to assess the severity of
arthritis and its therapeutic effect. WOMAC is the best
self-assessment scale for OA. It includes joint pain, joint
stiffness and daily activities. The total score was 20 points for
joint pain, 8 points for stiff and 68 points for daily activities.
Higher scores indicate more serious conditions. WOMAC scores were
evaluated before and at 3, 6 and 9 weeks after the beginning of
treatment to assess symptom improvement and functional
recovery.
Sample collection and detection
Fasting venous blood was extracted from each
participant at 1 week gap, before drug treatment, 3, 6 and 9 weeks
of treatment. The serum was separated by centrifugation at 3,000 ×
g (Hunan Pingfan Technology Co., Ltd., Changsha, China) and was
stored at −20°C. The concentrations of CTX-II and YKL-40 in serum
were detected by enzyme-linked immunosorbent assay (ELISA) using
human CTX-II ELISA kit (Shanghai Guye Biotechnology Co., Ltd.,
Shanghai, China) and human YKL-40 ELISA kit (Qingdao Jieshikang
Biotech Co., Ltd., Qingdao, China) according to the instructions of
the kit. The kit was kept at room temperature for 30 min before
use, and test sample, standard and blank wells were set.
Enzyme-labeled reagents and samples were not added into the black
wells. The remaining wells were added with 100 µl of the test
samples or standard samples. After mixing, the microtiter plates
were covered with membranes and incubated at 37°C for 2 h. After
that, the liquid was discarded. After air drying, 100 µl of working
solution A was added into each well. The wells were covered and
incubated for 1 h at 37°C. After that, the liquid was discarded.
After spin drying, the plate was washed three times with automatic
plate washer (Nanjing Detie Laboratory Equipment Co., Ltd.,
Nanjing, China). Then, 100 µl of working solution B was added into
each well and the wells were covered and incubated for 1 h at 37°C.
After that, the liquid was discarded. After spin dry, the plate was
washed 3 times and 90 µl of substrate solution was added into each
well. The wells were covered with membrane, followed by incubation
in the dark at room temperature for 20 min. Then, 50 µl of stop
solution was added into each well, and the OD value of each well
was immediately detected at 450 nm using an enzyme-labeled analyzer
(Shanghai Xinzhuang Instrument Co., Ltd., Shanghai, China) to
calculate the concentrations of CTX-II and YKL-40.
Statistical analysis
SPSS v.20.0 (Beijing Netscape Technology Co., Ltd.,
Beijing, China) was used for statistical analysis. Measured data
were expressed as mean ± standard deviation. t-test was used for
comparison of the measurement data between two groups. Chi-square
test was used to compare enumeration data between the groups.
One-way analysis of variance was used for comparisons among
multiple groups. Comparison of data at multiple time-points was
performed using the repeated measures analysis of variance and the
post hoc test was LSD. Intragroup comparisons were compared twice
by LSD t-test. Diagnostic performance of serum CTX-II and YKL-40
concentrations for OA was evaluated using receiver operating
characteristic (ROC) curves. Correlation analysis was performed
using Pearson's correlation coefficient. P<0.05 was considered
to indicate a statistically significant difference.
Results
General information
There were no significant differences in sex, age,
smoking habit, body mass index (BMI), creatinine (Cre), uric acid
(UA), alanine aminotransferase (ALT), aspartate aminotransferase
(AST), blood glucose (Glu), r-glutamyl transferase (r-GT) among the
study and control groups (P>0.05). WOMAC scores of the study
groups A-C were significantly higher than those of the control
group (t=28.310, P<0.001; Table
I).
 | Table I.Baseline data of the study and control
groups [n(%)]/(mean ± SD). |
Table I.
Baseline data of the study and control
groups [n(%)]/(mean ± SD).
| Indexes | Study group
(n=90) | Control group
(n=50) | t/χ2 | P-value |
|---|
| Sex |
|
| 0.187 |
0.723 |
| Male | 38 (42.22) | 23 (46.00) |
|
|
|
Female | 52 (57.78) | 27 (54.00) |
|
|
| Age, years | 58.8±6.7 | 59.2±5.1 |
0.367 |
0.714 |
| Smoking |
|
|
0.083 |
0.855 |
| Yes | 32 (35.56) | 19 (38.00) |
|
|
| No | 58 (64.44) | 31 (62.00) |
|
|
| BMI,
kg/m2 | 24.13±4.83 | 25.13±3.16 |
1.315 |
0.190 |
| Cre, mmol/l | 10.16±1.08 | 10.26±0.76 |
0.579 |
0.563 |
| UA, µmol/l | 193.23±22.14 | 186.14±23.47 |
1.777 |
0.077 |
| ALT, U/l | 19.41±8.46 | 21.62±8.04 |
1.507 |
0.134 |
| AST, U/l | 18.63±7.26 | 19.74±8.16 |
0.828 |
0.408 |
| Glu, mmol/l |
6.01±0.98 |
5.87±1.06 |
0.786 |
0.432 |
| r-GT, U/l |
43.53±17.63 |
45.15±16.78 |
0.529 |
0.597 |
| WOMAC score |
47.38±10.41 |
5.16±2.12 | 28.310 | <0.001 |
Serum CTX-II and YKL-40 concentrations
in study and control groups
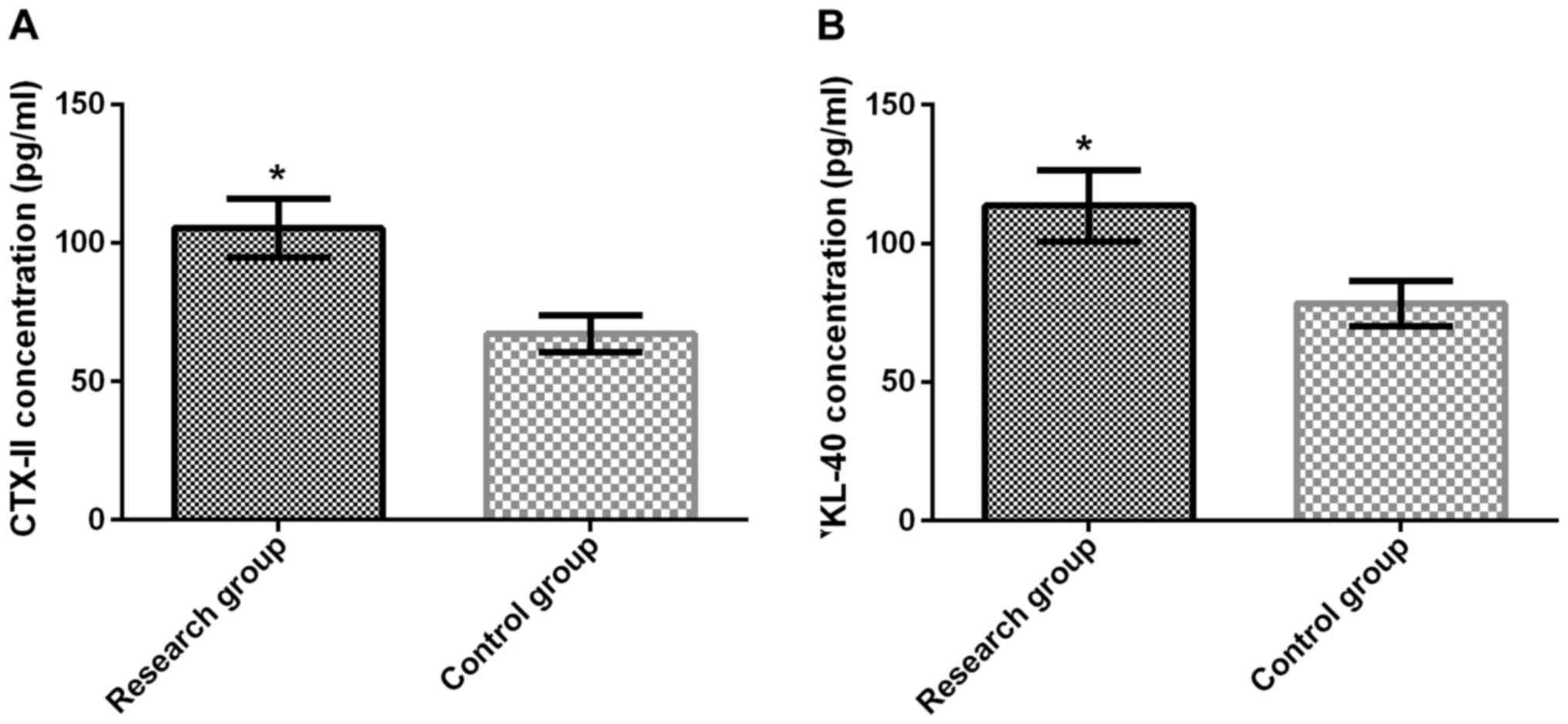
Concentrations of serum CTX-II and YKL-40 in the
study group were 105.41±10.63 pg/ml and 113.58±12.87 pg/ml,
respectively. Concentrations of serum CTX-II and YKL-40 in the
control group were 67.12±6.74 pg/ml and 78.26±8.12 pg/ml,
respectively. Serum CTX-II concentrations in the study group were
significantly higher than those in the control group (P<0.001).
Serum YKL-40 concentrations in the study group were also
significantly higher than those in the control group (P<0.001;
Fig. 1A and B).
Diagnostic value of serum CTX-II and
YKL-40 concentrations for OA
ROC curve of serum CTX-II and YKL-40 concentrations
in diagnosis of OA was plotted. Area under the curve (AUC) of serum
CTX-II in the diagnosis of OA was 0.886 [95% confidence interval
(CI): 0.930 to 0.942], optimal cut-off value for diagnosis of OA
was 0.70, diagnostic sensitivity was 84% and specificity was 86%.
AUC of serum YKL-40 in the diagnosis of OA was 0.880 (95% CI: 0.822
to 0.939), optimal cut-off value for diagnosis of OA was 0.62,
diagnostic sensitivity was 82%, and specificity was 80%. ROC curve
for the diagnosis of OA using combination of serum CTX-II and
YKL-40 was plotted. AUC for the diagnosis of OA by serum CTX-II
combined with YKL-40 was 0.880 (95% CI: 0.820 to 0.939), optimal
cutoff value for diagnosis of OA was 0.78, diagnostic sensitivity
was 90%, and specificity was 78% (Fig.
2).
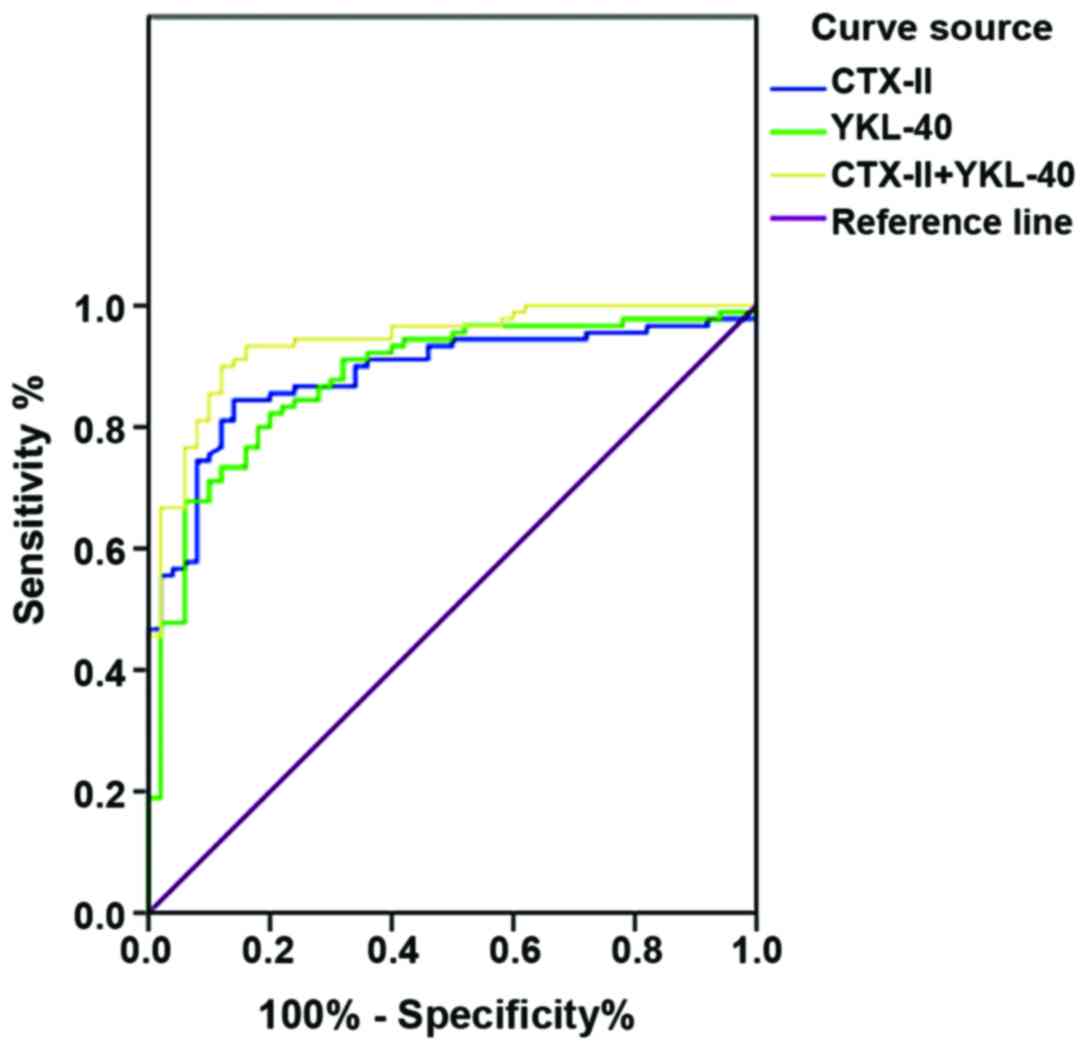 | Figure 2.Diagnostic value of serum CTX-II and
YKL-40 concentrations for OA. ROC curve showed that AUC of serum
CTX-II in the diagnosis of OA was 0.886 (95% CI: 0.930 to 0.942),
optimal cut-off value for diagnosis of OA was 0.70, diagnostic
sensitivity was 84% and specificity was 86%. AUC of serum YKL-40 in
the diagnosis of OA was 0.880 (95% CI: 0.822 to 0.939), optimal
cut-off value for diagnosis of OA was 0.62, diagnostic sensitivity
was 82%, and specificity was 80%. ROC curve for the diagnosis of OA
using combination of serum CTX-II and YKL-40 was plotted. AUC for
the diagnosis of OA by serum CTX-II combined with YKL-40 was 0.880
(95% CI: 0.820 to 0.939), optimal cut-off value for diagnosis of OA
was 0.78, diagnostic sensitivity was 90%, and specificity was 78%.
CTX-II, C-terminal telopeptides of collagen type II; OA,
osteoarthritis; ROC, receiver operating characteristic; AUC, area
under the curve; CI, confidence interval. |
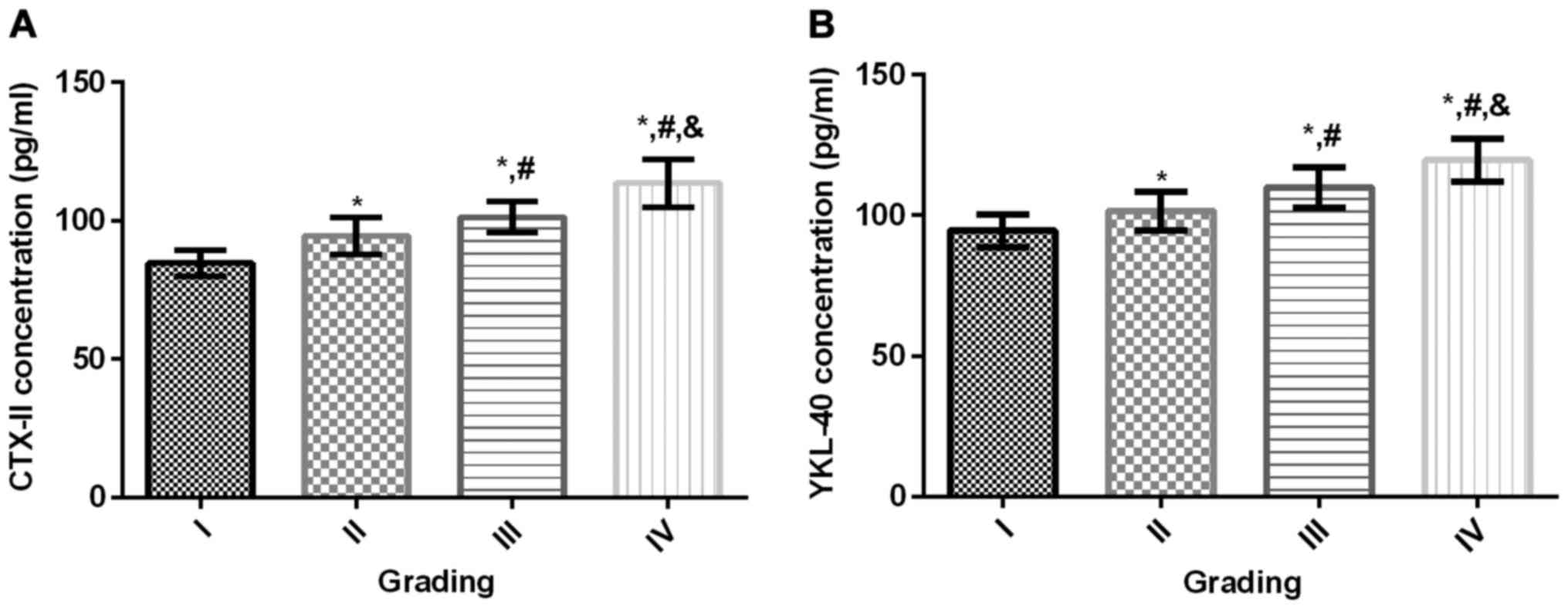
Serum CTX-II and YKL-40 levels of
different K-L grades
CTX-II and YKL-40 concentrations were significantly
higher in grades II–IV patients than in grade I (P<0.001).
CTX-II and YKL-40 concentrations were significantly higher in
patients with grade III and IV than in patients with K-L grade II
(P<0.001). The concentrations of CTX-II and YKL-40 in patients
with grade IV were significantly higher (P<0.001) than those
with grade III. Concentrations of CTX-II and YKL-40 increased with
the increase of K-L classification (Fig.
3A and B).
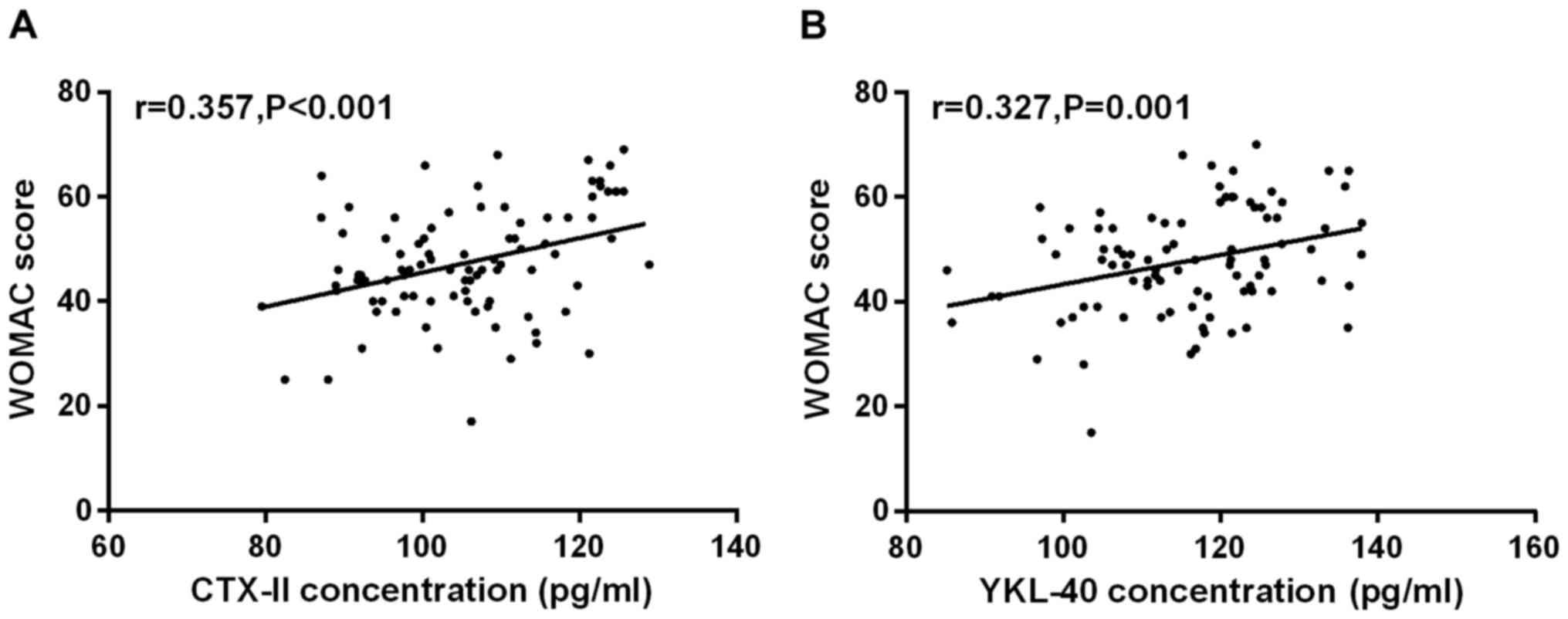
Correlation between serum CTX-II and
YKL-40 concentrations and WOMAC score before treatment in the study
group
Serum CTX-II concentrations in the study group was
positively correlated with WOMAC score (r=0.357, P<0.001). Serum
YKL-40 concentrations was positively correlated with WOMAC score
(r=0.327, P=0.001; Fig. 4A and
B).
WOMAC scores before and after
treatment in groups A-C
There was no significant difference in WOMAC scores
before treatment and at 3, 6 and 9 weeks after the beginning of
treatment among groups A-C (P>0.05). Compared with pre-treatment
scores, WOMAC scores decreased significantly at 3, 6, and 9 weeks
in groups A-C (P<0.001). Comparison of scores at 3 weeks after
the beginning of treatment, WOMAC scores of groups A-C decreased
significantly at 6 and 9 weeks (P<0.001) and the scores at 6
weeks after the beginning of treatment, WOMAC scores of groups A-C
decreased significantly at 9 weeks (P<0.001; Table II).
 | Table II.WOMAC scores before and after
treatment in groups A-C (mean ± SD). |
Table II.
WOMAC scores before and after
treatment in groups A-C (mean ± SD).
| Time-points | Group A (n=29) | Group B (n=29) | Group C (n=32) | F | P-value |
|---|
| Before
treatment | 45.63±9.58 |
46.17±10.22 |
50.26±11.25 | 1.830 | 0.166 |
| 3 weeks |
38.83±8.70a |
39.41±7.85a |
35.41±7.85a | 2.182 | 0.118 |
| 6 weeks |
30.16±6.45a,b |
32.10±5.72a,b |
29.74±5.74a,b | 1.324 | 0.271 |
| 9 weeks |
23.26±4.08a–c |
21.42±5.01a–c |
20.52±4.17a–c | 2.990 | 0.055 |
| F | 49.300 | 58.170 | 83.400 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
|
|
Changes of serum CTX-II concentrations
before and after treatment in groups A-C
There was no significant difference in serum CTX-II
concentrations before treatment and at 3, 6 and 9 weeks after the
beginning of treatment among groups A-C (P>0.05). Compared with
pre-treatment scores, serum CTX-II concentrations decreased
significantly at 3, 6 and 9 weeks in groups A-C (P<0.001).
Comparison of scores at 3 weeks after the beginning of treatment,
serum CTX-II concentrations of groups A-C decreased significantly
at 6 and 9 weeks (P<0.001). Comparison of scores at 6 weeks
after the beginning of treatment, serum CTX-II concentrations of
groups A-C decreased significantly at 9 weeks (P<0.001; Table III).
 | Table III.Serum CTX-II concentrations before
and after treatment in groups A-C (pg/ml)/(mean ± SD). |
Table III.
Serum CTX-II concentrations before
and after treatment in groups A-C (pg/ml)/(mean ± SD).
| Time-points | Group A (n=29) | Group B (n=29) | Group C (n=32) | F | P-value |
|---|
| Before
treatment | 101.11±11.62 | 104.26±10.89 | 105.43±12.17 | 1.113 | 0.333 |
| 3 weeks |
91.13±8.63a |
88.41±9.01a |
92.13±8.45a | 1.470 | 0.235 |
| 6 weeks |
83.56±7.15a,b |
82.18±6.71a,b |
86.12±7.27a,b | 2.465 | 0.090 |
| 9 weeks |
72.87±7.01a–c |
73.52±6.97a–c |
70.13±7.53a–c | 1.935 | 0.150 |
| F | 53.370 |
6.530 | 80.170 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
|
|
Changes of serum YKL-40 concentrations
before and after treatment in groups A-C
There was no significant difference in serum YKL-40
concentrations before treatment and at 3, 6 and 9 weeks after the
beginning of treatment among groups A-C (P>0.05). Compared with
pre-treatment scores, serum YKL-40 concentrations decreased
significantly at 3, 6 and 9 weeks in groups A-C (P<0.001).
Comparison of scores at 3 weeks after the beginning of treatment,
serum YKL-40 concentrations of groups A-C decreased significantly
at 6 and 9 weeks (P<0.001). Comparison of scores at 6 weeks
after the beginning of treatment, serum YKL-40 concentrations of
groups A-C decreased significantly at 9 weeks (P<0.001; Table IV).
 | Table IV.Serum YKL-40 concentrations before
and after treatment in groups A-C (pg/ml)/(mean ± SD). |
Table IV.
Serum YKL-40 concentrations before
and after treatment in groups A-C (pg/ml)/(mean ± SD).
| Time-points | Group A (n=29) | Group B (n=29) | Group C (n=32) | F | P-value |
|---|
| Before
treatment | 114.56±12.65 |
116.14±13.28 | 112.63±10.26 | 0.647 | 0.525 |
| 3 weeks |
103.14±11.63a |
105.41±9.78a |
101.74±10.03a | 0.940 | 0.394 |
| 6 weeks |
92.41±9.86a,b |
93.45±10.26a,b |
89.93±8.63a,b | 1.097 | 0.338 |
| 9 weeks |
83.74±8.41a–c |
84.11±8.45a–c |
80.17±9.12a–c | 1.942 | 0.149 |
| F | 44.610 | 50.410 | 66.360 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
|
|
Correlation between serum CTX-II and
YKL-40 concentration and WOMAC score at 3, 6 and 9 weeks of
treatment in OA patients
At 3 weeks of treatment, CTX-II was positively
correlated with YKL-40 concentration and WOMAC score (r=0.406,
P<0.001; r=0.430, P<0.001); CTX-II was positively correlated
with YKL-40 concentration and WOMAC score at 6 weeks of treatment
(r=0.350, P<0.001; r=0.358, P<0.001); At 9 weeks of
treatment, serum CTX-II was positively correlated with YKL-40
concentration and WOMAC score (r=0.370, P<0.001; r=0.394,
P<0.394; Fig. 5A-F).
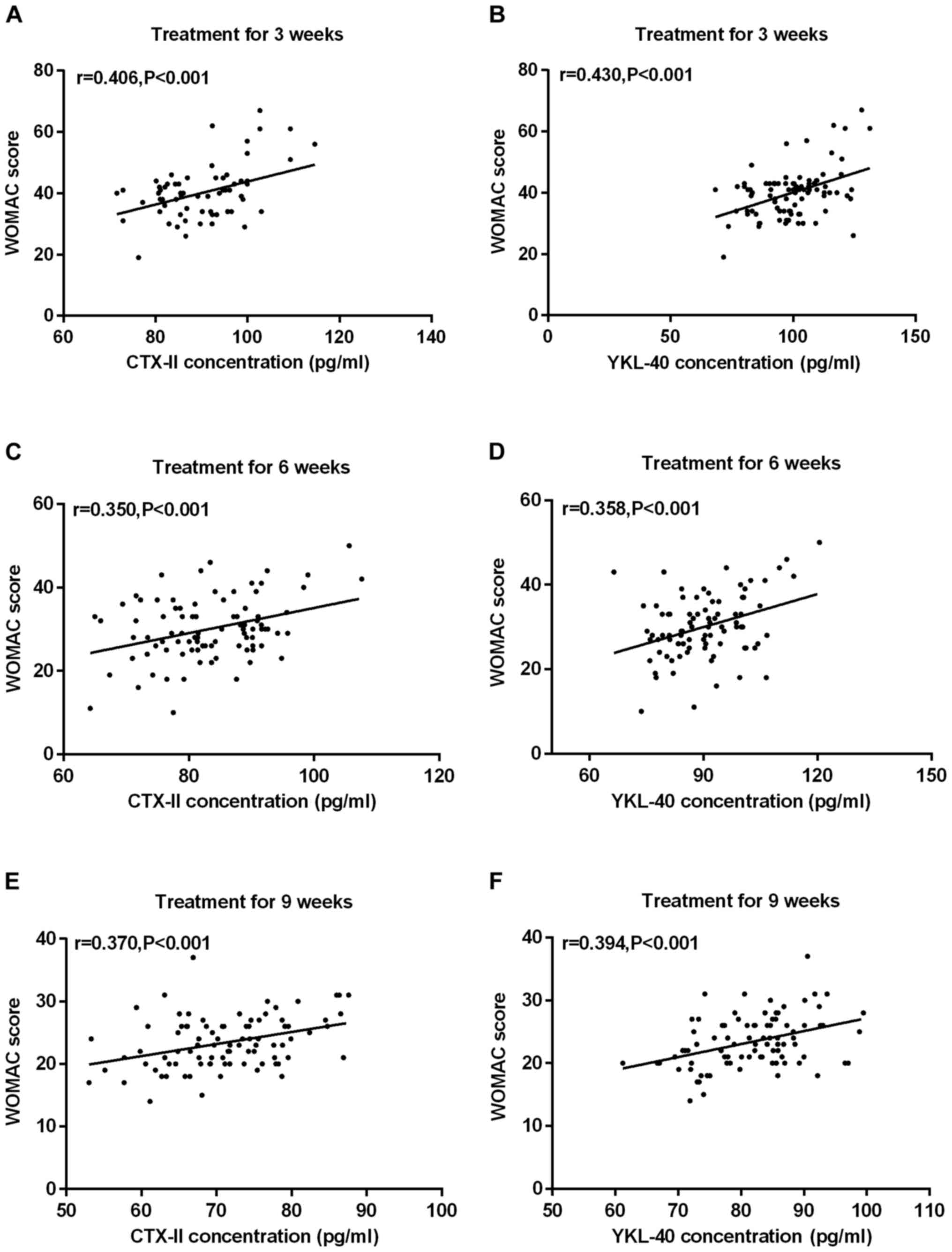 | Figure 5.Correlation between serum CTX-II and
YKL-40 concentration and WOMAC score in OA patients at 3, 6 and 9
weeks. (A) Pearson test results showed that the serum CTX-II
concentration of patients with OA was positively correlated with
the WOMAC score at 3 weeks of treatment (r=0.406, P<0.001). (B)
At 3 weeks of treatment, serum YKL-40 concentration in OA patients
was positively correlated with WOMAC score (r=0.430, P<0.001).
(C) The serum CTX-II concentration of patients with OA was
positively correlated with the WOMAC score at 6 weeks of treatment
(r=0.350, P<0.001). (D) At 6 weeks of treatment, serum YKL-40
concentration of OA patients was positively correlated with WOMAC
score (r=0.358, P<0.001). (E) At 9 weeks of treatment, serum
CTX-II concentration of OA patients was positively correlated with
WOMAC score (r=0.370, P<0.001). (F) At 9 weeks of treatment,
serum YKL-40 concentration in OA patients was positively correlated
with WOMAC score (r=0.394, P<0.001). CTX-II, C-terminal
telopeptides of collagen type II; WOMAC, Western Ontario and
McMaster Universities Osteoarthritis Index; OA, osteoarthritis. |
Safety analysis
None of the patients experienced any discomfort or
toxicity during the treatment of this study.
Discussion
OA is the most common joint disease in middle-aged
and elderly people. Pathological basis of OA mainly include
degenerative changes of articular cartilage and the hyperosteogeny.
Incidence of this disease increases with aging (16). OA causes irreversible damage to a
certain extent. OA not only brings inconvenience to the daily life
of patients, but also causes a heavy burden on their families.
Therefore, early diagnosis of OA has attracted increasing attention
(17). Compared with expensive MRI
and traumatic arthroscopy, molecular biology markers have the
advantages of affordable price and early detection. In recent
years, biomarkers have been increasingly used in the diagnosis of
OA.
Pathological changes of OA are manifested as the
loss or abnormal synthesis of glycoprotein in cartilage matrix,
which makes the base of the joint thinner and surface cartilage
softer, resulting in pathological hyperplasia and formation of
osteophytes (18). Collagen type II
is an important component of articular cartilage and is involved in
the reconstruction and repair of articular cartilage. When OA
occurs, the process of reconstruction and repair of articular
cartilage is accelerated, and the concentration of CTX-II in body
fluids also increases (19). CTX-II
is produced by the cleavage of mature type II collagen and passes
through joint blood, synovial fluid and urine in the form of
nano-collagen (20). YKL-40 is a
type of chitinase-like 3-protein that can reflect the state of
endothelial cell damage such as cell migration, adhesion and
reorganization (21). Concentration
of YKL-40 in normal individuals is low, but it is widely present in
articular chondrocytes and is mainly found in the surface and
middle layers of cartilage (22).
Results of this study showed that concentrations of serum CTX-II
and YKL-40 in the study group were significantly higher than those
of the control group, and concentrations of CTX-II and YKL-40
increased with the increase of k-l classification. Concentrations
of serum CTX-II and YKL-40 before treatment were positively
correlated with the WOMAC score, indicating that CTX-II and YKL-40
have important diagnostic value for early OA. Concentrations of
CTX-II and YKL-40 can be used as an objective reference for the
severity of OA. Meulenbelt et al (23) measured the concentration of CTX-II in
urine of patients with OA and found that the level of CTX-II in OA
patients was significantly increased, and there was a significant
correlation with the JOA score of hip, hand, articular surface and
knee joints, suggesting that CTX-II may be a sensitive marker of OA
activity. Väänänen et al (24) showed that expression of YKL-40 in the
synovial fluid of OA patients was significantly upregulated,
suggesting a significant correlation between YKL-40 and the degree
of inflammation in OA patients. Previous studies on CTX-II and
YKL-40 mainly focused on advanced OA. Our study further confirmed
the diagnostic value of CTX-II and YKL-40 for early OA. We further
found that the sensitivity of CTX-II combined with YKL-40 in the
diagnosis of OA was 90% and the specificity was 78%, while the
sensitivity of CTX-II alone in the diagnosis of OA was 84%, and the
specificity was 86%. For YKL-40 alone, sensitivity was 82% and
specificity was 80%. The combination of the two may improve the
sensitivity of early OA diagnosis.
Glucosamine and diacerein are commonly used
supplements in clinical treatment of OA patients. Diacerein can
suppress the vicious circle of joint inflammation by reducing the
production of inflammatory mediators, stabilize the articular
cartilage environment, thereby delaying the progression of OA and
improving the clinical symptoms of OA patients (25). Glucosamine is one of the components
of articular cartilage. It participates in glycosylation of lipids
and proteins in articular cartilage cells and metabolism of
articular chondrocytes. It promotes the production of bone marrow
mesenchymal stem cells and inhibits malignant cycle of joint
inflammation (26). The study of Wen
et al (27) showed that oral
glucosamine can delay the development of OA, relieve pain and
regulate the metabolism of chondrocytes in OA rats. Wilkens et
al (28) reported that
glucosamine has the properties of restoring cartilage and
anti-inflammation, and can reduce the pain related disability in
patients with degenerative lumbar OA. Pelletier et al
(29) confirmed that diacetaminophen
had good clinical efficacy for patients with OA, and believed that
the optimal dose of diacetaminophen was 100 mg/day. Therefore, it
was shown that glucosamine and diacetone have good clinical effects
on OA.
In this study, glucosamine, diacerein and their
combination were used to treat patients with early OA. Results
showed that there were no significant differences in WOMAC score,
serum CTX-II and YKL-40 concentrations before and at 3, 6 and 9
weeks after treatment among groups A, B and C. At 3, 6 and 9 weeks
after the beginning of treatment, WOMAC score and serum
concentrations of CTX-II and YKL-40 decreased significantly
(P<0.001). CTX-II was positively correlated with YKL-40
concentration and WOMAC score at 3, 6 and 9 weeks of treatment.
Glucosamine alone, diacerein alone and the combination showed
similar therapeutic effects, which may be explained by the short
treatment cycle.
Serum CTX-II and YKL-40 concentrations showed a
decreasing trend during the course of treatment. The degree of
decline was significantly correlated, so CTX-II and YKL-40 may
become biological indicators for the treatment effect evaluation of
OA patients. Manicourt et al (30) showed that oral administration of
salmon calcitonin in patients with knee OA can reduce the
expression of CTX-II, MMP-1 and MMP-3, and it is believed that the
expression level of these biomarkers can predict the change of knee
joint space. Väänänen et al (22) found that plasma YKL-40 levels are
associated with disease activity of rheumatoid arthritis during
treatment. Plasma YKL-40 is a biomarker for predicting RA disease
activity and can be used to guide RA remission therapy. Previous
studies mainly focused on CTX-II and YKL-40 in advanced OA, while
the use of CTX-II and YKL-40 for assessing treatment efficacy of
early OA patients is rare. In this study early OA patients were
included. Therefore, we confirmed that concentrations of serum
CTX-II and YKL-40 can be used as biological indicators for
evaluating the therapeutic effects of treatment of early OA
patients.
This study was conducted in strict accordance with
the inclusion and exclusion criteria. There was no difference in
sex, age, smoking habit, BMI, Cre, UA, ALT, AST, Glu, and r-GT
among the study subgroups A-C and the control group. Results
confirmed the potential of CTX-II and YKL-40 in the diagnosis of
the early stages of OA, determination of disease activity, and
treatment assessment. However, the regulatory mechanism of CTX-II
and YKL-40 in the development of OA has not yet been elucidated.
The treatment time is short and the sample size is small. In future
studies, we will expand the sample size, extend treatment time, and
conduct an in-depth investigation on the mechanisms of actions of
CTX-II and YKL-40 in OA.
In conclusion, combined detection of serum CTX-II
and YKL-40 can improve the sensitivity of OA diagnosis, and it has
an important diagnostic value for early OA patients. It can be used
as a biological indicator for OA diagnosis, severity assessment, as
well as evaluation of treatment effects.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW drafted the manuscript. PW and JS were mainly
devoted to collecting and interpreting the general data. PW, JS and
DQ performed ELISA. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital, Guangzhou Medical University
(Guangzhou, China). Signed informed consents were obtained from the
patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
MacDonald KV, Sanmartin C, Langlois K and
Marshall DA: Symptom onset, diagnosis and management of
osteoarthritis. Health Rep. 25:10–17. 2014.PubMed/NCBI
|
|
2
|
Chu CR, Millis MB and Olson SA:
Osteoarthritis: From Palliation to Prevention: AOA Critical Issues.
J Bone Joint Surg Am. 96:e1302014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hare KB, Lohmander Stefan L, Kise NJ,
Risberg MA and Roos EM: Middle-aged patients with an MRI-verified
medial meniscal tear report symptoms commonly associated with knee
osteoarthritis. Acta Orthop. 88:664–669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guermazi A, Roemer FW, Haugen IK, Crema MD
and Hayashi D: MRI-based semiquantitative scoring of joint
pathology in osteoarthritis. Nat Rev Rheumatol. 9:236–251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghavipour M, Sotoudeh G, Tavakoli E, Mowla
K, Hasanzadeh J and Mazloom Z: Pomegranate extract alleviates
disease activity and some blood biomarkers of inflammation and
oxidative stress in rheumatoid arthritis patients. Eur J Clin Nutr.
71:92–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Helgesson L, Johansson PK, Aurell Y,
Tiderius CJ, Kärrholm J and Riad J: Early osteoarthritis after
slipped capital femoral epiphysis. Acta Orthop. 89:222–228. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park YM, Kim SJ, Lee KJ, Yang SS, Min BH
and Yoon HC: Detection of CTX-II in serum and urine to diagnose
osteoarthritis by using a fluoro-microbeads guiding chip. Biosens
Bioelectron. 67:192–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karalilova R, Kazakova M, Batalov A and
Sarafian V: Correlation between protein YKL-40 and ultrasonographic
findings in active knee osteoarthritis. Med Ultrason. 1:57–63.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruyere O, Collette J, Kothari M, Zaim S,
White D, Genant H, Peterfy C, Burlet N, Ethgen D, Montague T, et
al: Osteoarthritis, magnetic resonance imaging, and biochemical
markers: A one year prospective study. Ann Rheum Dis. 65:1050–1054.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kongtharvonskul J, Woratanarat P, McEvoy
M, Attia J, Wongsak S, Kawinwonggowit V and Thakkinstian A:
Efficacy of glucosamine plus diacerein versus monotherapy of
glucosamine: A double-blind, parallel randomized clinical trial.
Arthritis Res Ther. 18:2332016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellamy N, Buchanan WW, Goldsmith CH,
Campbell J and Stitt LW: Validation study of WOMAC: A health status
instrument for measuring clinically important patient relevant
outcomes to antirheumatic drug therapy in patients with
osteoarthritis of the hip or knee. J Rheumatol. 15:1833–1840.
1988.PubMed/NCBI
|
|
12
|
Hunter AM, Leuchter AF, Cook IA, Abrams M,
Siegman BE, Furst DE and Chappell AS: Brain functional changes and
duloxetine treatment response in fibromyalgia: A pilot study. Pain
Med. 10:730–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazzuca SA, Brandt KD, Schauwecker DS,
Katz BP, Meyer JM, Lane KA, Bradley JD, Hugenberg ST, Wolfe F,
Moreland LW, et al: Severity of joint pain and Kellgren-Lawrence
grade at baseline are better predictors of joint space narrowing
than bone scintigraphy in obese women with knee osteoarthritis. J
Rheumatol. 32:1540–1546. 2005.PubMed/NCBI
|
|
14
|
Pavelká K, Gatterová J, Olejarová M,
Machacek S, Giacovelli G and Rovati LC: Glucosamine sulfate use and
delay of progression of knee osteoarthritis: A 3-year, randomized,
placebo-controlled, double-blind study. Arch Intern Med.
162:2113–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pavelka K, Trc T, Karpas K, Vítek P,
Sedlácková M, Vlasáková V, Böhmová J and Rovenský J: The efficacy
and safety of diacerein in the treatment of painful osteoarthritis
of the knee: A randomized, multicenter, double-blind,
placebo-controlled study with primary end points at two months
after the end of a three-month treatment period. Arthritis Rheum.
56:4055–4064. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park G, Horie T, Fukasawa K, Ozaki K,
Onishi Y, Kanayama T, Iezaki T, Kaneda K, Sugiura M and Hinoi E:
Amelioration of the development of osteoarthritis by daily intake
of β-cryptoxanthin. Biol Pharm Bull. 40:1116–1120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirakura M, Kram V, Robinson J, Sikka S,
Kilts TM, Wadhwa S and Young MF: Extracellular matrix mediates
BMP-2 in a model of temporomandibular joint osteoarthritis. Cells
Tissues Organs. 204:84–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto K, Santamaria S, Botkjaer KA,
Dudhia J, Troeberg L, Itoh Y, Murphy G and Nagase H: Inhibition of
shedding of low-density lipoprotein receptor-related protein 1
reverses cartilage matrix degradation in osteoarthritis. Arthritis
Rheumatol. 69:1246–1256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ok SM, Lee SM, Park HR, Jeong SH, Ko CC
and Kim YI: Concentrations of CTX I, CTX II, DPD, and PYD in the
urine as a biomarker for the diagnosis of temporomandibular joint
osteoarthritis: A preliminary study. Cranio. 36:366–372.
2018.PubMed/NCBI
|
|
20
|
Duclos ME, Roualdes O, Cararo R, Rousseau
JC, Roger T and Hartmann DJ: Significance of the serum CTX-II level
in an osteoarthritis animal model: A 5-month longitudinal study.
Osteoarthritis Cartilage. 18:1467–1476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dündar Ü, Aşık G, Ulaşlı AM, Sınıcı Ş,
Yaman F, Solak Ö, Toktaş H and Eroğlu S: Assessment of pulsed
electromagnetic field therapy with Serum YKL-40 and ultrasonography
in patients with knee osteoarthritis. Int J Rheum Dis. 19:287–293.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Väänänen T, Vuolteenaho K, Kautiainen H,
Nieminen R, Möttönen T, Hannonen P, Korpela M, Kauppi MJ, Laiho K,
Kaipiainen-Seppänen O, et al: NEO-RACo Study Group: Glycoprotein
YKL-40: A potential biomarker of disease activity in rheumatoid
arthritis during intensive treatment with csDMARDs and infliximab.
Evidence from the randomised controlled NEO-RACo trial. PLoS One.
12:e01832942017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meulenbelt I, Kloppenburg M, Kroon HM,
Houwing-Duistermaat JJ, Garnero P, Hellio Le, Graverand MP, Degroot
J and Slagboom PE: Urinary CTX-II levels are associated with
radiographic subtypes of osteoarthritis in hip, knee, hand, and
facet joints in subject with familial osteoarthritis at multiple
sites: The GARP study. Ann Rheum Dis. 65:360–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Väänänen T, Koskinen A, Paukkeri EL,
Hämäläinen M, Moilanen T, Moilanen E and Vuolteenaho K: YKL-40 as a
novel factor associated with inflammation and catabolic mechanisms
in osteoarthritic joints. Mediators Inflamm. 2014:2151402014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pavelka K, Bruyère O, Cooper C, Kanis JA,
Leeb BF, Maheu E, Martel-Pelletier J, Monfort J, Pelletier JP,
Rizzoli R, et al: Erratum to: Diacerein: benefits, risks and place
in the management of Osteoarthritis. An opinion-based report from
the ESCEO. Drugs Aging. 34:4132017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roman-Blas JA, Castañeda S,
Sánchez-Pernaute O, Largo R and Herrero-Beaumont G: CS/GS Combined
Therapy Study Group: Combined treatment with chondroitin sulfate
and glucosamine sulfate shows no superiority over placebo for
reduction of joint pain and functional impairment in patients with
knee osteoarthritis: A six-month multicenter, randomized,
double-blind, placebo-controlled clinical trial. Arthritis
Rheumatol. 69:77–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen ZH, Tang CC, Chang YC, Huang SY, Hsieh
SP, Lee CH, Huang GS, Ng HF, Neoh CA, Hsieh CS, et al: Glucosamine
sulfate reduces experimental osteoarthritis and nociception in
rats: association with changes of mitogen-activated protein kinase
in chondrocytes. Osteoarthritis Cartilage. 18:1192–1202. 2012.
View Article : Google Scholar
|
|
28
|
Wilkens P, Scheel IB, Grundnes O, Hellum C
and Storheim K: Effect of glucosamine on pain-related disability in
patients with chronic low back pain and degenerative lumbar
osteoarthritis: a randomized controlled trial. JAMA. 304:45–52.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pelletier JP, Yaron M, Haraoui B, Cohen P,
Nahir MA, Choquette D, Wigler I, Rosner IA and Beaulieu AD: The
Diacerein Study Group: Efficacy and safety of diacerein in
osteoarthritis of the knee: A double-blind, placebo-controlled
trial. Arthritis Rheum. 43:2339–2348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Manicourt DH, Azria M, Mindeholm L, Thonar
EJ and Devogelaer JP: Oral salmon calcitonin reduces Lequesne's
algofunctional index scores and decreases urinary and serum levels
of biomarkers of joint metabolism in knee osteoarthritis. Arthritis
Rheum. 54:3205–3211. 2006. View Article : Google Scholar : PubMed/NCBI
|