Introduction
Endometrial cancer was the fourth most common cancer
in women, and ~61,380 new cases and ~23,110 associated deaths were
registered in the USA in 2017 (1).
While surgery has a high success rate for endometrial cancer at the
early stage, the subset of patients with an advanced stage at
diagnosis or with recurrent disease has poor treatment outcomes and
low survival rates (2). Early
detection of endometrial cancer may significantly improve the
prognosis of affected patients. Therefore, the clinical
implementation of screening for early-stage cancer prior to the
onset of symptoms is expected to significantly improve the overall
survival (OS). However, at present, effective methods for the early
diagnosis of endometrial cancer are lacking and routine screening
is not performed (3). A large
quantity of studies have indicated that tumor markers may be used
as indicators for the diagnosis of cancer and prognostication of
affected patients, which may provide information that may be useful
regarding their clinical management and follow-up (4).
The huge public transcriptome database provides a
valuable resource for the analysis of whole-genome co-expression
networks, screening of tumor markers associated with prognosis and
phenotypes and investigation of the molecular mechanisms of
pathogenesis (5). The weighted gene
co-expression network analysis (WGCNA) method was developed by
Langfelder and Horvath (6) in 2008.
WGCNA has been proved to be an effective systems biology method to
describe the correlation patterns among genes across microarray
samples and to group genes into a model or network based on highly
correlated expression profiles. Module eigengenes may be used to
correlate modules with clinical traits to screen genes that may be
used to identify candidate biomarkers or therapeutic targets. These
methods have been successfully implemented in various biological
contexts (6). In the present study,
the WGCNA algorithm was applied to identify genes associated with
clinical parameters of endometrial cancer, and 24 gene
co-expression modules including 2,414 genes were retrieved,
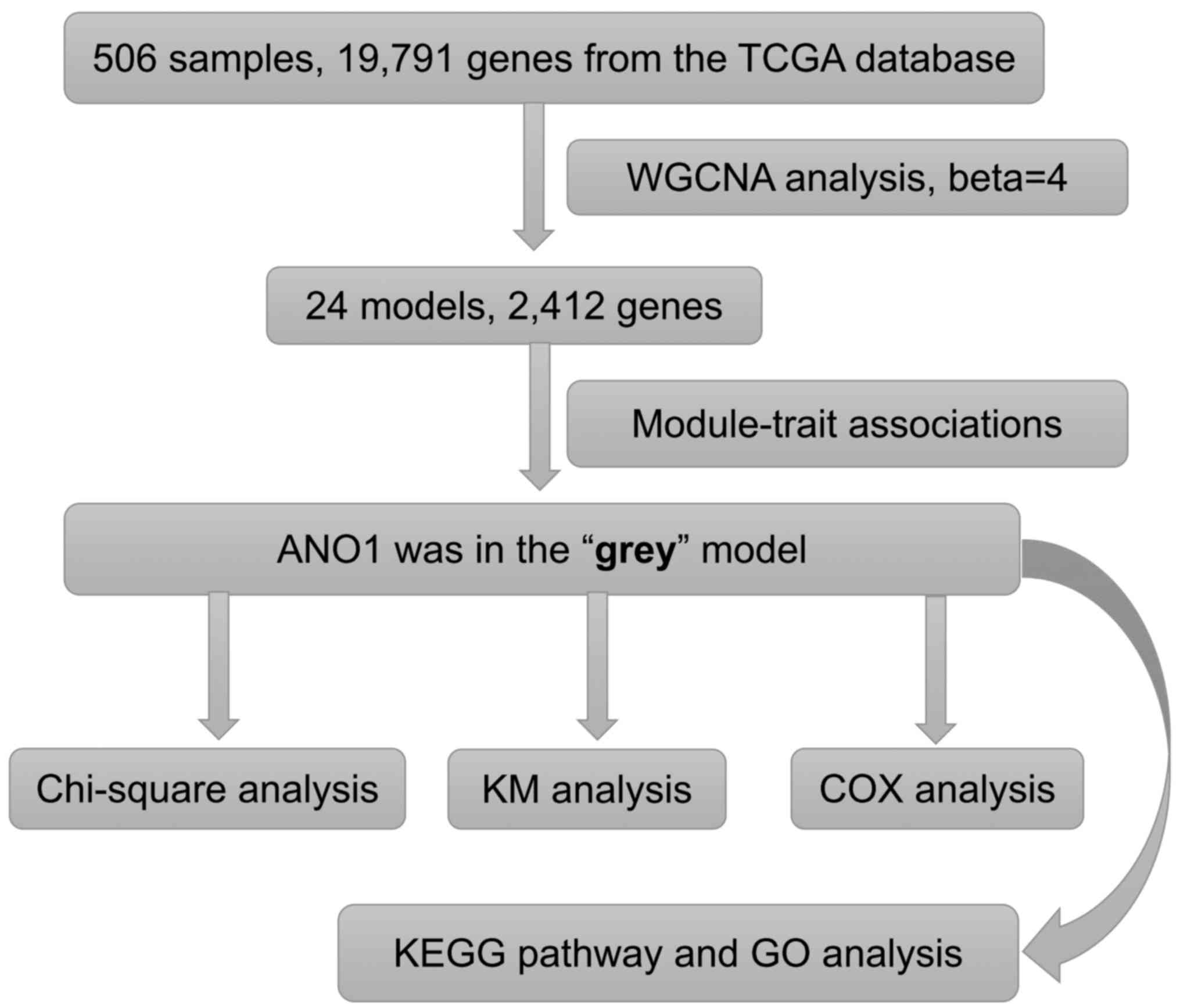
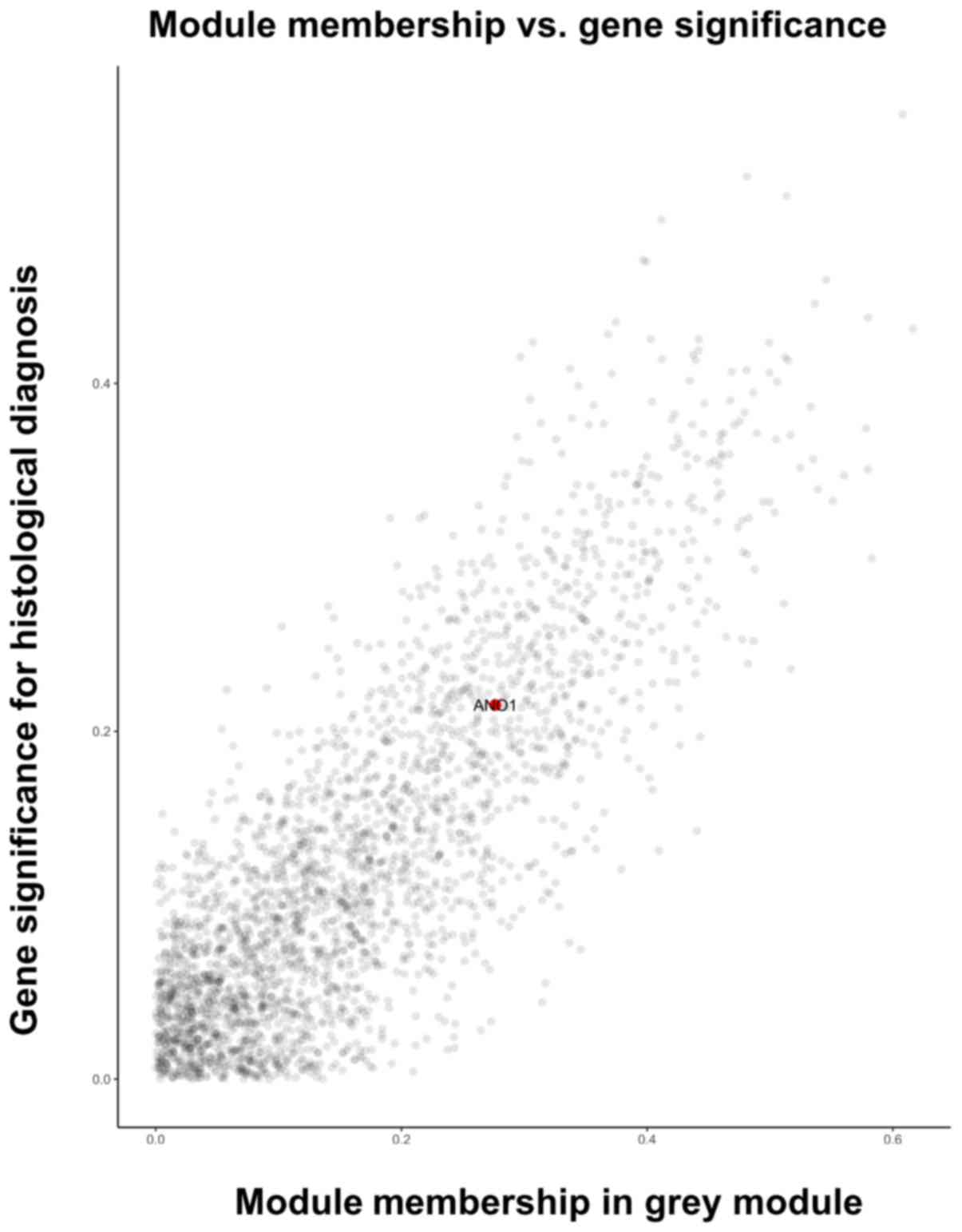
containing the anoctamin 1 (ANO1) gene (Fig. 1).
ANO1, also known as transmembrane member 16A
(TMEM16A), oral cancer overexpressed 2, DOG1, TAOS2, FLJ10261 or
Ca2+-activated Cl− channel, is one of the 10
members of the transmembrane protein family (TMEM16A-K or ANO1-10)
and was identified in 2008 (7). The
ANO1 gene is localized on 11q13 (8),
one of the most frequently amplified regions in human cancers. It
has recently been indicated that ANO1/TMEM16A is amplified or
overexpressed in several human cancer types and associated with a
poor prognosis, including gastrointestinal stromal tumors (9,10),
pancreatic ductal adenocarcinoma (11), prostate cancer (12), head and neck squamous cell carcinoma
(HNSCC) (13), breast cancer
(14–16), lung cancer (17) and colorectal cancer (18). To date, only two studies in the
pubmed database have examined the expression of ANO1 in endometrial
cancer, one of which detected 10 endometrioid adenocarcinoma
tissues by immunohistochemistry (IHC) with the new antibody K9, and
only 4 of them were positive (19).
The other study reported that no ANO1 expression was present in all
14 endometrial stromal sarcoma samples with the tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein
epsilon (YWHAE)-NUT family member 2 (NUTM2)A/B genetic fusion
(20). However, the correlation
between the expression of ANO1 in endometrial carcinoma and the
clinical phenotype and prognosis have remained elusive. Therefore,
in the present study, the expression of ANO1 was investigated and
its clinical significance in endometrial cancer was determined.
Materials and methods
Gene expression profiles
mRNA expression data obtained through RNA-sequencing
and clinical follow-up information from 547 endometrial cancer
samples were downloaded from The Cancer Genome Atlas (TCGA) data
portal (https://tcga-data.nci.nih.gov/tcga/) in March 2018,
including 19,791 human genes. A total of 41 patients were excluded
due to missing information.
WGCNA
Screening of genes associated with the endometrial
cancer phenotype was performed based on the constructed weighted
correlation matrices according to the WGCNA protocols. The detailed
steps are in accordance with those described by Langfelder and
Horvath (6).
Functional enrichment analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG)
is a tool for the systematic analysis of gene function, connecting
genomic information with higher-order functional information. Gene
Ontology (GO) anaylsis was performed to analyze ANO1 at the
functional level, included biological processes, cellular component
and molecular function. In the present study, GO and KEGG pathway
analysis was performed using the database for annotation,
visualization and integrated discovery (DAVID) platform (https://david.ncifcrf.gov/).
Statistical analysis
The Chi-square test was performed to evaluate the
association between the RNA expression of ANO1 and clinical
characteristics. Survival analysis was performed using the
Kaplan-Meier method and significant differences between subgroups
were determined using the log-rank test. Univariate/multivariate
Cox proportional hazard regression analysis was performed to
identify factors influencing survival. The cut-off values for the
genes were determined using X-tile software (version 3.6.1)
(21). P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using SPSS software version 22.0 (IBM
Corp., Armonk, NY, USA). WGCNA was performed using R software
version 3.4.1 (https://www.r-project.org/). The ‘WGCNA’ package was
downloaded from https://mran.microsoft.com/package/WGCNA.
Results
Patient characteristics
All of the 506 patients included in the present
study were clinically and pathologically diagnosed with endometrial
cancer between 1995 and 2013. The mean age of these patients was 64
years (range, 31–90 years). According to the International
Federation of Gynaecology and Obstetrics staging guidelines from
2009 (22), the cohort included 310
patients (61.26%) with stage I, 48 (9.49%) with stage II, 121
(23.91%) with stage III and 27 (5.35%) with stage IV endometrial
cancer. Among the 506 cases, 376 (74.31%) were histologically
classified as endometrioid endometrial adenocarcinoma, 22 (4.35%)
were mixed serous and endometrioid adenocarcinoma and 108 (21.34%)
were serous endometrial adenocarcinoma. The distribution of
histological grades was as follows: A total of 104 cases (20.55%)
were of grade 1, 110 (21.74%) were of grade 2 and 292 (57.71%) were
of grade 3.
Screening of genes associated with the
phenotype of endometrial carcinoma
To identify genes that may be significantly
associated with the endometrial carcinoma phenotype, a WGCNA was
employed to identify modules of highly interconnected genes. A gene
co-expression network was constructed on the basis of pairwise
correlations of the expression of highly interconnected genes with
a significantly correlated co-expression by using the total of
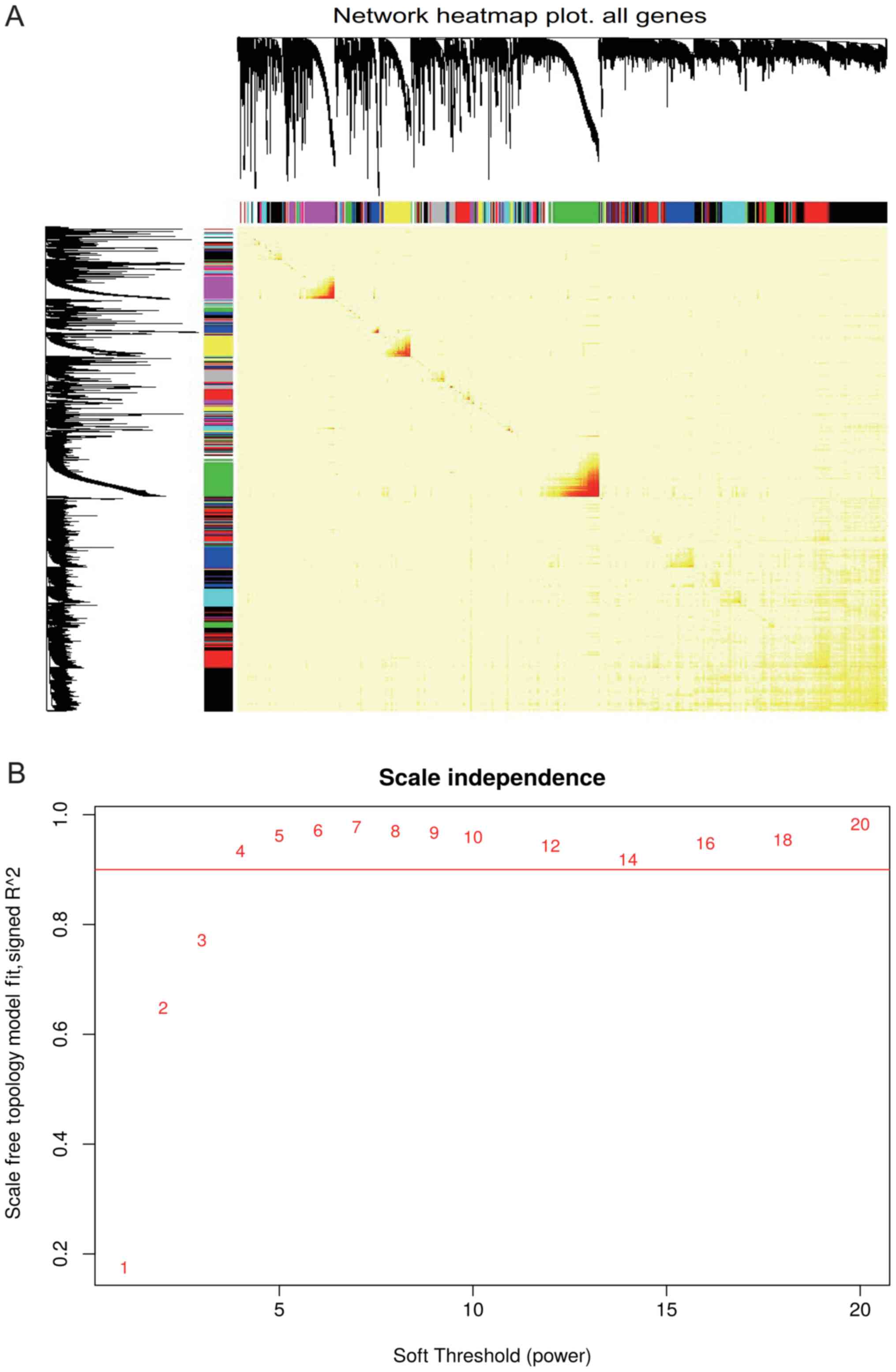
19,791 genes (Fig. 2A). Though the
function ‘PickSoftThreshold’ in R software, the optimum soft
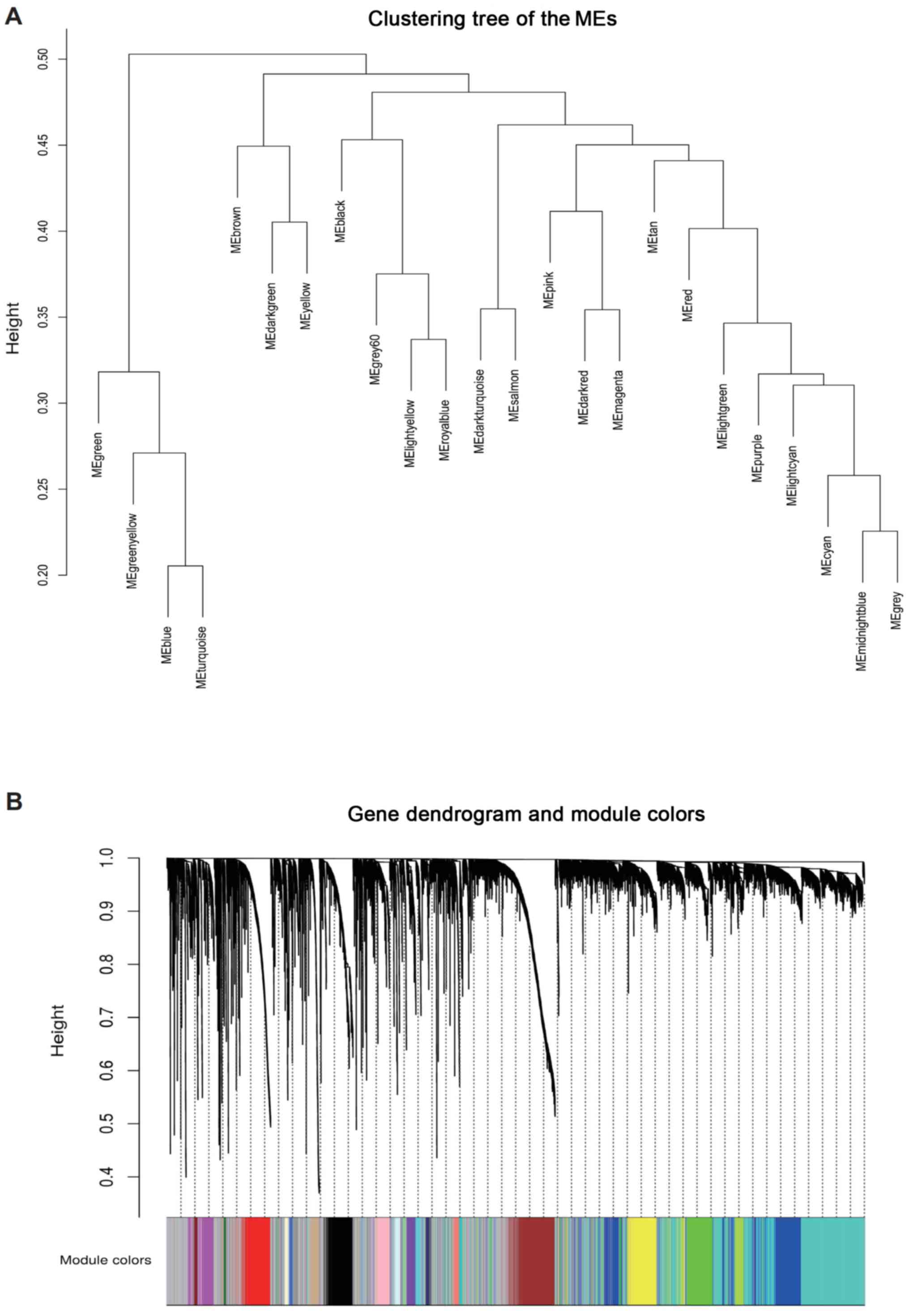
thresholding power was calculated as 4 (Fig. 2B). Subsequently, a total of 2,414
expressed genes were screened from 19,791 genes in 506 samples,
which were divided into 24 modules according to the different
expression patterns via average linkage hierarchical clustering
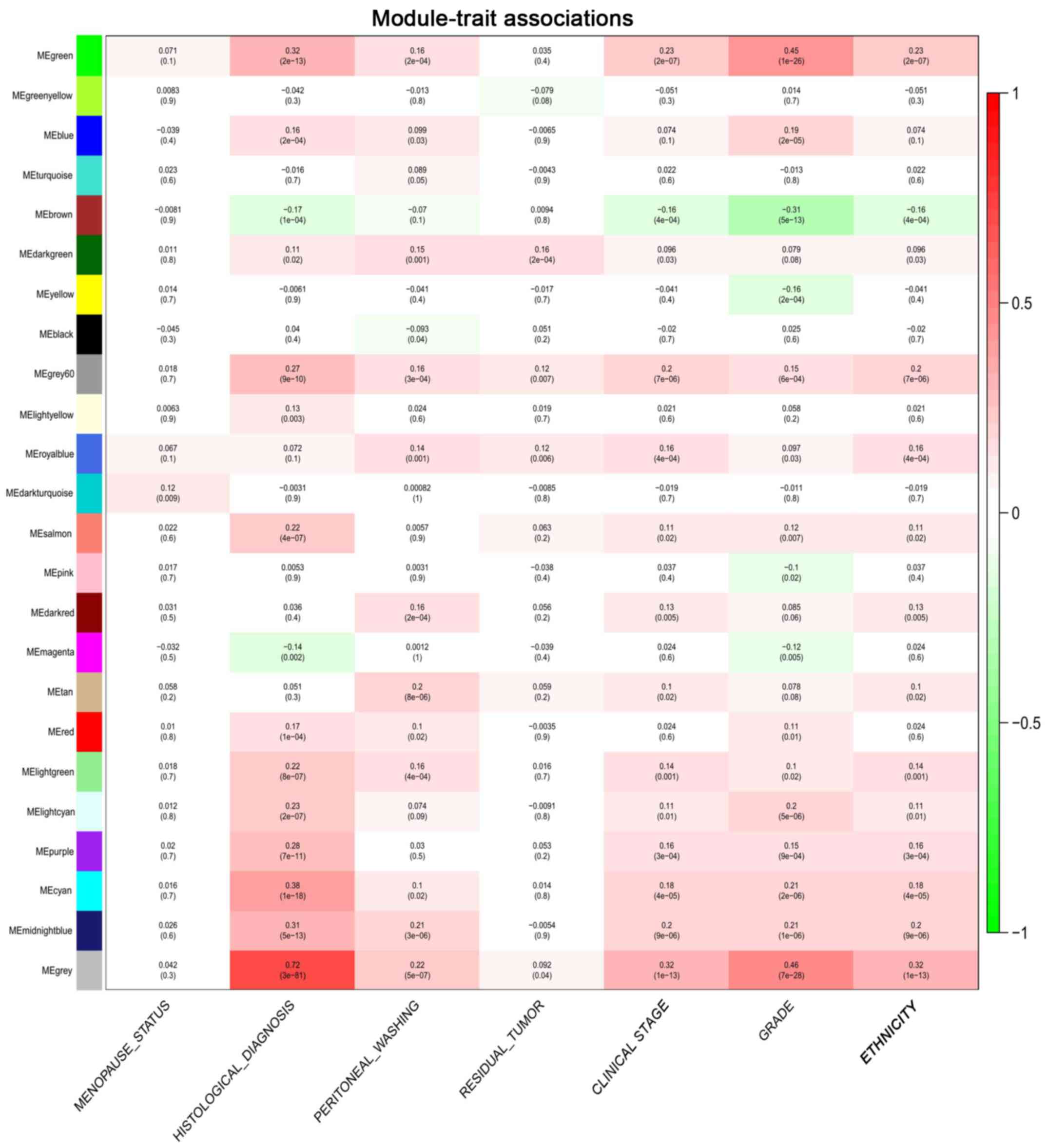
(Fig. 3A and B). The association
between seven clinical phenotypes (histological type, clinical
stage, pathological grade, positive peritoneal washing, ethnicity,
residual tumor and menopause status) and genome-wide expression
profiles were investigated using the tool ‘Eigengene Networks’
(Fig. 4). As depicted in Fig. 4, the ‘grey’ module was associated
with each clinical phenotype and was most highly correlated with
the histological type (coefficient r=0.72; P<0.001). By
consulting the literature, ANO1 was selected for further study as
it as determined to be involved in endometrial cancer (7–20). The
ANO1 gene was in the ‘grey’ module and closely correlated with the
clinical phenotype of the endometrial cancer patients (Figs. 1 and 5).
Clinicopathological
characteristics
The results of the Chi-square analysis further
confirmed the prediction results of WCGNA, as ANO1 mRNA expression
was significantly associated with age (P=0.047), histological type
(P<0.001), clinical stage (P<0.001), pathological grade
(P<0.001) and positive peritoneal washing (P<0.001) of
endometrial carcinoma. However, no correlation with the patients'
menopause status was identified (P=0.893; Table I).
 | Table I.Association of ANO1 with
clinicopathological characteristics in endometrial cancer patients
(n=506). |
Table I.
Association of ANO1 with
clinicopathological characteristics in endometrial cancer patients
(n=506).
|
| Risk based on ANO1
expressiona |
|
|---|
|
|
|
|
|---|
| Parameter | High (n=233) | Low (n=273) | P-value |
|---|
| Age (years) |
|
| 0.047 |
| ≤64 | 112 (41.8) | 156 (58.2) |
|
|
>64 | 119 (50.6) | 116 (49.4) |
|
| Not
available | 2 (66.7) | 1 (33.3%) |
|
| Histological
type |
|
| <0.001 |
|
Endometrioid endometrial
adenocarcinoma | 134 (35.6) | 242 (64.4) |
|
| Mixed
serous and endometrioid | 11 (50.0) | 11 (50.0) |
|
| Serous
endometrial adenocarcinoma | 88 (81.5) | 20 (18.5) |
|
| Grade |
|
| <0.001 |
| G1 | 25 (24.0) | 79 (76.0) |
|
| G2 | 30 (27.3) | 80 (72.7) |
|
| G3 | 178 (61.0) | 114 (39.0) |
|
| FIGO stage |
|
| <0.001 |
| I | 116 (37.4) | 194 (62.6) |
|
| II | 20 (41.7) | 28 (58.3) |
|
|
III | 74 (61.2) | 47 (38.8) |
|
| IV | 23 (85.2) | 4 (14.8) |
|
| Peritoneal
washing |
|
| 0.001 |
|
Negative | 138 (42.7) | 185 (57.3) |
|
|
Positive | 38 (66.7) | 19 (33.3) |
|
| Not
available | 57 (45.2%) | 69 (54.8) |
|
| Menopausal
status |
|
| 0.893 |
|
Period | 7 (43.8) | 9 (56.3) |
|
|
Post | 192 (46.4) | 222 (53.6) |
|
|
Pre | 14 (42.4) | 19 (57.6) |
|
| Not
available | 29 (67.4) | 14 (32.6) |
|
Prognostic significance
To estimate the clinical prognostic significance of
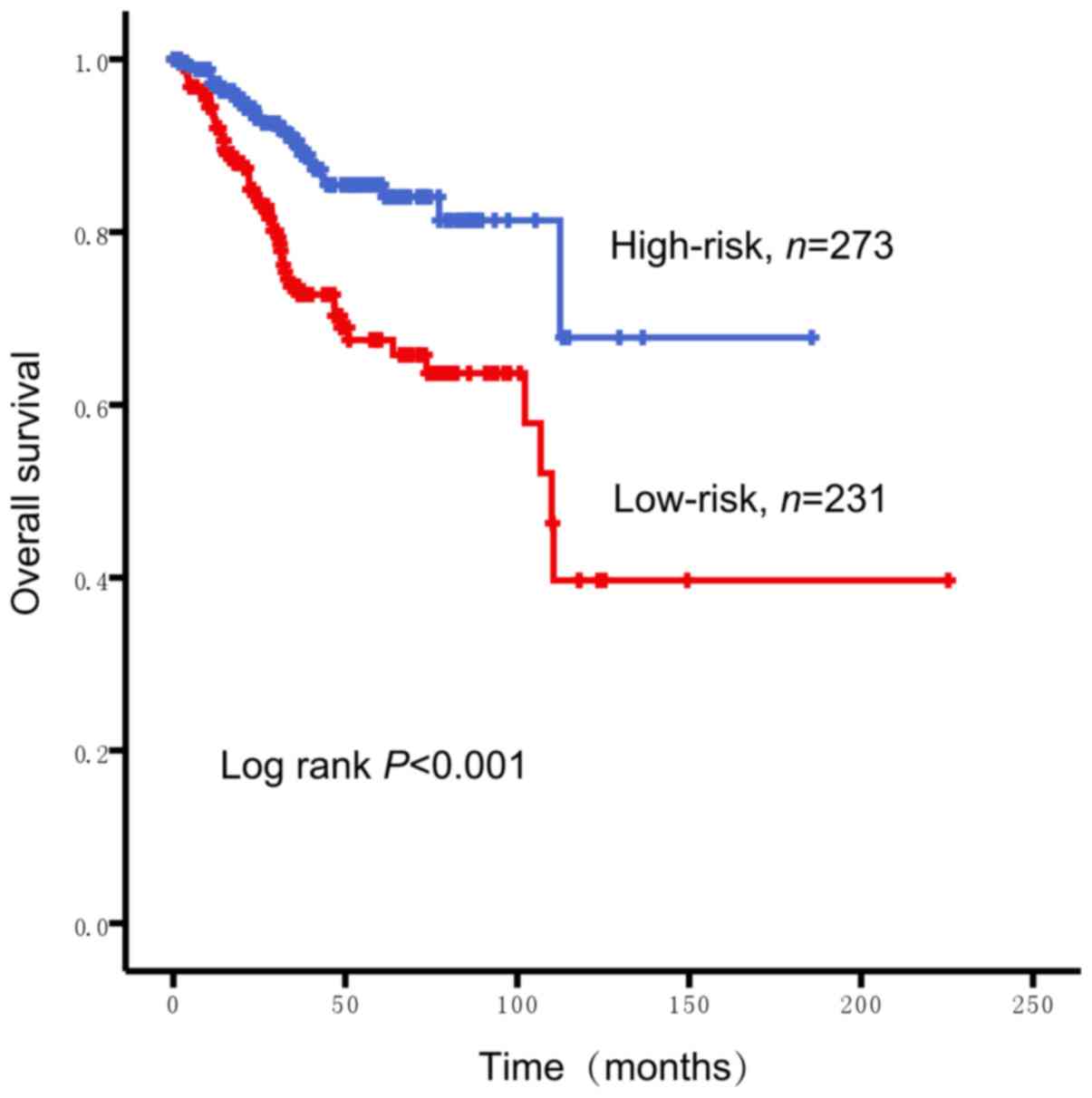
ANO1 mRNA expression, a Kaplan-Meier survival analysis was
performed. The cut-off value (0.5) of the relative mRNA expression
of ANO1 was computed by X-tile; patietns with endometrial cancer
were stratified into low- and high-risk groups. The number of
subjects in the ANO1 high-risk group and low-risk group was 273 and
231, respectively. As presented in Fig.
6, patients with a lower expression of ANO1 had a worse
prognosis (P<0.001). To assess whether the prognostic power of
ANO1 was independent of other clinical features, univariate and
multivariable Cox regression analyses were performed, the results
demonstrated that the prognostic power of ANO1 for the prediction
of survival rate was independent of these clinical features (age,
histological type, clinical stage and pathological grade) for
patients with endometrial cancer (High-risk group vs. Low-risk
group, HR=1.904, P=0.011; Table
II).
 | Table II.Univariate and multivariate Cox
regression analysis in endometrial cancer patients regarding
overall survival. |
Table II.
Univariate and multivariate Cox
regression analysis in endometrial cancer patients regarding
overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≤64 vs. >64
years) | 1.568
(1.018–2.416) | 0.041 | 1.738
(1.103–2.738) | 0.017 |
| Histological type
(EEA vs. SEA) | 2.785
(1.772–4.377) | <0.001 |
| 0.223 |
| Grade (G1 vs.
G2+G3) | 3.002
(1.448–6.225) | 0.003 |
| 0.90 |
| FIGO stage (I/II
vs. III/IV) | 4.026
(2.605–6.223) | <0.001 | 0.297
(0.186–0.476) | <0.001 |
| Risk based on ANO1
(high vs. low)a | 0.397
(0.252–0.626) | <0.001 | 1.904
(1.158–3.130) | 0.011 |
Functional analysis
To gain a primary understanding of the biological
relevance of ANO1, KEGG pathway and GO enrichment analyses of the
differentially expressed genes determined from the data from TCGA
database were performed. Further characterization of the most
relevant module containing the ANO1 gene with the DAVID tool
indicated that genes co-expressed with ANO1 were mainly enriched in
metabolic pathways (Fig. 7A). In
Fig. 7B, significantly enriched GO
terms in the categories biological process, cellular component and
molecular function are listed. In the category biological process,
genes co-expressed with ANO1 were mainly enriched in the term
‘homophilic cell adhesion via plasma membrane adhesion molecule’.
In the category cellular component, genes co-expressed with ANO1
were mainly enriched in the term ‘integral component of membrane’.
In the category molecular function, genes co-expressed with ANO1
were mainly enriched in the term ‘bitter taste receptor activity’
and also enriched in ‘calcium ion binding’.
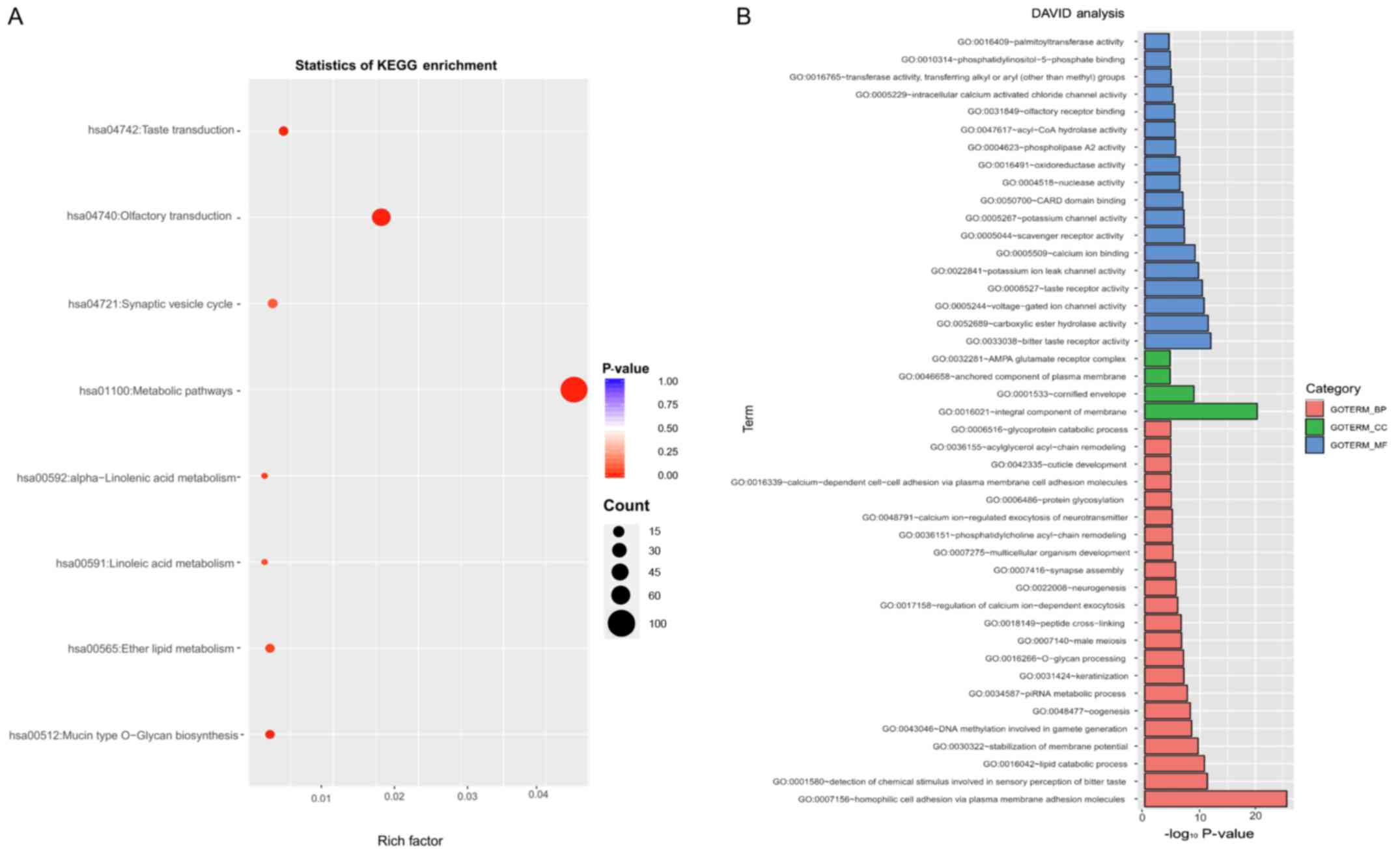 | Figure 7.Functional analysis. (A) The
significantly enriched KEGG pathways of the genes co-expressed with
ANO1; (B) significantly enriched GO terms in the categories BP, CC
and MF for the genes co-expressed with ANO1. ANO1, anoctamin 1;
KEGG, Kyoto Encyclopedia of Genes and Genomes; hsa, Homo
sapiens; GO, Gene Ontology; DAVID, database for annotation,
visualization and integrated discovery; BP, Biological Process; CC,
Cellular Component; MF, Molecular Function. |
Discussion
The purpose of the present study was to screen out
the factors associated with the endometrial carcinoma phenotype by
WCGAN analysis using the resources in TCGA public database and to
further verify whether they were associated with the phenotype and
progression of endometrial cancer by statistical methods. Although
several genes were identified following the literature review
(7–20), ANO1 was selected for further
analysis. The ANO1 gene was identified as an independent prognostic
factor, providing a basis for further experimental verification
using tissue and cell tests in the laboratory.
The present analysis of TCGA database by WCGNA
indicated that 2,414 genes, including ANO1, were associated with
endometrial cancer; these were stratified into 24 subgroups.
Subsequently, the Chi-square analysis revealed that the expression
status of ANO1 was significantly associated with patient age,
histological type, clinical stage, pathological grade and positive
peritoneal washing of endometrial carcinoma. High ANO1 levels were
significantly correlated with a good prognosis of endometrial
cancer patients. Wu et al (15) assessed the expression of ANO1 in 431
breast cancer patients with invasive ductal carcinoma and
identified that ANO1 overexpression was associated with good
prognosis in progesterone receptor-positive or human epidermal
growth factor receptor 2-negative patients following tamoxifen
treatment. However, ANO1 has previously been reported to be
overexpressed in numerous types of cancer and to be associated with
poor prognosis. Bae et al (16) investigated the expression of ANO1 in
139 breast cancer patients and identified that ANO1 was
significantly associated with poor prognosis in terms of a shorted
OS and relapse-free survival rate of breast cancer patients. In
gastrointestinal stromal tumors with mutations in KIT or PDGFRA,
for which ANO1 had been previously indicated to be a specific
biomarker, ANO1 was reported to be highly associated with tumor
size and to be of predictive value (10). Jia et al (17) demonstrated that ANO1 overexpression
contributes to tumor growth and invasion of lung cancer. In
esophageal squamous cell carcinoma, Shi et al (23) identified that ANO1 mRNA expression
was elevated in samples with moderate dysplasia compared with that
in normal esophageal epithelium; ANO1 was overexpressed in
esophageal squamous cell carcinoma, and positively correlated with
lymph node metastasis and advanced clinical stage determined by
IHC. By analyzing gnomic amplification and protein expression of
ANO1 in a large number of HNSCC patients, Ruiz et al
(13) revealed that ANO1 protein
expression in cancer tissue was associated with poor OS. The
abovementioned studies indicated that the role of the ANO1 gene may
vary among different types of cancer.
The ANO1 gene was first discovered in gastric
stromal cancer and was initially named DOG1 (24). ANO1, as an ion channel, has a crucial
role in sensing and transmitting extracellular signals to the
intracellular machinery of epithelial cells, and a dysfunction of
ANO1 may be involved in the development and progression of cancer
of the epithelium by causing alterations of ion homeostasis and
volume regulation (25,26). The ANO1 gene is involved in the
development of cancer based on regulating intracellular free
Ca2+ levels (12,27). In the present study, GO functional
enrichment and KEGG pathway analysis indicated that genes
co-expressed with ANO1 were mainly enriched in the term ‘homophilic
cell adhesion via plasma membrane adhesion molecule’ and involved
in the Ca2+ ion metabolism pathway. This result in the
current study was consistent with the existing literature.
The expression of ANO1 in endometrial cancer has
been assessed by only two previous studies. One of the studies
examined 10 endometrioid adenocarcinoma tissues with the new
antibody K9 and the results indicated that only 4 of them were
positive (19). The other study
suggested that ANO1 was negatively expressed in all 14 endometrial
stromal sarcomas with the YWHAE-NUTM2A/B genetic fusion (20). The Human Protein Atlas database
(https://www.proteinatlas.org/search/ANO1) (28) contains data obtained by IHC analysis
of 11 endometrial carcinoma samples using an anti-ANO1 antibody
(cat. no. HPA032148; Sigma-Aldrich; Merck KGaA). The results
indicated high expression in 2 cases, very high expression in 6
cases and weak expression in 3 cases, and the intensity of ANO1
expression was different in endometrial cancer tissues of different
stages. To the best of our knowledge, the correlation between ANO1
and the clinical phenotype and prognosis of endometrial carcinoma
has not been previously assessed. In the present study, the
expression of ANO1 was determined from data of endometrial cancer
tissues downloaded from TCGA and its clinical significance was
assessed. In addition, the number of subjects included in the two
previous studies was relatively small; one comprised 10
endometrioid adenocarcinoma tissues and the other 14 endometrial
stromal sarcomas (19,20). The present study included a
relatively large number of subjects, namely 506 patients with
endometrial cancer. Based on the public data from TCGA database,
factors associated with the clinical characteristics of endometrial
cancer were screened out, and ANO1 was further investigated. It was
revealed that high expression of ANO1 was associated with a good
prognosis of endometrial carcinoma patients. This result provides a
theoretical basis for further verifying the role and the potential
prognostic value of ANO1 in endometrial cancer in laboratory
studies to be performed in the future. These may include polymerase
chain reaction and IHC analyses of a large number of cancer
samples.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are
available in the TCGA repository, https://tcga-data.nci.nih.gov/tcga/; DAVID repository
https://david.ncifcrf.gov/; R
repository, https://www.r-project.org/ and the Human Prtotein
Atlas repository, https://www.proteinatlas.org/search/ANO1.
Authors' contributions
Data curation: FzW, BW and JL; formal analysis: FmW
and PW; drafting of the manuscript: BW; critical review and editing
of the manuscript: FzW and JL.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing of
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berek JS: Novak's Gynecology 13th edition.
Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2002
|
|
4
|
Ueda Y, Enomoto T and Kimura T, Miyatake
T, Yoshino K, Fujita M and Kimura T: Serum biomarkers for early
detection of gynecologic cancers. Cancers (Basel). 2:1312–1327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roychowdhury S and Chinnaiyan AM:
Translating cancer genomes and transcriptomes for precision
oncology. CA Cancer J Clin. 66:75–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caputo A, Caci E, Ferrera L, Pedemonte N,
Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O and
Galietta LJ: TMEM16A, a membrane protein associated with
calcium-dependent chloride channel activity. Science. 322:590–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katoh M and Katoh M: FLJ10261 gene,
located within the CCND1-EMS1 locus on human chromosome 11q13,
encodes the eight-transmembrane protein homologous to C12orf3,
C11orf25 and FLJ34272 gene products. Int J Oncol. 22:1375–1381.
2003.PubMed/NCBI
|
|
9
|
Berglund E, Akcakaya P, Berglund D,
Karlsson F, Vukojevic V, Lee L, Bogdanović D, Lui WO, Larsson C,
Zedenius J, et al: Functional role of the Ca2+-activated
Cl− channel DOG1/TMEM16A in gastrointestinal stromal
tumor cells. Exp Cell Res. 326:315–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rizzo FM, Palmirotta R, Marzullo A, Resta
N, Cives M, Tucci M and Silvestris F: Parallelism of DOG1
expression with recurrence risk in gastrointestinal stromal tumors
bearing KIT or PDGFRA mutations. BMC Cancer. 16:872016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sauter DR, Novak I, Pedersen SF, Larsen EH
and Hoffmann EK: ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma
(PDAC). Pflugers Arch. 467:1495–1508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu W, Lu M, Liu B, Huang Y and Wang K:
Inhibition of Ca(2+)-activated Cl(−) channel ANO1/TMEM16A
expression suppresses tumor growth and invasiveness in human
prostate carcinoma. Cancer Lett. 326:41–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruiz C, Martins JR, Rudin F, Schneider S,
Dietsche T, Fischer CA, Tornillo L, Terracciano LM, Schreiber R,
Bubendorf L and Kunzelmann K: Enhanced expression of ANO1 in head
and neck squamous cell carcinoma causes cell migration and
correlates with poor prognosis. PLoS One. 7:e432652012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ubby I, Bussani E, Colonna A, Stacul G,
Locatelli M, Scudieri P, Galietta L and Pagani F: TMEM16A
alternative splicing coordination in breast cancer. Mol Cancer.
12:752013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu H, Guan S, Sun M, Yu Z, Zhao L, He M,
Zhao H, Yao W, Wang E, Jin F, et al: Ano1/TMEM16A overexpression is
associated with good prognosis in PR-positive or HER2-negative
breast cancer patients following tamoxifen treatment. PLoS One.
10:e01261282015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae JS, Park JY, Park SH, Ha SH, An AR,
Noh SJ, Kwon KS, Jung SH, Park HS, Kang MJ and Jang KY: Expression
of ANO1/DOG1 is associated with shorter survival and progression of
breast carcinomas. Oncotarget. 9:607–621. 2017.PubMed/NCBI
|
|
17
|
Jia L, Liu W, Guan L, Lu M and Wang K:
Inhibition of calcium-activated chloride channel ANO1/TMEM16A
suppresses tumor growth and invasion in human lung cancer. PLoS
One. 10:e01365842015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G,
Zhong L, Ma Z, Zheng J, Fang X and Ma T: Inhibition of TMEM16A
expression suppresses growth and invasion in human colorectal
cancer cells. PLoS One. 9:e1154432014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hemminger J and Iwenofu OH: Discovered on
gastrointestinal stromal tumours 1 (DOG1) expression in
non-gastrointestinal stromal tumour (GIST) neoplasms.
Histopathology. 61:170–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee CH, Hoang LN, Yip S, Reyes C,
Marino-Enriquez A, Eilers G, Tao D, Chiang S, Fletcher JA, Soslow
RA, et al: Frequent expression of KIT in endometrial stromal
sarcoma with YWHAE genetic rearrangement. Mod Pathol. 27:751–757.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Creasman W: Revised FIGO staging for
carcinoma of the endometrium. Int J Gynaecol Obstet. 105:1032009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi ZZ, Shang L, Jiang YY, Hao JJ, Zhang
Y, Zhang TT, Lin DC, Liu SG, Wang BS, Gong T, et al: Consistent and
differential genetic aberrations between esophageal dysplasia and
squamous cell carcinoma detected by array comparative genomic
hybridization. Clin Cancer Res. 19:5867–5878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robert B, Corless CL, Chen X, Rubin BP,
Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R,
et al: The novel marker, DOG1, is expressed ubiquitously in
gastrointestinal stromal tumors irrespective of KIT or PDGFRA
mutation status. Am J Pathol. 165:107–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu B, Jin X, Min L, Li Q, Deng L, Wu H,
Lin G, Chen L, Zhang H, Li C, et al: Chloride channel-3 promotes
tumor metastasis by regulating membrane ruffling and is associated
with poor survival. Oncotarget. 6:2434–2450. 2015.PubMed/NCBI
|
|
26
|
Kim TH, Kim JS, Kim ZH, Huang RB, Chae YL
and Wang RS: Khz (Fusion Product of Ganoderma lucidum and Polyporus
umbellatus Mycelia) induces apoptosis in human colon carcinoma
HCT116 cells, accompanied by an increase in reactive oxygen
species, activation of caspase 3, and increased intracellular
Ca2+. J Med Food. 18:332–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazzone A, Eisenman ST, Strege PR, Yao Z,
Ordog T, Gibbons SJ and Farrugia G: Inhibition of cell
proliferation by a selective inhibitor of the Ca2(+)-activated
Cl(−) channel, Ano1. Biochem Biophys Res Commun. 427:248–253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pontén F, Jirström K and Uhlen M: The
human protein Atlas-a tool for pathology. J Pathol. 216:387–393.
2008. View Article : Google Scholar : PubMed/NCBI
|