Introduction
Peripheral nerve lesions represent a serious problem
in traumatic injuries (1,2). Current strategies to repair the
transected peripheral nerve are limited to end-to-end coaptation or
autologous nerve grafting, if there are long nerve gaps after
devastating injuries (3). Autologous
nerve bridges are the most effective method for repairing nerve
gaps. However, the use of this method is limited by additional
morbidity and by restricted nerve resources. To address this issue,
numerous synthetic and natural biomaterials have been tested to
function as artificial nerve conduits for regrowing axons (4–6). Several
experimental studies have demonstrated that the regeneration of
artificial guidance channels is enhanced when the channel is seeded
with Schwann cells (SCs) (7,8). SCs appear to have a crucial role in the
process of nerve regeneration (9).
The regenerating axons do not grow through longer acellular nerve
grafts if SC migration is impeded (10).
After nerve injury in adult mammals, Wallerian
degeneration occurs at the distal end of the transected peripheral
nerve (11). SCs depleted of their
axon counterpart revert back to a non-differentiated, proliferative
phenotype, become activated and start to multiply (12). This injury-induced cell plasticity
has been proposed to be a transdifferentiation that generates a
specialized repair cell, also termed a Büngner cell, which may be
distinguished from the SCs present in the developing nerve. It has
been proposed that these repair cells guide the regrowth of the
injured axons and eventually remyelinate them to facilitate
functional recovery of the regenerated nerve fibers.
In vitro, it is possible to generate high
quantities of SCs from peripheral nerves of newborn rats (13,14).
However, the purification and enrichment of SCs from adult tissues
is much more challenging. Several methods to isolate SCs from adult
mammalian nerves have been reported. Most of the protocols
published use a predegeneration step that enhances the effectivity
of cultivation (15–20). The process of predegeneration appears
to activate the proliferative abilities of SCs and diminish the
number of fibroblasts by their migration out of the connective
tissues during the predegeneration phase. However, different
protocols use various lengths of predegeneration that vary from 1
week to 4–5 weeks and the optimal time has remained to be
determined. In order to address this question, the present study
systematically assessed the effect of different predegeneration
periods on SC yield in culture.
Materials and methods
Animals
A total of 8 adult female Wistar rats (body weight,
200–250 g; age, 12–16 weeks) were used in the present study (Velaz
Ltd., Czechia, Prague). The animals were divided into four groups
with different lengths of predegeneration periods. In group A
(n=2), nerves were dissociated immediately after harvesting. The
in vitro predegeneration period prior to dissociation lasted
for 2 weeks in group B (n=2), 4 weeks in group C (n=2) and 6 weeks
in group D (n=2).
Peripheral nerve tissue
harvesting
Animals were deeply anesthetized by isoflurane
inhalation and killed by cervical dislocation. The skin of the
hindlimbs was shaved and disinfected. Under sterile conditions,
sciatic nerves on each side were removed and transferred into a
Petri dish with Dulbecco's modified Eagle's medium (DMEM; BioSera,
Shanghai, China) with antibiotics (ATB; 100 U/ml penicillin and 100
µg/ml streptomycin; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Under the operating microscope, the epineurium was removed and the
sciatic nerves were weighed. The nerves were then placed into a
Petri dish with fresh cultivation medium and cut into pieces of 1–2
mm in length. Pieces from a pair of nerves were collected and
placed in one well of a 6-well culture plate.
In vitro predegeneration
Nerve explants were cultivated at 37°C and 5%
CO2 in a predegeneration medium [SC medium (SCM)]
according to Haastert-Talini (21),
which contained melanocyte growth medium (PromoCell, Heidelberg,
Germany) supplemented with 10% fetal bovine serum (Sigma-Aldrich;
Merck KGaA), ATB, 10 ng/ml fibroblast growth factor 2
(Sigma-Aldrich; Merck KGaA), 2 µM forskolin (Sigma-Aldrich; Merck
KGaA) and 5 µg/ml bovine pituitary extract (Sigma-Aldrich; Merck
KGaA). The medium was changed twice a week. Nerve fragments were
transferred into a new well after 6 or 7 days, when the migrating
fibroblasts had formed a near-confluent layer of cells around the
nerve fragments.
Dissociation and primary plating
The nerve fragments were dissociated either
immediately after harvesting (group A) or after the predegeneration
periods (groups B-D).
Nerve fragments were placed into test tubes with
DMEM containing 0,125% collagenase I (Sigma-Aldrich; Merck KGaA)
and 1,25 U/ml dispase (Roche Diagnostics, Basel, Switzerland) for
enzymatic digestion. They were incubated for 24 h and then
mechanically triturated with micropipette, and the cell suspension
was filtrated into a test tube through a 40-µm cell strainer. The
test tubes were then centrifuged at 235 × g for 6 min, 20°C. The
supernatant was removed, the pellet was resuspended with fresh
medium and the process of centrifugation-resuspension was repeated
once more. Cells in the suspension were counted and seeded into
6-well culture plates, at ~1 milion cells per well (5–7 wells from
each animal). The wells were coated with laminin (6 µg/ml;
Sigma-Aldrich; Merck KGaA) and poly-L-ortnithine hydrobromide (1
mg/ml; Sigma-Aldrich; Merck KGaA) for better adherence of SCs.
Initially, cells were cultivated in SCM with addition of 1% bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA) for 24 h.
Subsequently, the medium was replaced with SCM without BSA. The
medium was changed every second day. When the proliferating cells
reached counflency (days 3–4), the SC population was enriched by
means of the cold jet (CJ) technique (17,21).
The SCM was removed from the wells and ice-cold PBS
was slowly added and aspirated 2 times to cool down the culture and
loosen SCs that grow on top of the fibroblasts. Subsequently,
ice-cold SCM was added in a moderate stream to detach SCs. This
process was repeated once more if the SCs were not detached after
the first flush with SCM. During this process, the culture was
examined under the microscope in order to make sure that
fibroblasts were still attached to the surface of the well and SCs
were floating in the medium. The SCM containing the suspended SCs
was removed, added to a test tube and centrifuged (at a speed of
235 × g for 6 min at 20°C. The supernatant was removed and the
pellet was resuspended with fresh SCM. The cells were then seeded
onto a new coated well and the SCM was changed every second day.
When the proliferating cells in culture achieved confluency (20
days), imunocytochemical staining was performed in order to
distinguish SCs from other cells.
Immunocytochemistry
After removal of the medium, 4% paraformaldehyde in
0.1 PBS was added to fix the cells at 4°C for 30 min. The
paraformaldehyde was then removed and wells were washed with 0,1 M
PBS twice.
A solution of 0,1 M PBS with 0,2% Triton-X-100
(Sigma-Aldrich; Merck KGaA) and 10% normal goat serum (NGS;
Sigma-Aldrich; Merck KGaA) was added for blocking non-specific
protein reactions. Cultures were incubated for 1 h at room
temperature. The solution was then removed and substituted by a
solution of the primary antibody S100 (dilution, 1:1,000;
Sigma-Aldrich; Merck KgaA; cat. no. Z0311) in 0,1 M PBS with 0,2%
Triton-X-100 and 1% NGS. After overnight incubation at 4°C, the
solution was removed and the wells were rinsed with PBS, followed
by incubation with secondary antibody (fluorescein
isothiocyanate-conjugated anti-rabbit immunoglobulin G; dilution,
3:1,000; DAKO, Glostrup, Denmark; cat. no. F0382) and DAPI
(dilution, 1 µl:10 ml; Sigma-Aldrich; Merck KGaA, D9542) in 0,1 M
PBS with 0,2% Triton-X-100 and 1% NGS in the dark for 1 h at 20°C.
Subsequently, the samples were washed with distilled water, the
wells were left to dry for 2 h at room temperature and cells then
were embedded with Mowiol (Sigma-Aldrich; Merck KgaA; cat. no.
81381) and mounted with coverslips. Prior to observation, the
stained wells were kept in a refrigerator.
Cell counting
Immediately after dissociation, the cell suspension
was sampled and the concentration of cells was determined by
counting in a Burker chamber.
At the end of cultivation, the cell cultures were
fixed and immunostained. The number of total cells and the number
of SCs were determined by counting DAPI-positive nuclei and
S100-positive cells in ten random fields per well (magnification,
×10) in three different wells per animal. Counting was performed
using ImageJ software, version 1.51q (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All quantitative data are expressed as the mean ±
standard error of the mean. Statistical differences between groups
without predegeneration (group A) and groups with predegeneration
(groups B, C and D) were evaluated by one-way analysis of variance
with post-hoc Bonferroni and Holm multiple comparisons.
Calculations were made using Microsoft Excel (Microsoft Corp.,
Redmond, WA, USA), Online Web Statistical Calculators (www.astatsa.com) and the GraphPad online application
QuickCalcs (https://www.graphpad.com/quickcalcs/). P<0.05 was
considered to indicate a statistically significant difference.
Results
General observation
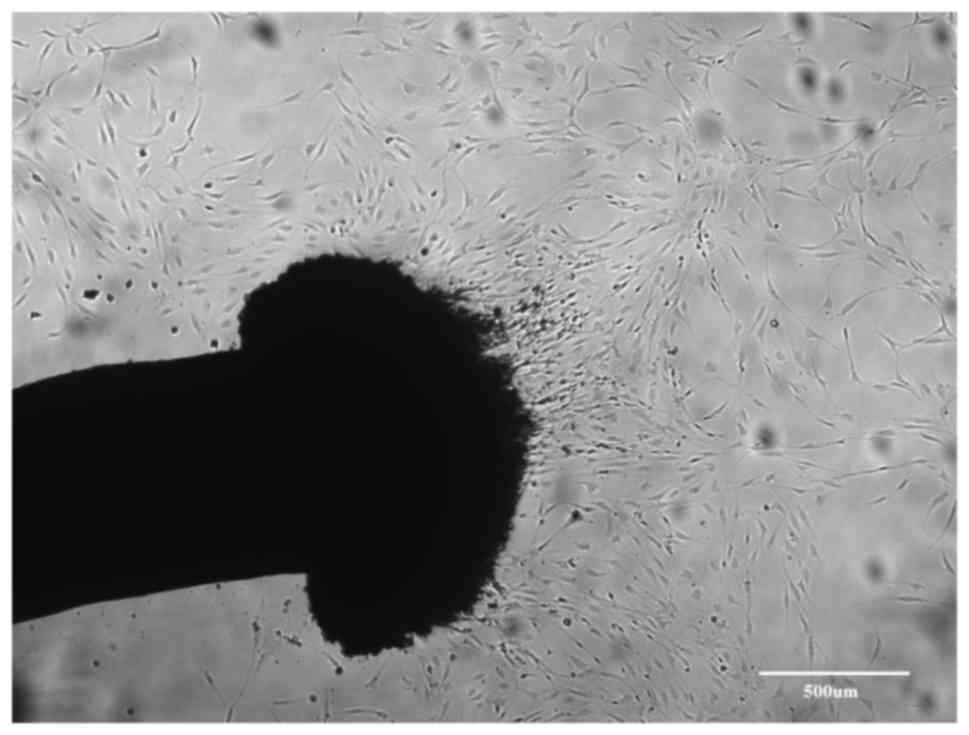
During the predegeneration period, fibroblasts
migrated out of the cut ends of nerve fragments (Fig. 1). Migration of fibroblasts was high
during the first two weeks of in vitro predegeneration. The
flattened cells were attached to the plastic and within one week,
they formed a near-confluent layer. After repeated transfers of
nerve fragments, the number of fibroblasts decreased. In addition,
numerous clusters of small spherical cells appeared around nerve
fragments. These cells were not adherent to the surface, but
floated freely in the medium. Apparently, with prolonged in
vitro predegeneration, the number of the migrating fibroblasts
further decreased and the number of the aggregates of spherical
cells in the medium increased. These spherical cells were SCs.
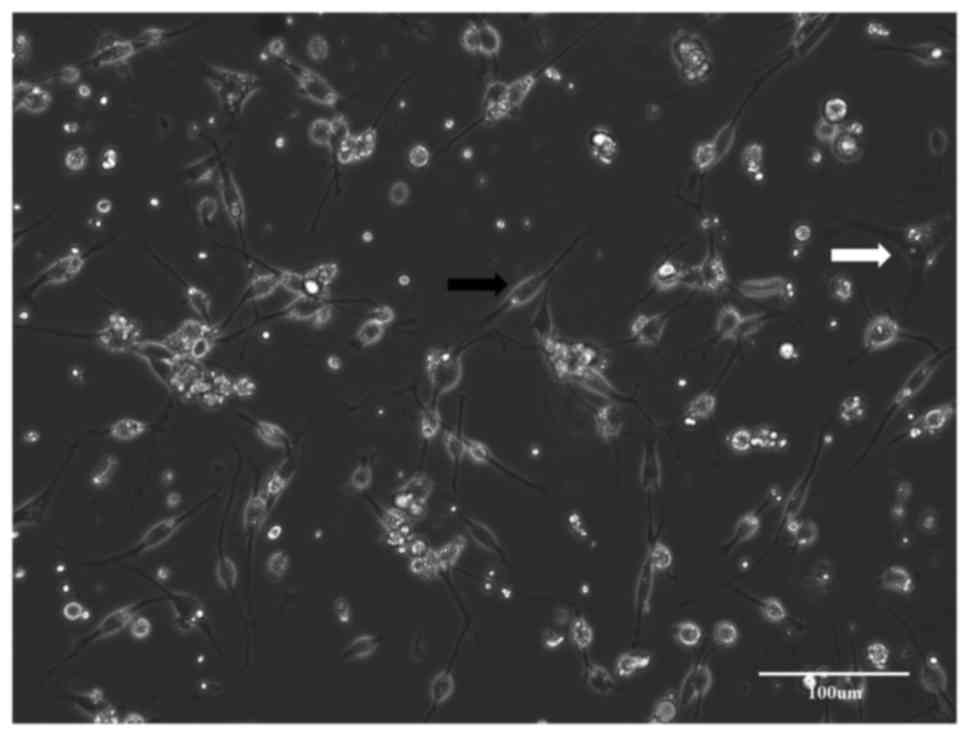
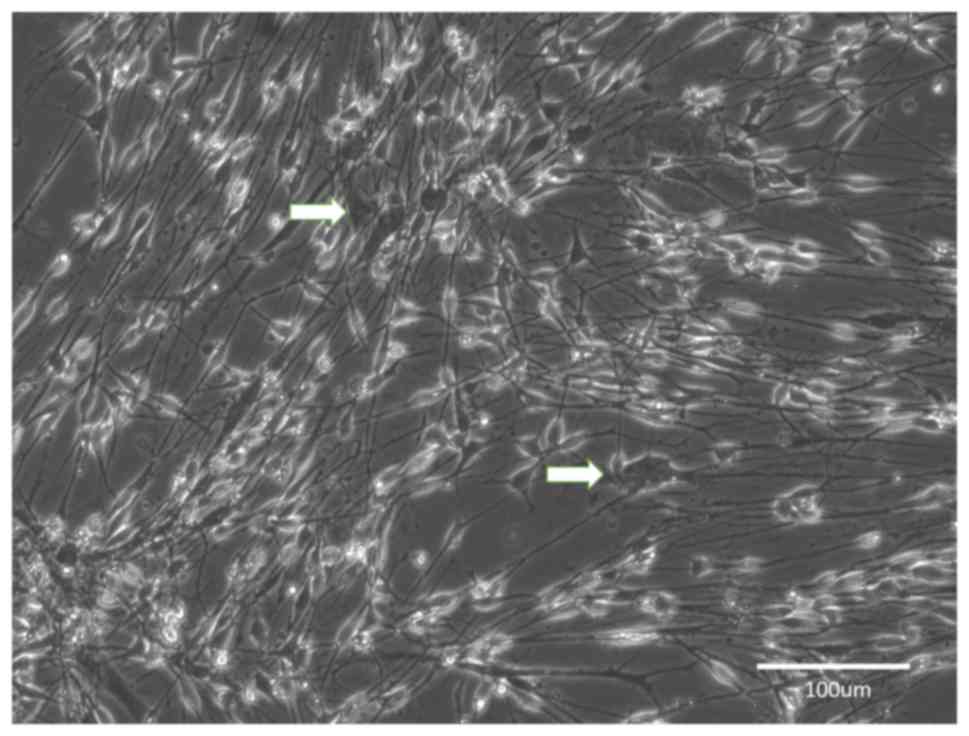
Group A
The nerve fragments were dissociated immediately
after their isolation. After incubation, the cells adhered to the
bottom of the dish and started to proliferate (Fig. 2). Within 3–4 days, the culture
achieved confluency. Based on their morphology, most of the cells
were fibroblasts and only few spindle-like SCs grew on the top of
them. It was attempted to achieve SC enrichment by CJ application;
however, it was not possible to completely remove the fibroblasts
and the newly passaged cultures were again rapidly overgrown by the
contaminating fibroblasts. During this process, it was obvious that
the proliferation of SCs was halted when the number of fibroblasts
prevailed in the growing culture (Fig.
3).
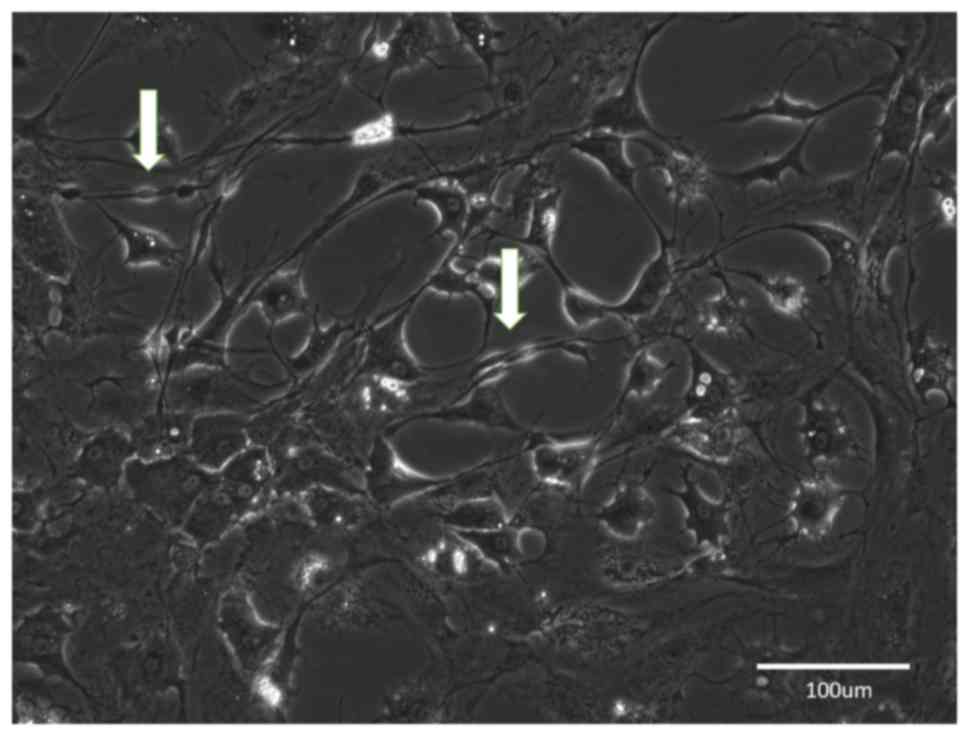
Group B
After 2 weeks of in vitro predegeneration,
the time course of cellular proliferation in the culture was
similar to that in group A. After dissociation and seeding, the
predominant cell type were fibroblasts that rapidly outgrew the
minority of SCs in the culture. However, within the first days in
culture, the number of SCs appeared to be higher than that in group
A. However, during the cultivation, fibroblasts overgrew in the
culture (Fig. 4) and the CJ
application had only a minimal effect on this process.
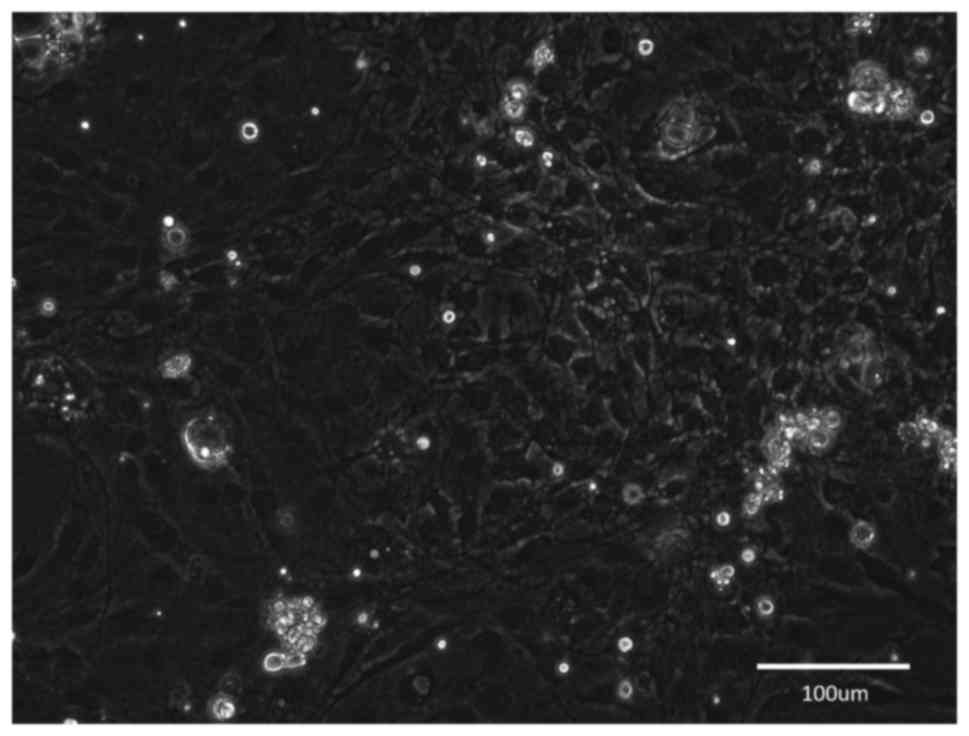
Group C
After 4 weeks of predegeneration, the early cultures
contained SCs and fibroblasts, but it appeared that most of the
cells had an SC-like morphology. After prolonged cultivation, the
cultures developed into two types: Type 1 formed a near-homogeneous
population of SCs with a minority of scattered fibroblasts
(Fig. 5) and in type 2, large areas
of near-homogeneous fibroblast populations intermingled with
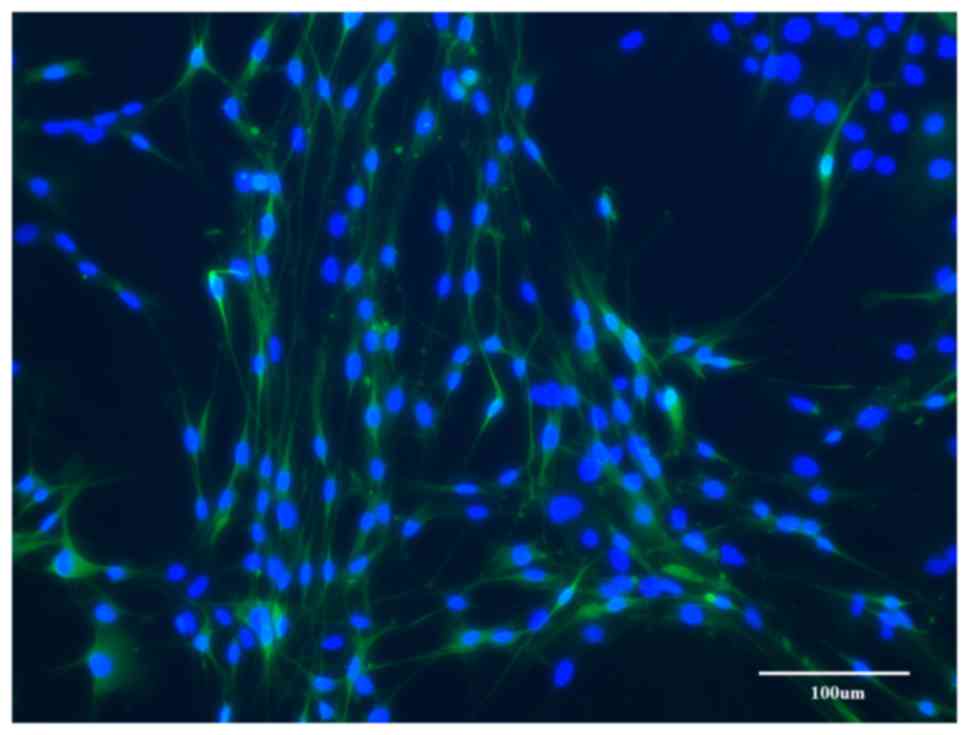
smaller islets of proliferating SCs (Fig. 6).
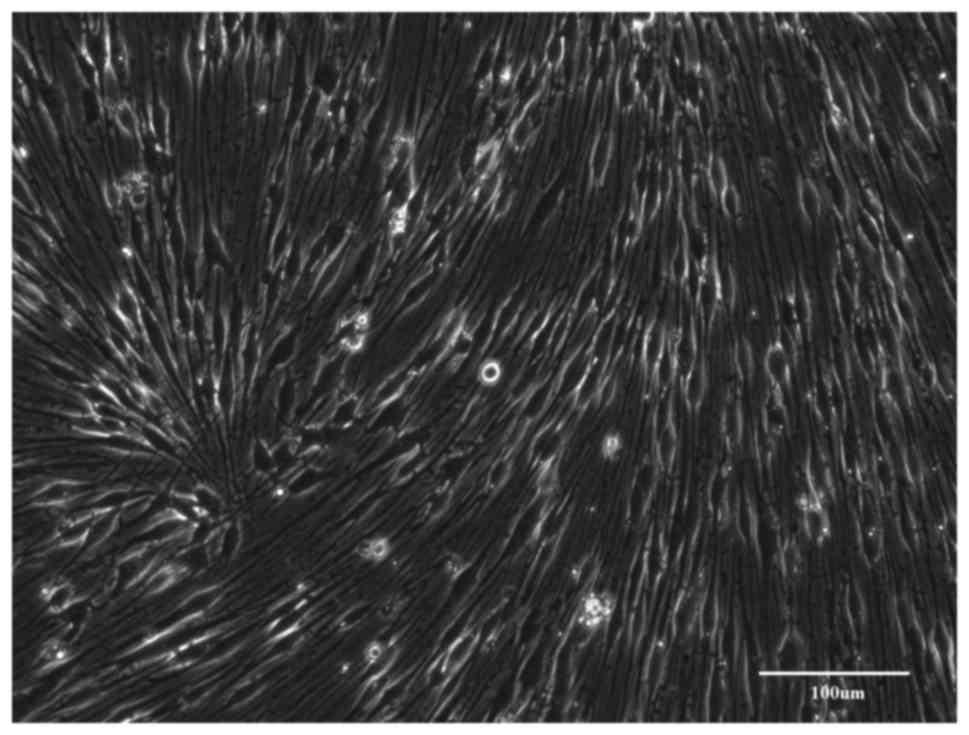
Group D
The nerve fragments were predegenerated for 6 weeks.
After dissociation, the cultures contained mostly SCs with only few
fibroblasts. When the proliferating cells achieved confluency, they
contained an almost homogeneous population of SCs and the
monolayers of paralelly arranged SCs had formed typical waves
(Fig. 7). Only in certain dishes,
among the monolayer of SCs, small islands of homogeneous fibroblast
populations were observed, particularly in the areas where the
density of SCs was low.
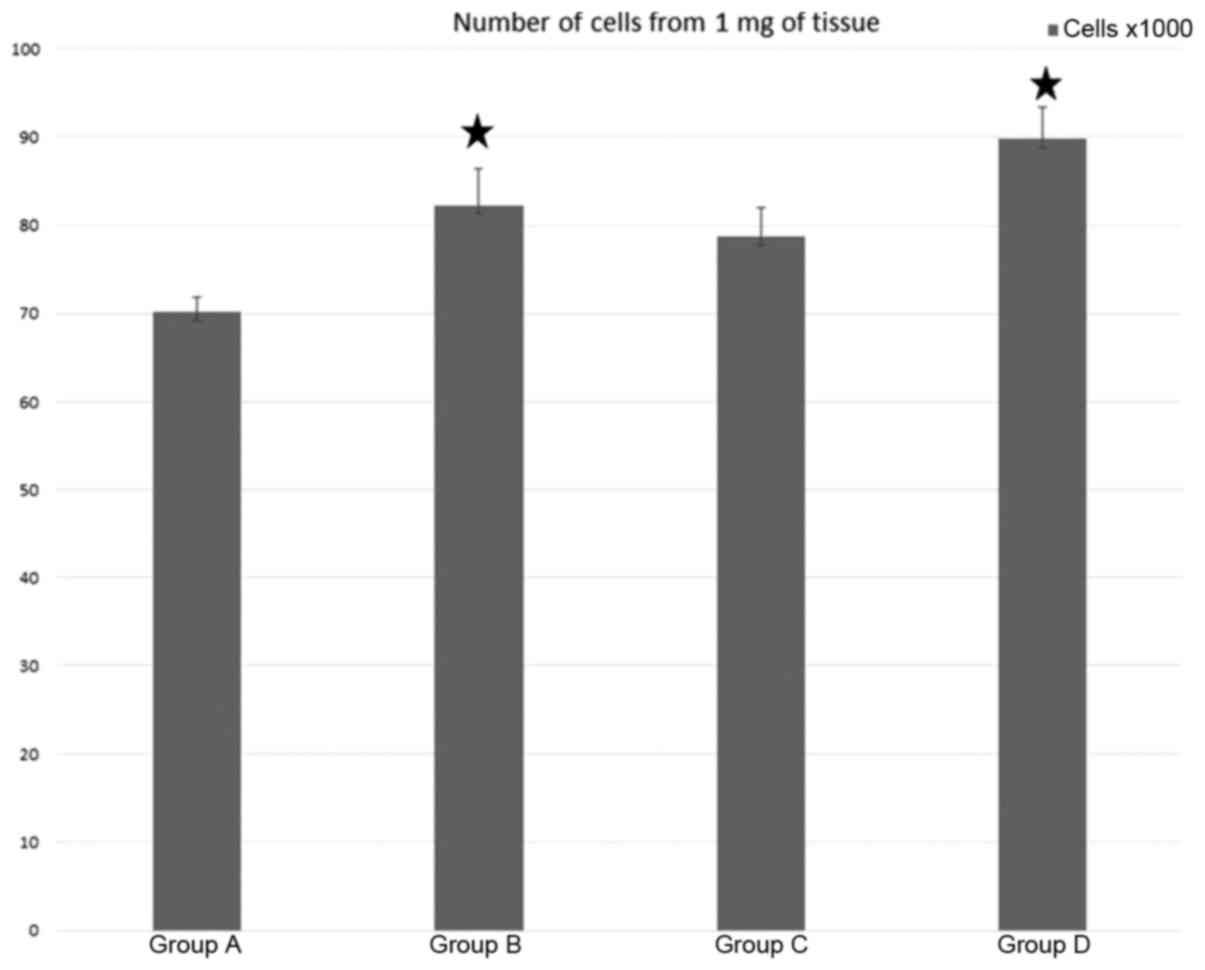
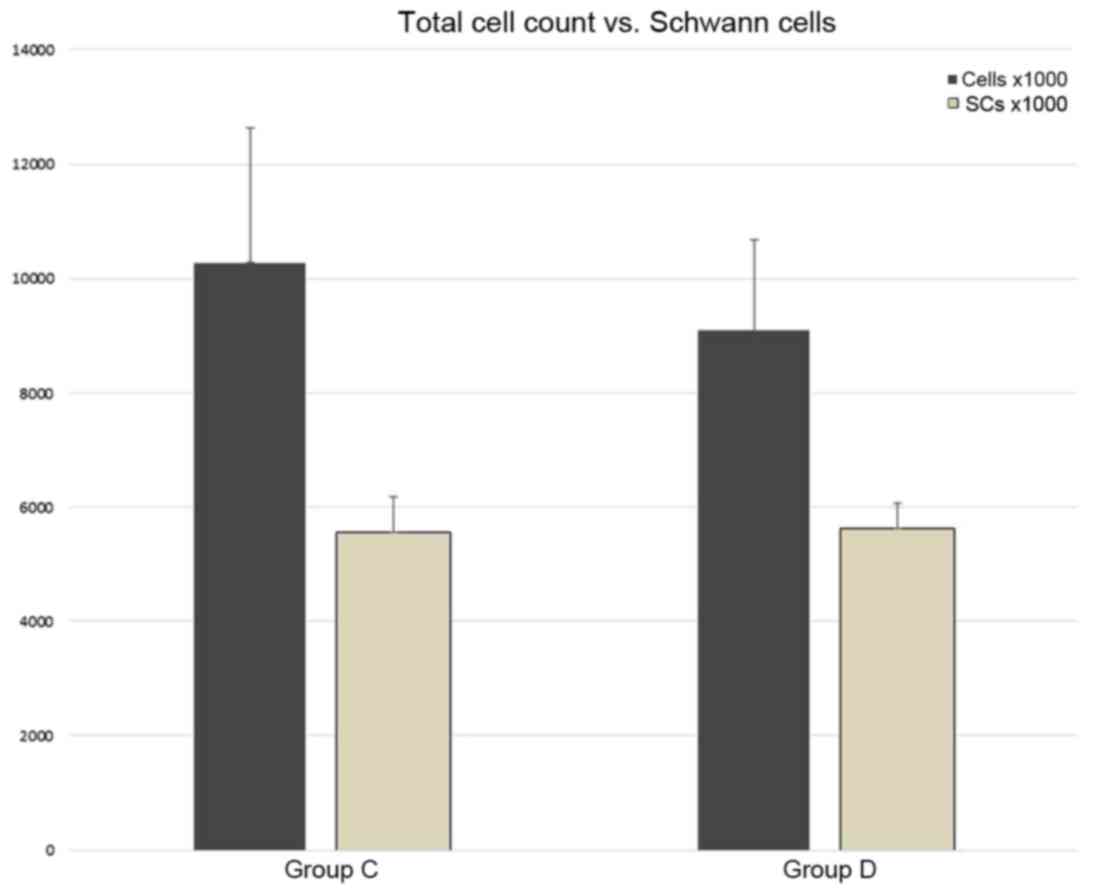
Quantification
Cell counts after dissociation are presented in
Fig. 8. The yield of cells was
expressed as a number of cells per mg of wet weight of nerve after
epineurium removal. Compared to the control (group A), the
predegeneration appeared to increase the number of dissociated
cells, but the intergroup differences were relatively modest.
Statisical analysis indicated significant differences between
groups A and B and also between groups A and D.
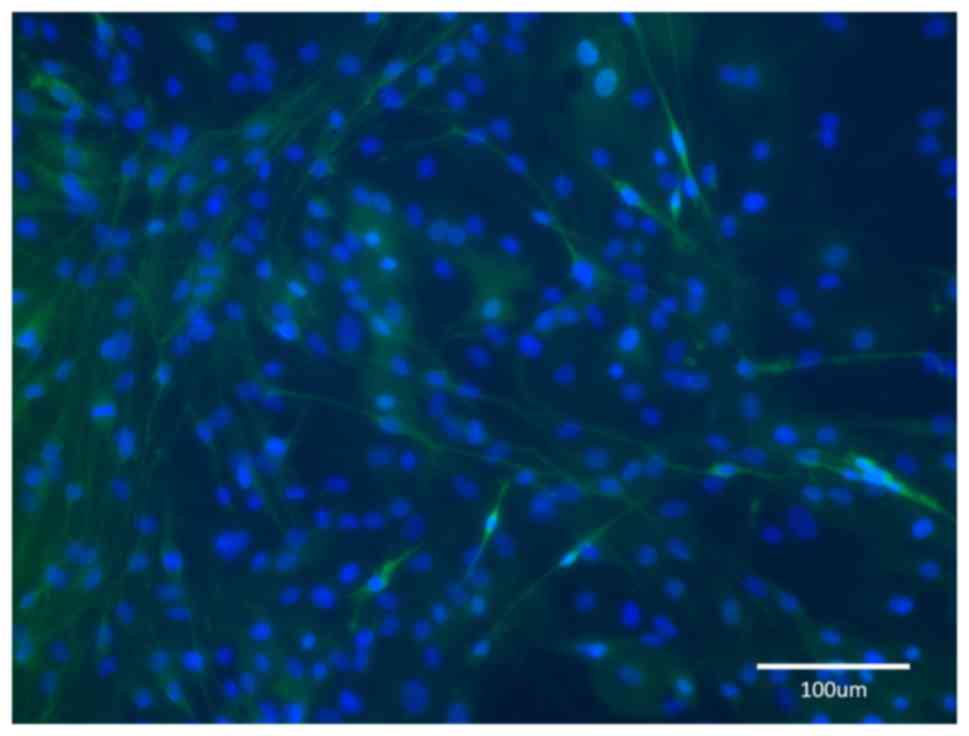
In immunostained cultures, the spindle-shaped cells
were S100-positive (Fig. 9. The cell
counts in the final cultures are presented in Fig. 10. In groups A and B, the SCs were
essentially absent, and the cell numbers are therefore presented
for groups C and D only.
Discussion
The predegenerated peripheral nerves form an
environment that is supportive of axonal growth in the regenerating
peripheral nerves and even in the central nervous system, as
demonstarted by David and Aguayo (22). More recently, a concept of artificial
SC-filled guidance channels has been proposed as a promising tool
for bridging peripheral nerve defects (6,23,24). For
this purpose, production of highly enriched adult SC cultures is
required. It has been reported that it is possible to obtain high
quantities of enriched SCs from peripheral nerves of newborn rats
(13,25). However, the purification and
enrichment of SCs from adult peripheral nerve tissue is much more
challenging. The problems associated with adult SC cultivation
include i) the low yield of viable cells from the nerve tissue, ii)
their poor proliferation capacity and iii) progressive overgrowth
by the contaminating fibroblasts.
A study published by Kaewkhaw et al (26) appears to solve these problems by
using a special medium containing D-valine. However, in all
previosly used protocols, predegeneration of peripheral nerves is
utilized (15,18–20). The
present study was based on the predegeneration protocol by Morissey
et al (15) and its later
modifications. The reasons for this decision were twofold: i)
Problems with the availability of the medium (DMEM with D-valine)
at the time of the experiments and ii) in the past, the
predegeneration protocols were repeatedly used for producing SCs
for transplantation experiments. It was documented that, even after
several passages, the cultivated SCs maintain their ability to
myelinize the regenerating axons. The known conditions were used in
order to ensure that functional SCs were obtained in the subsequent
transplantation experiments.
The predegeneration brings SCs into a mitotically
activated state and enriches the cultures in favor of SCs as
compared to fibroblasts. Initially, predegeneration in vivo
was tested by transecting the peripheral nerve and leaving it in
vivo for up to 21 days prior to harvesting and subsequent
dissociation (19). From a clinical
perspective, the in vivo predegeneration is questionable, as
it requires two consequent surgical interventions. In addition, the
degenerating nerves are also colonized by non-glial cells,
particularly activated macrophages that may interfere with the
subsequent cultivation. For these reasons, it is advisable to use
in vitro predegeneration, which imitates Wallerian
degeneration under controlled conditions. In previous studies,
various durations of predegeneration have been used, varying
between 1 and 5 weeks (12,15,27,28). In
an original study by Morrissey et al (15), a long predegeneration time (4–5
weeks) was advised. However, several other studies have used much
shorter predegeneration periods (18–20,28).
Kraus et al (27) have
assessed the effect of different predegeneration times on the cell
count and purity of SC cultures. They performed predegeneration for
2, 7 and 14 days, and reported the highest SCs counts after 7 days
of predegeneration. Similar results have been reported by Niapour
et al (12) in a study with
canine SCs. The initial experiments on SC cultivation in our group
used shorter predegeneration times (up to 2 weeks), but resulted in
rapid debasement of the culture by proliferating fibroblasts. It
was therefore decided to systematically assess the effect of
different predegeneration times on the quality of SC cultures.
In the present study, minimal differences were
observed between the cultures prepared immediately after harvesting
and after 2 weeks of predegeneration. In those cases, the cultures
were rapidly overgrown by fibroblasts. In experimental groups A and
B, the initial number of SCs, as identified by their typical
morphology, was low. In addition, the rapidly growing population of
fibroblasts had a detrimental effect on the proliferation of the SC
population and thus, the SCs were almost completely eliminated from
the cultures at the end of the cultivation period. In group C, the
cultures contained either a highly enriched population of SCs, or
in certain wells, the fibroblast population prevailed. In group D,
all of the wells contained cultures highly enriched with SCs.
These results indicate a gradual improvement of SC
purity associated with long-term predegeneration. It is known that
denervated SCs produce and secrete large amounts of neurotrophic
factors after nerve injury (29).
Upregulation of neurotrophic factors and their receptors may
contribute to the proliferation of SCs during Wallerian
degeneration, and also in primary cultures of pre-activated SCs.
After transection of the peripheral nerve, marked changes in the
levels of tumor necrosis factor (TNF)-α, interferon γ and
interleukin-1 have been described after 7,14 and 35 days (30). Interleukin-1 enhances fibroblast
growth (31) and tumor necrosis
factor α is a chemotactic agent for fibroblasts (32).
In the developing in vitro cultures in the
present study, these two populations (fibroblasts and SCs) were
influencing each other during the proliferation phase. When the
fibroblasts prevailed, they had a tendency to form a monolayer and
almost completely eliminate SCs from the culture. When the SCs
prevailed, they proliferated rapidly and formed a near-pure culture
that was intermingled with solitary fibroblasts. However, with
prolonged cultivation, the number of fibroblasts in the SC culture
slowly increased. For this reason, in the 21 day-old cultures of
the present study, the percentage of SCs (54,02% in group C and
61,95% in group D) was lower than that reported in previous studies
(12,15,17–19,21,26,27).
The present results are consistent with those
reported by Morissey et al (15), who recommend 4–5 weeks of
predegeneration and appear to contradict the previous observations
indicating that the predegeneration periods of >7 days are
associated with worse cell counts and purity of the final culture
(26). This difference may be
influenced by the fact that animals older than 3 months were used
in the present study. It is known that in newborn animals, the
process of Wallerian degeneration is faster (33) and that the recovery of the transected
nerves is slower in older animals (34).
In conclusion, the major result of the present study
is that when preparing pimary SC cultures from adult rats, long
predegeneration periods are critical in order to produce enriched
SC cultures. In our hands, the prolonged predegeneration only
modestly improved cell yields after dissociation. It may thus be
hypothesized that the prolonged predegeneration minimizes the
proliferation activity of fibroblasts in favor of SCs.
The primary goal of the present study was to produce
a population of cultured SCs that may be further used for
autotransplantation studies. While this has been achieved, it was
also revealed that the populations of cultivated SCs undego dynamic
changes. In further studies, the proliferation index of the SCs in
culture will be assessed and optimal periods of cultivation with
respect to the purity of the SC population will be determined.
Acknowledgements
Preliminary results have been presented as a poster
at FENS meeting, Copenhagen 2016.
Funding
The present study was supported by grants
APVV-14-0847 (Slovak research and development agency) and VEGA
2/0145/16 (Scientific Grant Agency, Ministry of education, science,
research and sport of the Slovak republic and Slovak Academy of
Sciences).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
PT was responsible for the concept and design of the
study, and contributed to the literature search. LS contributed to
the literature search, analysed the data and reviewed the
manuscript. IV contributed to the design of the study and the
literature search. The final version of the manuscript has been
read and approved by all authors, and each author believes that the
manuscript represents honest work.
Ethical approval and consent to
participate
This study was performed with the approval and
according to the guidelines of the Institutional Animal Care and
Use Committee of the Slovak Academy of Sciences (Košice, Slovakia)
and with the European Communities Council Directive (2010/63/EU)
regarding the use of animals in research and Slovak Law for Animal
Protection 377/2012 and 436/2012.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATB
|
antibiotics
|
|
CJ
|
cold jet
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
NGS
|
normal goat serum
|
|
SCM
|
SC medium
|
|
SCs
|
Schwann cells
|
References
|
1
|
Noble J, Munro CA, Prasad VS and Midha R:
Analysis of upper and lower extremity peripheral nerve injuries in
a population of patients with multiple injuries. J Trauma.
45:116–122. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robinson LR: Traumatic injury to
peripheral nerves. Muscle Nerve. 23:863–873. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans-Jones G, Kay SP, Weindling AM,
Cranny G, Ward A, Bradshaw A and Hernon C: Congenital brachial
palsy: Incidence, causes, and outcome in the United Kingdom and
Republic of Ireland. Arch Dis Child Fetal Neonatal Ed.
88:F185–F189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson EO and Soucacos PN: Nerve repair:
Experimental and clinical evaluation of biodegradable artificial
nerve guides. Injury. 3 (39 Suppl):S30–S36. 2008. View Article : Google Scholar
|
|
5
|
Evans GR: Peripheral nerve injury: A
review and approach to tissue engineered constructs. Anat Rec.
263:396–404. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kehoe S, Zhang XF and Boyd D: FDA approved
guidance conduits and wraps for peripheral nerve injury: A review
of materials and efficacy. Injury. 43:553–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guénard V, Kleitman N, Morrissey TK, Bunge
RP and Aebisher P: Syngeneic Schwann cells derived from adult
nerves seeded in semipermeable guidance channels enhance peripheral
nerve regeneration. J Neurosci. 12:3310–3320. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calancie B, Madsen PW, Wood P, Marcillo
AE, Levi AD and Bunge RP: A guidance channel seeded with autologous
Schwann cells for repair of cauda equina injury in a primate model.
J Spinal Cord Med. 32:379–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HA, Mindos T and Parkinson DB: Plastic
fantastic: Schwann cells and repair of the peripheral nervous
system. Stem Cells Transl Med. 2:553–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hall SM: Regeneration in cellular and
acellular autografts in the peripheral nervous system. Neuropathol
Appl Neurobiol. 12:27–46. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kocsis JD and Waxman SG: Schwann cells and
their precursors for repair of central nervous system myelin.
Brain. 130:1978–1980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niapour N, Mohammadi-Ghalehbin B,
Golmohammadi MG, Amani M, Salehi H and Niapour A: Efficacy of
optimized in vitro predegeneration period on the cell count and
purity of canine Schwann cell culture. Iran J Basic Med Sci.
18:307–311. 2015.PubMed/NCBI
|
|
13
|
Brockes JP, Fields KL and Raff MC: Studies
on cultured rat Schwann cells. I. Establishment of purified
populations from cultures of peripheral nerve. Brain Res.
165:105–118. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Timmer M, Robben S, Müller-Ostermeyer F,
Nikkah G and Grothe C: Axonal regeneration across long gaps in
silicone chambers filled with Schwann cells overexpressing high
molecular weight FGF-2. Cell Transplant. 12:265–277. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morissey TK, Kleitman N and Bunge RP:
Isolation and functional characterization of Schwann cells derived
from adult peripheral nerve. J Neurosci. 11:2433–2442. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rutkowski JL, Tennekoon GI and McGilicuddy
JE: Selective culture of mitotically active human Schwann cells
from adult sural nerves. Ann Neurol. 31:580–586. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jirsová K, Soddar P, Mandys V and Bär PR:
Cold jet: A method to obtain pure Schwann cell cultures without the
need for cytotoxic, apoptosis-inducing drug treatment. J Neurosci
Methods. 78:133–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keilhoff G, Fansa H, Schneider W and Wolf
G: In vivo predegeneration of peripheral nerves: An effective
technique to obtain activated Schwann cells for nerve conduits. J
Neurosci Methods. 89:17–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komiyama T, Nakao Y, Toyama Y, Asou H,
Vacanti CA and Vacanti MP: A novel technique to isolate adult
Schwann cells for an artificial nerve conduit. J Neurosci Methods.
122:195–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verdú E, Rodríguez FJ, Gudiño-Cabrera G,
Nieto-Sampedro M and Navarro X: Expansion of adult Schwann cells
from mouse predegenerated peripheral nerves. J Neurosci Methods.
99:111–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haastert-Talini K: Culture and
proliferation of highly purified adult Schwann cells from rat, dog,
and man. Methods Mol Biol. 843:189–200. 2012. View Article : Google Scholar
|
|
22
|
David S and Aguayo AJ: Axonal elongation
into peripheral nervous system ‘bridges’ after central nervous
system injury in adult rats. Science. 214:931–933. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu X, Ding F and Williams DF: Neural
tissue engineering options for peripheral nerve regeneration.
Biomaterials. 35:6143–6156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Faroni A, Smith RJ, Lu L and Reid AJ:
Human Schwann-like cells derived from adipose-derived mesenchymal
stem cells rapidly de-differentiate in the absence of stimulating
medium. Eur J Neurosci. 43:417–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wood PM: Separation of functional Schwann
cells and neurons from normal peripheral nerve tissue. Brain Res.
115:361–375. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaewkhaw R, Scutt AM and Haycock JW:
Integrated culture and purification of rat Schwann cells from
freshly isolated adult tissue. Nat Protoc. 7:1996–2004. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kraus A, Täger J, Kohler K, Manoli T,
Haerle M, Werdin F, Hoffman J, Schaller HE and Sinis N: Efficacy of
various durations of in vitro predegeneration on the cell count and
purity of rat Schwann-cell cultures. J Neurotrauma. 27:197–203.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mauritz C, Grothe C and Haastert K:
Comparative study of cell culture and purification methods to
obtain highly enriched cultures of proliferating adult rat Schwann
cells. J Neurosci Res. 77:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fawcett JW and Keynes RJ: Peripheral nerve
regeneration. Annu Rev Neurosci. 13:43–60. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruohonen S, Khademi M, Jagodic M, Taskinen
HS, Olsson T and Röyttä M: Cytokine responses during chronic
denervation. J Neuroinflammation. 2:262005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh JP, Adams LD and Bonin PD: Mode of
fibroblast growth enhancement by human interleukin-1. J Cell Biol.
106:813–819. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Postlethwaite AE and Seyer JM: Stimulation
of fibroblast chemotaxis by human recombinant tumor necrosis factor
alpha (TNF-alpha) and a synthetic TNF-alpha 31-68 peptide. J Exp
Med. 172:1749–1756. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vyas A, Li Z, Aspalter M, Feiner J, Hoke
A, Zhou C, O'Daly A, Abdullah M, Rohde C and Brushart TM: An in
vitro model of adult mammalian nerve repair. Exp Neurol.
223:112–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi SJ, Harii K, Lee MJ, Furuya F and
Ueda K: Electrophysiological, morphological, and morphometric
effects of aging on nerve regeneration in rats. Scand J Plast
Reconstr Surg Hand Surg. 29:133–140. 1995. View Article : Google Scholar : PubMed/NCBI
|