Introduction
Wogonin is a flavonoid compound first isolated from
Scutellaria baicalensis and is used in Chinese herbal
medicine (1). It has been recognized
as a potent anticancer agent due to its broad toxicity in various
types of cancer cell lines, including human breast cancer, liver
cancer, lung cancer and human gastric cancer cells (2–5). The
underlying mechanisms of the growth-suppressive effects of wogonin
on tumor cells are considered to be associated with inhibition of
cell proliferation (6), induction of
apoptosis (7), antiangiogenesis
(8–12) and promotive effects on tumor cell
differentiation (13). In addition,
wogonin further exhibited pharmacologic properties, including
neuroprotective, antiviral, anti-inflammatory and antioxyradical
effects (14–16). Previously, various studies focused on
exploring the underlying cellular pathways responsible for the
energy metabolism in tumorigenesis. Increased catabolic glucose
metabolism is one of the primary metabolic changes observed in
proliferating cells (17). The shift
in energy production in tumor cells from oxidative phosphorylation
to glycolysis, regardless of the oxygen concentration, is a
phenomenon termed ‘Warburg effect’ (18). Although the mechanisms and benefits
of this metabolic behavior in tumor cells remain unclear,
disturbance of the glycolysis emerges as a promising strategy for
cancer therapy (19,20). The effects of wogonin on
antiproliferative and apoptotic activities have been documented
using various human cancer cells; however, its effects on energy
metabolism-associated enzymes and adenosine triphosphate (ATP)
generation in SGC-7901 and A549, human gastric cancer and human
lung adenocarcinoma cell lines, respectively, remains to be
elucidated.
Tumor cells have a unique aerobic glycolysis.
Abnormal changes in glucose metabolism may exist in tumor cells and
even in the presence of oxygen, glucose metabolism is transformed
from oxidative phosphorylation to glycolysis, which consumes large
quantities of glucose and generates lactic acid (21). In line with these characteristics,
the present study attempted to evaluate different effects of
wogonin on proliferation inhibition of SGC-7901 and A549 cells and
further explored the sensitivity of these cell lines to wogonin,
based on changes observed for various enzymes involved in the
energy metabolism. The results suggested that in SGC-7901 cells,
wogonin inhibited the growth of tumor cells by interfering with the
energy metabolism. Furthermore, decreased hypoxia inducible
factor-1α (HIF-1α) and monocarboxylate transporter-4 (MCT-4)
expression induced by wogonin may be partially responsible for
inhibitory effects in the tumor metabolism. In A549 cells, wogonin
demonstrated little influence on the energy metabolism. Since
sensitivity to wogonin may be not the same in certain types of
tumor cell, different anti-tumor therapy should therefore be
considered when wogonin is used alone or in combination. The
present study aimed to provide a guide for further studies on
targeted therapy for different tumors types.
Materials and methods
Reagents and antibodies
Wogonin (Chengdu Institute of Biology, Chinese
Academy of Science, Chengdu, China) was dissolved in dimethyl
sulfoxide (DMSO; 100 mg/ml) and stored at −20°C. The solution was
diluted as required using RPMI-1640 medium. 5-Fluorouracil (5-Fu)
and MTT were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). SGC-7901 and A549 cell lines were obtained from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). RPMI-1640 medium, Fetal Bovine Serum
(cat. no. 16000-044) and trypsin-EDTA 0.25% (cat. no. 25200-072)
were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Bicinchoninic acid (BCA) Protein Assay kit (cat. no. P0010),
RIPA Lysis Buffer (cat. no. P0013B) and Trypan blue Staining Cell
Viability Assay kit (cat. no. C0011) were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). Hexokinase (HK) assay
kit (cat. no. A007-1), pyruvate kinase (PK) assay kit (cat. no.
A076-1), lactate dehydrogenase (LDH) assay kit (cat. no. A020-1)
and succinate dehydrogenase (SDH) activity assay kit (cat. no.
A022). ATP assay kit (cat. no. A059-1) and Hematoxylin-Eosin stain
kit (cat. no. D006) were obtained from Nanjing Jiancheng
Bioengineering Institute (Nanjing, Jiangsu, China). Rabbit HIF-1α
(cat. no. PB0245) and MCT-4 (cat. no. PB0269) antibodies were
obtained from Boster Biological Technology (Pleasanton, CA, USA).
Mouse β-actin (cat. no. 60008-1), horseradish peroxidase-conjugated
goat anti-rabbit IgG (cat. no. SA00001-2) and goat anti-mouse IgG
(cat. no. SA00001-1) antibodies were obtained from ProteinTech
Group, Inc. (Chicago, IL, USA). The diaminobenzidine (DAB) kit
(cat. no. ZLI-9018) was obtained from Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd. (OriGene Technologies, Inc., Beijing.
China). The enhanced chemiluminescence (ECL) kit (cat. no. 32109)
was obtained from Thermo Fisher Scientific, Inc.
Measurements of cell growth and
viability
SGC-7901 and A549 cells were cultured in RPMI-1640
medium supplemented with 10% FBS and 1% penicillin/streptomycin in
a humidified incubator in an atmosphere of 5% CO2 at
37°C as previously described (22,23).
Cells were seeded in 96-well plates at a density of
5×104 cells/well and incubated with 5% CO2 at
37°C for 24 h. Subsequently, the cells were treated with wogonin at
varying concentrations (5, 10, 15, 20, 25 and 30 µg/ml) at 37°C for
48 h. An untreated control experiment was also performed. Cell
viability was assessed by adding MTT in PBS to a final
concentration of 5 mg/ml. Plates were incubated at 37°C for a
further 4 h. DMSO (150 µl) was added to each well and incubated for
10 min at room temperature. The amount of MTT-formazan was directly
proportional to the number of living cells and was determined by
measuring the optical density at 490 nm. Cell survival rate was
calculated using the following equation: Cell survival rate (%) =
(Average ODwogonin - Average
ODcontrol)/(Average ODcontrol - Average
ODblank) × 100%. IC50 were calculated using
SPSS 20.0 (IBM Corp., Armonk, NY, USA). Each experiment was
repeated 4 times.
Trypan blue exclusion assay
SGC-7901 and A549 cells were seeded into 24-well
plates at a density of 5×104 cells/well and incubated
with 5% CO2 at 37°C for 24 h. Following pretreatment
with wogonin (15 µg/ml) or 5-Fu (10 µg/ml) at 37°C for 24 h, a cell
suspension was prepared at a suitable dilution (1.0×105
cells/ml) in PBS. A total of 50 µl of cell suspension was taken and
mixed with an equal volume of 0.4% Trypan blue. The number of
viable cells was counted under a light microscope at a
magnification of ×20 once daily for 7 days, and the growth curve
was constructed. An untreated control group was analyzed
additionally. Each experiment was repeated 4 times and the results
were expressed as the mean ± standard deviation for each treatment
group.
Cell morphological assessment
SGC-7901 and A549 cells were seeded at a density of
5×104 cells/well on coverslips, which were placed in
6-well plates and incubated with 5% CO2 at 37°C for 24
h. Subsequently, the cells were treated with wogonin (15 µg/ml) at
37°C for 48 h. An untreated control experiment was performed in
parallel. Cells were washed with ice-cold PBS and fixed with 95%
ethanol for 15 min at room temperature, followed by staining with
hematoxylin (5 mg/ml) for 5 min and eosin (5 mg/ml) for 3 min at
room temperature. Then the coverslips were then dehydrated with
graded ethanol for 1–2 sec at room temperature and soaked in xylene
for 5 min, mounted at room temperature and dried at 37°C for 24 h.
Morphological changes of the cells were observed under a light
microscope at a magnification of ×40. Furthermore, the number of
cells in each group were counted at a magnification of ×20 and
evaluated. Each experiment was repeated 4 times.
Enzyme activity assays
After SGC-7901 and A549 cells were seeded into
6-well plates at a density of 5×105 cells/well, cells
were pretreated with wogonin (15 µg/ml) for 48 h at 37°C. An
untreated control experiment was also performed. Following
pretreatment, cells were trypsinized with Trypsin-EDTA and
suspended in 0.9% physiological saline, then centrifuged at 2,000 ×
g for 5 min at 4°C. HK, PK, LDH and SDH activities were assessed
with activity assay kits by measuring the absorbance at 340, 440
and 600 nm, respectively. Enzyme activities were normalized against
the protein concentration, determined using the BCA Protein Assay
kit at 562 nm. Each experiment was repeated 4 times.
Western blot analysis
HIF-1α and MCT-4 expression were examined by western
blot analysis. After SGC-7901 and A549 cells reached a confluence
of 70%, they were incubated with wogonin (15 µg/ml) for 48 h with
5% CO2 at 37°C in cell culture flasks. Cells were then
washed with ice-cold PBS 3 times and the total protein was
extracted with RIPA Lysis Buffer. Samples were then centrifuged at
13,500 × g for 15 min at 4°C to remove cell debris. An untreated
control experiment was also performed. The protein concentration
was measured using the BCA Protein Assay kit. Protein (~30 µg) was
loaded and separated on 10% SDS-PAGE gels and transferred to
nitrocellulose membranes. Following 1 h of blocking with 5% non-fat
dried milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T)
at room temperature, the membranes were incubated with primary
antibodies with gentle shaking overnight at 4°C; HIF-1α (1:100),
MCT-4 (1:100) and β-actin (1:1,000) prepared in 5% non-fat dried
milk in TBS-T. Membranes were further incubated with secondary
antibodies for 1 h at room temperature, then developed using an ECL
kit and scanned densitometry. The band densities were quantified
via densitometry using Quantity One Software 4.4 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The intensities of the
protein bands were normalized against β-actin. Each experiment was
repeated 4 times.
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical analysis was performed with SPSS (version 20.0; IBM
Corp., Armonk, NY, USA). Differences between the conditions tested
were identified using one-way analysis of variance and differences
among groups were analyzed for significance using a Tukey's test.
Comparisons between two groups were performed using the Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
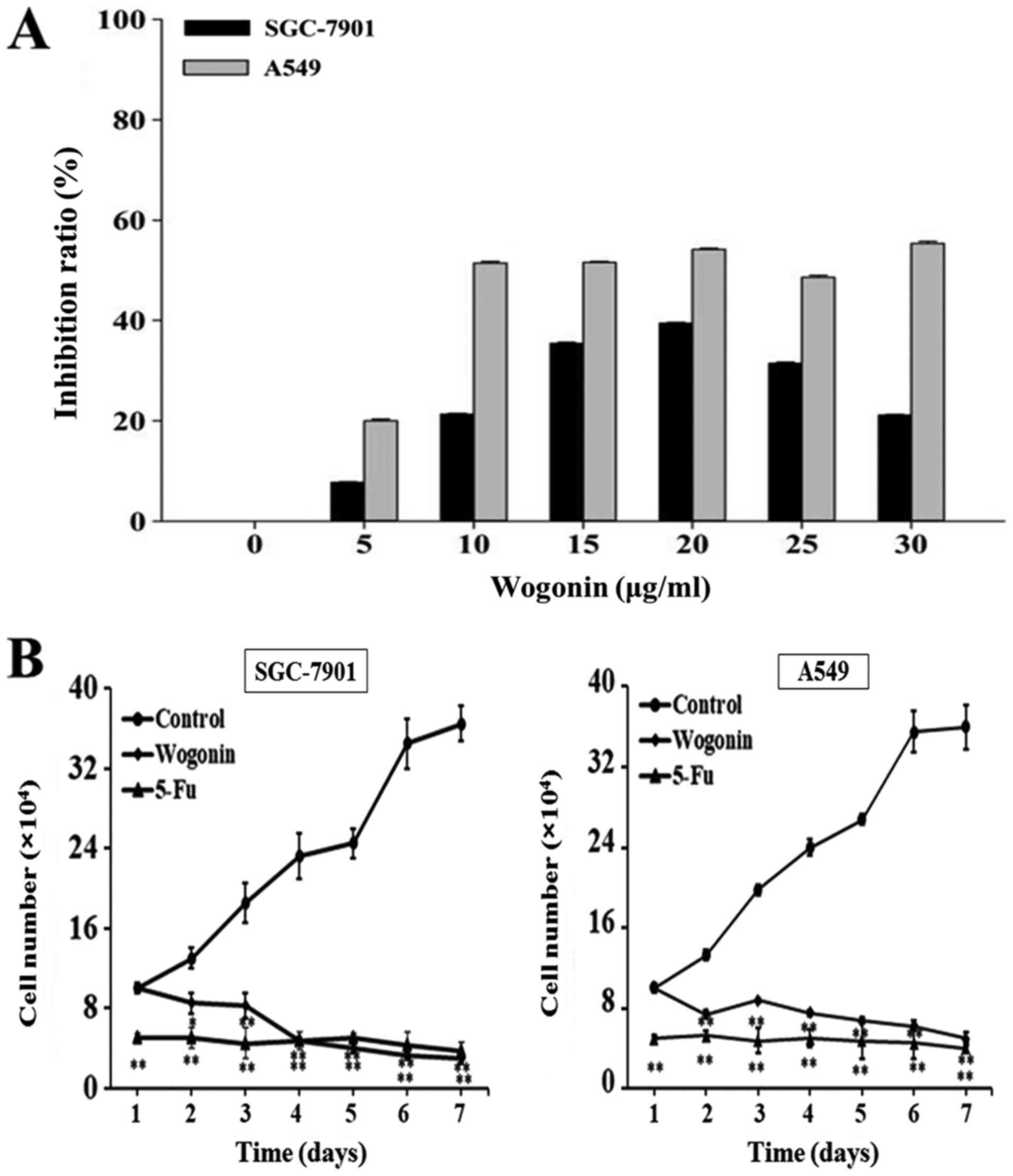
Wogonin affects the proliferation of
SGC-7901 and A549 cells
To evaluate the effect of wogonin on cell
proliferation, SGC-7901 and A549 cells were treated with varying
concentrations of wogonin and viability was evaluated by MTT
assays. As presented in Fig. 1A, the
results suggested that the inhibition rates of wogonin in A549
cells were stable at high concentrations (10–30 µg/ml). Measured
rates were all similar to the IC50 (18.17 µg/ml) for
A549 cells. To achieve high efficiency and low toxicity, 15 µg/ml
wogonin were used in subsequent experiments with A549 cells. In
SGC-7901 cells, the inhibitory effect of wogonin on cell
proliferation was dose-dependent between 5 and 20 µg/ml. Although
the inhibitory rate was higher at 20 µg/ml compared with the 15
µg/ml treatment, to achieve lower toxicity and to be consistent
with the A549 cells, 15 µg/ml wogonin in SGC-7901 cells was
selected for further research. The inhibitory rates of SGC-7901 and
A549 cells following wogonin treatment (15 µg/ml) were 35.00±0.12
and 54.17±0.24%, respectively, indicating a stronger inhibitory
effect of wogonin on A549 cell proliferation compared with SGC-7901
cells.
To investigate whether the effect of wogonin on cell
proliferation was time-dependent, SGC-7901 and A549 cells were
treated with 15 µg/ml wogonin for 7 days and the cell numbers were
counted daily. Growth curves were prepared to describe the effect
of wogonin on cell proliferation over time. As presented in
Fig. 1B, wogonin decreased the cell
proliferation in the cell lines in a time-dependent manner compared
with the untreated control. Treatment with wogonin markedly
decreased cell number in a time-dependent manner in each cell line,
which was similar in strength to the effect exhibited by 10 µg/ml
5-Fluorouracil (5-Fu). 5-Fu is a potent anti-tumor agent that
affects pyrimidine synthesis by inhibiting thymidylate synthetase,
thus depleting intracellular dTTP pools (24). The results suggested that wogonin
significantly inhibited the proliferation of SGC-7901 and A549
cells over time compared with an untreated control.
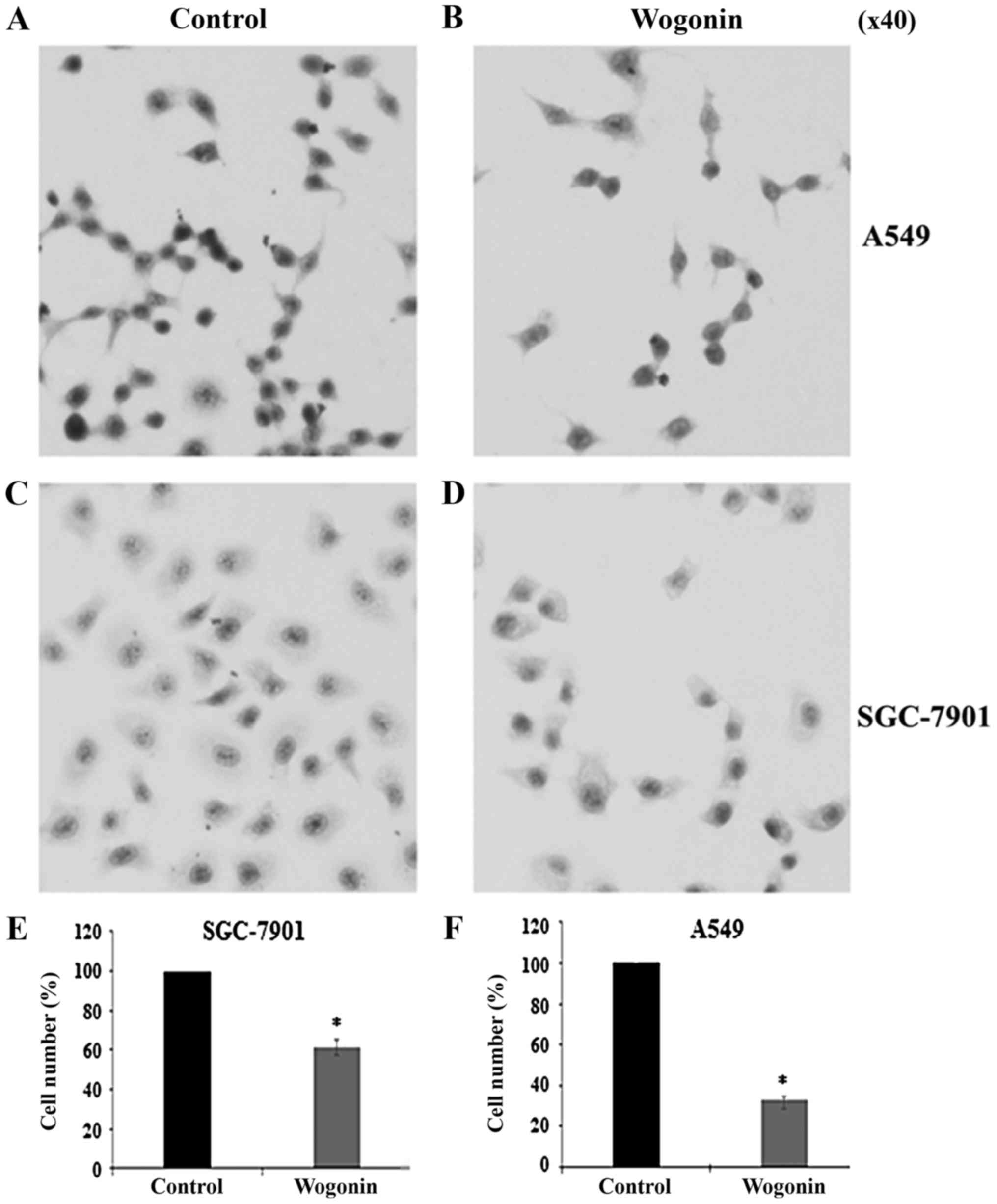
Cell morphological assessment
following wogonin treatment
Morphological changes induced by Wogonin (15 µg/ml)
were observed by H&E staining. As presented in Fig. 2, the majority of treated cells
exhibited a round in shape, loss of cell volume, hyperchromatic
nuclei and cytoplasm agglutination compared with the untreated
controls (Fig. 2A-D). Compared with
controls, the cell numbers were significantly decreased following
treatment with wogonin in SGC-7901 and A549 cells (Fig. 2E and F). These results indicated that
wogonin may induce cell morphological changes in the studied cell
lines.
Wogonin affects ATP generation and the
activities of energy metabolism-associated enzymes
To investigate the effects of wogonin on energy
metabolism, activities of enzymes participating in glucose
anaerobic glycolysis, including HK, PK and LDH, and the activity of
SDH, a contributor to the tricarboxylic acid cycle (TAC), were
measured. As presented in Table I,
SDH activity and ATP levels were significantly decreased by wogonin
in SGC-7901 cells when compared with the untreated control
(P<0.05). The inhibitory effect of wogonin significantly
impacted on LDH activity when compared to the untreated control
(P<0.01). Compared with the controls, A549 cells treated with
wogonin significantly inhibited LDH activity (P<0.01) and ATP
content was decreased (P<0.05). HK, SDH and PK activities were
not significantly affected. The results indicated that wogonin may
affect lactic acid and cell energy generation in SGC-7901 cells.
Furthermore, wogonin treatment only regulated lactic acid and ATP
generation in A549 cells.
 | Table I.Energy metabolism-related enzymes
activities and ATP level. |
Table I.
Energy metabolism-related enzymes
activities and ATP level.
| Enzyme
activity | Wogonin
(SGC-7901) | Control
(SGC-7901) | Wogonin (A549) | Control (A549) |
|---|
| HK (U/g prot) | 49.80±5.41 | 56.56±6.49 | 48.61±0.41 | 47.09±6.49 |
| PK (U/g prot) |
2,156.56±135.61 | 1,975.76±94.71 |
2,507.98±191.61 |
3,206.05±469.47 |
| LDH (U/g prot) |
216.22±8.95b | 3301.54±263.39 |
8406.95±506.35b |
12,805.51±803.25 |
| SDH (U/mg
prot) |
239.07±6.83a | 382.46±15.89 | 525.27±46.54 | 511.90±36.24 |
| ATP (µmol/g
prot) |
3,090.50±456.35a |
4,595.84±563.52 |
1,219.45±314.87a |
2,428.03±203.99 |
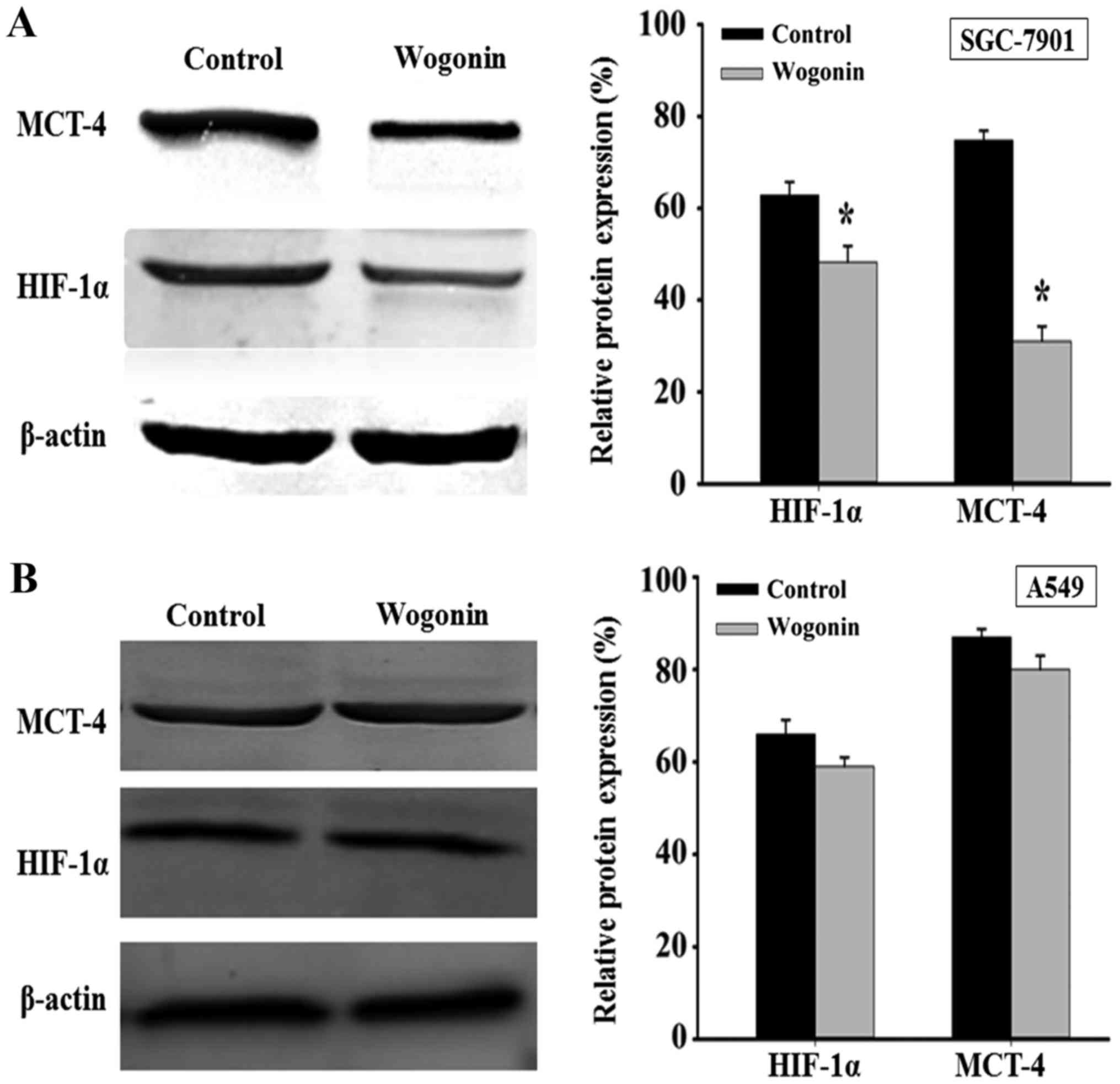
Wogonin affects HIF-1α and MCT-4
expressions
To further investigate the effect of wogonin on
energy metabolism-associated enzymes, HIF-1α and MCT-4 expression
were determined by western blot. As presented in Fig. 3, following treatment with wogonin for
48 h, HIF-1α and MCT-4 expression significantly decreased in
SGC-7901 cells compared with the control (P<0.01; Fig. 3A), but no significant changes were
observed in A549 cells (Fig.
3B).
Discussion
Scutellaria has been used in Chinese herbal
medicine for the treatment of inflammation, cancer, allergies and
bacterial and viral infections of the respiratory and
gastrointestinal tract (25).
Wogonin is one of the effective compounds extracted from
Scutellaria, exhibiting multiple pharmacological effects,
including cytotoxic effects in human cancer cell lines, inhibitory
effects on tumor angiogenesis and neoplasm metastasis (26).
The present study performed MTT assays to measure
the inhibitory rates of wogonin in several cancer cell lines and it
was demonstrated that human gastric cancer cells and human lung
adenocarcinoma cells were sensitive to wogonin treatment. The
inhibitory effects of wogonin on cell proliferation were evaluated
and the maximal inhibitory rate was induced by 15–20 µg/ml wogonin,
suggesting a high inhibitory efficiency on cell proliferation.
Furthermore, according to the inhibitory rates exerted by wogonin
(15 µg/ml), a stronger effect on A549 cell proliferation was
observed when compared with SGC-7901 cells, thus it is necessary
and essential to launch a contrastive study on the effects of
wogonin in these two cell lines. Growth curves and H&E staining
results suggested that, wogonin (15 µg/ml) may inhibit the cell
proliferation in a time-dependent manner. 5-Fu is a first-line
adjuvant chemotherapeutic agent that is often administered as part
of a regimen with other cytotoxic drugs, including Cisplatin. It is
also a potent antitumor agent that affects pyrimidine synthesis by
inhibiting thymidylate synthetase (27,28). In
the current study, 5-Fu was selected as a positive control to
further assess the inhibitory effects of wogonin on SGC-7901 and
A549 cell proliferation. The observed effects on proliferation
induced by wogonin were not significantly different compared with
the effects exhibited by 5-Fu treatment.
Cancer is considered a metabolic disease, which
requires a lot of energy for proliferation, metastasis,
infiltration and other physiological functions (29). Even in the presence of ample oxygen,
cancer cells preferentially metabolize glucose by glycolysis, which
is a less efficient pathway for producing ATP when compared with
oxidative phosphorylation (30). In
highly proliferating tumors, blood supply may become insufficient
and cancer cells are exposed to hypoxia, which may upregulate
HIF-1, which in turn mediates overexpression of glycolytic enzymes
and the upregulation of glucose transporters (GLUT) (31,32). In
addition, HIF stimulates angiogenesis by upregulating several
factors, including the vascular endothelial growth factor, GLUT-1
and LDH (33–35). In tumor cells, glucose is metabolized
to lactate and the latter is exported from cells by MCT-4, which
results in an accumulation of lactate, lowering the pH in the tumor
microenvironment (36,37). Tumor cells primarily perform
glycolysis instead of oxidative phosphorylation and it is well
known that this metabolic alteration is important for tumor
development and progression and is a hallmark of cancer (38). HK and PK are two rate-limiting
enzymes in the glycolysis, which catalyze the initial step of
glycolysis and the dephosphorylation of phosphoenolpyruvate to
pyruvate, respectively (39). LDH is
a glycolytic enzyme, which catalyzes the reversible conversion of
lactate to pyruvic acid, therefore contributing to the acidic
microenvironment (40). SDH
participates in the TAC and is located on the inner mitochondrial
membrane (41). Functions of
mammalian SDH extent from mitochondrial energy generation to oxygen
sensing and tumor suppression (42).
In the current study, activities of three glycolytic
enzymes, HK, PK and LDH, and SDH, HIF-1α and MCT-4 expression, and
ATP levels were measured in SGC-7901 and A549 cells treated with
wogonin. Wogonin exhibited different effects in the two cell lines.
In SGC-7901 cells, wogonin significantly decreased the activities
of LDH and SDH, and the ATP level compared with an untreated
control. In cancer progression, increasing glucose consumption
leads to the accumulation of lactate, which lowers the pH in the
tumor microenvironment (43).
Wogonin may improve the acidity of the microenvironment by reducing
lactate generation and potentially affect glycolysis through the
inhibition of different glycolytic enzymes. In comparison, wogonin
inhibited the activity of LDH and the generation of ATP in A549
cells, and exhibited no significant effects on activity of the
other enzymes. In summary, wogonin may decrease lactic acid and ATP
generation in A549 cells, but may not affect the other elements to
the energy generation process.
The current study evaluated effects of wogonin on
various enzymes involved in glycolysis, but only one enzyme
participated in the process of aerobic oxidation. Further key
enzymes, including citrate synthase, isocitrate dehydrogenase and
α-oxoglutarate dehydrogenase, involved in TAC may be analyzed in
future experiments to evaluate the effects of wogonin on aerobic
oxidation.
In addition, it has been suggested that the
phosphatidylinositol-4, 5-bisphosphate 3-kinase (PI3K) signaling
pathway serves a critical role in cancer metabolism and progression
(44). The PI3K signaling pathway
regulates glucose uptake via protein kinase B by regulating GLUT-1
expression, enhancing glucose capture and stimulating
phosphofructokinase activity, this may cause a dependency of the
cells on high levels of glucose (45). Intracellular mammalian target of
rapamycin, which is an upstream mediator of HIF-1 activation, is
further activated via the PI3K signaling pathway (46). HIF-1 activation upregulates GLUTs and
HK expression in tumor cells (47).
The pathways through which wogonin affected the energy metabolisms
in the current study require further verification. Furthermore, in
order to demonstrate the antitumor activity of wogonin and to
evaluate the role wogonin serves in the energy metabolisms, tumor
animal models may be established for in vivo
experiments.
In summary, the findings of the present study
indicated that wogonin may affect the energy metabolism and the
acidic microenvironment in SGC-7901 cells by decreasing HIF-1α and
MCT-4 expressions. In A549 cells, wogonin exhibited no significant
effects on the energy metabolism, indicating that the strong
inhibitory effect of wogonin on cells proliferation may be induced
by other mechanisms, but not by the inhibition of the energy
metabolism. The growth of cells cannot continue without the supply
of energy. While inhibiting cell proliferation, wogonin interferes
with changes in certain proteins and key enzymes during cellular
energy metabolism. The current study hypothesizes that the
combination of wogonin and certain enzyme inhibitors in energy
metabolism may prevent the supply of energy to tumor cells and
therefore inhibit tumor cell proliferation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the scientific
research project of Harbin University of Commerce (grant no.
17XN073).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SJW and JNZ conceived and designed the experiments
of the current study. JNZ, JKZ, SR, WWS and WJZ performed the
experiments. JNZ, WWS, JKZ, SR and WJZ contributed
reagents/materials/analysis tools. JNZ wrote the manuscript and SW
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tai MC, Shui YT, Chang LY and Xue H:
Therapeutic potential of wogonin: A naturally occurring flavonoid.
CNS Drug Rev. 11:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang KF, Zhuang Y, Huang YQ and Diao Y:
Experimental study on inhibitory effect of wogonin on proliferation
and invasion of breast cancer cells. Zhongguo Zhong Yao Za Zhi.
39:1485–1489. 2014.(In Chinese). PubMed/NCBI
|
|
3
|
Li XD: Inhibitory effects of wogonin on
lung cancer A549 cell line in vitro. J Chongqing Med Univ.
7:790–793. 2011.(In Chinese).
|
|
4
|
Ding J, Polier G, Köhler R, Giaisi M,
Krammer PH and Li-Weber M: Wogonin and related natural flavones
overcome tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL) protein resistance of tumors by down-regulation of c-FLIP
protein and up-regulation of TRAIL receptor 2 expression. J Biol
Chem. 287:641–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong ZP, Wang LG, Wang HJ, Ye WF and Wang
XZ: Wogonin exacerbates the cytotoxic effect of oxaliplatin by
inducing nitrosative stress and autophagy in human gastric cancer
cells. Phytomedicine. 39:168–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu X, Wu XL, Li Y, Peng Y and Chen PF:
Inhibitory effect of wogonin on gastric cancer SGC 7901 cell line
in vitro. J Chin Pharm Sci. 46:512–517. 2011.
|
|
7
|
Bauman S, Fas SC, Giaisi M, Müller WW,
Merling A, Gülow K, Edler L, Krammer PH and Li-Weber M: Wogonin
preferentially kills malignant lymphocytes and suppresses T-cell
tumor growth by inducing PLCC1 and Ca2+ dependent
apoptosis. Blood. 11:2354–2363. 2008. View Article : Google Scholar
|
|
8
|
Lin CM, Chang H, Chen YH, Wu IH and Chiu
JH: Wogonin inhibits IL6 induced angiogenesis via downregulation of
VEGF and VEGFR-1, not VEGFR-2. Planta Med. 72:1305–1312. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu N, Gao Y, Ling Y, Chen Y, Yang Y, Gu
HY, Qi Q, Liu W, Wang XT, You QD and Guo QL: Wogonin suppresses
tumor growth in vivo and VEGF induced angiogenesis through
inhibiting tyrosine phosphorylation of VEGFR-2. Life Sci.
82:956–963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HW, Lin CP, Chiu JH, Chow KC, Kuo KT,
Lin CS and Wang LS: Reversal of inflammation as sociated
dihydrodiol dehydrogenases (AKR1C1 and AKR1C2) overexpression and
drug resistance in nonsmall cell lung cancer cells by wogonin and
chrysin. Int J Cancer. 120:2019–2027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chow KC, Lu MP and Wu MT: Expression of
dihydrodiol dehydrogenase plays important roles in apoptosis and
drug resistance of A431 squamous cell carcinoma. J Dermatol Sci.
41:205–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Penning TM, Jin Y, Steckelbroeck S,
Lanisnik Rizner T and Lewis M: Structure function of human 3
alpha-hydroxysteroid dehydrogenases: Genes and proteins. Mol Cell
Endocrinol. 215:63–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HW, Yang Y, Zhang K, Qiang L, Yang
L, Yang L, Hu Y, Wang XT, You QD and Guo QL: Wogonin induced
differentiation and G1 phase arrest of human U-937 leukemia cells
via PKC delta phosphorylation. Eur J Pharmacol. 591:7–12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho J and Lee HK: Wogonin inhibits
excitotoxic and oxidative neuronal damage in primary cultured rat
cortical cells. Eur J Pharmacol. 485:105–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma SC, Du J, But PP, Deng XL, Zhang YW,
Ooi VE, Xu HX, Lee SH and Lee SF: Antiviral Chinese medicinal herbs
against respiratory syncytial virus. J Ethnopharmacol. 79:205–211.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao WM, Bu P and Gong WJ: Advance in
studies on antitumor and immunomodulatory effects of wogonin.
Zhongguo Zhong Yao Za Zhi. 39:3004–3009. 2014.(In Chinese).
PubMed/NCBI
|
|
17
|
Cantor JR and Sabatini DM: Cancer cell
metabolism: One hallmark, many faces. Cancer Discov. 2:881–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong B and Zhu YM: Molecular-tareted
therapy for cancer. Chin J Cancer. 29:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Batool S, Joseph TP, Hussain M, Vuai MS,
Khinsar KH, Din SRU, Padhiar AA, Zhong M, Ning A, Zhang W, et al:
LP1 from Lentinula edodes C91-3 induces autophagy, apoptosis and
reduces metastasis in human gastric cancer cell line SGC-7901. Int
J Mol Sci. 19(pii): E29862018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park YJ, Lee SR, Kim DM, Yu JS,
Beemelmanns C, Chung KH and Kim KH: The inhibitory effects of
cyclodepsipeptides from the entomopathogenic fungus beauveria
bassiana on myofibroblast differentiation in A549 alveolar
epithelial cells. Molecules. 23(pii): E25682018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EH, Shim B, Kang S, Jeong G, Lee JS,
Yu YB and Chun M: Anti-inflammatory effects of Scutellaria
baicalensis extract via suppression of immune modulators and MAP
kinase signaling molecules. J Ethnopharmacol. 126:320–331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu
F, Zhang Y, Dong X and Sun B: HIF-1α promoted vasculogenic mimicry
formation in hepatocellular carcinoma through LOXL2 up-regulation
in hypoxic tumor microenvironment. J Exp Clin Cancer Res.
36:602017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shirota Y, Stoehlmacher J, Brabender J,
Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg
PV and Lenz HJ: ERCC1 and thymidy-late synthase mRNA levels predict
survival for colorectal cancer patients receiving combination
oxaliplatin and fluorouracil chemotherapy. J Clin Oncol.
19:4298–4304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced col-orectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartrons R and Caro J: Hypoxia, glucose
metabolism and the Warburg's effect. J Bioenerg Biomembr.
39:223–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang BY, Qu QX and Gu AK: Expression and
clinical significance of CD147, MCT4 in cervical lesions. J Int
Obstet Gynecol. 42:194–196. 2015.
|
|
32
|
Pietras A, Johnsson AS and Påhlman S: The
HIF-2α-driven pseudo-hypoxic phenotype in tumor aggressiveness,
differentiation, and vascularization. Curr Top Microbiol Immunol.
345:1–20. 2010.PubMed/NCBI
|
|
33
|
Wolf A, Agnihotri S, Micallef J, Mukherjee
J, Sabha N, Cairns R, Hawkins C and Guha A: Hexokinase 2 is a key
mediator of aerobic glycolysis and promotes tumor growth in human
glioblastoma multiforme. Cell. 208:313–326. 2011.
|
|
34
|
Chen J, Xie J, Jiang Z, Wang B, Wang Y and
Hu X: Shikonin and its analogs inhibit cancer cell glycolysis by
targeting tumor pyruvate kinase-M2. Oncogene. 30:4297–4306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trufelli DC, Moraes TV, Lima AA and Giglio
AD: Epidemiological profile and prognostic factors in patients with
lung cancer. Rev Assoc Med Bras (1992). 62:428–433. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancers Achilles heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukumura D, Xu L, Chen Y, Gohongi T, Seed
B and Jain RK: Hypoxia and acidosis independently upregulate
vascular endothelial growth factor transcription in brain tumors in
vivo. Cancer Res. 61:6020–6024. 2001.PubMed/NCBI
|
|
38
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chan AK, Bruce JI and Siriwardena AK:
Glucose metabolic phenotype of pancreatic cancer. World J
Gastroenterol. 22:3471–3485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mansouri S, Shahriari A, Kalantar H, Moini
Zanjani T and Haghi Karamallah M: Role of malate dehydrogenase in
facilitating lactate dehydrogenase to support the glycolysis
pathway in tumors. Biomed Rep. 6:463–467. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ghezzi D, Goffrini P, Uziel G, Horvath R,
Klopstock T, Lochmüller H, D'Adamo P, Gasparini P, Strom TM,
Prokisch H, et al: SDHAF1, encoding a LYR complex-II specific
assembly factor, is mutated in SDH-defective infantile
leukoencephalopathy. Nat Genet. 41:654–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pecsi I, Hards K, Ekanayaka N, Berney M,
Hartman T, Jacobs WR Jr and Cook GM: Essentiality of succinate
dehydrogenase in Mycobacterium smegmatis and its role in the
generation of the membrane potential under hypoxia. MBio. 5(pii):
e01093–14. 2014.PubMed/NCBI
|
|
43
|
Pucino V, Cucchi D and Mauro C: Lactate
transporters as therapeutic targets in cancer and inflammatory
diseases. Expert Opin Ther Targets. 22:735–743. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han K, Xu X, Chen G, Zeng Y, Zhu J, Du X,
Zhang Z, Cao B, Liu Z and Mao X: Identification of apromising PI3K
inhibitor for the treatment of multiple myeloma throughthe
structural optimization. J Hematol Oncol. 7:92014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wallace DC: Mitochondria and cancer:
Warburg addressed. Cold Spring Harb Symp Quant Biol. 70:363–374.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hudson CC, Liu M, Chiang GG, Otterness DM,
Loomis DC, Kaper F, Giaccia AJ and Abraham RT: Regulation of
hypoxiainducible factor 1alpha expression and function by the
mammalian target of rapamycin. Mol Cell Biol. 22:7004–7014. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|