Introduction
Vascular calcification (VC) is a common event in
patients with diabetes and/or chronic kidney disease (CKD)
(1,2). It is strongly associated with
cardiovascular morbidity and mortality (3,4).
Previous studies have revealed that hyperphosphatemia caused by CKD
triggers the transformation of vascular smooth muscle cells (VSMCs)
into chondrocytes or osteoblast-like cells, and induces medial
calcification deposits (5–7). It has also been demonstrated that
hyperglycemia induces VC (8,9). The expression of VSMC differentiation
marker genes, including smooth muscle 22α (SM22α) and smooth muscle
α-actin (SMα-actin), decrease in smooth muscle cell phenotypic
transition (10). Expression of the
phenotypic osteoblast gene, runt-related transcription factor 2
(Runx2), also known as core binding factor alpha-1, is increased in
VC (11,12). Phosphate transport into cells is
primarily mediated by sodium-phosphate (NaPi) cotransporters, of
which there are 3 types. Type III sodium-dependent phosphate
cotransporter-1 (Pit-1) is the predominant NaPi cotransporter in
human VSMCs (13). It has been
identified as a pivotal transporter in phosphate-induced VSMC
calcification. PiT-1 may promote vascular calcification via
modulation of anti-calcification proteins (such as matrix Gla
protein) or modification of kinases that phosphorylate secreted
matrix proteins (such as osteopontin) (14). Pit-1 has been a focus of previous VC
research, and its regulation serves a significant role in the
pathogenesis of VC (15,16). However, the exact mechanisms of VC
remain unclear.
Two experimental CKD rat models are typically used
to research VC: An adenine-induced CKD model and a partial
nephrectomy model. However, these are not the predominant causes of
CKD in patients (17,18). Research into the interactions between
hyperphosphatemia and hyperglycemia in VC and the underlying
mechanisms is limited. In the present study, the effects of
hyperphosphatemia and hyperglycemia on the phenotypic transition
and calcification of cultured human aortic smooth muscle cells
(HASMCs) were investigated, and the associated mechanisms were
examined.
Materials and methods
Cell culture and calcification
model
HASMCs were purchased from Procell Life Science and
Technology Co., Ltd. (Wuhan, China). Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) with 10% (v/v) fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and 1%
streptomycin/penicillin in 5% (v/v) CO2 at 37°C in a
humidified atmosphere. Cells at passages 4–6 were used for further
experimentation. When 80% confluence was reached, cells were
incubated in calcifying media containing 2.5 mM Pi (inorganic
phosphorus) and/or 30 mM glucose for up to 14 days to induce
calcification. Medium was replaced every 3 days.
Na2HPO4·12H2O,
NaH2PO4·2H2O and/or glucose were
added to the serum-supplemented DMEM to create various high
phosphate and glucose environments, according to our experimental
groups, with a pH between 7.2 and 7.4. There were four experimental
groups (n=9 per group): i) Control (CNT), normal Pi (0.9 mM) and
glucose (5.5 mM); ii) HPi, high Pi (2.5 mM) and normal glucose;
iii) HG, normal Pi and high glucose (30 mM); and iv) HGHPi: High
glucose (30 mM) and high Pi (2.5 mM).
Quantification of HASMC
calcification
HASMCs were grown in six-well plates and treated
with growth or calcifying medium. On days 2, 8 and 14, the culture
medium in several six-well plates was removed and washed with PBS,
and cells were subsequently treated with 0.6N HCl overnight at 4°C.
Calcium concentration in the supernatant was determined by the
o-cresolphthalein complexone method, using a Calcium Assay kit
(Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing,
China). A Bicinchoninic Acid (BCA) Protein Assay kit (Aspen
Biotechnology Co., Ltd., Wuhan, China) was used to evaluate protein
concentration, in order to normalize the calcium concentration.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HASMCs with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA USA), according to the manufacturer's
instructions. cDNA was generated using a PrimeScript™ RT reagent
kit with gDNA eraser (Takara Bio, Inc., Otsu, Japan). qPCR was
performed on a StepOne™ Real-Time PCR system (Thermo Fisher
Scientific, Inc.) with the SYBR® Premix Ex Taq™ reagent
kit (Takara Bio, Inc.). The PCR conditions were as follows: 95°C
initial denaturation for 1 min., 40 cycles with 95°C denaturation
for 15 sec, 58°C annealing for 20 sec, 72°C elongation for 45 sec.
The program for analytic melting was followed by an increase in
temperature from 60°C to 95°C with a 0.05°C/sec ramp rate. GAPDH
was used as the reference gene. PCR primers were purchased from
Genecreate Bioengineering Co., Ltd. (Wuhan, China) and the
sequences were as follows: Runx2, 5′-TACTCTGCCGAGCTACGAAATG-3′
(forward), 5′-TGAAACTCTTGCCTCGTCCG-3′ (reverse); SM22α,
5′-ATCCAAGCCAGTGAAGGTGC-3′ (forward), 5′-ACTCCCTCTTATGCTCCTGGG-3′
(reverse); SMα-actin, 5′-GTGACGAGGACGAGACCACC-3′ (forward),
5′-GGGTCAGGATACCTCGCTTG-3′ (reverse); GAPDH,
5′-CGCTAACATCAAATGGGGTG-3′ (forward), 5′-TTGCTGACAATCTTGAGGGAG-3′
(reverse). The specificity of the PCR products was confirmed by
melting curve analysis. Relative expression levels were determined
using the 2−ΔΔCq method (19).
Western blotting
HASMCs were lysed using radioimmunoprecipitation
assay buffer (Aspen Biotechnology Co., Ltd.) and protein
concentrations were measured with a BCA protein assay. Total
protein (40 µg/lane) was separated by 8 or 10% SDS-PAGE and
transferred to a polyvinylidene difluoride membrane. Following
blocking in 5% non-fat milk in Tris-buffered saline and Tween 20
for 1 h at room temperature, membranes were incubated with the
following primary antibodies overnight at 4°C: Rabbit anti-GAPDH
(dilution 1:10,000; cat. no. ab37168; Abcam, Cambridge, UK) and
rabbit anti-Pit-1 (dilution 1:2,000; cat. no. ab177147; Abcam).
Membranes were then incubated with horseradish peroxidase-labeled
secondary antibody for 30 min at room temperature (dilution
1:10,000; cat. no. AS1107; Aspen Biotechnology Co., Ltd.).
Immunoreactive proteins were detected using enhanced
chemiluminescent reagents (Aspen Biotechnology Co., Ltd.).
Quantitative densitometry analysis was performed using AlphaEaseFC
software Version 3.3.0 (ProteinSimple, San Jose, CA, USA).
Alizarin red staining
HASMCs in six-well plates were washed three times
with PBS and fixed with 10% (v/v) formaldehyde for 10 min at room
temperature. The slides of cells were subsequently washed with PBS
three times. Cells were exposed to 1% (w/v) Alizarin red for 30 min
at 37°C and then washed with 0.2% (v/v) acetic acid. Red indicated
positive staining of calcium nodules using a light microscope
(Olympus Corporation, Tokyo, Japan; magnification, ×200).
Statistical analysis
All numerical data are expressed as the mean ±
standard deviation. The mixed-effects model of repeated measures
(MMRM) was used to analyze every parameter. The model included
group, time point and group-by-time point interaction as fixed
factors. The restricted maximum likelihood (REML) was used. The
covariance structure to model the within-sample errors was
unstructured. The Kenward-Roger method was used to estimate the
denominator degrees of freedom. Type III tests for the
least-squares means were used for statistical comparisons.
Comparisons between each group at every time point were reported.
PROC MIXED in SAS Version 9.2 (SAS Institute, Cary, North Carolina,
USA) was used for MMRM. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of hyperphosphatemia and
hyperglycemia on calcification
To determine the effects of hyperphosphatemia and
hyperglycemia on the calcification of cultured HASMCs, Alizarin red
staining intensities (Fig. 1A) and
Ca concentrations (Fig. 1B) were
determined on days 2, 8 and 14. The results revealed that no
calcification occurred under normal conditions. Significant
calcification was present in hyperphosphatemia or hyperglycemia
medium on days 8 and 14, compared with the CNT (P<0.01), and
calcification in HG media was more severe than in HPi media
(P<0.01). When cultured in both hyperphosphatemia and
hyperglycemia media, the most severe calcification occurred as
early as day 2 compared with CNT, HPi and HG (P<0.01). Thus, it
was concluded that there were combined effects of hyperglycemia and
hyperphosphatemia on the calcification of HASMCs.
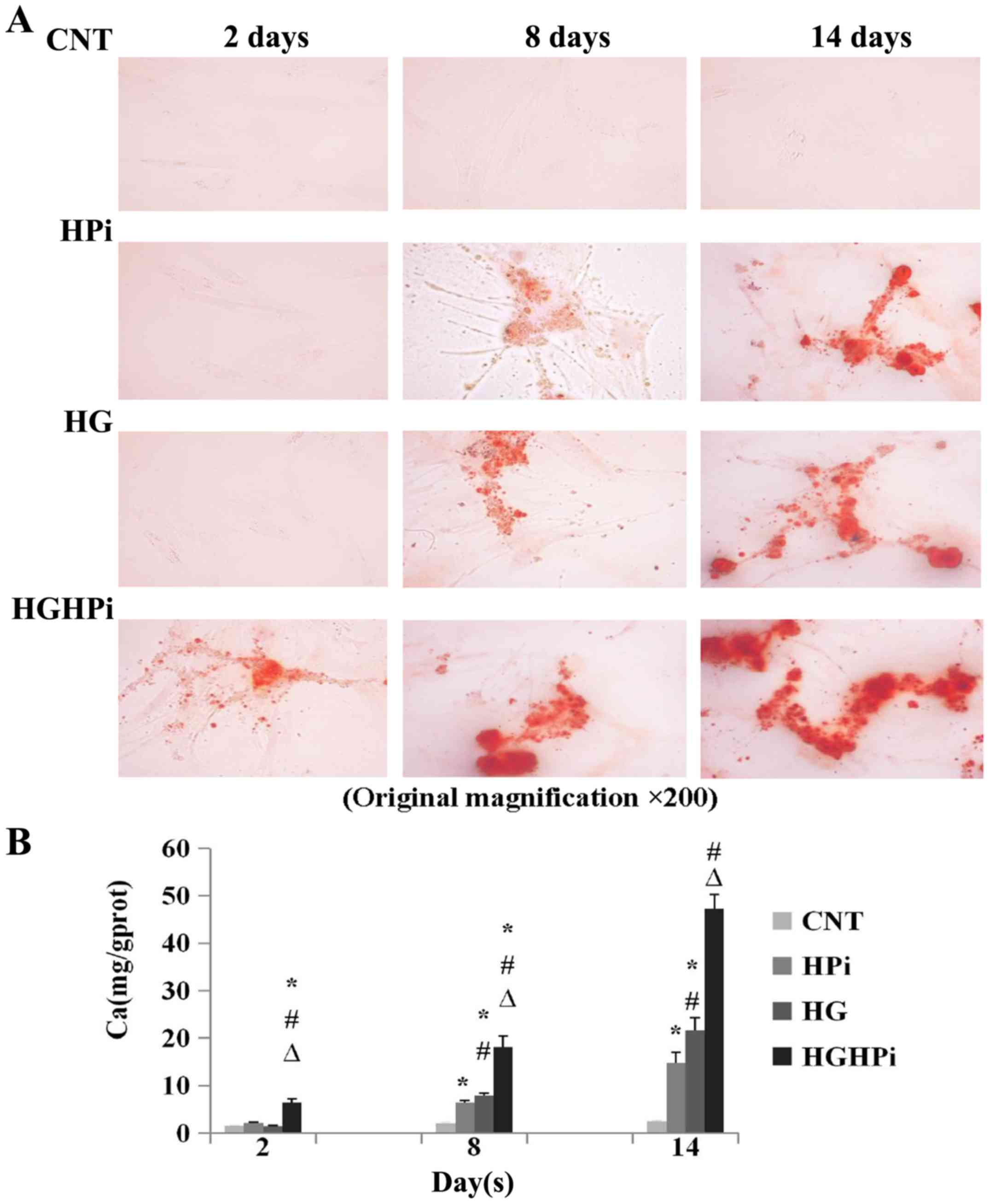 | Figure 1.(A) Images of Alizarin red staining
sections (original magnification, ×200). HASMCs were cultured in
Dulbecco's modified Eagle's medium supplemented with CNT (0.9 mM
Pi, 5.5 mM glucose), HPi (2.5 mM Pi) and/or HG (30 mM glucose) for
14 days to induce calcification. Sections exhibited no staining
when cultured in normal medium, and significant calcification was
observed in HPi or HG medium. The most severe calcification was
observed in the HGHPi group. (B) Quantification of HASMC
calcification induced by HPi and/or HG. Calcium content was
normalized to total cellular protein, as determined by a
bicinchoninic acid protein assay. The combined effects of
hyperphosphatemia and hyperglycemia on HASMC calcification were
observed. Values are expressed as the mean ± standard deviation
(n=9). *P<0.01 vs. CNT; #P<0.01 vs. HPi;
∆P<0.01 vs. HG. HASMC, human aortic smooth muscle
cell; Pi, inorganic phosphorus; CNT, control; HPi, high Pi; HG,
high glucose; HGHPi, high Pi and glucose. |
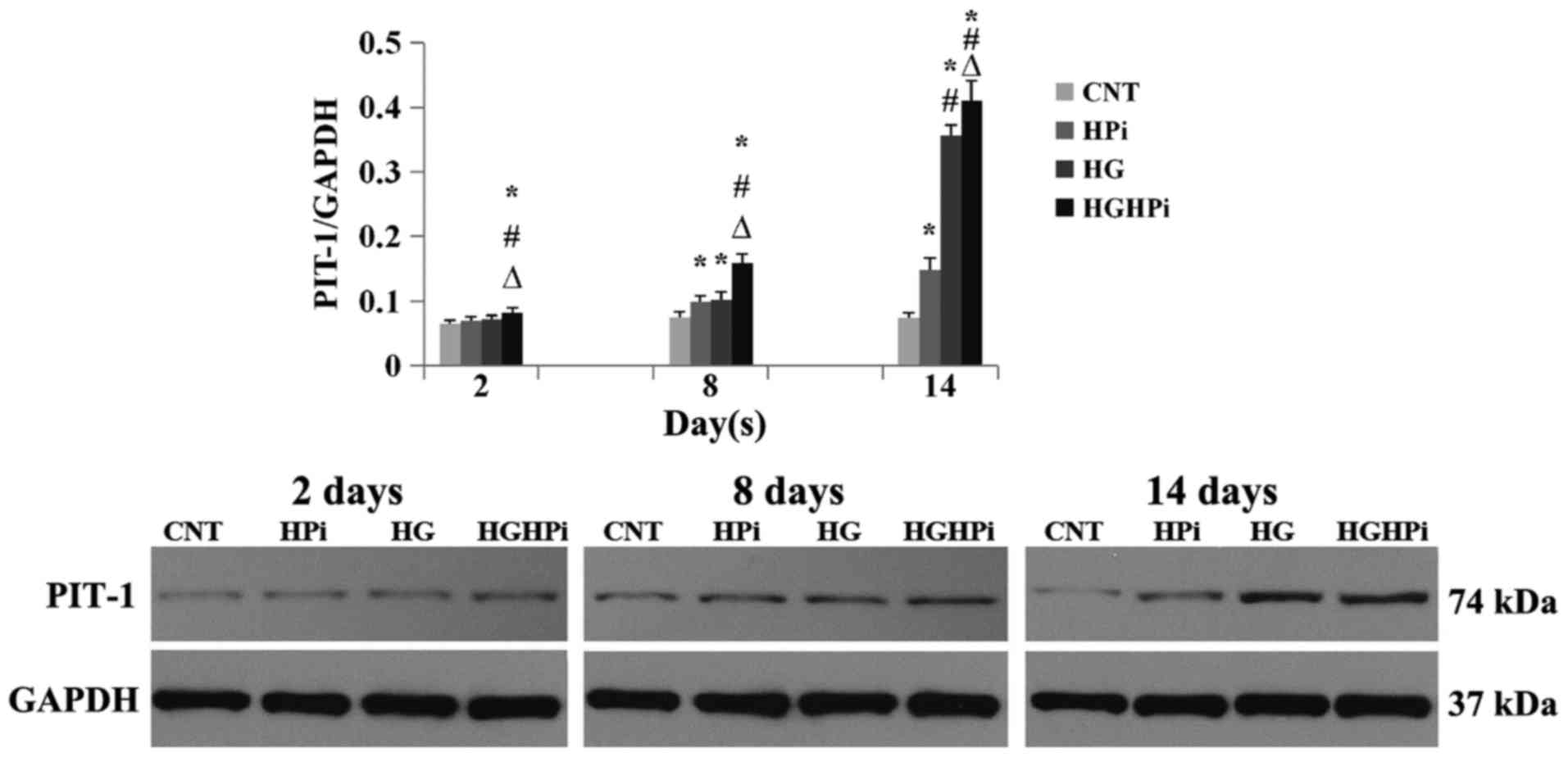
Effects of hyperphosphatemia and
hyperglycemia on the expression of Pit-1 proteins
Pit-1 protein expression on day 2, 8 and 14 was
detected by western blot analysis (Fig.
2). The results demonstrated that HPi and HG elevated the
expression of Pit-1 on days 8 and 14, compared with the CNT
(P<0.01). The combined effects of hyperphosphatemia and
hyperglycemia on Pit-1 expression were also observed: HASMCs
cultured in hyperphosphatemia and hyperglycemia medium expressed
the most Pit-1 protein from day 2, compared with that in the CNT,
HPi and HG groups (P<0.01).
Effects of hyperphosphatemia and
hyperglycemia on phenotypic transition
The relative expression of Runx2, SM22α and
SMα-actin mRNA in cultured HASMCs was evaluated by RT-qPCR analysis
(Fig. 3). Upregulation of Runx2, as
well as downregulation of SM22α and SMα-actin was observed in the
HPi, HG and HGHPi groups, compared with the CNT. The differences in
the expression of SM22α and Runx2 mRNAs on day 14 were significant
between the HGHPi and HPi or HG groups (P<0.01). The differences
in the expression of SMα-actin mRNA on day 14 between the HGHPi and
HG groups were not significant (P=0.106), but both groups exhibited
significantly decreased expression compared with the HPi group
(P<0.01). Therefore, it was concluded that there were combined
effects of hyperglycemia and hyperphosphatemia on the phenotypic
transition of HASMCs from vascular smooth muscle cells to
osteoblast-like cells.
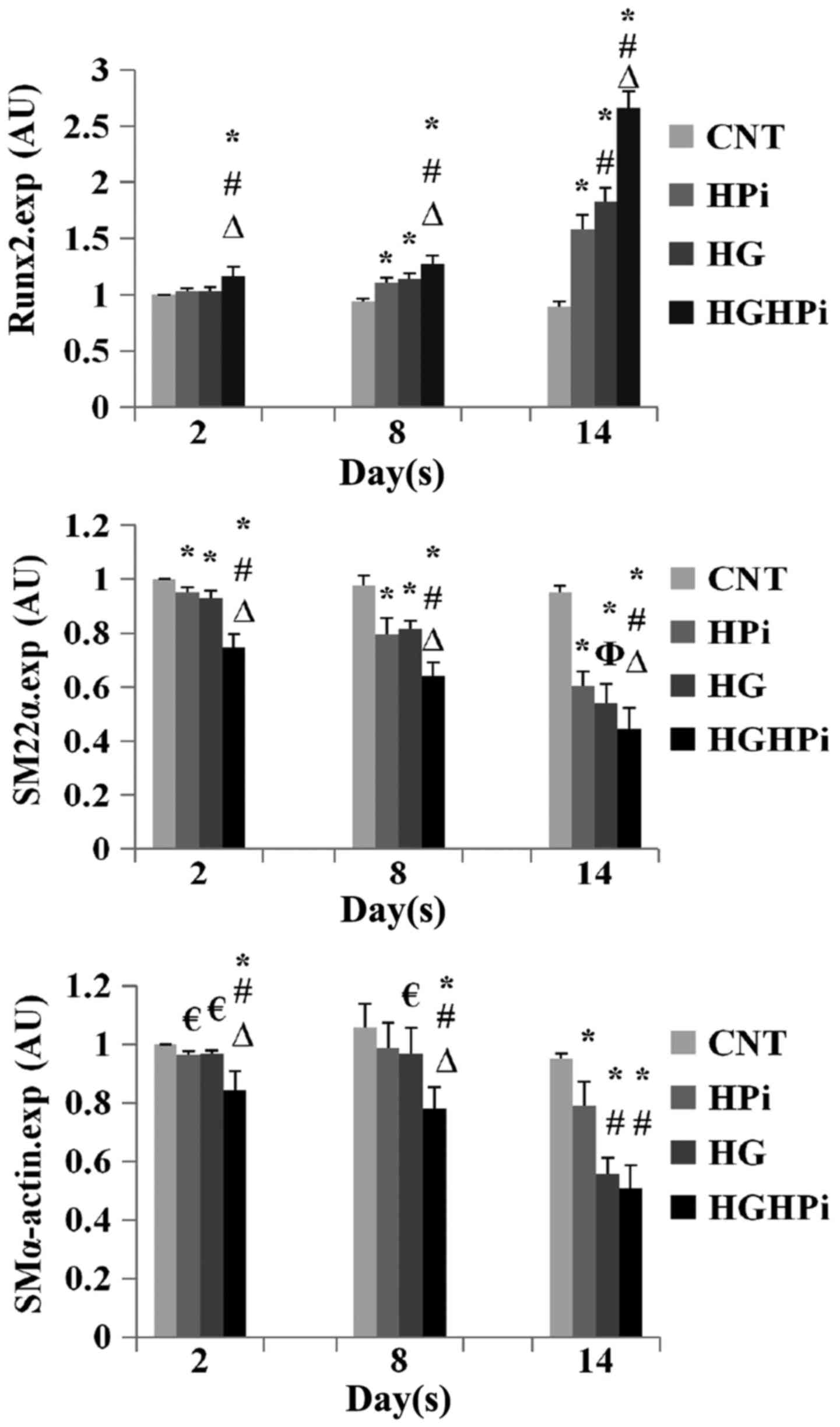 | Figure 3.Relative expression of Runx2, SM22α
and SMα-actin mRNA of HASMCs cultured in normal, HPi and/or HG
medium. Upregulation of Runx2 and downregulation of SM22α and
SMα-actin was observed in the HPi, HG and HGHPi groups. The
combined effects of HPi and HG on HASMC phenotypic transition were
also observed. Values are expressed as the mean ± standard
deviation (n=9). €P<0.05 vs. CNT; *P<0.01 vs. CNT;
ΦP<0.05 vs. HPi; #P<0.01 vs. HPi;
∆P<0.01 vs. HG. AU, arbitrary units; HASMC, human
aortic smooth muscle cell; Pi, inorganic phosphorus; CNT, control;
HPi, high Pi; HG, high glucose; HGHPi, high Pi and glucose; Runx2,
runt-related transcription factor 2; SM22α, smooth muscle 22α;
SMα-actin, smooth muscle α-actin. |
Discussion
Clinically, hyperphosphatemia and hyperglycemia are
common comorbidities of patients with CKD. The results of the
present study indicated that both hyperphosphatemia and
hyperglycemia induced HASMC calcification and the phenotypic
transition from vascular smooth muscle cells to osteoblast-like
cells. These results are consistent with previous reports (10,20). It
is unclear what occurs when hyperphosphatemia and hyperglycemia are
present at the same time; however, these are likely to be the
conditions that occur in patients with diabetic kidney disease
(DKD) (17). It is well established
that CKD patients with hyperglycemia have a shorter survival time
and/or higher mortality rate (21,22).
Yoshida et al (23) incubated
rat and human aortic SMCs with various concentrations of phosphate
and glucose, and demonstrated that calcium accumulation is
increased by high phosphate concentration in a dose-dependent
manner, but not by high glucose concentration. However, this was
inconsistent with the findings of the present study and others
(9,24). The exact reasons for these
differences are unclear; they may be due to the complicated
mechanisms of VC, or perhaps there were some overlooked details in
these previous experiments, which resulted in insufficient
induction of VC by hyperglycemia. Rat aortic vascular rings were
cultured in hyperphosphatemia and hyperglycemia media in our
previous work, and these results also supported the findings of the
present study (unpublished data). Hence, it is reasonable to
propose that there are combined effects of hyperglycemia and
hyperphosphatemia on VC, which may contribute to the mortality of
CKD patients with hyperglycemia. The current results provide
experimental evidence for this hypothesis and suggest that it would
be beneficial to advocate stricter control on the serum phosphorus
and glucose levels of patients with DKD. In addition, conditions of
hyperphosphatemia combined with hyperglycemia should be considered
an appropriate experimental model to study VC in DKD.
There are three types of NaPi cotransporter in
humans, and Pit-1 is the predominant type in VSMCs (15,25). In
recent decades, an increasing number of studies have demonstrated
that Pit-1 regulation serves a significant role in the pathogenesis
of VC (16,26,27). The
present study suggested that Pit-1 expression was upregulated not
only by hyperphosphatemia but also by hyperglycemia, and the level
of expression appeared to be associated with Ca deposition in
calcified HASMCs. Hence, Pit-1 may be a promising index for VC.
However, the present study had certain limitations.
The model did not represent the structure and/or matrix of a
vessel, and HASMCs were cultured in vitro. Thus, the results
may differ in vivo. More detailed research, in both animals
and clinical trials, will be required in order to verify the
underlying mechanisms of and effective control strategies for
VC.
In conclusion, it was observed that there were
combined effects of hyperphosphatemia and hyperglycemia on HASMC
calcification. Hyperphosphatemia medium combined with hyperglycemia
medium should be considered an appropriate experimental model to
study VC in DKD. Pit-1 may be a promising index for VC. These
findings may aid in making clinical decisions for patients with
CKD.
Acknowledgements
The authors would like to thank Professor Xicheng
Hong for assistance in examining the data.
Funding
Not applicable.
Availability of data and materials
All data generated and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors conceived and designed the research. PW
and PZ performed the experiments and drafted the manuscript. DP
analyzed the data and revised the manuscript. WC reviewed the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Górriz JL, Molina P, Cerverón MJ, Vila R,
Bover J, Nieto J, Barril G, Martínez-Castelao A, Fernández E,
Escudero V, et al: Vascular calcification in patients with
nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 10:654–666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Emerging Risk Factors Collaboration, ;
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio
E, Ingelsson E, Lawlor DA, Selvin E, et al: Diabetes mellitus,
fasting blood glucose concentration, and risk of vascular disease:
A collaborative meta-analysis of 102 prospective studies. Lancet.
375:2215–2222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
London GM, Guérin AP, Marchais SJ,
Métivier F, Pannier B and Adda H: Arterial media calcification in
end-stage renal disease: Impact on all-cause and cardiovascular
mortality. Nephrol Dial Transplant. 18:1731–1740. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizobuchi M, Tower D and Slatopolsky E:
Vascular calcification: The killer of patients with chronic kidney
disease. J Am Soc Nephrol. 20:1453–1464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hruska K, Mathew S, Lund R, Qiu P and
Pratt R: Hyperphosphatemia of chronic kidney disease. Kidney Int.
74:148–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Demer L and Tintut Y: Vascular
calcification: Pathobiology of a multifaceted disease. Circulation.
117:2938–2948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giachelli CM: Vascular calcification
mechanisms. J Am Soc Nephrol. 15:2959–2964. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhan JK, Tan P, Wang YJ, Wang Y, He JY,
Tang ZY, Huang W and Liu YS: Exenatide can inhibit calcification of
human VSMCs through the NF-kappaB/RANKL signaling pathway.
Cardiovasc Diabetol. 13:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu F, Zhong H, Liang JY, Fu P, Luo ZJ,
Zhou L, Gou R and Huang J: Effect of high glucose levels on the
calcification of vascular smooth muscle cells by inducing
osteoblastic differentiation and intracellular calcium deposition
via BMP-2/Cbfα-1 pathway. J Zhejiang Univ Sci B. 11:905–911. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steitz SA, Speer MY, Curinga G, Yang HY,
Haynes P, Aebersold R, Schinke T, Karsenty G and Giachelli CM:
Smooth muscle cell phenotypic transition associated with
calcification: Upregulation of Cbfα1 and downregulation of smooth
muscle lineage markers. Circ Res. 89:1147–1154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Speer MY, Li X, Hiremath PG and Giachelli
CM: Runx2/Cbfα1, but not loss of myocardin, is required for smooth
muscle cell lineage reprogramming toward osteochondrogenesis. J
Cell Biochem. 110:935–947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Zheng B, Zhou PP, Zhang RN, He M,
Yang Z and Wen JK: Vascular calcification is coupled with
phenotypic conversion of vascular smooth muscle cells through
Klf5-mediated transactivation of the Runx2 promoter. Biosci Rep.
34:e001482014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Yang H and Giachelli CM: Role of the
sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth
muscle cell calcification. Circ Res. 98:905–912. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Villa-Bellosta R, Levi M and Sorribas V:
Vascular smooth muscle cell calcification and SLC20 inorganic
phosphate transporters. Effects of PDGF, TNF-alpha, and Pi Pflugers
Arch. 458:1151–1161. 2009. View Article : Google Scholar
|
|
15
|
Lau WL, Festing MH and Giachelli CM:
Phosphate and vascular calcification: Emerging role of the
sodium-dependent phosphate co-transporter Pit-1. Thromb Haemost.
104:464–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Curinga G and Giachelli CM:
Elevated extracellular calcium levels induce smooth muscle cell
matrix mineralization in vitro. Kidney Int. 66:2293–2299. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for
the evaluation and management of chronic kidney disease. Kidney
Int. (Suppl 3):S1–S150. 2013.
|
|
18
|
Yokozawa T, Zheng PD, Oura H and Koizumi
F: Animal model of adenine-induced chronic renal failure in rats.
Nephron. 44:230–234. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida T, Yamashita M and Hayashi M:
Kruppel-like factor 4 contributes to high phosphate-induced
phenotypic switching of vascular smooth muscle cells into
osteogenic cells. J Biol Chem. 287:25706–25714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jardine MJ, Hata J, Woodward M, Perkovic
V, Ninomiya T, Arima H, Zoungas S, Cass A, Patel A, Marre M, et al:
Prediction of kidney-related outcomes in patients with type 2
diabetes. Am J Kidney Dis. 60:770–778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan EYF, Fong DYT, Fung CSC, Yu EYT, Chin
WY, Chan AKC and Lam CLK: Prediction of new onset of end stage
renal disease in Chinese patients with type 2 diabetes mellitus-a
population-based retrospective cohort study. BMC Nephrol.
18:2572017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida T, Yamashita M, Horimai C and
Hayashi M: High glucose concentration does not modulate the
formation of arterial medial calcification in experimental uremic
rats. J Vasc Res. 50:512–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen NX, Duan D, O'Neill KD and Moe SM:
High glucose increases the expression of Cbfa1 and BMP-2 and
enhances the calcification of vascular smooth muscle cells. Nephrol
Dial Transplant. 21:3435–3442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyamoto K, Haito-sugino S, Kuwahara S,
Ohi A, Nomura K, Ito M, Kuwahata M, Kido S, Tatsumi S, Kaneko I and
Segawa H: Sodium-dependent phosphate cotransporters: Lessons from
gene knockout and mutation studies. J Pharm Sci. 100:3719–3730.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki A, Ghayor C, Guicheux J, Magne D,
Quillard S, Kakita A, Ono Y, Miura Y, Oiso Y, Itoh M and Caverzasio
J: Enhanced expression of the inorganic phosphate transporter Pit-1
is involved in BMP-2-induced matrix mineralization in
osteoblast-like cells. J Bone Miner Res. 21:674–683. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voelkl J, Alesutan I, Leibrock CB,
Quintanilla-Martinez L, Kuhn V, Feger M, Mia S, Ahmed MS,
Rosenblatt KP, Kuro-O M and Lang F: Spironolactone ameliorates
PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice.
J Clin Invest. 123:812–822. 2013.PubMed/NCBI
|