Introduction
Renal cell carcinoma (RCC), which has the highest
mortality rate among all types of genitourinary cancers, represents
a serious problem to public health (1). Over 300,000 patients are diagnosed with
RCC each year worldwide and the incidence rate of RCC still
exhibits an increasing trend (2).
Although various drugs, including axitinib, sorafenib, nivolumab
and everolimus have been developed to treat patients with RCC
(3,4), treatment of advanced RCC has typically
failed to provide satisfactory outcomes due to a series of
problems, such as drug tolerance and individual difference in
response to drugs (5). It has
previously been demonstrated that, although recurrence will occur
in a minority group of patients, RCC is generally curable in early
stages (1). Therefore, early
diagnosis and treatment is the key to the treatment of RCC.
Nephritis occurs in tubules, glomeruli and interstitial tissue
surrounding the tubules and glomeruli. It has been demonstrated
that the onset and development of RCC may be associated with
certain risk factors of kidney inflammation, including oxidative
stress (6). Therefore, proper
treatment of nephritis will inhibit the progression of RCC, and
therefore potentially reduce the mortality rate.
Phosphatase and Tensin Homolog
(PTEN)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)
pathway serves essential roles in the regulation of the balance
between survival and apoptosis as well as cell migration (7,8). The
pathway also has inhibitory effects on a number of human diseases
including different types of cancer, such as prostate cancer
(9), breast cancer (10), hepatocellular carcinoma (11) and RCC (12). PTEN-Long, which is a translational
variant of PTEN produced by in-frame alternative translation, has
been demonstrated to have similar roles to PTEN in inhibiting the
growth of tumors by inhibiting PI3K signaling through a
phosphatase-dependent manner (13).
Compared with PTEN, PTEN-Long protein contains an additional
N-terminal region, which gives it the ability to cross the cell
membrane (14), and due to its
functions, PTEN-Long has been selected as a therapeutic target for
the treatment of pancreatic cancer (15,16). A
previous study has demonstrated that expression levels of PTEN-Long
were increased in patients with clear cell RCC (ccRCC), and that
PTEN-Long overexpression can inhibit cell proliferation, migration
and invasion, which in turn leads to the inhibition of tumor growth
(14), indicating the inhibitory
effects of PTEN-Long on ccRCC. Although functions of PTEN-Long have
been studied previously in various cancers including RCC, its
specific roles in the progression of nephritis have not yet been
investigated, to the best of our knowledge. Due to the pathogenesis
shared by RCC and nephritis, it may be hypothesized that PTEN-Long
also has important functions in the progression of nephritis.
CRISPR/Cas9 is a novel gene-editing tool derived
from prokaryotic adaptive immune system against phage/virus and
foreign DNA invasion. It is widely used in mammalian genomic
editing, including repressing gene expression (17), gene knock-in (18), repairing disease-associated genes
(19) and development of animal
models of cancer (20). In the
present study, effects of PTEN-Long on nephritis have been
investigated in patients and animal models. In addition, effects of
PTEN-Long on inflammatory factors were also evaluated using
PTEN-Long knock-in and knock-out mice established by the
CRISPR/Cas9 technique.
Materials and methods
Specimen collection
Blood samples from patients with nephritis, RCC and
normal healthy controls (n=60 each) treated at the Institute of
Urology of The Affiliated Yantai Yuhuangding Hospital of Qingdao
University (Yantai, China) between May 2014 to December 2016 were
collected. No patient received chemoradiotherapy or other antitumor
therapy prior to surgery. The median age is 58 years (range, 45–77
years) and the ratio of male to female was 0.68 (73/107). The
present study was approved by the Ethics Committee of The
Affiliated Yantai Yuhuangding Hospital of Qingdao University, and
all participants provided written informed consent. All surgery was
performed according to the Declaration of Helsinki.
Preparation of mouse nephritis
model
All mice used in the present study were 8 weeks old
with a body weight of 20–25 g and were housed in a pathogen-free
environment with 12-h light/dark cycles at room temperature
(20±2°C) with a relative humidity of 50–70%. Animals had free
access to food and water. PTEN-Long knock-in and knock-out mice
were constructed using CRISPR-Cas9 technique by Cyagen Biosciences,
Inc. (Santa Clara, CA, USA). The efficiency of knock-in and
knock-out was detected by western blotting. Female transgenic
BALB/c mice (Cyagen Biosciences), including 10 knock-in mice and 10
knock-out mice, were used in the present study. A total of 20
transgenic mice (10 knock-in mice and 10 knock-out mice) were
treated with intraperitoneal injection of lipopolysaccharide (30
mg/kg, 50 µl; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
renal homogenate (50 µl; renal tissue:saline, 1:6 g/ml; Cyagen
Biosciences) and wild type BALB/c mice (n=10) in the control group
were administered 100 µl saline. The present study was approved by
the Ethics Committee of The Affiliated Yantai Yuhuangding Hospital
of Qingdao University.
Determination of serum levels of
inflammatory factors
Following intraperitoneal injection, blood samples
(0.2 ml) were extracted from the fundus vein at 12, 24 and 48 h
later. Blood samples were injected into EDTA anticoagulant tubes
and maintained at 4°C for 30 min, followed by centrifugation at 800
× g for 10 min at 4°C to collect supernatant. A Bio-Plex system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to compare
the secretion of tumor necrosis factor (TNF)-α (cat. no. ab6671),
interleukin (IL)-6 (cat. no. 100712), IL-1β (cat. no. ab100704) and
IL-18 (cat. no. ab216165; all Abcam, Cambridge, UK) in serum of
different groups. All kits used to detect inflammatory markers were
purchased from Abcam and the manufacturer's protocols were
followed.
Detection of inflammatory factors'
gene expression in renal tissue by reverse transcription
semi-quantitative polymerase chain reaction (PCR)
Mice were sacrificed at 48 h following
intraperitoneal injection. Left kidneys were harvested and cut,
added into normal saline prior to preparing a renal tissue
homogenate using an electric homogenizer. Total RNA was extracted
from tissue homogenate using TRIzol (Life Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Purity and integrity of total RNA were
detected and cDNA was synthesized via reverse transcription using
PrimeScript™ RT reagent kit (cat. no. RR037Q; Takara
Biotechnology Co., Ltd., Dalian, China). SYBR Green Master Mix kit
(Thermo Fisher Scientific, Inc.) and 1 µl cDNA were used to prepare
the PCR reaction system. All primers used for PCR reaction are
listed in Table I. PCR reaction
conditions were as follows: 95°C for 5 min, and 40 cycles of 95°C
for 20 sec, 60°C for 30 sec and 72°C for 30 sec. Subsequently, PCR
product was subjected to 1.2% agarose gel horizontal
electrophoresis. Ethidium bromide was visualized using the Gel Doc
XR gel imaging system (Bio-Rad Laboratories, Inc.) that allowed
images to be captures, and ImageJ software 1.48u (National
Institutes of Health, Bethesda, MD, USA) was used to analyze the
results. The relative expression level of TNF-α, IL-6, IL-1β and
IL-18 mRNA were quantified according to the endogenous control
(β-actin) using the aforementioned software.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward | Reverse |
|---|
| TNF-α |
5′-TACTCCCAGGTTCTCTTCAAGG-3′ |
5′-GGAGGCTGACTTTCTCCTGGTA-3′ |
| IL-6 |
5′-GAGTTGTGCAATGGCAATTC-3′ |
5′-ACTCCAGAAGACCAGAGCAG-3′ |
| IL-1β |
5′-CACCTCTCAAGCAGAGCACAG-3′ |
5′-GGGTTCCATGGTGAAGTCAAC-3′ |
| IL-18 |
5′-AAACCCGCCTGTGTTCGA-3′ |
5′-TCAGTCTGGTCTGGGATTCGT-3′ |
| β-actin |
5′-TTGTTACCAACTGGGACG-3′ |
5′-GGCATAGAGGTCTTTACGG-3′ |
Observation of pathological changes by
hematoxylin and eosin staining
At room temperature, mouse renal tissue was fixed in
4% paraformaldehyde for 12–24 h, followed by paraffin-embedding for
1 h. The embedded tissue was then cut into 4-µm sections. Dewaxing
was performed twice with xylene for 15 min at 24°C. Following
hydration by passing a series of graded ethanol concentrations (95%
ethanol for 5 min, 90% ethanol for 5 min, 70% ethanol for 2 min and
distilled water for 5 min), hematoxylin staining was performed for
10 min, followed by washing with water for 10 min at room
temperature. Following treatment with 95% ethanol for 5 sec, eosin
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) staining was performed for 2 min at 24°C. Following
staining, dehydration was performed by passing a series of graded
ethanol concentrations (70% ethanol for 2 min, 80% ethanol for 2
min, 90% ethanol for 5 min and twice with 100% ethanol for 5 min).
Then, tissue sections were then treated twice with xylene, 10 min
each. Neutral gum was used to seal the sections and optical
microscopy (magnification, ×200; Olympus Corporation, Tokyo, Japan)
was used to observe the signals.
Western blotting
Total protein was extracted from human blood samples
and mice renal tissue by TPE™ kit (Beijing Solarbio
Science & Technology Co., Ltd.) and quantified using the
bicinchoninic acid protein assay method. Protein samples were
boiled for 3 min and then loaded into 10–20% Ready Gels (40
µg/lane; Bio-Rad Laboratories, Inc.) for electrophoresis, followed
by transferring to a polyvinylidene difluoride membrane. The
membrane was blocked with 5% skimmed milk for 2 h at room
temperature. Following washing, membrane was incubated with
PTEN-Long primary antibody (1:500; cat. no. ABM-2052; Cascade
Bioscience, Inc., Winchester, MA, USA), β-actin polyclonal antibody
(1:500; cat. no. orb129534; Biorbyt Ltd., Cambridge, UK) overnight
at 4°C. Following washing with TBS-Tween-20 (3X), the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (1:1,000; cat. no. ab150084;
Abcam) at room temperature for 2 h. Enhanced chemiluminescence
(cat. no. 34580; Thermo Fisher Scientific, Inc.) was used to detect
the signals. ImageJ software 1.48u was used to analyze the data and
the relative expression level of PTEN-Long was quantified relative
to the endogenous control, β-actin.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
statistical software (IBM, Corp., Armonk, NY, USA). Data are
presented as mean + standard deviation. All data were analyzed
using one-way analysis of variance with Bonferroni's correction for
multiple group comparisons and Student's t-test for the comparisons
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Serum levels of PTEN-Long protein in
serum of patients with nephritis, RCC and normal controls
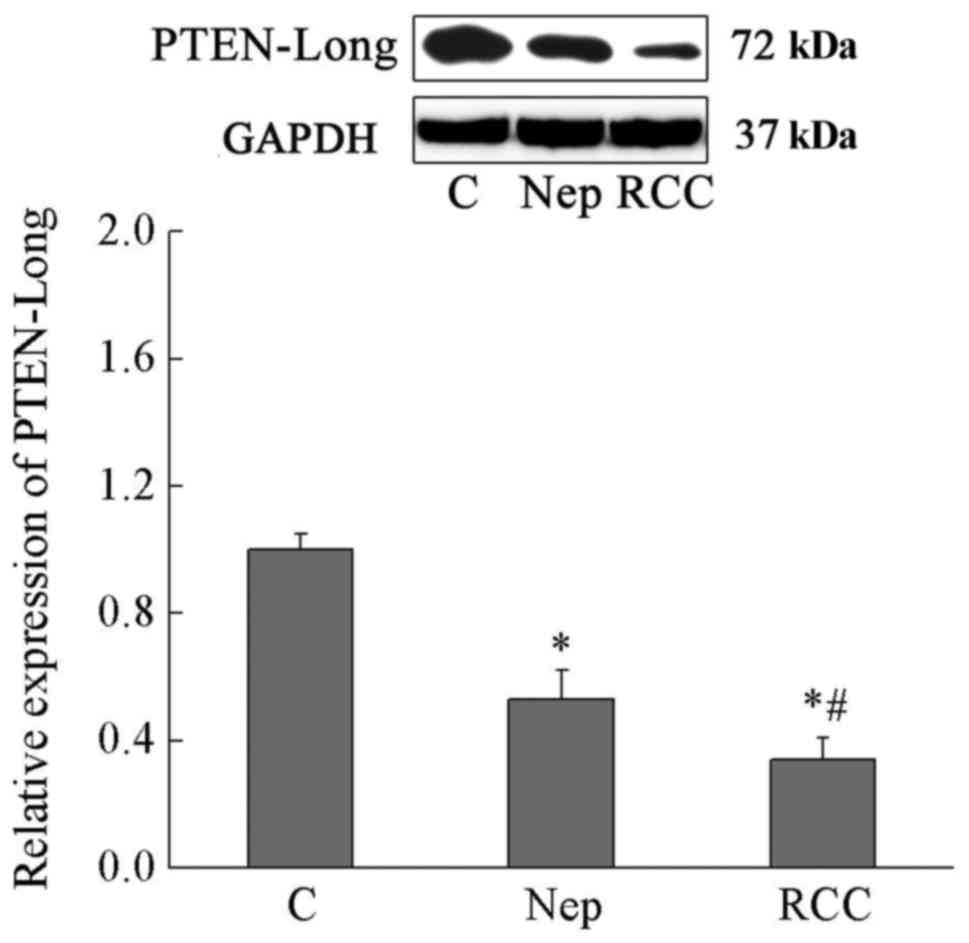
As presented in Fig.
1, Serum levels of PTEN-Long in patients with nephritis and RCC
carcinoma were significantly reduced by 47 and 66% compared with
normal controls (P<0.01). In addition, serum levels of PTEN-Long
in patients with nephritis was significantly higher than that of
patients with RCC (P<0.01). These data suggest that serum level
of PTEN-Long was negatively associated with the severity of renal
disease (Fig. 1).
Expression of PTEN-Long protein in
serum and rental tissue of different groups
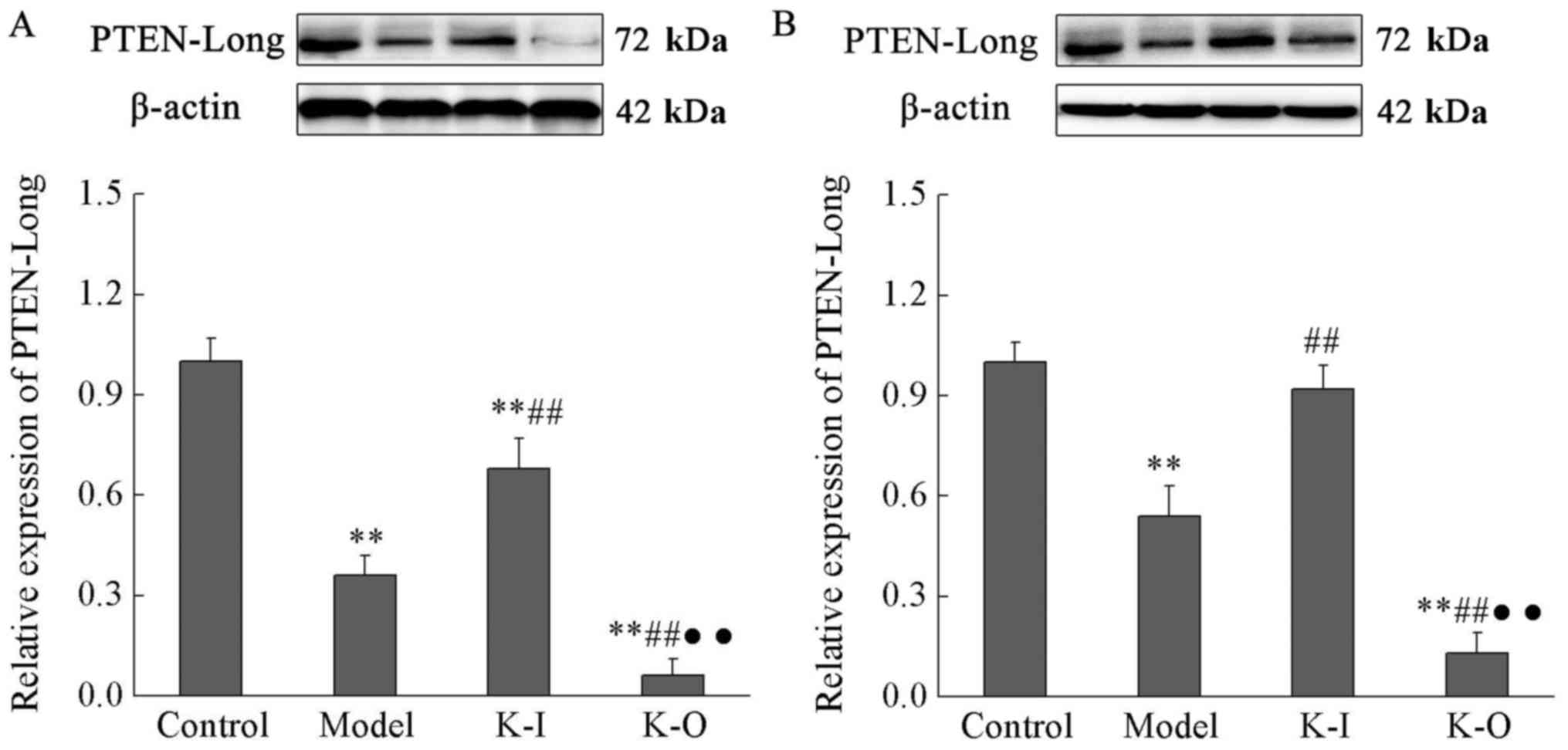
As presented in Fig.
2, expression levels of PTEN-Long protein were significantly
lower in serum than in rental tissue. Expression levels of
PTEN-Long protein in serum and renal tissue of the model group were
significantly reduced by 64 and 36%, respectively, compared with
the control group (P<0.01). Compared with the model group,
expression levels of PTEN-Long protein were significantly increased
in serum and rental tissue of knock-in group, but remained lower
than those of control group (68 and 92% of the control group,
respectively; P<0.01). Compared with the model group, expression
levels of PTEN-Long protein were significantly reduced in knock-out
group (P<0.01). The data suggested that low expression of
PTEN-Long protein may promote the progression of nephritis, whereas
PTEN-Long protein overexpression may inhibit it (Fig. 2).
Effects of PTEN-Long knock-in and
knock-out on serum levels of inflammatory factors
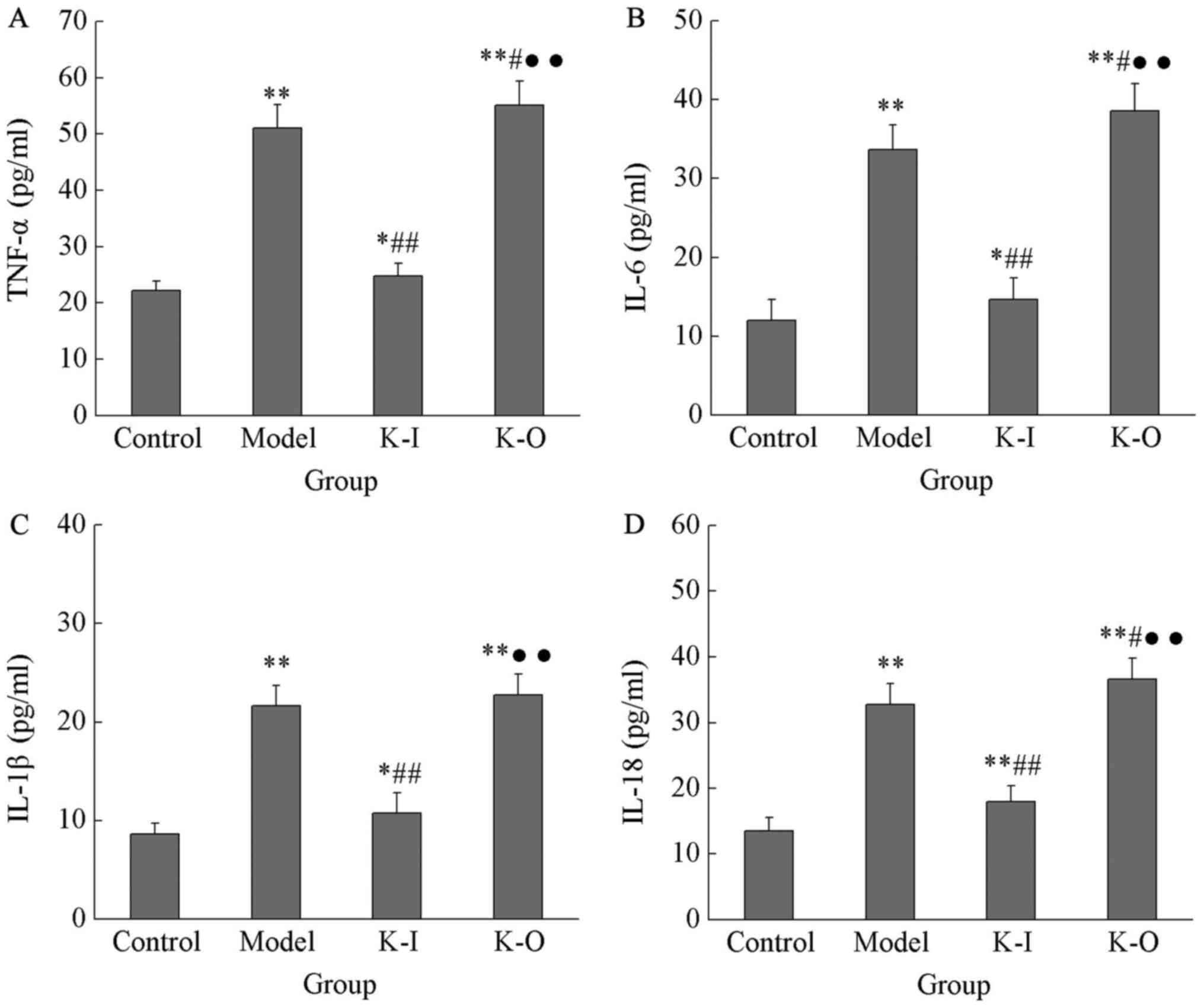
In order to investigate the effects of PTEN-Long on
serum levels of inflammatory factors, PTEN-Long knock-in and
knock-out mice were constructed, and serum levels of several
selected inflammatory factors were determined. As presented in
Fig. 3, serum levels of TNF-α, IL-6,
IL-1β and IL-18 in the model group were 51.07±4.18, 33.64±3.12,
21.63±2.0 and 32.75±3.19 pg/ml, respectively, which were
significantly higher than those in the control group (22.15±1.69,
11.98±2.64, 8.65±1.09 and 13.46±2.03 pg/ml, respectively;
P<0.05). Compared with the model group, PTEN-Long knock-out
further significantly increased serum levels of TNF-α, IL-6, IL-1β
and IL-18 to 55.17±4.23, 38.59±3.46, 22.76±2.09 and 36.62±3.21
pg/ml, respectively (P<0.05). Compared with PTEN-Long knock-out,
PTEN-Long knock-in significantly decreased serum levels of TNF-α,
IL-6, IL-1β and IL-18 to 24.72±2.36, 14.61±2.79, 10.76±2.05 and
17.93±2.46, respectively (P<0.01). These results suggested that,
PTEN-Long can inhibit inflammation caused by nephritis through its
interactions with inflammatory factors (Fig. 3).
Expression of inflammatory factors in
renal tissue of mice with different backgrounds
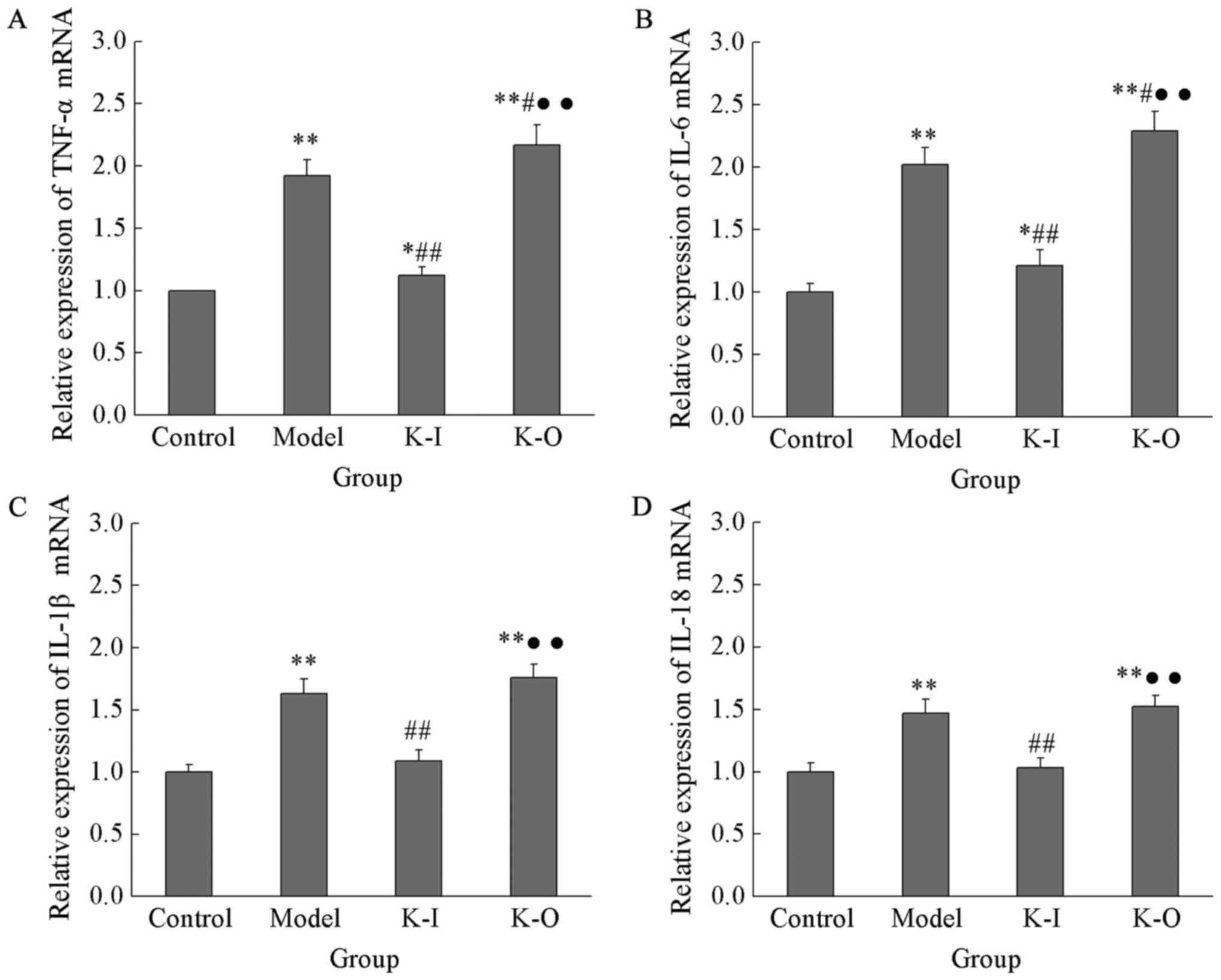
As presented in Fig.
4, expression levels of TNF-α, IL-6, IL-1β and IL-18 in the
model group were 1.92±0.13, 2.02±0.14, 1.63±0.12 and 1.47±0.11,
respectively, which were significantly higher than those of the
control group (P<0.05). Compared with the model group,
expression levels of TNF-α and IL-6 mRNA in the knock-out group
were significantly increased to 2.17±0.16 and 2.29±0.16,
respectively, and no significant differences were found in
expression levels of IL-1β and IL-18 between those two groups. In
contrast, compared with the model group, expression levels of
TNF-α, IL-6, IL-1β and IL-18 in PTEN-Long knock-in group were
significantly reduced to 1.12±0.07, 1.21±0.13, 1.09±0.09 and
1.03±0.08, respectively (P<0.05). Expression levels of TNF-α and
IL-6 mRNA were significantly higher in the PTEN-Long knock-in group
than in the control group, but no significant differences in
expression levels of IL-1β and IL-18 mRNA were observed between
these groups. These data demonstrated that PTEN-Long can inhibit
the expression of inflammatory factors to protect kidney from the
injuries caused by nephritis (Fig.
4).
Histological changes of kidney of mice
in different groups
As presented in Fig.
5, the structure of glomerular cells in the control group is
clear, no interstitial inflammatory cell infiltration and edema
were exhibited and no proliferation of mesangial cells and matrix
was observed. In the model group, glomerular adhesions were
observed, glomerular blood vessels and basement membrane were
markedly thickened, and inflammatory cell infiltration was also
observed; compared with the model group, structure of the
glomerular was improved, the thickening of glomerular blood vessels
and basement membrane and inflammatory cell infiltration were
reduced in PTEN-Long knock-in group; in PTEN-Long knock-out group,
renal tissue structure was destroyed, no clear glomerular was
observed and severe inflammatory cell infiltration was observed.
The results indicate that PTEN-Long can protect renal tissue
structure in patients with nephritis.
Discussion
It is well known that the PI3K/Akt pathway is
closely associated with the onset and progression of a variety of
cancers (21). PI3Ks can
phosphorylate phosphatidylinositol (4,5)-bisphosphate (PIP2) to produce
phosphatidylinositol 3, 4, 5 triphosphate (PIP3), and the produced
PIP3 can active Akt to phosphorylate a variety of target proteins
associated with the regulation of cell growth, survival,
proliferation and other cellular processes (22). PTEN protein, which is a tumor
suppressor, serves essential roles in the regulation of the
PI3K/Akt pathway. PTEN can convert PIP3 to the inactive form of
PIP2 through hydrolyzation, so as to inhibit signaling transduction
of PI3k/Akt pathway (23).
PTEN-Long, which is derived from PTEN, is the permeable form of
PTEN that can be secreted by one cell to enter neighboring cells.
Similar to the functions of PTEN, PTEN-Long can also
dephosphorylate PIP3 to reduce the signaling transduction of the
PI3K-Akt pathway, which in turn induces cell death and inhibit
tumor growth (24). Previous studies
have demonstrated that PTEN-Long serves pivotal roles in the
development and progression of various cancers (13,25,26). A
previous study on the treatment of pancreatic cancer have
demonstrated that PTEN-Long targeted therapy combined with
chemotherapy may significantly extend overall survival in mice
model (15). In the clinical study
of ccRCC, expression levels of PTEN-Long protein were observed to
be significantly reduced in patients with ccRCC, and the reduced
level of PTEN-Long protein caused the increased phosphorylation
levels of Akt (16). In contrast,
PTEN-Long overexpression in the ccRCC cell line 786-0 decreased the
phosphorylation level of Akt, which in turn inhibit the
proliferation, migration and invasion of cancer cells or may induce
cells death (16). Therefore,
expression level of PTEN-Long protein was negatively associated
with the development of ccRCC. In the present study, reduced serum
level of PTEN-Long protein was observed in patients with RCC
compared with healthy controls. In addition, serum levels of
PTEN-Long protein in patients with nephritis were lower than that
of healthy controls but higher than that of patients with RCC.
These data suggest that PTEN-Long is also associated with the
development of nephritis and its expression level is negatively
associated with the severity of kidney diseases.
The development of nephritis is a complex process
with a variety of associated factors, particularly inflammatory
factors. Expression levels of TNF-α, IL-6, IL-12 and IL-18, which
are proinflammatory cytokines, may be used to sensitively reflect
the status of inflammation (27,28). It
has previously been demonstrated that expression levels of IL-1β,
IL-18 and IL-6 were significantly increased in patients with acute
renal failure (29). TNF-α can
mediate the expression of chemokines and cytokines to interact with
inflammatory responses caused by renal injury (30). In the present study, serum levels of
TNF-α, IL-6, IL-12 and IL-18 were significantly increased in mice
with nephritis than in healthy control mice. In addition,
expression levels of TNF-α, IL-6, IL-12 and IL-18 mRNA in renal
tissue were also increased in nephritis mice compared with
controls, indicating the inflammation response caused by nephritis.
Furthermore, PTEN knock-in significantly decreased the serum levels
of TNF-α, IL-6, IL-12 and IL-18 and their expression in retinal
tissue. In contrast, PTEN knock-out served an opposite role. The
development of nephritis is accompanied by histological changes in
renal tissue that can significantly reduce renal function (31). In the present study, PTEN knock-in
was demonstrated to improve the histological changes caused by
nephritis, but PTEN knock-out promoted those changes. These data
suggest that PTEN-Long can protect kidney from nephritis injuries
by inhibiting the expression of inflammatory factors.
Based on these findings, PTEN-Long expression was
significantly reduced due to kidney diseases, and the reduced level
of PTEN-Long expression was positively correlated with the severity
of disease. Therefore, PTEN-Long protein can potentially serve as a
biomarker for the diagnosis of nephritis. The protein levels of
PTEN-Long in serum and renal tissue of mice in different groups
were evaluated, and protein levels of PTEN-Long were observed to be
significantly lower in serum than in retinal tissue. Therefore,
serum level of PTEN-Long may more sensitively reflect the status of
kidney diseases.
In conclusion, serum level of PTEN-long was
negatively associated with the severity of kidney disease, and
PTEN-long may protect retinal tissue from the damage caused by
nephritis. Therefore, PTEN-long may potentially be a target for the
diagnosis and treatment of nephritis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HW and QY conceived and designed the experiments.
HW, QY, LW, YLi, MX, YLu and YC performed the experiments and
prepared the manuscript. HW, QY, LW, YLi, MX, YLu and YC analyzed
the data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao
University, and all participants provided written informed
consent.
Patient consent for publication
All participants provided written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cairns P: Renal cell carcinoma. Cancer
Biomarkers. 9:461–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Escudier B, Tomczak P, Hutson
TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J,
Hariharan S, et al: Axitinib versus sorafenib as second-line
treatment for advanced renal cell carcinoma: Overall survival
analysis and updated results from a randomised phase 3 trial.
Lancet Oncol. 14:552–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomita Y, Fukasawa S, Shinohara N,
Kitamura H, Oya M, Eto M, Tanabe K, Kimura G, Yonese J, Yao M, et
al: Nivolumab versus everolimus in advanced renal cell carcinoma:
Japanese subgroup analysis from the CheckMate 025 study. Jpn J Clin
Oncol. 47:639–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanriverdi O: Review on targeted treatment
of patients with advanced-stage renal cell carcinoma: A medical
oncologist's perspective. Asian Pac J Cancer Prev. 14:609–617.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perry JM, He XC, Sugimura R, Grindley JC,
Haug JS, Ding S and Li L: Cooperation between both
Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive
hematopoietic stem cell self-renewal and expansion. Genes Dev.
25:1928–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dasari VR, Kaur K, Velpula KK, Gujrati M,
Fassett D, Klopfenstein JD, Dinh DH and Rao JS: Upregulation of
PTEN in glioma cells by cord blood mesenchymal stem cells inhibits
migration via downregulation of the PI3K/Akt pathway. PLoS One.
5:e103502010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carver BS, Chapinski C, Wongvipat J,
Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J,
Scher H, et al: Reciprocal feedback regulation of PI3K and androgen
receptor signaling in PTEN-deficient prostate cancer. Cancer Cell.
19:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esteva FJ, Guo H, Zhang S, Santa-Maria C,
Stone S, Lanchbury JS, Sahin AA, Hortobagyi GN and Yu D: PTEN,
PIK3CA, p-AKT, and p-p70S6K status: Association with trastuzumab
response and survival in patients with HER2-positive metastatic
breast cancer. Am J Pathol. 177:1647–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Yang Z, Song W, Zhou L, Li Q, Tao K,
Zhou J, Wang X, Zheng Z, You N, et al: Overexpression of Bmi-1
contributes to the invasion and metastasis of hepatocellular
carcinoma by increasing the expression of matrix metalloproteinase
(MMP)-2, MMP-9 and vascular endothelial growth factor via the
PTEN/PI3K/Akt pathway. Int J Oncol. 43:793–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brenner W, Färber G, Herget T, Lehr HA,
Hengstler JG and Thüroff JW: Loss of tumor suppressor protein PTEN
during renal carcinogenesis. Int J Cancer. 99:53–57. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hopkins BD, Fine B, Steinbach N, Dendy M,
Rapp Z, Shaw J, Pappas K, Yu JS, Hodakoski C, Mense S, et al: A
secreted PTEN phosphatase that enters cells to alter signaling and
survival. Science. 341:399–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed RA, Hopkins B, Palermo C, Sastra SA,
Rapp Z, Parsons R and Olive KP: Abstract A86: PTEN-Long and
gemcitabine combination treatment as a therapeutic for pancreatic
cancer. Cancer Res. 75 (13 Suppl):A862015. View Article : Google Scholar
|
|
16
|
Wang H, Zhang P, Lin C, Yu Q, Wu J, Wang
L, Cui Y, Wang K, Gao Z and Li H: Relevance and therapeutic
possibility of PTEN-long in renal cell carcinoma. PLoS One.
10:e1142502015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang R, Miner JJ, Gorman MJ, Rausch K,
Ramage H, White JP, Zuiani A, Zhang P, Fermandez E, Zhang Q, et al:
A CRISPR screen defines a signal peptide processing pathway
required by flaviviruses. Nature. 535:164–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Wang C, Zhang K, Wang Y, Gong X,
Wang Y, Li S and Luo Y: Generation of human embryonic stem cell
line expressing zsGreen in cholinergic neurons using CRISPR/Cas9
system. Neurochem Res. 41:2065–2074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y,
Wu Q, Park A, Yang J, Suresh S, Bizhanova A, et al: Therapeutic
genome editing by combined viral and non-viral delivery of CRISPR
system components in vivo. Nat Biotechnol. 34:328–333. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuckermann M, Kawauchi D and Gronych J:
Applications of the CRISPR/Cas9 system in murine cancer modeling.
Brief Funct Genomics. 16:25–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carnero A and Paramio JM: The
PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front Oncol.
4:2522014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bassi C, Ho J, Srikumar T, Dowling RJ,
Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B and Stambolic V:
Nuclear PTEN controls DNA repair and sensitivity to genotoxic
stress. Science. 341:395–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Q, Li Z and Liu Q: Treatment with
PTEN-Long protein inhibits hepatitis C virus replication. Virology.
511:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan MW and Hou LL: Relationship and tumor
inhibition between PTEN and PTEN-long. Sheng Li Ke Xue Jin Zhan.
47:355–360. 2016.(In Chinese). PubMed/NCBI
|
|
27
|
Krishnan SM, Sobey CG, Latz E, Mansell A
and Drummond GR: IL-1β and IL-18: Inflammatory markers or mediators
of hypertension? Br J Pharmacol. 171:5589–5602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daniele G, Guardado Mendoza R, Winnier D,
Fiorentino TV, Pengou Z, Cornell J, Andreozzi F, Jenkinson C,
Cersosimo E, Federici M, et al: The inflammatory status score
including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and
adiponectin underlies whole-body insulin resistance and
hyperglycemia in type 2 diabetes mellitus. Acta Diabetol.
51:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Faubel S, Lewis EC, Reznikov L, Ljubanovic
D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA and
Edelstein CL: Cisplatin-induced acute renal failure is associated
with an increase in the cytokines interleukin (IL)-1beta, IL-18,
IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp
Ther. 322:8–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramesh G and Reeves WB: TNF-alpha mediates
chemokine and cytokine expression and renal injury in cisplatin
nephrotoxicity. J Clin Invest. 110:835–842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Risdon RA, Sloper JC and De Wardener HE:
Relationship between renal function and histological changes found
in renal-biopsy specimens from patients with persistent glomerular
nephritis. Lancet. 2:363–366. 1968. View Article : Google Scholar : PubMed/NCBI
|