Introduction
German physicist Walter Bradford was the first who
discovered X-rays and their properties in November 1885. Their
properties attracted medical researchers to use them in the medical
field (1). To increase the
sensibility and specificity of the radiological techniques,
contrast media have been introduced into the medical practice. They
are currently being used worldwide to increase the visibility of
the investigated structures. Contrast agents were first used in the
beginning of the 20th century, but with high incidence of toxicity
and poor results. In the 1950's the use of contrast agents
increased due to new formulations that became available. In the
1970's non-ionic dimeric contrast agents were developed and today
they play a major role in diagnosis, being the most used contrast
agents in daily radiology practice (2).
Radiocontrast agents are typically iodine-,
gadolinium- or barium-sulphate-based compounds and they can be
administered orally or parenterally. Oral contrast agents are
mostly used in the radiological diagnosis of the gastrointestinal
tract. Contrast media for the bowel are classified as positive or
negative, depending on whether the material is hyperattenuating or
hypoattenuating relative to the walls of the gastrointestinal tract
(3).
For the investigation of parenchymal organs and
blood vessels, parenterally administered contrast media are used as
iodinated contrast media (ICM) and gadolinium contrast media
(4). Their administration is not
totally safe, the adverse effects of the contrast media may be due
to the type of administration, or to the type of agent used. The
most common side effects after parenteral administration of
contrast media are organ toxicity (with kidney being the most
affected organ) (5) and
hypersensitivity reactions (6).
The local side effect is represented by the contrast
extravasation of the parenterally administrated agent, depending on
the type of administration. As administration typically involves
small volumes, it seldom leads to serious injuries.
Non-communicative patients such as children or debilitated
patients, multiple injections in the same vein, or friable vessels
are considered to be risk factors (7).
The systemic side effects of the contrast media may
occur early, usually in less than 20 min, or late (over 20 min),
and the cause may be an anaphylactoid reaction or effects due to
the osmolarity and chemotoxicity of the substance. The
concentration, volume and rate of injection are also risk factors
to be taken into consideration. Clinical reactions vary from minor,
to intermediate, to severe and skin manifestations are the most
frequent and sometimes the only manifestations that can be
misinterpreted. The severe adverse reactions need an accurate
diagnosis even if they are very rare because their evolution can be
life-threatening (8,9).
The aim of the study was to examine through a
systematic analysis the studies published in the last 10 years
regarding the incidence of immediate and delayed hypersensitivity
reactions with skin manifestations, to identify the skin patterns
that are characteristic in these types of reactions and to analyze
the risk factors and comorbidities that can influence their
appearance.
Materials and methods
Search for studies
We performed multiple PubMed searches using all
possible combinations between the following keywords: Contrast
agents or contrast media and immediate, non-immediate, late,
delayed, allergic reactions, allergic effects, skin lesions,
adverse effects, skin hypersensitivity and side effects. After
analyzing the results only studies relevant to the subject were
included in this systematic analysis. For a more accurate search we
also checked the references of excluded studies.
Inclusion criteria were: Large observational studies
that analyze the incidence of immediate and/or delayed
hypersensitivity reactions to different class of contrast media
and/or their association with the comorbidities, written in English
language in the last 10 years, i.e., from January, 2008 till
January, 2018.
Exclusion criteria were: Articles written in
languages other than English, studies published before January
2008, non-human studies, studies published only as abstracts, as
reviews, meta-analyses, commentaries, editorials, and case-reports
but only after a careful check of those references. After reading
the abstract and entire article we excluded from the systematic
review the articles that did not clearly present the manifestations
observed in the immediate and delayed reactions to the contrast
media and studies that presented the incidence of other types of
adverse reactions, as the purpose of this review was the skin
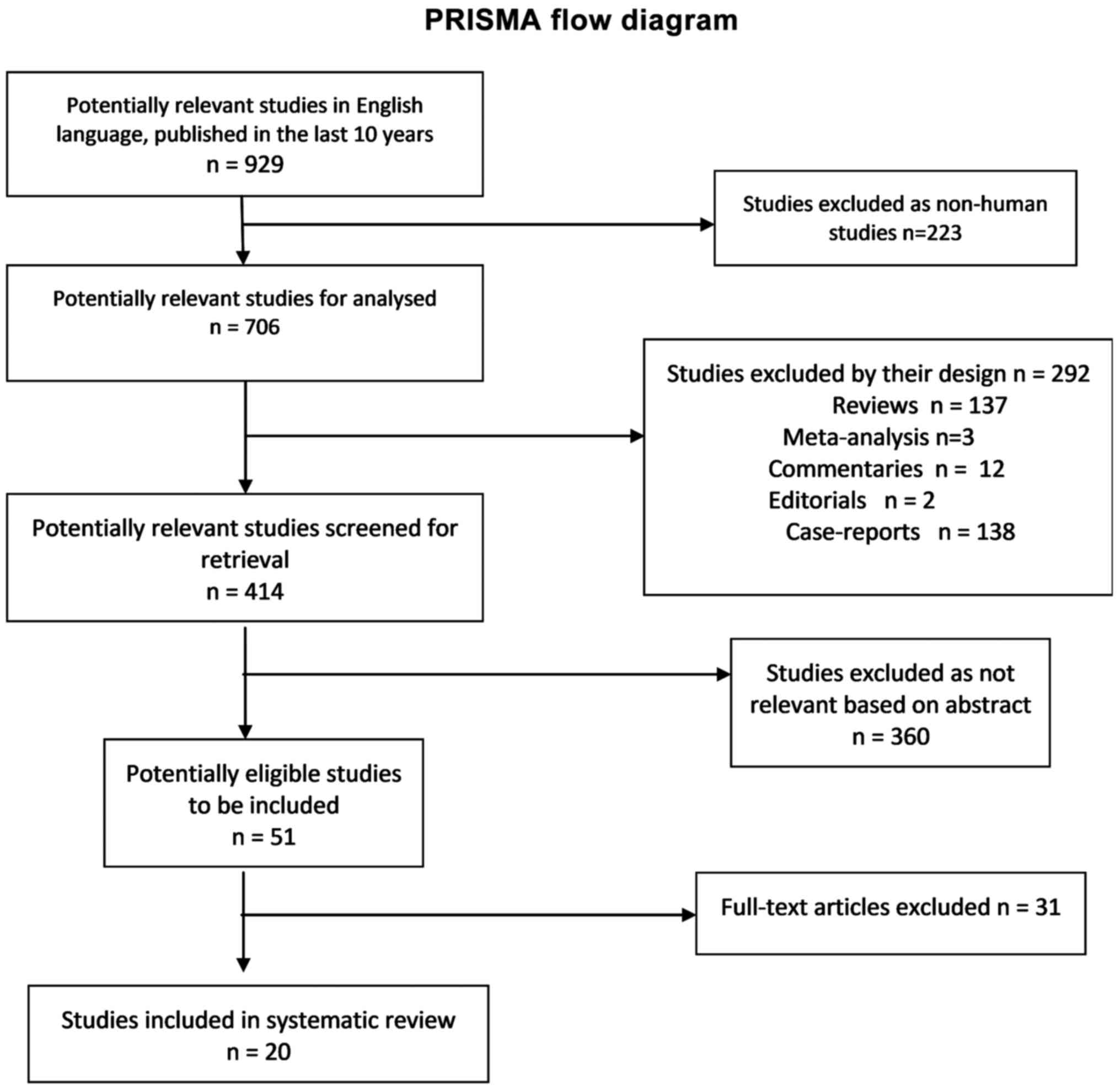
manifestations in contrast media adverse reactions (Fig. 1).
Immediate hypersensitivity reactions to contrast
media are defined as an allergic reaction that occurs within 30 min
of contrast media administration. Delayed hypersensitivity
reactions to contrast media are defined as reactions that appear
between 1 h and 7 days after administration of the contrast agent
(6).
Results
General
The initial research for the studies published in
English between January 2008 and January 2018 yielded 929 articles.
After excluding non-human studies and the reviews, meta-analyses,
commentaries, editorials, and case-reports, 414 articles remained
to be screened for their content by reading the abstract. In total,
51 full-articles were assessed for eligible studies to be included.
Finally, 20 articles were included in the analysis. The selection
procedure was carried out according to the inclusion and exclusion
criteria and is presented in the PRISMA flow chart (Fig. 1). We identified 11 studies that
described immediate skin reactions to ICM, 9 studies that described
immediate skin reactions to other contrast media, 6 studies that
described delayed skin adverse reactions to ICM, and 3 studies that
described delayed skin adverse reactions to other contrast media.
Regarding the risk factors associated with the incidence of
immediate and delayed allergic reactions from the studies included
in the systematic review, 7 studies analyzed the risk factors
associated with immediate allergic reactions to ICM and 4 studies
analyzed the risk factors associated with immediate allergic
reactions to other contrast agents. No study analyzed the risk
factors associated with delayed allergic reactions.
Incidence of immediate skin adverse
reactions to ICM
The epidemiological studies published in the last 10
years that evaluate the incidence of immediate adverse reactions to
ICM with skin manifestations identify a prevalence of immediate
hypersensitivity reactions between 0.16 and 2.24%, from which the
percentage of skin manifestation was between 33.33 and 87.7%
(Table I) (9–19).
Severe hypersensitivity reactions were between 0.010 and 0.024%,
with anaphylaxis being the most frequent reaction (Table I) (9,10,18).
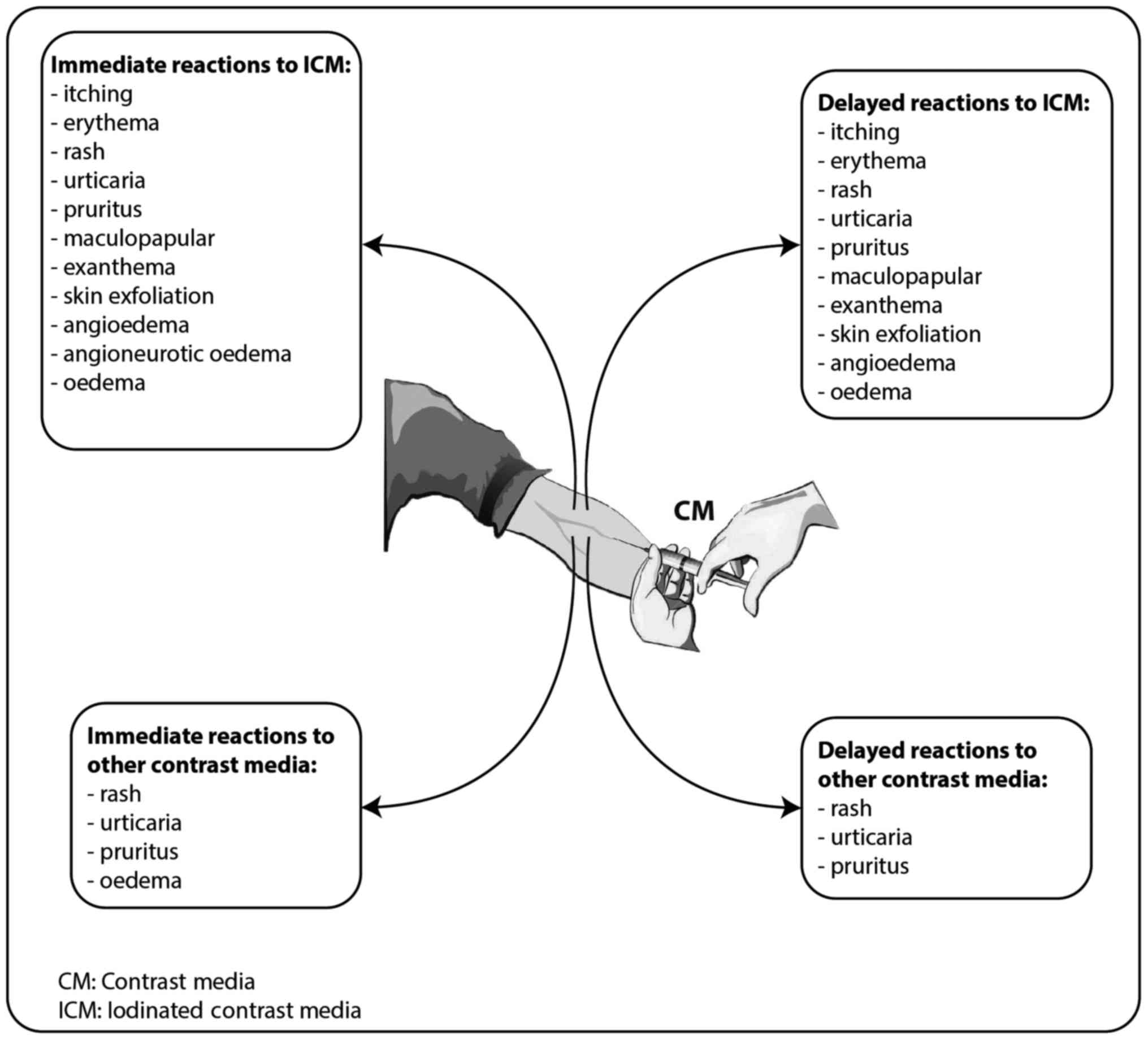
Regarding the skin patterns observed in these cases, the most
frequent were urticaria (between 30.77 and 83.78% of the cases with
skin manifestations) (10,13,15,17–19),
rash (38.46–85.3%) (9,10,13,19),
itching sensation/pruritus (12.82–100%) (10,13–15,17–19),
oedema (6.25–17.3%) (12,18,19),
erythemas (36.54–100%) (12,14,17–19),
angioedema (8%-13.51%) (15,17), and angioneurotic oedema (3.84%)
(18) (Fig. 2).
 | Table I.Studies that evaluated the incidence
of immediate skin adverse reactions to iodinated contrast
media. |
Table I.
Studies that evaluated the incidence
of immediate skin adverse reactions to iodinated contrast
media.
| Studies, year | Study
type/samples | Iodinated contrast
media | Overall skin
reactions (%) | Skin reactions from
total immediate adverse reactions (%) | Severe reactions
(%) | Refs. |
|---|
| Morales-Cabeza
et al, 2017 | Prospective
study/161,319 procedures | Iopamidol,
Ioversol, Iomeprol | 0.18 | 87.7 | 0.010 | (9) |
| Kim et al,
2017 | Retrospective
study/286,087 procedures | Iobitridol,
Iohexol, Iopamidol, Iopromide | 0.69 | Not specify | 0.024
(anaphylaxis) | (10) |
| Zhang et al,
2014 | Prospective
study/20,185 subjects | Iodixanol | 0.19 | 33.33 | 0.009 | (11) |
| García et
al, 2014 | Retrospective
study/Iopromide investigation 72,887; Iomeprol investigations
37,154 | Iopromide,
Iomeprol | Iopromide (0.27)
Iomeprol (0.19) | Iopromide (56.7)
Iomeprol (41.1) | Iopromide (0.014)
Iomeprol (0.045) | (12) |
| Kalaiselvan et
al, 2014 | Retrospective
study/59,915 subjects | Diatrizoate,
Iothalmate, Ioxaglate Iobitridol, Iodixanol, Iohexol Iomeprol,
Ioversol, Iopromide Iopamidol, Iodized oil | 0.47 | Not specify | 0.07 | (13) |
| Pradubpongsa et
al, 2013 | Retrospective
study/55,286 subjects | Low osmolarity CM
(non-ionic iodinated monomer), high osmolarity CM (ionic iodinated
monomer and dimer), and iso-osmolarity CM (non-ionic iodinated
dimer) | 0.83 | 74.2 | 0.024 | (14) |
| Ho et al,
2012 | Prospective
study/29,962 subjects | Iopromide | 0.12 | 78.72 | 0.003 | (15) |
| Mitchell et
al, 2011 | Prospective
study/633 patients | Iopamidol | 0.47 | 60 | 0 | (16) |
| Goksel et
al, 2011 | Retrospective
study/1,131 subjects | Iohexol, Iomeprol,
Ioxilan, Iopamidol, Iodixanol, Iobitridol | 1.15 | Not specify | – | (17) |
| Häussler, 2010 | Retrospective
study/9,515 patients | Iodixanol | 0.31 | Not specify | 0.02 | (18) |
| Lapi et al,
2008 | Prospective
study/1,514 patients | Iopromide,
Iodixanol, Iomeprol, Iobitridol | 1.05 | 47 | 0.06 | (19) |
Incidence of immediate skin adverse reactions to
other contrast media. In Table II
are presented the studies published in the last 10 years that
evaluate the incidence of immediate adverse reactions to other
contrast media except ICM with skin manifestations. We identified 6
studies that evaluated the immediate reactions to gadolinium-based
contrast agents, 2 studies that evaluated the immediate reactions
to sulfur hexafluoride microbubbles, a contrast agent used for
contrast-enhanced ultrasound and 1 study that evaluated the
immediate hypersensitivity reactions to sodium fluorescein.
 | Table II.Studies that evaluated the incidence
of immediate skin adverse reactions to other contrast media. |
Table II.
Studies that evaluated the incidence
of immediate skin adverse reactions to other contrast media.
| Studies, year | Study
type/samples | Other contrast
media | Overall skin
reactions (%) | Skin reactions from
total immediate adverse reactions (%) | Severe reactions
(%) | Refs. |
|---|
| Kalaiselvan et
al, 2014 | Retrospective
study/59,915 patients | Gadopentetate,
Gadoterate, Gadolinium, Gadodiamide, | 0.01 | 100 | 0.005 | (13) |
| Torres et
al, 2017 | Retrospective
study/173 pediatric patients that underwent 287 CEUS | Sulfur hexafluoride
microbubbles | 0 | 0 | 0 | (20) |
| Yusuf et al,
2017 | Retrospective
study/305 pediatric patients that underwent CEUS | Sulfur hexafluoride
microbubbles | 0 | 0 | 0 | (21) |
| Granata et
al, 2016 | Retrospective
study/10,608 Caucasian patients | Gd-BOPTA
(Gadobenate dimeglumine) Gd-EOB-DTPA (gadoxetic acid disodium)
Gd-DOTA (gadoterate meglumine) Gd-BT-DO3A (gadobutrol) Gd-DTPA
(gadopentetate dimeglumine) | 0.23 | 75 |
| (22) |
| Power et al,
2016 | Observational
study/30,373 gadobutrol investigations | Gadobutrol | 0.27 | 100 | 0.003 | (23) |
| Jung et al,
2012 | Retrospective study
between/141,623 administrations | Gadoteric acid,
Gadobutrol, Gadopentetate, Dimeglumine, Gadobenate, Dimeglumine,
Gadoxetic acid, Gadodiamide, | 0.076 | 96.42 |
| (24) |
| Emond et al,
2011 | Observational
study/104 neonates and infants (<18 months) | Gadoteric acid | 0 | 0 | 0 | (25) |
| Prince et
al, 2011 | Retrospective
study/158,796 gadolinium-based contrast agent enhanced
examination | Gadodiamide,
Gadopentetate, Dimeglumine, Gadoteridol, Gadobenate Dimeglumine,
Gadoxetate | 0.06 | 100 | 0.002 |
(26) |
| Wallace et
al, 2010 | A cross-sectional
survey of 16 International Academic Medical Centres/2,272
gastrointestinal CLE procedures | 10% Sodium
fluorescein | 0.39 | 28.12 | 0 | (27) |
Two studies investigated the immediate adverse
reactions to sulfur hexafluoride microbubbles in pediatric
patients. No immediate hypersensitivity effects were evident after
the administration (20,21).
Regarding the gadolinium contrast media the
incidence of immediate hypersensitivity reactions was between 0.01
and 0.3%. The skin manifestations were observed in 75–100% of these
reactions (13,22–27). The
most frequent skin manifestations associated with hypersensitivity
reactions to gadolinium contrast agents were urticaria (63–91.1%)
(13,22–24),
rash (20.4%) (13,22,23),
pruritus (22.2%) (23), limited
facial edema (6.17%) (23).
Regarding the life-threatening severe hypersensitivity reactions
only Kalaiselvan et al reported one case manifested by
laryngospasm (13) (Fig. 2).
Wallace et al evaluated the safety of
fluorescein, a contrast agent utilized for confocal laser
endomicroscopy. They determined an incidence of 1.4% of immediate
side effects from which 28.12% had skin manifestations. The skin
patterns observed were injection side erythema 88 and rash 12%
(from the cases with skin effects) (27).
Incidence of delayed skin adverse
reactions to ICM
The delayed hypersensitivity reactions were also
reported in the studies published in the last 10 years that
investigated the safety of ICM (Table
III). A prevalence of 0.42 and 14.3% was observed, from which
the incidence of skin manifestations were between 43.05 and 100%
(11,14,17–19,28). The
most frequent skin pattern was angioedema (between 11.1 and 43.7%)
(13,16), itching/pruritus (18.7–55.6%)
(10,13,16–18,27) and
maculopapular exanthema (33.3–37.5%) (14,17).
Other reactions with low incidence were erythema, rash, urticarial,
and oedema (11,14,17–19,28)
(Fig. 2).
 | Table III.Studies that evaluated the incidence
of delayed skin adverse reactions to iodinated contrast media. |
Table III.
Studies that evaluated the incidence
of delayed skin adverse reactions to iodinated contrast media.
| Studies, year | Study
type/samples | Iodinated contrast
media | Overall reactions
(%) | Severe reactions
(%) | Skin reactions from
total delayed adverse reactions (%) | Refs. |
|---|
| Goksel et
al, 2011 | Retrospective
study/1,131 subjects | Iohexol, Iomeprol,
Ioxilan, Iopamidol, Iodixanol, Iobitridol | 1.41 | 100 | 0 | (17) |
| Pradubpongsa et
al, 2013 | Retrospective
study/55,286 subjects | Low osmolarity CM
(non-ionic iodinated monomer), high osmolarity CM (ionic iodinated
monomer and dimer), and iso-osmolarity CM (non-ionic iodinated
dimer) | 0.03 | 100 | 0 | (14) |
| Zhang et al,
2014 | Prospective
study/20,185 subjects | Iodixanol | 0.68 | 100 | 0 | (11) |
| Häussler et
al, 2010 | Retrospective
study/9,515 patients | Iodixanol | 0.42 | 100 | 0.03 | (18) |
| Loh et al,
2010 | Prospective
study/258 patients | Iohexol | 10.1 | 70.27 | 0 | (28) |
| Lapi et al,
2008 | Prospective
study/1,514 patients | Iopromide,
Iodixanol, Iomeprol, Iobitridol | 4.09 | 43.05 | 0 | (19) |
In a study by Häussler 3 cases (0.03%) of severe
delayed hypersensitivity reactions were reported: i) One
59-year-old female that experienced hypersensitivity, pruritus,
urticarial, increased blood pressure, vomiting, swelling of the
face, eyelid edema, facial edema, diarrhea and tachycardia; ii) one
53-year-old female that presented hypersensitivity, swelling of the
face, erythema, and skin irritation and; iii) one 79-year-old male
that presented hypersensitivity, rash, pruritus, dermatitis, and
erythema. All 3 cases needed hospitalization (18).
Incidence of delayed skin adverse
reactions to other contrast media
Delayed skin reactions to other contrast media
except ICM were investigated in only 3 studies published in the
last 10 years (Table IV). Two
studies evaluated the safety of sulfur hexafluoride microbubbles in
pediatric population and reported no delayed adverse effects
(20,21). Power et al reported an
incidence of 0.05% delayed sensitivity reactions to
gadolinium-based contrast agents with skin lesions. The symptoms
observed were urticaria (66%), rash (33%) and pruritus (6.6%).
Delayed reactions occurred on the same day in 46% of cases, on the
following day in 20% of cases and in 33% of cases the moment of
manifestation was uncertain (23)
(Fig. 2).
 | Table IV.Studies that evaluated the incidence
of delayed skin adverse reactions to other contrast media. |
Table IV.
Studies that evaluated the incidence
of delayed skin adverse reactions to other contrast media.
| Studies, year | Study
type/samples | Iodinated contrast
media | Overall reactions
(%) | Skin reactions from
total delayed adverse reactions (%) | Severe reactions
(%) | Refs. |
|---|
| Torres et
al, 2017 | Retrospective
study/173 pediatric patients that underwent 287 CEUS | Sulfur hexafluoride
microbubbles | 0 | 0 | 0 | (20) |
| Yusuf et al,
2017 | Retrospective
study/305 pediatric patients that underwent CEUS | Sulfur hexafluoride
microbubbles | 0 | 0 | 0 | (21) |
| Power et al,
2016 | Observational
study/30,373 gadobutrol investigations | Gadobutrol | 0.05 | 100 | 0 | (23) |
Risk factors associated with immediate
allergic reactions to ICM
Several risk factors were associated with the
appearance of immediate hypersensitivity reactions to ICM. We
identified 7 studies there of (Table
V). The female sex was associated with increased risk for
immediate allergic reactions to ICM between 51.44 and 65.95%
(9,10,14,15,17,18).
Other risk factors identified included history of previous
reactions to ICM (1.2–11.6%) (9,12–15,17),
atopy (14.3%) (8), asthma
(2.1–12.7%) (9,14,15,17),
drug allergy (3.6–25%) (9,14,15,17), and
allergic rhinitis (1.5–4%) (14,15,17).
 | Table V.Studies that evaluated the risk
factors associated with immediate allergic reactions to iodinated
contrast media. |
Table V.
Studies that evaluated the risk
factors associated with immediate allergic reactions to iodinated
contrast media.
| Studies, year | Study
type/samples | History of
allergy | History of previous
reaction to CM | Age (years) | Concomitant
treatment | Sex | History of cardiac
disease | Refs. |
|---|
| Morales-Cabeza
et al, 2017 | Prospective
study/161,319 procedures | Atopy (14,3%)
Asthma (5,9%) Drug allergy (13,2%) | 7% | – | β-blockers (7,9%)
ACEI (13,2%) | Female (52.3%) | – | (9) |
| Kim et al,
2017 | Retrospective
study/286,087 procedures | – | – | 20–50 (34.4%) | – | Females
(51.44%) | – | (10) |
| Goksel et
al, 2011 | Retrospective
study/1,131 subjects | Drug allergies
(3.6%) Asthma (4.4%) Allergic rhinitis (1.5%) Chronic
urticaria/Angioedema (1%) Contact dermatitis (1%) Food allergy
(0.4%) | 2.92% |
|
| Females
(55.7%) | 33.2% | (17) |
| Pradubpongsa et
al, 2013 | Retrospective
study/55,286 subjects | Drug allergy
(13.1%) Seafood allergy (7.1%) Allergic rhinitis (2.8%) Asthma
(2.1%) Chronic urticaria (0.9%) | 11.6% | – | – | Females
(58.7%) | 27.6% | (14) |
| García et
al, 2014 | Retrospective
study/Iopromide investigation 72,887 Iomeprol investigations
37,154 | Iopromide (6.5%)
Iomeprol (16.3%) - | Iopromide (4%)
Iomeprol (1.2%) - | – | – | – | – | (12) |
| Häussler et
al, 2010 | Retrospective
study/9,515 patients | – | – | 18–50 (0.66%)
compared to only 0.22% >51 | – | Females
(65.45%) | – | (18) |
| Ho et al,
2012 | Prospective
study/29,962 subjects | Asthma (12.7%)
Allergy to other substances (25%) Allergic rhinitis (4%) | 4% | – | – | Females
(65.95%) | – | (15) |
The study of Morales-Cabeza et al associated
the immediate allergic reactions to concomitant treatment with
β-blockers (7.9%) or angiotensin-converting enzyme inhibitors
(ACEI) (13.2%) (9).
Risk factors associated with immediate
allergic reactions to other contrast media
Being female was associated with gadolinium-based
contrast agents and immediate adverse reactions in 65.2–81.25% of
cases (22–24,26).
Immediate adverse reactions to other contrast media were associated
with previous reaction to gadolinium contrast media (7.31–8.5%)
(23,26), asthma (2.9–11%) (23,24,26),
drug hypersensitivity (2%) (24),
allergic rhinitis (2.9%) (24), and
chronic urticaria (2%) (24)
(Table VI).
 | Table VI.Risk factors associated with
immediate allergic reactions to other contrast media than ICM. |
Table VI.
Risk factors associated with
immediate allergic reactions to other contrast media than ICM.
| Studies, year | Study
type/samples | History of
allergy | History of previous
reaction to CM | Age (years) | Concomitant
treatment | Sex | History of cardiac
disease | Refs. |
|---|
| Granata et
al, 2016 | Retrospective
study/10,608 Caucasian patients gadolinium-based CM | – | – | – | – | Females
(81.25%) | – | (22) |
| Power et al,
2016 | Observational
study/30,373 gadobutrol investigations | Asthma (11%) | 7.31% previous
reaction to gadolinium-based contrast agents 10.97% previous
reaction to ICM | – | – | Females
(81.7%) | – | (23) |
| Jung et al,
2012 | Retrospective study
between/141,623 administrations gadolinium-based CM | Asthma (2.9%)
Allergic rhinitis (2.9%) Drug hypersensitivity (2%) Chronic
urticaria (2%) | – | – | – | Females
(65.2%) | – | (24) |
| Prince et
al, 2011 | Retrospective
study/158,796 gadolinium-based contrast agent enhanced
examination | Asthma (8.5%)
Previous allergic event (40.4%) | 8.5% previous
reaction to gadolinium-based contrast agents | – | – | Females
(76,6%) | – | (26) |
Discussion
In our systematic literature search the incidence of
overall skin immediate reactions to ICM had an incidence between
1.15 and 0.12%, depending on the cohort analyzed in the studies.
Goksel et al (17) analyzed
only a cohort of 1,131 patients and observed an incidence of 1.15%
of immediate reactions with skin manifestations in comparison with
Ho et al (15) who analyzed a
cohort of 29,962 patients and determined an incidence of 0.12%. The
percentage of cutaneous manifestations in the cohort that
experienced immediate hypersensitivity reactions was between 33.33
and 87.7%, with most of the studies reporting percentages above
50%. The most frequent symptoms observed were urticaria, rash,
itching and erythemas. Severe immediate adverse reactions,
anaphylactic manifestations being the most frequent, were also
identified. Those results are in accordance with the older studies,
which reported a prevalence of 3.8 and 12.7% of mild immediate CM
reactions after using an ionic-iodinated CM and between 0.7 and
3.1% for non-ionic-iodinated CM (29–32).
Most of the studies discussed in the present systematic analysis
reported results after exposure to non-ionic ICM.
The mechanism of CM-induced allergic reaction is
unclear and multifactorial and it is considered that more
mechanisms are involved. The main mechanism considered is type-I
hypersensitivity mechanism. Other proposed mechanisms are
associated with histamine release from mast cells and basophils or
activation of the complement system. Studies have shown that
osmolarity or chemical structure of ICM can act directly on the
cells and determine direct membrane effects that lead to these
types of reactions (33). Previous
findings showed that some ICM activate one of the true
hypersensitivity pathways. The lower rate of adverse reactions to
gadolinium-based contrast media is probably due to limited data
available (34). Therefore, most
immediate adverse reactions to contrast media probably occur
through different pathways, supported by high level of histamine
observed in the serum of patients with this type of reactions
(33).
Non-iodinated contrast agents have a safer profile
compared with ICM, the incidence of immediate adverse reactions
being very low in gadolinium-based contrast agents and other agents
used for contrast-enhanced ultrasound (CEUS). Previous studies
showed that gadolinium-based contrast agents determine mild,
moderate and severe immediate adverse reactions with an incidence
of 0.07 and 3.1% (35–37), which is in accordance with our
findings. The incidence of immediate adverse reactions was
identified as 0.01 and 1.3%, from which the skin manifestations
were presented in 75 and 100% of cases. The most frequent skin
manifestations were urticaria, rashes, pruritus and limited facial
edema. Regarding other contrast agents, the ones used for CEUS are
considered a safer alternative, especially in pediatric population
where CT and MRI investigations present numerous disadvantages with
regard to sedation or general anesthesia. The incidence of
immediate adverse reactions to CEUS agents was very low, around
0.0086% (38). In our systematic
analysis, we identified 2 studies that reported no immediate
adverse reaction to sulfur hexafluoride microbubbles used as CM for
CEUS in pediatric population.
In the studies analyzed in our systematic analysis
the incidence of delayed reactions to ICM was between 10.1 and
0.03%. The higher incidence observed can also be determined because
of the low cohort investigated of only 258 patients compared with
the study where the lowest incidence was observed, which analyzed
55,286 subjects. Older studies showed an incidence of delayed
reactions to ICM of 2 and 5% (39–42). In
the studies we analyzed the main adverse reactions due to delayed
hypersensitivity phenomena were cutaneous manifestations that were
present between 70.27 and 100% of the cases analyzed. The skin
manifestations in the mild and moderate reactions were rash,
urticaria, pruritus or erythema and angioedema. The severe
reactions are very rare but life threatening, being a significant
problem for both the patients and physicians involved. Between the
skin patterns observed in the severe delayed reactions there were
reported multiform erythema (43),
cutaneous vasculitis (44), fixed
drug eruption (45), Stevens-Johnson
syndrome (46), and toxic epidermal
necrolysis (47). We identified only
one study that reported 3 cases of severe delayed reactions (0.03%)
from 9,515 patients exposed to contrast agents (18). The skin manifestations observed in
these patients were pruritus, urticarial, swelling of the face,
eyelid edema, facial edema, rash and erythema (18).
The mechanism of appearance of these delayed
reactions is mediated via T cells. This mechanism is supported by
the studies that showed the presence of dermal infiltrates of T
cells in affected skin and at positive skin test sites, the
reappearance of the eruption after provocation testing and by the
ability of CM to stimulate the proliferation of peripheral T cells
from patients with CM-induced skin eruptions (48).
The delayed sensitivity reactions to other contrast
media except ICM are rarely reported, also because these products
are on the market for a short period (49). We identified only one study that
reported an incidence of 0.05% delayed sensitivity reactions and
the skin manifestations observed were urticarial, rash and pruritus
(23). No severe reactions were
reported (23).
The hypersensitivity reactions to ICM are associated
with certain associated factors that may be risk factors and a
correct investigation of the patient before the use of a certain
contrast agent can be beneficial for decreasing the risk of its
manifestation. History of allergy, history of previous reactions to
CM, age less than 50 years, female sex, history of cardiac disease,
and concomitant treatment with other drugs such as β-blockers or
ACEI were identified as risk factors, which is in accordance with
older studies (50,51). History of asthma was associated with
severe reactions, thus in these patients other alternatives could
be suggested.
Regarding the risk factors for developing immediate
adverse reactions to gadolinium-based CM, the studies showed a
similar pattern as those that appear after exposure to ICM, female
gender being a predisposing factor, a percentage between 65.2 and
81.7% from those that developed immediate hypersensitivity
reactions to gadolinium-based contrast agents were women, findings
that are in concordance with the older literature (26,52).
These differences are not well explained, but animal studies
suggested that specific sex hormones may be involved in the
increased incidence in females (53). History of allergy and history of
previous reaction to CM is an important determinant factor. In the
studies analyzed, age and concomitant treatment were not found to
be risk factors, even if other studies reported them as such
(54).
In conclusion, contrast agents are extensively used
at present for a large range of imagistic investigations and their
utilization is not totally safe. Irrespective of the class of
contrast agents to which they belong, immediate or delayed adverse
reactions could appear, ICM presenting a higher incidence compared
with new agents as gadolinium-based contrast agents or others
utilized for CEUS. Skin manifestations are the most frequent
manifestations in all the type of allergic reactions and a precise
diagnosis can be very helpful in practice. Several risk factors
were associated with the appearance of immediate hypersensitivity
reactions to contrast media. An accurate anamnesis of the patients
and a correctly conducted pretreatment can limit the incidence and
the severity of the adverse reactions and also can avoid the
occurrence of life threatening reactions.
Acknowledgements
This study is part of the PhD theses of Andrei Mihai
Iordache from the University of Medicine and Pharmacy of Craiova,
Craiova, Romania.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AMI, AOD, AMB, RM and DC contributed to the
interpretation of study, performed the systematic search of the
literature, writing the manuscript and revising it critically for
important intellectual content. DA, OZ, SI, GI, DN, MS, OCR and DEB
are responsible for the acquisition, analysis and interpretation of
the data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Filler A: The History, development and
impact of computed imaging in neurological diagnosis and
neurosurgery: CT, MRI, and DTI. Nat Preced. 7:pp. 1–76. 2009,
https://doi.org/10.1038/npre.2009.3267
|
|
2
|
Singh J and Daftary A: Iodinated contrast
media and their adverse reactions. J Nucl Med Technol. 36:69–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hasegawa M and Gomi T: Radiological
contrast agents and radiopharmaceuticals. Side Effects of Drugs
Annual. Ray SD: 37. 1st. Elsevier; Amsterdam: pp. 583–593. 2015,
View Article : Google Scholar
|
|
4
|
Iyer RS, Schopp JG, Swanson JO, Thapa MM
and Phillips GS: Safety essentials: Acute reactions to iodinated
contrast media. J Can Assoc Radiol. 64:193–199. 2013. View Article : Google Scholar
|
|
5
|
Mamoulakis C, Tsarouhas K, Fragkiadoulaki
I, Heretis I, Wilks MF, Spandidos DA, Tsitsimpikou C and Tsatsakis
A: Contrast-induced nephropathy: Basic concepts, pathophysiological
implications and prevention strategies. Pharmacol Ther. 180:99–112.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sodagari F, Mozaffary A, Wood CG III,
Schmitz B, Miller FH and Yaghmai V: Reactions to both nonionic
iodinated and gadolinium-based contrast media: Incidence and
clinical characteristics. AJR Am J Roentgenol. 210:715–719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicola R, Shaqdan KW, Aran S, Prabhakar
AM, Singh AK and Abujudeh HH: Contrast media extravasation of
computed tomography and magnetic resonance imaging: Management
guidelines for the radiologist. Curr Probl Diagn Radiol.
45:161–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fok JS and Smith WB: Hypersensitivity
reactions to gadolinium-based contrast agents. Curr Opin Allergy
Clin Immunol. 17:241–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morales-Cabeza C, Roa-Medellin D, Torrado
I, De Barrio M, Fernández-Álvarez C, Montes-Aceñero JF, De La Riva
I and Prieto-García A: Immediate reactions to iodinated contrast
media. Ann Allergy Asthma Immunol. 119:553–557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SR, Lee JH, Park KH, Park HJ and Park
JW: Varied incidence of immediate adverse reactions to low-osmolar
non-ionic iodide radiocontrast media used in computed tomography.
Clin Exp Allergy. 47:106–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang BC, Hou L, Lv B and Xu YW:
Post-marketing surveillance study with iodixanol in 20 185 Chinese
patients from routine clinical practices. Br J Radiol.
87:201303252014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García M, Aguirre U, Martinez A, Ruiz B,
Lertxundi U and Aguirre C: Acute adverse reactions to iopromide vs
iomeprol: A retrospective analysis of spontaneous reporting from a
radiology department. Br J Radiol. 87:201305112014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalaiselvan V, Sharma S and Singh GN:
Adverse reactions to contrast media: An analysis of spontaneous
reports in the database of the pharmacovigilance programme of
India. Drug Saf. 37:703–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pradubpongsa P, Dhana N,
Jongjarearnprasert K, Janpanich S and Thongngarm T: Adverse
reactions to iodinated contrast media: Prevalence, risk factors and
outcome-the results of a 3-year period. Asian Pac J Allergy
Immunol. 31:299–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho J, Kingston RJ, Young N, Katelaris CH
and Sindhusake D: Immediate hypersensitivity reactions to IV
non-ionic iodinated contrast in computed tomography. Asia Pac
Allergy. 2:242–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitchell AM, Jones AE, Tumlin JA and Kline
JA: Immediate complications of intravenous contrast for computed
tomography imaging in the outpatient setting are rare. Acad Emerg
Med. 18:1005–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goksel O, Aydın O, Atasoy C, Akyar S,
Demirel YS, Misirligil Z and Bavbek S: Hypersensitivity reactions
to contrast media: Prevalence, risk factors and the role of skin
tests in diagnosis - a cross-sectional survey. Int Arch Allergy
Immunol. 155:297–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Häussler MD: Safety and patient comfort
with iodixanol: A postmarketing surveillance study in 9515 patients
undergoing diagnostic CT examinations. Acta Radiol. 51:924–933.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lapi F, Cecchi E, Pedone C, Attanasio F,
Banchelli G, Vannacci A, Di Pirro M, Moschini M, Berni V, Matucci
R, et al: Safety aspects of iodinated contrast media related to
their physicochemical properties: A pharmacoepidemiology study in
two Tuscany hospitals. Eur J Clin Pharmacol. 64:723–737. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Torres A, Koskinen SK, Gjertsen H and
Fischler B: Contrast-enhanced ultrasound using sulfur hexafluoride
is safe in the pediatric setting. Acta Radiol. 58:1395–1399. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yusuf GT, Sellars ME, Deganello A,
Cosgrove DO and Sidhu PS: Retrospective analysis of the safety and
cost implications of pediatric contrast-enhanced ultrasound at a
single center. AJR Am J Roentgenol. 208:446–452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Granata V, Cascella M, Fusco R,
dell'Aprovitola N, Catalano O, Filice S, Schiavone V, Izzo F, Cuomo
A and Petrillo A: Immediate adverse reactions to gadolinium-based
MR contrast media: A retrospective analysis on 10,608 examinations.
BioMed Res Int. 2016, pp. 39182922016.
doi.org/10.1155/2016/3918292. PubMed/NCBI
|
|
23
|
Power S, Talbot N, Kucharczyk W and
Mandell DM: Allergic-like reactions to the MR imaging contrast
agent gadobutrol: A prospective study of 32 991 consecutive
injections. Radiology. 281:72–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung JW, Kang HR, Kim MH, Lee W, Min KU,
Han MH and Cho SH: Immediate hypersensitivity reaction to
gadolinium-based MR contrast media. Radiology. 264:414–422. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emond S and Brunelle F: Gd-DOTA
administration at MRI in children younger than 18 months of age:
Immediate adverse reactions. Pediatr Radiol. 41:1401–1406. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prince MR, Zhang H, Zou Z, Staron RB and
Brill PW: Incidence of immediate gadolinium contrast media
reactions. AJR Am J Roentgenol. 196:138–143. 2011. View Article : Google Scholar
|
|
27
|
Wallace MB, Meining A, Canto MI, Fockens
P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, Löhr M,
et al: The safety of intravenous fluorescein for confocal laser
endomicroscopy in the gastrointestinal tract. Aliment Pharmacol
Ther. 31:548–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loh S, Bagheri S, Katzberg RW, Fung MA and
Li CS: Delayed adverse reaction to contrast-enhanced CT: A
prospective single-center study comparison to control group without
enhancement. Radiology. 255:764–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katayama H, Yamaguchi K, Kozuka T,
Takashima T, Seez P and Matsuura K: Adverse reactions to ionic and
nonionic contrast media. A report from the Japanese committee on
the safety of contrast media. Radiology. 175:621–628. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wolf GL, Arenson RL and Cross AP: A
prospective trial of ionic vs nonionic contrast agents in routine
clinical practice: Comparison of adverse effects. AJR Am J
Roentgenol. 152:939–944. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Palmer FJ: The RACR survey of intravenous
contrast media reactions. Final report. Australas Radiol.
32:426–428. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Caro JJ, Trindade E and McGregor M: The
risks of death and of severe nonfatal reactions with high- vs
low-osmolality contrast media: A meta-analysis. AJR Am J
Roentgenol. 156:825–832. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Idée JM, Pinès E, Prigent P and Corot C:
Allergy-like reactions to iodinated contrast agents. A critical
analysis. Fundam Clin Pharmacol. 19:263–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boyd B, Zamora CA and Castillo M: Managing
adverse reactions to contrast agents. Magn Reson Imaging Clin N Am.
25:737–742. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kirchin MA and Runge VM: Contrast agents
for magnetic resonance imaging: Safety update. Top Magn Reson
Imaging. 14:426–435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ersoy H and Rybicki FJ: Biochemical safety
profiles of gadolinium-based extracellular contrast agents and
nephrogenic systemic fibrosis. J Magn Reson Imaging. 26:1190–1197.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nelson KL, Gifford LM, Lauber-Huber C,
Gross CA and Lasser TA: Clinical safety of gadopentetate
dimeglumine. Radiology. 196:439–443. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Piscaglia F and Bolondi L: Italian society
for ultrasound in medicine and biology (SIUMB) study group on
ultrasound contrast agents: The safety of Sonovue in abdominal
applications: Retrospective analysis of 23188 investigations.
Ultrasound Med Biol. 32:1369–1375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yasuda R and Munechika H: Delayed adverse
reactions to nonionic monomeric contrast-enhanced media. Invest
Radiol. 33:1–5. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Munechika H, Hiramatsu Y, Kudo S, Sugimura
K, Hamada C, Yamaguchi K and Katayama H: A prospective survey of
delayed adverse reactions to iohexol in urography and computed
tomography. Eur Radiol. 13:185–194. 2003.PubMed/NCBI
|
|
41
|
Rydberg J, Charles J and Aspelin P:
Frequency of late allergy-like adverse reactions following
injection of intravascular non-ionic contrast media. A
retrospective study comparing a non-ionic monomeric contrast medium
with a non-ionic dimeric contrast medium. Acta Radiol. 39:219–222.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Panto PN and Davies P: Delayed reactions
to urographic contrast media. Br J Radiol. 59:41–44. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akiyama M, Nakada T, Sueki H, Fujisawa R
and Iijima M: Drug eruption caused by nonionic iodinated X-ray
contrast media. Acad Radiol. 5:159–161. 1998. View Article : Google Scholar
|
|
44
|
Reynolds NJ, Wallington TB and Burton JL:
Hydralazine predisposes to acute cutaneous vasculitis following
urography with iopamidol. Br J Dermatol. 129:82–85. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Good AE, Novak E and Sonda LP III: Fixed
eruption and fever after urography. South Med J. 73:948–949. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Savill JS, Barrie R, Ghosh S, Muhlemann M,
Dawson P and Pusey CD: Fatal Stevens-Johnson syndrome following
urography with iopamidol in systemic lupus erythematosus. J
Postgrad Med. 64:392–394. 1988. View Article : Google Scholar
|
|
47
|
Rosado A, Canto G, Veleiro B and Rodríguez
J: Toxic epidermal necrolysis after repeated injections of iohexol.
AJR Am J Roentgenol. 176:262–263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kanny G, Pichler W, Morisset M, Franck P,
Marie B, Kohler C, Renaudin JM, Beaudouin E, Laudy JS and
Moneret-Vautrin DA: T cell-mediated reactions to iodinated contrast
media: Evaluation by skin and lymphocyte activation tests. J
Allergy Clin Immunol. 115:179–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Voth M, Rosenberg M and Breuer J: Safety
of gadobutrol, a new generation of contrast agents: Experience from
clinical trials and postmarketing surveillance. Invest Radiol.
46:663–671. 2011.PubMed/NCBI
|
|
50
|
Lang DM, Alpern MB, Visintainer PF and
Smith ST: Elevated risk of anaphylactoid reaction from radiographic
contrast media is associated with both beta-blocker exposure and
cardiovascular disorders. Arch Intern Med. 153:2033–2040. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gonzalo-Garijo MA, de Argila D, Pimentel
JJ, Alejo M and García-Menaya JM: Skin reaction to contrast medium.
Allergy. 52:875–876. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dillman JR, Ellis JH, Cohan RH, Strouse PJ
and Jan SC: Frequency and severity of acute allergic-like reactions
to gadolinium-containing i.v. contrast media in children and
adults. AJR Am J Roentgenol. 189:1533–1538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hox V, Desai A, Bandara G, Gilfillan AM,
Metcalfe DD and Olivera A: Estrogen increases the severity of
anaphylaxis in female mice through enhanced endothelial nitric
oxide synthase expression and nitric oxide production. J Allergy
Clin Immunol. 135:729–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sze G, Brant-Zawadzki M, Haughton VM,
Maravilla KR, McNamara MT, Kumar AJ, Aisen AM, Dreisbach JN,
Bradley WG Jr and Weinreb JC: Multicenter study of gadodiamide
injection as a contrast agent in MR imaging of the brain and spine.
Radiology. 181:693–699. 1991. View Article : Google Scholar : PubMed/NCBI
|