Introduction
Experimental models serve important roles in
otological research, and animal models of the middle ear are
required for otorhinolaryngologists to gain experience and
competence, and to develop novel surgical techniques (1–3). The
most valuable animal models exhibit the greatest degree of
similarity with humans (4,5). At present, guinea pigs and rats are
mainly used in otological research, including ototoxictiy research
(6), sensorineural hearing loss
research (7), cochlear implantation
research (8) and gene therapy
research (9). However, the tympanic
cavity of these animals is frequently too small to perform middle
ear surgeries, thus it has been hypothesized that larger animal
models, including rabbits, sheep and pigs will more accurately
reflect the anatomy of the human ear and it will be easier to
perform middle ear surgeries (10–15). The
rabbit middle ear has been widely used for middle ear surgeries in
certain procedures, including osscicular chain restoration
(11,16,17) and
stapes surgery (18,19). The retroauricular surgical approach
is the most frequently applied method in rabbit middle ear surgery
(11). However, the anatomical
structure of the rabbit middle ear has not been well documented and
it has been reported that gaining access to this area via the
retroauricular surgical approach is challenging (11,16).
Thus, the current study aimed to investigate the middle ear
structure in rabbits and to develop a surgical approach appropriate
for middle ear surgery in rabbits.
Materials and methods
Eight New Zealand rabbits (age, 6 months; four males
and four females; weight, 2.0–2.5 kg), were obtained from the
Zhejiang Academy of Medical Sciences (Hangzhou, China). Animals
were maintained in a conventional animal environment (temperature,
26±2°C; humidity, 55±10%) with a 12-h light/dark cycle. Animals had
free access to water and were fed three times per day. Rabbits were
deeply anesthetized by intravenous injection of sodium pentothal
(~30 mg/kg) into the auricular vein of the left ear. In addition,
disappearance of the conjunctival reflex and the decrease in muscle
tension of four limbs were used as signs to stop the injection.
Rabbits were stabilized on a surgical table. The preauricular skin
was shaved and disinfected with 5% iodine in 95% ethanol. First, an
incision was made along the axis of the rabbit ear from the
auricular notch to the connective line between the cartilaginous
part and the bony part of the external auditory canal, and then the
auricle and the cartilaginous part of the external auditory canal
were dissected to clearly expose the tympanic membrane.
Subsequently, the external auditory canal skin flap was dissected
and the tympanic cavity was observed under the microscope. Lastly
the posterior and superior walls of the bony external auditory
canal were removed to investigate the anatomical structures in the
rabbit middle ear. The surgical procedures were approved by the
Institutional Animal Care and Use Committee of the Zhejiang Academy
of Medical Sciences. Operations were performed under a surgical
microscope and digital photomicrographs were captured using the
attached camera. Anatomical structures were measured using a (Shang
Guang, Inc., Shanghai, China), microscope graticules (Shang Guang,
Inc., Shanghai, China) and Image-Pro Plus software (version 6;
Media Cybernetics, Inc., Rockville, MD, USA). Data were analyzed
using SPSS software (version 13.0; SPSS, Inc., Chicago, IL,
USA).
Results
Anatomical structure of the external
auditory canal and the surgical approach into the middle ear in
rabbits
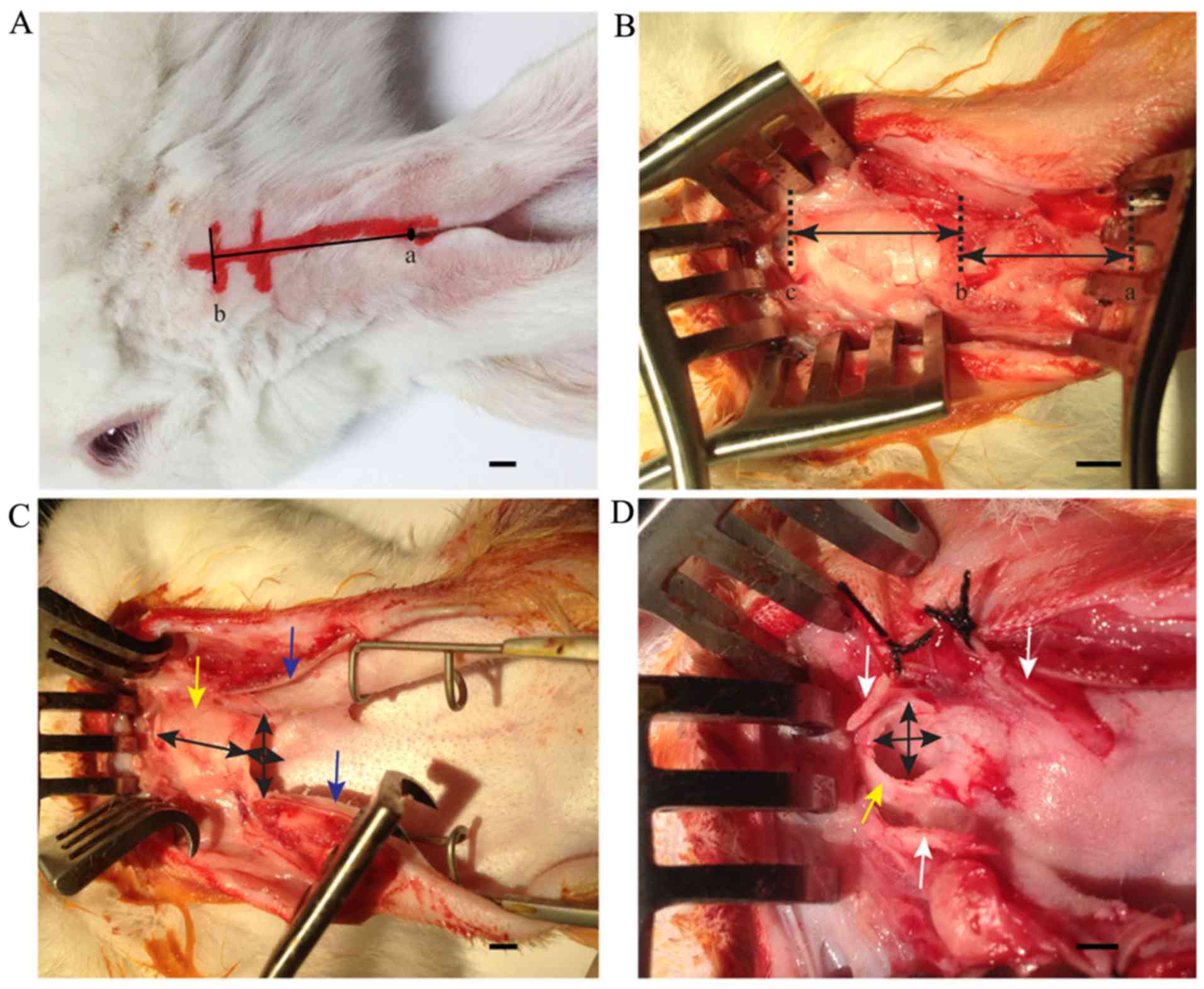
The transcanal surgical approach was applied to
study the middle ear anatomy in rabbits through an incision along
the axis of the rabbit ear from the auricular notch to the
connective line between cartilaginous part and bony part of the
external auditory canal (Fig. 1A).
The length of the auricular cartilage and the auditory canal
cartilage was measured (Fig. 1B).
Subsequently, the connection between the auricular cartilage and
the auditory canal cartilage was separated, and the auricular
cartilage was cut to measure the aperture of the cartilaginous
auditory canal (Fig. 1C). The
auditory canal cartilage was cut to measure the aperture of the
bony auditory canal (Fig. 1D).
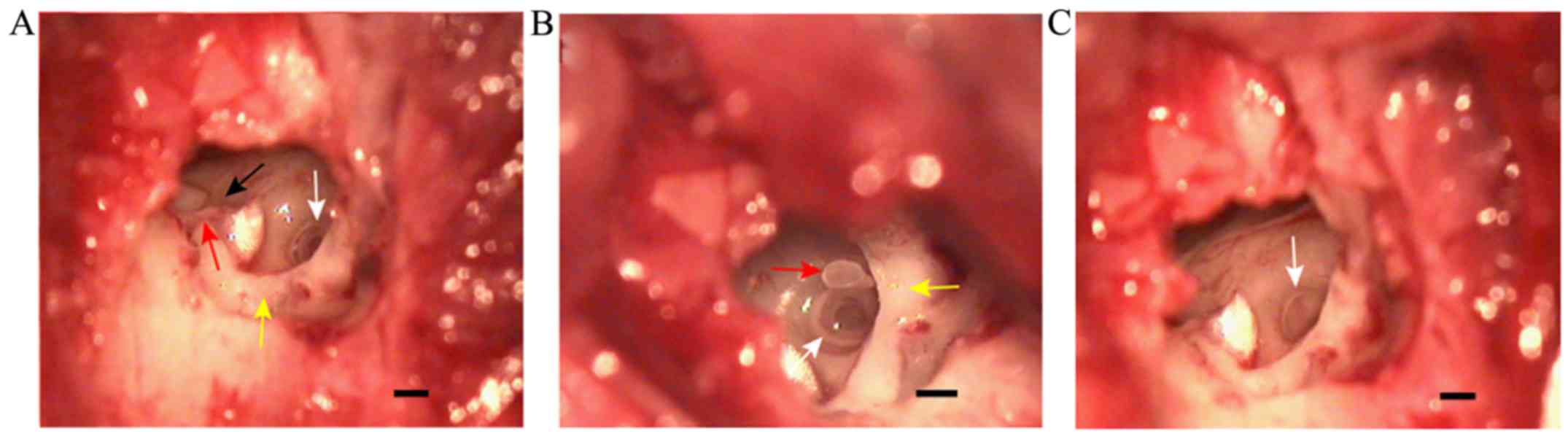
Observation of the tympanic membrane
in rabbits under a surgical microscope
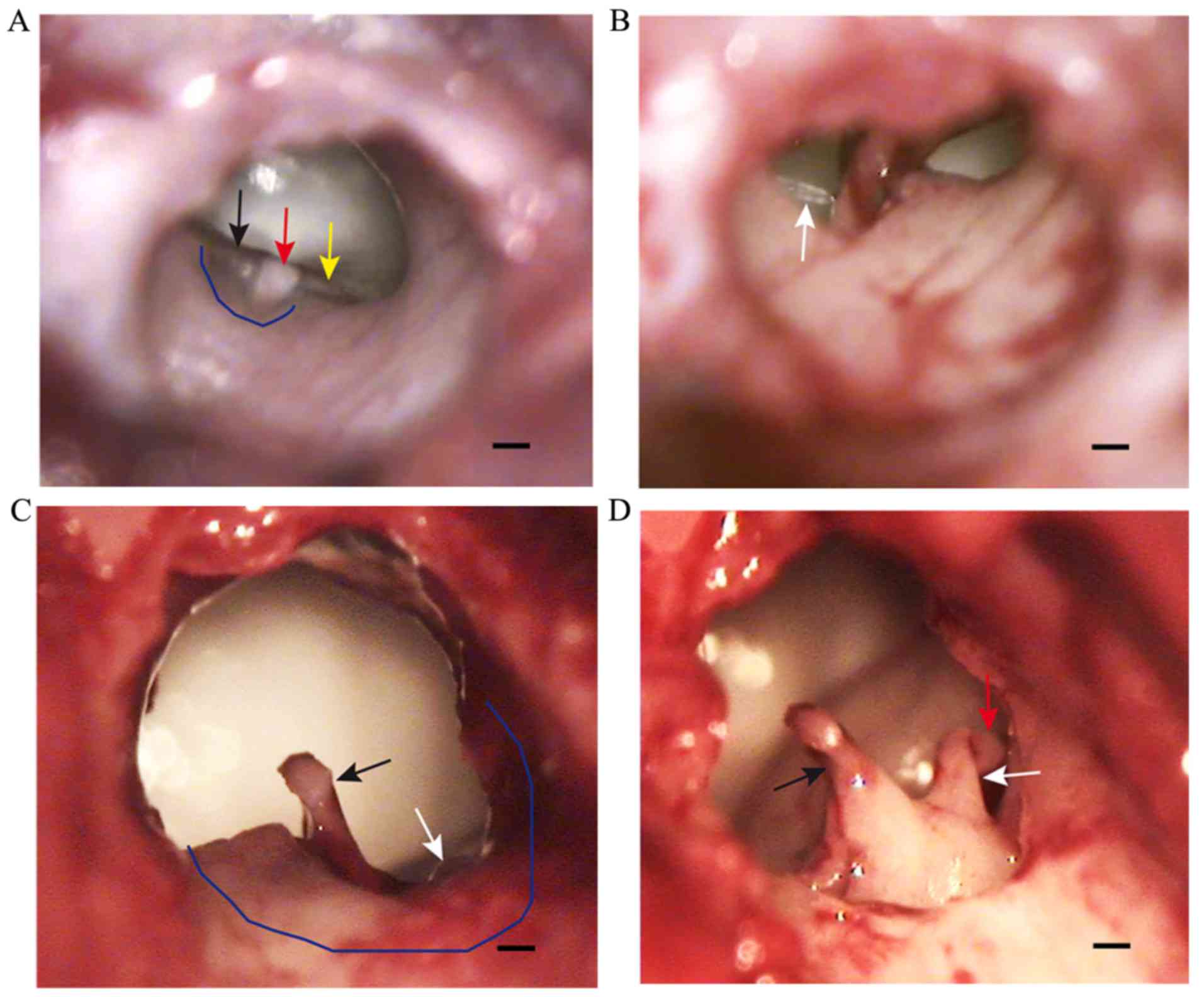
The tympanic incisure in the posterosuperior part of
the bony auditory canal was covered by pars flaccida, which was
thick and exhibited movement with respiration (Fig. 2A). Pars tensa was semicircular and
fixed, forming a sharp angle with the external auditory canal. This
portion of the tympanic membrane was thin and tightly connected
with the malleus, and the handle of malleus was observed through it
(Fig. 2A). The interface between
pars tensa and pars flaccida formed the anterior malleolar fold and
the posterior malleolar fold, which were visible beyond pars tensa
(Fig. 2A). After lifting the skin of
the external auditory canal, the handle of the malleus and the
chorda tympani nerve were observed (Fig.
2B). Furthermore, a part of the lenticular process of the incus
and the incudostapedial joint were observed following adjustment of
the microscopic angle (Fig. 2C). A
part of the posterosuperior wall of the auditory canal was removed
to completely expose the ossicular chain composed of malleus, incus
and stapes (Fig. 2D).
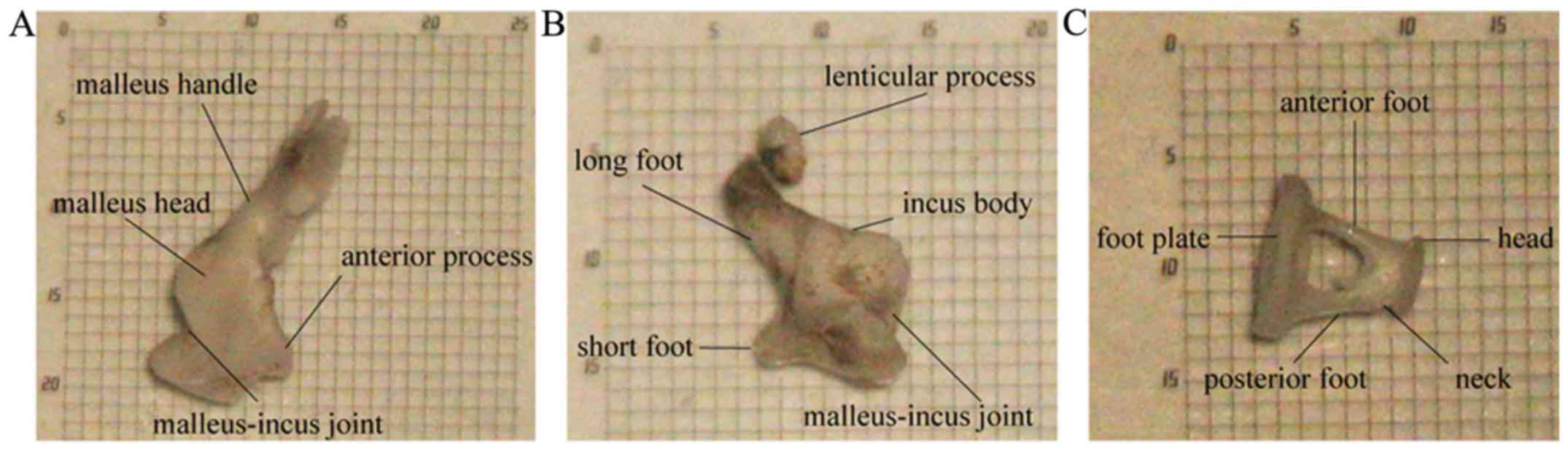
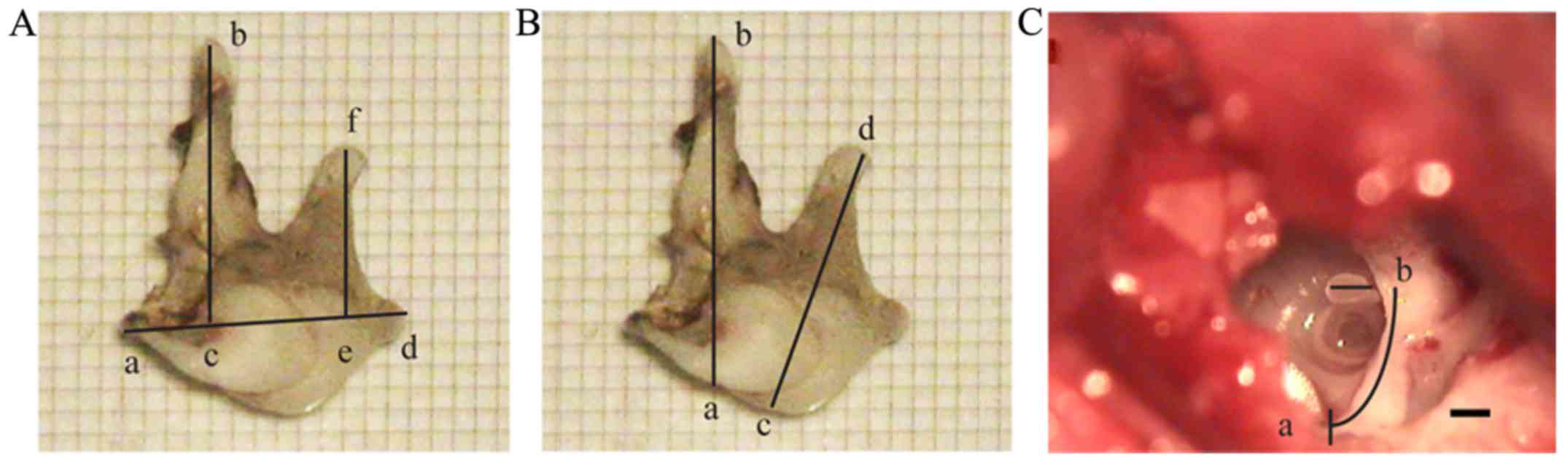
Morphology of the rabbit ossicular
chain
The structure of malleus included the head, neck,
handle and anterior process. The end of handle was bent outward
where the pars tensa connected (Fig.
3A). The incus consisted of the body, long foot, short foot and
lenticular process (Fig. 3B). The
stapes consisted of the head, neck, anterior foot, posterior foot
and footplate (Fig. 3C). The
stapedial footplate was arched, and its superior border was thick
on both sides and thin in the middle (Fig. 3C). The articular surface of the
malleus-incus joint was curved and tightly connected (Figs. 2D and 3A
and B). The incudostapedial joint was formed between the
lenticular process of the incus and the head of stapes and was
easily separated compared with humans (Figs. 2D and 3B).
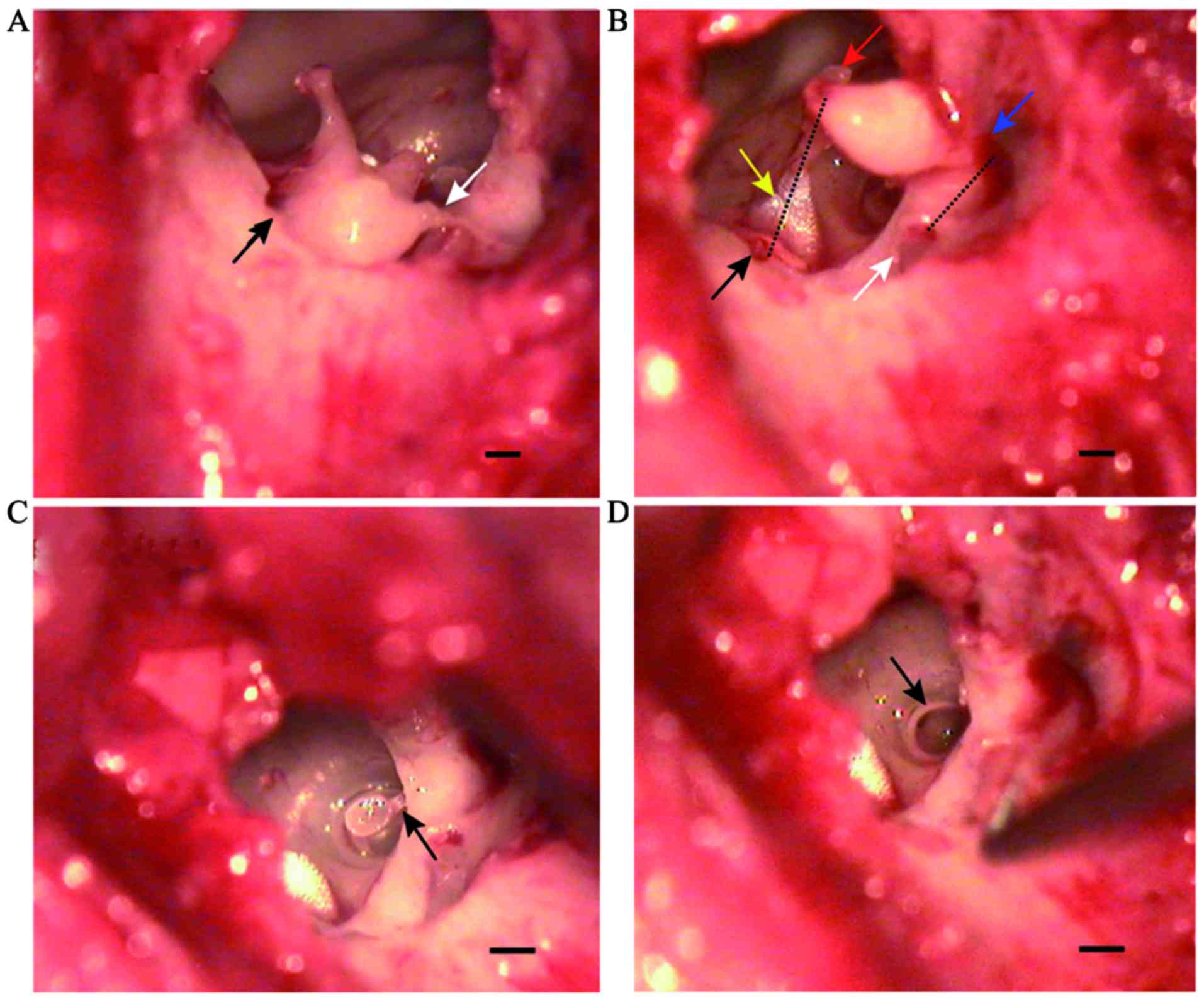
Ossicular ligament and ossicular
muscle in the middle ear
After separating the incudostapedial joint and
moving the auditory ossicular chain, the ligaments and muscles
connecting the auditory ossicles could be observed, including the
anterior ligament of malleus, posterior ligament of incus, annular
ligament of stapes, tensor tympani and stapedius. The anterior
ligament of malleus connected the end of the anterior process of
the malleus to the superior wall of the tympanic cavity.
Furthermore, the posterior ligament of the incus connected the end
of the short process of the incus to the posterior wall of the
incudal fossa located in close proximity to the facial nerve
(Fig. 4A and B). Tensor tympani
arose from the medial wall of the tympanic cavity located superior
to the tympanic ostium of the eustachian tube and ended at the
distal handle of the malleus (Fig.
4B). Stapedius arose from the posterior wall of the tympanic
cavity and ended in the posterior region of the stapes neck
(Fig. 4C). The annular ligament of
stapes was located around the footplate and connected to the
vestibular window (Fig. 4D).
Facial nerve, vestibular window and
tympanic ostium of the eustachian tube in the rabbit tympanic
cavity
Following dislocation of the incus and stapes, and
removal of the posterosuperior meatal wall, the facial nerve,
vestibular window and tympanic ostium of eustachian tube were
observed. The tympanic segment of the facial nerve was located
superior to the vestibular window (Fig.
5A). The mastoid segment of the facial nerve was located
posterior to the vestibular window and stapes (Fig. 5B). The vestibular window in the
medial wall of the tympanic cavity was oval-shaped (Fig. 5C). The tympanic ostium of the
eustachian tube was inferior to the semicanal of the tensor
tympani, which was open like a horn mouth (Fig. 5A).
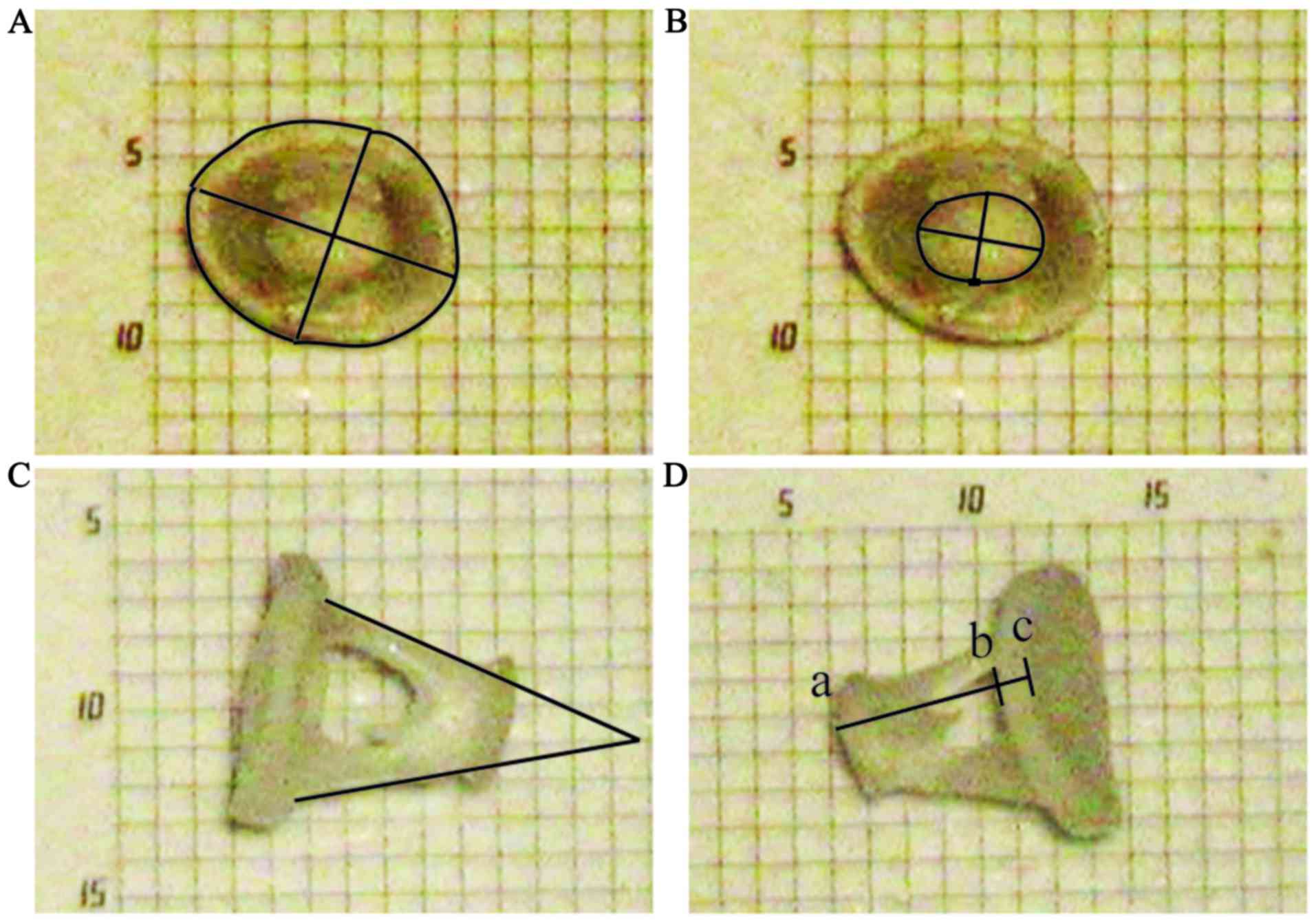
Measurement of anatomical structures
in the rabbit ear
The anatomical structures of the external auditory
canal in rabbits were measured as indicated in Fig. 1, and data are presented in Table I. The results demonstrated that the
length of the auditory canal auricular cartilagious part was
19.94±0.41 mm, the auditory canal cartilagious part was 19.23±0.82
mm and the auditory canal bony part was 10.50±0.50 mm.
Additionally, the height and transverse diameter of the auditory
canal cartilagious part were 6.39±0.61 and 8.96±0.55 mm,
respectively, and the height and transverse diameter of the
auditory canal bony part were 5.04±0.34 and 5.61±0.20 mm,
respectively. In addition, the anatomical structure of the middle
ear was measured as illustrated in Figs.
6 and 7, and the results are
presented in Table II. The results
demonstrated that the length of the lever arm of malleus was
2.57±0.05 mm, while the length of the lever arm of incus was
1.55±0.05 mm; therefore the leverage ratio of the ossicular chain
was 1.66:1. The malleus length was 3.22±0.07 mm, the incus length
was 2.49±0.03 mm, and the footplate length, width, area, perimeter
and thickness were 1.44±0.01, 1.10±0.02 mm, 1.14±0.02
mm2, 4.04±0.03 and 0.18±0.01 mm, respectively. The
stapes foot angle, height, head area, length and head width were
76.28±2.54°, 1.02±0.01 mm, 0.21±0.01 mm2, 0.66±0.01 and
0.46±0.02 mm, respectively. Additionally, the facial nerve canal
diameter and length were 0.79±0.03 and 2.74±0.07 mm,
respectively.
 | Table I.External auditory canal structure in
rabbits. |
Table I.
External auditory canal structure in
rabbits.
| Structure | Dimensions (mm) |
|---|
| Length of the
auditory canal auricular cartilagious part | 19.94±0.41
(19.25–20.85) |
| Length of the
auditory canal cartilagious part | 19.23±0.82
(17.90–20.87) |
| Length of the
auditory canal bony part | 10.50±0.50
(9.50–11.10) |
| Height of the
auditory canal cartilagious part | 6.39±0.61
(5.34–7.34) |
| Transverse diameter
of the auditory canal cartilagious part | 8.96±0.55
(8.11–9.89) |
| Height of the
auditory canal bony part | 5.04±0.34
(4.45–5.74) |
| Transverse diameter
of the auditory canal bony part | 5.61±0.20
(5.25–6.03) |
 | Table II.Middle ear structure in rabbits. |
Table II.
Middle ear structure in rabbits.
| Structure | Dimensions |
|---|
| Malleus lever
arm | 2.57±0.05
(2.51–2.62) |
| Incus lever arm | 1.55±0.05
(1.49–1.60) |
| Malleus length | 3.22±0.07
(3.08–3.32) |
| Incus length | 2.49±0.03
(2.40–2.54) |
| Footplate length | 1.44±0.01
(1.43–1.47) |
| Footplate width | 1.10±0.02
(1.08–1.14) |
| Footplate area | 1.14±0.02
(1.10–1.16)b |
| Footplate
perimeter | 4.04±0.03
(3.96–4.09) |
| Footplate
thickness | 0.18±0.01
(0.16–0.20) |
| Stapes foot
angle | 76.28±2.54
(72.41–80.82)c |
| Stapes height | 1.02±0.01
(1.01–1.04) |
| Stapes head
area | 0.21±0.01
(0.20–0.23)b |
| Stapes head
length | 0.66±0.01
(0.64–0.68) |
| Stapes head
width | 0.46±0.02
(0.42–0.48) |
| Facial nerve canal
diameter | 0.79±0.03
(0.76–0.85) |
| Facial nerve canal
lengtha | 2.74±0.07
(2.60–2.89) |
Discussion
The surgical approach to the middle ear was
performed transcanally in the present study. Rabbit skin was cut
vertically from the auricular notch to the boundary between the
cartilaginous and the bony part of the external auditory canal. The
auricle and the cartilaginous part of the external auditory canal
were dissected to clearly expose the tympanic membrane. Secondly,
we dissected the external auditory canal skin flap and observed the
tympanic cavity under the microscope. The posterior and superior
walls of the bony external auditory canal were removed to
investigate the malleus, incus and stapes. These surgical
procedures were performed without complications and have numerous
advantages. The anatomical landmarks in the auricle and the
external auditory canal were apparent, making it easily to locate
the incision, which was along the axis of the rabbit ear from the
auricular notch to the connective line between the cartilaginous
part and the bony part of the external auditory canal, and repair
the wound. No large vessels were present in the surgical zone and
the bleeding during surgery was minor. Furthermore, removal of
large bony sections of the external auditory canal or the mastoid
was not required, which was performed in the retroauricular
surgical approach but not the trancanal surgical approach, thus
shortening the duration of surgery and avoiding severe tissue
injury. There results indicate that the transcanal surgical
approach was a suitable option to study the middle ear anatomy in
rabbits.
The anatomical structure of the external and middle
ear in rabbits was similar to the human anatomy (11); however, certain unique features were
identified. The external ear could be separated into three parts,
including the auricular cartilage part, auditory canal
cartilaginous part and auditory canal bony part, respectively. The
cross-section shape of the auditory canal was oval and markedly
narrower inside than outside. The height of the cartilaginous part
of the auditory canal was ~6.39±0.61 mm and the height of the bony
part was ~5.04±0.34 mm (Table I).
The tympanic membrane was hardly accessible unless the
cartilaginous part of the auditory canal was opened and
retracted.
The tympanic membrane in rabbits and humans
consisted of two parts, including pars tensa and pars flaccida.
Pars tensa in rabbits was fixed and formed a sharp angle with the
external auditory canal, which was thin and tightly attached to the
malleus, making it harder to separate them. Pars flaccida was
moving with respiration, and, therefore, it was hypothesized that
the eustachian tube may be constantly open in rabbits, and the
movement of pars flaccida may balance the air pressure in the
tympanic cavity. Furthermore, the authors of the current study
hypothesized that the accessible eustachian tube increased the
susceptibility of rabbits to media otitis, suggesting that rabbits
may be used to establish animal models of media otitis. The
interface between pars tensa and pars flaccida was more visible
compared with the human equivalent, as the anterior malleolar fold
and posterior malleolar fold projected beyond pars tensa.
Furthermore, a tympanic incisure was identified in the
posterosuperior zone of the bony part of the external auditory
canal covered by pars flaccida.
The incus and stapes remained invisible until the
posterosuperior bony wall of the auditory canal was partially
removed. The connection between the incus and stapes was easily
discontinued, enabling the dislocation of the stapes from the
vestibular window. Therefore, the auditory ossicles should be
manipulated with care.
The constitution and leveraging capabilities of the
middle ear ossicles in rabbits were similar to humans. As in
humans, the ossicular chain in rabbits was composed of the malleus,
incus and stapes. The shapes of these three auditory ossicles in
rabbits and human were also quite similar. The structure of the
malleus could be divided into the head, neck, handle and anterior
process. The incus was composed of the body, long foot, short foot
and lenticular process. Furthermore, the stapes consisted of the
head, neck, anterior foot, posterior foot and footplate. The
leverage ratio of the ossicular chain in rabbits was ~1.66:1,
similar to the 1.31:1 ratio of the human ossicular chain (20). The area of the footplate in rabbits
was ~1.14 mm2, markedly smaller compared with 2.97–3.03
mm2 in humans (21–23);
however, larger compared with 0.79 mm2 in guinea pigs
(21). The ossicular chain in guinea
pigs is fused at the incus-malleus level (24). Furthermore, the carotid artery in
humans is located in anterior and inferior aspect of basal turn of
the cochlea, whereas the carotid artery in rats passes along the
base of the cochlea;, thus, exposure of the oval window by removing
the stapes foot in rats can cause hemorrhages and animal mortality
(25–27). The conduction of sound is the main
function of the ossicular chain (27). Due to the similarities in the
constitution, leverage ratio and morphology of the ossicular chain
between rabbits and humans, rabbits may serve as a model for stapes
surgeries, including stapedectomies and stapedotomies, and
ossiculoplasties. This model may be used to investigate novel
surgical techniques or test new materials for reconstruction of the
middle ear.
Ligaments were also observed in the middle ear
cavity of rabbits used in the current study. The anterior ligament
of malleus connected the end of the short process of malleus to
tegmen tympani. The posterior ligament of incus connected the end
of the short process of incus to the posterior wall of incudal
fossa located in close proximity to the facial nerve. The annular
ligament of stapes connected the foot plate of stapes to the
vestibular window. Furthermore, two muscles of auditory ossicles
were identified in the middle ear cavity of animals used in the
current study. Tensor tympani began at the medial wall of the
tympanic cavity superior to the tympanic ostium of eustachian tube
and ended at the distal handle of malleus. The stapedius began at
the posterior wall of the tympanic cavity and ended at the
posterior region of the neck of stapes. Like in humans, these
ligaments and muscles in rabbits may contribute to the
stabilization of the ossicular chain and protection of the inner
ear from loud sounds.
The facial nerve canal was exposed following removal
of the bony part of the posterosuperior wall of the auditory canal,
as there was space between the mastoid segment of the facial nerve
and the posterior wall of the external auditory canal. The facial
nerve canal was prominent and identified easily. Therefore, the
rabbit middle ear may serve as a model for facial nerve research,
including facial nerve decompression and facial nerve grafting. The
vestibular window in rabbits was located inferior to the facial
nerve tympanic segment and anterior to the facial nerve mastoid
segment as in humans, enabling its complete exposure.
In conclusion, the transcanal surgical approach to
the middle ear in rabbits was performed without complications and
was suitable to study the middle ear. The anatomical structure of
the middle ear in rabbits was similar to the human anatomy;
however, the facial nerve canal was more prominent and easily
identifiable. The results of the current study indicated that the
rabbit middle ear may serve as a model for ossicular surgery and
facial nerve research.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Medical and Health Foundation of Zhejiang Province (grant no.
2012KYB146) and the Medical and Health Foundation of Hangzhou City
(grant no. 2013A11).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XG and YL designed the experiments. MG, JZ, YJ, XC,
and XL performed the experiments. MG, JZ, YJ, XC, XL analyzed the
data. MG, JZ, XG, and YL wrote the manuscript.
Ethics approval and consent to
participate
Surgical procedures were approved by the
Institutional Animal Care and Use Committee of the Zhejiang Academy
of Medical Sciences (Hangzhou, China).
Patient consent for publication
Not applicable.
Competing of interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamamoto-Fukuda T, Takahashi H and Koji T:
Animal models of middle ear cholesteatoma. J Biomed Biotechnol.
2011:3942412011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergin MJ, Bird PA, Vlajkovic SM and
Thorne PR: High frequency bone conduction auditory evoked
potentials in the guinea pig: Assessing cochlear injury after
ossicular chain manipulation. Hear Res. 330:147–154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park MK and Lee BD: Development of animal
models of otitis media. Korean J Audiol. 17:9–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seibel VA, Lavinsky L and De Oliveira JA:
Morphometric study of the external and middle ear anatomy in sheep:
A possible model for ear experiments. Clin Anat. 19:503–509. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okada DM, de Sousa AM, Huertas Rde A and
Suzuki FA: Surgical simulator for temporal bone dissection
training. Braz J Otorhinolaryngol. 76:575–578. 2010.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang ZJ, Guan HX, Yang K, Xiao BK, Liao
H, Jiang Y, Zhou T and Hua QQ: Dose-dependent effects of ouabain on
spiral ganglion neurons and Schwann cells in mouse cochlea. Acta
Otolaryngol. 137:1017–1023. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kujawa SG and Liberman MC: Synaptopathy in
the noise-exposed and aging cochlea: Primary neural degeneration in
acquired sensorineural hearing loss. Hear Res. 330:191–199. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Attias J, Hod R, Raveh E, Mizrachi A,
Avraham KB, Lenz DR and Nageris BI: Hearing loss patterns after
cochlear implantation via the round window in an animal model. Am J
Otolaryngol. 37:162–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isgrig K, Shteamer JW, Belyantseva IA,
Drummond MC, Fitzgerald TS, Vijayakumar S, Jones SM, Griffith AJ,
Friedman TB, Cunningham LL and Chien WW: Gene therapy restores
balance and auditory functions in a mouse model of usher syndrome.
Mol Ther. 25:780–791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turck C, Brandes G, Krueger I, Behrens P,
Mojallal H, Lenarz T and Stieve M: Histological evaluation of novel
ossicular chain replacement prostheses: An animal study in rabbits.
Acta Otolaryngol. 127:801–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stieve M, Hedrich HJ, Battmer RD, Behrens
P, Müller P and Lenarz T: Experimental middle ear surgery in
rabbits: A new approach for reconstructing the ossicular chain. Lab
Anim. 43:198–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto K, Hama T, Yamato M, Uchimizu H,
Sugiyama H, Takagi R, Yaguchi Y, Okano T and Kojima H: The effect
of transplantation of nasal mucosal epithelial cell sheets after
middle ear surgery in a rabbit model. Biomaterials. 42:87–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cordero A, Benitez S, Reyes P, Vaca M,
Polo R, Pérez C, Alonso A and Cobeta I: Ovine ear model for fully
endoscopic stapedectomy training. Eur Arch Otorhinolaryngol.
272:2167–2174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Péus D, Dobrev I, Prochazka L, Thoele K,
Dalbert A, Boss A, Newcomb N, Probst R, Röösli C, Sim JH, et al:
Sheep as a large animal ear model: Middle-ear ossicular velocities
and intracochlear sound pressure. Hear Res. 351:88–97. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoffstetter M, Lugauer F, Kundu S, Wacker
S, Perea-Saveedra H, Lenarz T, Hoffstetter P, Schreyer AG and
Wintermantel E: Middle ear of human and pig: A comparison of
structures and mechanics. Biomed Tech (Berl). 56:159–165. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ráth G, Kereskai L, Bauer M, Bakó P,
Bányavölgyi V and Gerlinger I: Should the ossicle be denuded prior
to the application of glass ionomer cement? An experimental study
on rabbit. Eur Arch Otorhinolaryngol. 269:773–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun JJ and Li XS: A study on
reconstruction of ossicular chain by an in situ bone tissue
engineering technique. Acta Otolaryngol. 129:507–511. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peacock J, Pintelon R and Dirckx J:
Nonlinear vibration response measured at umbo and stapes in the
rabbit middle ear. J Assoc Res Otolaryngol. 16:569–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lupo JE, Koka K, Holland NJ, Jenkins HA
and Tollin DJ: Prospective electrophysiologic findings of round
window stimulation in a model of experimentally induced stapes
fixation. Otol Neurotol. 30:1215–1224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hemilä S, Nummela S and Reuter T: What
middle ear parameters tell about impedance matching and high
frequency hearing. Hear Res. 85:31–44. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sim JH, Röösli C, Chatzimichalis M, Eiber
A and Huber AM: Characterization of stapes anatomy: Investigation
of human and guinea pig. J Assoc Res Otolaryngol. 14:159–173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sim JH, Chatzimichalis M, Lauxmann M,
Röösli C, Eiber A and Huber AM: Complex stapes motions in human
ears. J Assoc Res Otolaryngol. 11:329–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sim JH, Lauxmann M, Chatzimichalis M,
Röösli C, Eiber A and Huber AM: Errors in measurement of
three-dimensional motions of the stapes using a laser Doppler
vibrometer system. Hear Res. 270:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mason MJ: Of mice, moles and guinea pigs:
Functional morphology of the middle ear in living mammals. Hear
Res. 301:4–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Judkins RF and Li H: Surgical anatomy of
the rat middle ear. Otolaryngol Head Neck Surg (1979).
1179:557–558. 1998.
|
|
26
|
Pinilla M, Ramírez-Camacho R, Jorge E,
Trinidad A and Vergara J: Ventral approach to the rat middle ear
for otologic research. Otolaryngol Head Neck Surg. 124:515–517.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Albuquerque AA, Rossato M, Oliveira JA and
Hyppolito MA: Understanding the anatomy of ears from guinea pigs
and rats and its use in basic otologic research. Braz J
Otorhinolaryngol. 75:43–49. 2009. View Article : Google Scholar : PubMed/NCBI
|