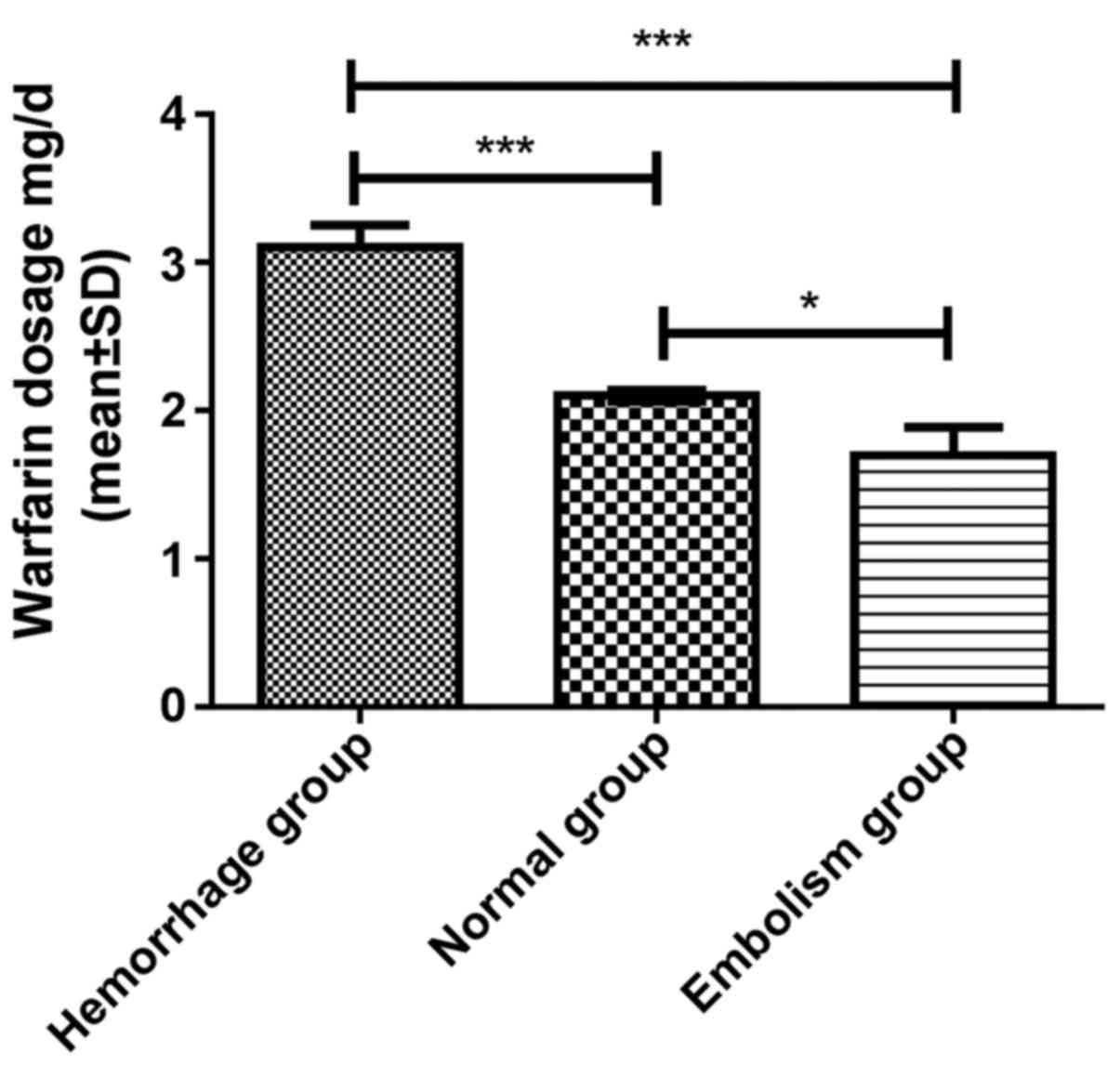
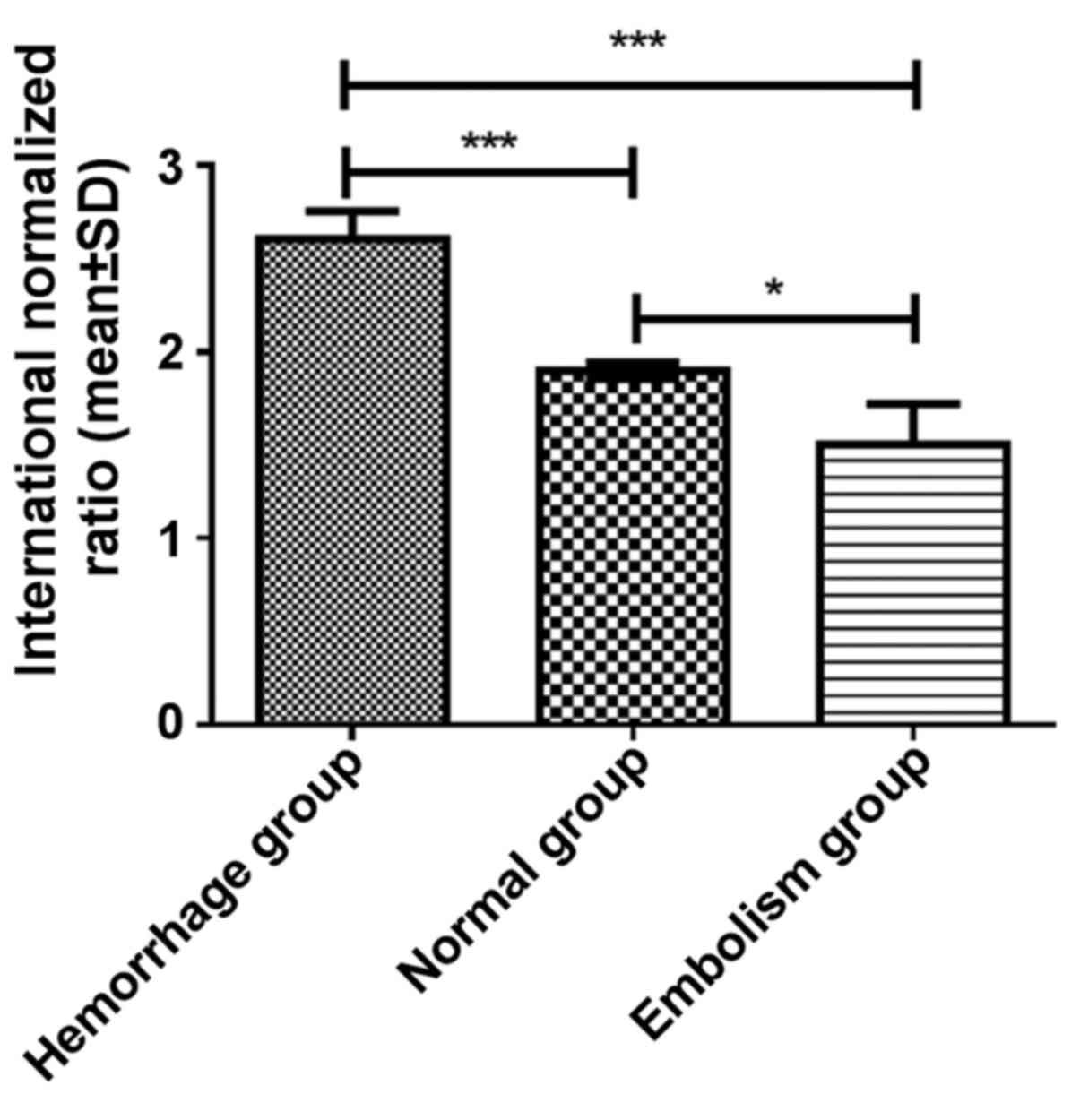
|
1
|
Coffey S, Harper AR, Cairns BJ, Roberts IS
and Prendergast BD: Clinical information has low sensitivity for
postmortem diagnosis of heart valve disease. Heart. 103:1031–1035.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chambers JB, Prendergast B, Iung B,
Rosenhek R, Zamorano JL, Piérard LA, Modine T, Falk V, Kappetein
AP, Pibarot P, et al: Standards defining a ‘Heart Valve Centre’:
ESC Working Group on Valvular Heart Disease and European
Association for Cardiothoracic Surgery Viewpoint. Eur Heart J.
38:2177–2183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schaefer A, Sill B, Schoenebeck J,
Schneeberger Y, Reichenspurner H and Gulbins H: Failing stentless
Bioprostheses in patients with carcinoid heart valve disease. J
Cardiothorac Surg. 10:412015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duffy N, Xue YQ and Hughes L: Prosthetic
heart valve devices and methods of valve repair. US Patent
US9795482B2. Filed April 27, 2010; issued October 24, 2017.
|
|
5
|
Chikwe J, Chiang YP, Egorova NN, Itagaki S
and Adams DH: Survival and outcomes following bioprosthetic vs
mechanical mitral valve replacement in patients aged 50 to 69
years. JAMA. 313:1435–1442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spenser B, Benichou N, Bash A and Zakai A:
Prosthetic heart valve and method. US Patent US 20170224481 A1.
Filed April 24, 2017; issued August 10, 2017.
|
|
7
|
Eikelboom JW, Connolly SJ, Brueckmann M,
Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K,
Friedman J, Guiver K, et al: Dabigatran versus warfarin in patients
with mechanical heart valves. N Engl J Med. 369:1206–1214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwann TA, Habib RH, Suri RM, Brennan JM,
He X, Thourani VH, Engoren M, Ailawadi G, Englum BR, Bonnell MR and
Gammie JS: Variation in warfarin use at hospital discharge after
isolated bioprosthetic mitral valve replacement: An analysis of the
society of thoracic surgeons adult cardiac surgery database. Chest.
150:597–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gudmundsdottir BR, Jonsson PI and
Onundarson PT: INR and vitamin K Dependent coagulation factor
fluctuation during warfarin initiation and stable therapy in
patients dosed with the fiix-prothrombin time or the quick
prothrombin time. The Fiix Trial. Blood. 124:42782014.
|
|
10
|
Qian X: Influence of pharmacist
intervention on the knowledge of anticoagulation therapy with
warfarin by the patient. Pharmacotherapy. 35:2015.
|
|
11
|
Nakano T, Nakamura T, Nakamura Y, Irie K,
Sato K, Matsuo K, Imakyure O, Ogata K, Mishima K and Kamimura H:
Effects of Teicoplanin on the PT-INR controlled by warfarin in
infection patients. Yakugaku Zasshi. 137:909–916. 2017.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang X, Li Z, Zhao X, Wang C, Liu L, Wang
C, Pan Y, Li H, Wang D, Hart RG, et al: Use of warfarin at
discharge among acute ischemic stroke patients with nonvalvular
atrial fibrillation in China. Stroke. 47:464–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong L, Shi YK, Xu JP, Zhang EY, Liu JC,
Li YX, Ni YM, Yang Q, Han T, Fu B, et al: The multicenter study on
the registration and follow-up of low anticoagulation therapy for
the heart valve operation in China. Zhonghua Yi Xue Za Zhi.
96:1489–1494. 2016.(In Chinese). PubMed/NCBI
|
|
14
|
Parks T, Mirabel MM, Kado J, Auckland K,
Nowak J, Rautanen A, Mentzer AJ, Marijon E, Jouven X, Perman ML, et
al: Association between a common immunoglobulin heavy chain allele
and rheumatic heart disease risk in Oceania. Nat Commun.
8:149462017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zuhlke L, Engel ME, Karthikeyan G,
Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A,
Daniels R, et al: Characteristics, complications, and gaps in
evidence-based interventions in rheumatic heart disease: The Global
Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J.
36:1115–1122a. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matchar DB, Love SR, Jacobson AK, Edson R,
Uyeda L, Phibbs CS and Dolor RJ: The impact of frequency of patient
self-testing of prothrombin time on time in target range within VA
Cooperative Study #481: The Home INR Study (THINRS), a randomized,
controlled trial. J Thromb Thrombolysis. 40:17–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mcdowell TY, Lawrence J, Florian J,
Southworth MR, Grant S and Stockbridge N: Relationship between
International normalized ratio and outcomes in modern trials with
warfarin controls. Pharmacotherapy. 38:899–906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van de Werf F, Brueckmann M, Connolly SJ,
Friedman J, Granger CB, Härtter S, Harper R, Kappetein AP, Lehr T,
Mack MJ, et al: A comparison of dabigatran etexilate with warfarin
in patients with mechanical heart valves: THE Randomized, phase II
study to evaluate the safety and pharmacokinetics of oral
dabigatran etexilate in patients after heart valve replacement
(RE-ALIGN). Am Heart J. 163:931–937.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Huang X and Bao J: Application
and discussion of health education in Warfarin therapy of atrial
fibrillation patients. China Med Herald (Zhong Guo Yi Yao Dao Bao).
5:0992011.(In Chinese).
|
|
20
|
Liu Y, Yu XY, Zhong SL, Yang M, Tan HH,
Fei HW and Chen JY: Clinical application of anticoagulation
treatment with warfarin after prosthetic heart valve replacement: A
single center-based survey. Nan Fang Yi Ke Da Xue Xue Bao.
30:2242–2245. 2010.(In Chinese). PubMed/NCBI
|
|
21
|
Go AS, Singer DE, Toh S, Cheetham TC,
Reichman ME, Graham DJ, Southworth MR, Zhang R, Izem R, Goulding
MR, et al: Outcomes of Dabigatran and warfarin for atrial
fibrillation in contemporary practice: A retrospective cohort
study. Ann Intern Med. 167:845–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan Y, Dong L, Zhang D, Xiang D and Li Y:
Correlation between demographic factors and warfarin stable dosage
in population of Western China. Biomed Res. 28:8249–8253. 2017.
|
|
23
|
Yu Z, Ding YL, Lu F, Miao LY, Shen ZY and
Ye WX: Warfarin dosage adjustment strategy in Chinese population.
Int J Clin Exp Med. 8:9904–9910. 2015.PubMed/NCBI
|
|
24
|
You JH, Chan FW, Wong RS and Cheng G: Is
INR between 2.0 and 3. 0 the optimal level for Chinese patients on
warfarin therapy for moderate-intensity anticoagulation? Br J Clin
Pharmacol. 59:582–587. 2005.PubMed/NCBI
|
|
25
|
Volpp KG, Loewenstein G, Troxel AB, Doshi
J, Price M, Laskin M and Kimmel SE: A test of financial incentives
to improve warfarin adherence. BMC Health Serv Res. 8:2722008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larsen TB, Rasmussen LH, Skjøth F, Due KM,
Callréus T, Rosenzweig M and Lip GY: Efficacy and safety of
dabigatran etexilate and warfarin in ‘real-world’ patients with
atrial fibrillation: A prospective nationwide cohort study. J Am
Coll Cardiol. 61:2264–2273. 2013. View Article : Google Scholar : PubMed/NCBI
|