Introduction
Interruption of blood supply to tissues results in
ischemic injury. However, restoration of the blood supply initiates
a chain of events that may result in additional cell injury, which
is known as reperfusion injury (1,2).
Myocardial ischemia-reperfusion (I/R) injury has crucial roles in
inducing cardiomyocyte apoptosis, necrosis and left ventricular
remodeling, which can result in irreversible consequences,
including myocardial infarction, progressive deterioration of
cardiac function and heart failure (3,4). In
myocardial I/R injury, redox imbalances trigger a number of
signaling pathways mediated by free radicals including reactive
nitrogen species (RNS) and reactive oxygen species (ROS), such as
superoxide anions, hydroxyl radicals, hydrogen peroxide, nitric
oxide and peroxynitrite (5–7). In myocardial ischemia, hypoxia and
reoxygenation, which may induce the excessive production of RNS and
ROS in cardiac tissues, are principal causes of reperfusion injury
(8). Free radicals are typically
scavenged by antioxidant enzymes, including superoxide dismutase
(SOD), catalase and glutathione peroxidase (GPx), which serve
important roles in the prevention of lipid peroxidation (9,10).
Furthermore, previous studies have revealed that the antioxidant
activities of these enzymes may be reduced in patients following
myocardial infarction or in those with ischemic heart disease
(11,12).
Previous studies have reported some effective
strategies for protecting the heart against I/R injury in rat
models; however, these strategies have been mostly unsatisfactory
in limiting oxidative damage in clinical trials (13–15).
Ebselen [2-phenyl-1, 2-benzisoselenazol-3(2H)-one] is a
selenium-containing organic compound that is described as a GPx
mimic and has protective effects against oxidative injury (16,17).
Since oxygen free radical scavengers serve an important role in the
attenuation of I/R injury, the present study evaluated the effects
of ebselen, a potent scavenger of peroxynitrite, against myocardial
I/R injury in a rat model.
Materials and methods
Animals and myocardial I/R model
A total of 32 male Sprague-Dawley rats (260–300 g,
3–4 weeks old) were obtained from the Laboratory Animal Center of
Wuhan University, Wuhan, China. The rats were maintained with
standard feeding conditions including adequate food and water ad
libitum, a 12 h light/dark, 20–25°C and 50–65% humidity.
Following a 12-h fasting period, all rats were anesthetized with
10% ketamine (80 mg/kg; Jiangsu Hengrui Pharmaceutical Co., Ltd.,
Lianyungang, China) and 2% xylazine (5 mg/kg; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) by intraperitoneal injection.
Subsequently, surgery was performed on rats at room temperature
(22–24°C). Following skin incision, the hearts were exposed through
a left thoracotomy in the fourth intercostal space. A 6-0 silk
suture was placed l-2 cm from the root of the left anterior
descending coronary artery. The suture was loosened following
occlusion for 30 min, which was followed by 2 h of reperfusion. The
same surgical procedures without ligation were performed on rats in
a sham group. Following this, the skin was sutured, anesthesia was
discontinued and the rats were put into pre-warmed cages for
recovery. Rats were sacrificed by cervical dislocation under
anesthesia with 5.0% isoflurane at the end of reperfusion ~3 h
after surgery. Blood samples and heart tissues were collected for
further analysis.
All experiments were performed in accordance with
protocols approved by the Laboratory Animal Ethics Committee of
Wuhan University. All experimental animals received care in
compliance with the Guide for the Care and Use of Laboratory
Animals of the National Institute of Health (18).
Experimental procedures
Rats were equally and randomly divided into four
groups as follows: A sham-operated group (sham group, n=8); an I/R
group (n=8); an ebselen + I/R group (20 mg/kg ebselen treatment
prior to I/R, n=8); and an ebselen control group (20 mg/kg ebselen
treatment with sham operation, n=8). As aforementioned, in the sham
group the rats underwent sham operation, and in the I/R group the
rats were subjected to 30 min of ischemia followed by 2 h of
reperfusion. In the ebselen + I/R group, the rats received 20 mg/kg
ebselen (MedChemExpress, Monmouth Junction, NJ, USA)
intragastrically 24 h prior to the I/R-inducing surgery and
throughout the experimental period. In the ebselen control group,
rats received ebselen intragastrically and underwent the sham
operation.
Determination of myocardial infarct
size
Myocardial infarct size was estimated using
triphenyltetrazolium chloride (TTC; Sigma-Aldrich; Merck KGaA)
staining. Heart tissues were perfused with saline to wash out the
remaining blood from the coronary vasculature. Then, each heart was
sectioned horizontally into 1 mm-thick slices, which were stained
with 1% TTC for 20 min at 37°C and fixed by immersion in 10%
neutral buffered formalin for 6 h at room temperature. Finally, the
infarct area (IA) and area at risk (AAR) were determined using
Image J software V1.8.0 (National Institutes of Health, Bethesda,
MD, USA). The infarct size was presented as a percentage of the AAR
(IA/AAR).
Echocardiography
Echocardiography was performed in rats for
assessment of cardiac function prior to and following myocardial
I/R injury. Rats were anesthetized with 5.0% isoflurane and placed
supinely on a heating pad, which maintained rat body temperature at
36–37°C. Anesthesia was maintained by inhalation of 2% isoflurane,
which was driven by 100% oxygen flow and ventilated using a small
animal respirator. Two-dimensional M-mode echocardiograms and
pulsed-wave Doppler spectral tracings were obtained using a Vivid 7
echocardiography machine (General Electric Healthcare Corporation,
Waukesha, WI, USA) equipped with a 10 MHz-phased array transducer.
Following this, diastolic left ventricular posterior wall
thickness, systolic left ventricular posterior wall thickness, left
ventricle diastolic dimension and left ventricle systolic dimension
were measured using M-mode tracings. In addition, ejection fraction
(EF%) and percent fractional shortening (%FS) were calculated.
Histopathological examination of
myocardium
Heart tissue samples were obtained and preserved in
2% glutaraldehyde solution for histopathological examination at the
end of myocardial reperfusion. For this, heart tissue specimens
were transferred into 70% ethanol, embedded in paraffin, sectioned
into 5 µm-thick sections, stained with hematoxylin for 5 min and
eosin for 3 min at room temperature, and examined by light
microscopy.
Electron microscopy analysis
Heart tissue samples were obtained at the end of
reperfusion and immediately preserved in 2% glutaraldehyde
solution. Samples were fixed in 1% osmic acid for 2–3 h at 4°C,
embedded in Epon 812 epoxy resin overnight at 37°C and cut into 5
µm-thick sections. Sections were prepared with acetate double
oxygenic uranium for 30 min and citrate lead stain for 10 min at
room temperature. Following this, the sections were examined using
a JEM-1230 transmission electron microscope (BD Biosciences, Tokyo,
Japan).
Terminal dUTP nick end-labelling
(TUNEL) assay
The TUNEL assay was used to examine myocardial
apoptosis. An in situ cell death detection kit (Roche
Diagnostics, Indianapolis, IN, USA) was used according to the
manufacturer's instructions. The excised heart tissues were fixed
for 24 h using 10% formic acid solution at room temperature and
then cut horizontally into 5 µm-thick sections. After washing with
phosphate-buffered saline (PBS) twice, the tissue sections were
preprocessed with 0.1% Triton X-100 and 0.1% sodium citrate
(freshly prepared) for 15 min. Following this, the sections were
incubated for 1 h at 37°C with the commercially prepared labelling
mixture and stained with 4′,6-diamidino-2-phenylindole for 5 min at
room temperature. Three slides from each block were evaluated under
a fluorescence microscope to determine the percentage of apoptotic
cells. In addition, four fields on each slide were examined at the
border areas using a defined rectangular field area with a
magnification of ×20. The apoptosis rate of cardiomyocytes was
presented as the percentage of total cells counted.
Assessment of serum cardiac
enzymes
At the end of myocardial reperfusion and prior to
sacrifice, 3–5 ml blood samples were collected from the abdominal
aorta. Subsequently, the blood samples were centrifuged at 2,000 ×
g for 5 min at room temperature, and the serum obtained was assayed
for the activity of cardiac enzymes [creatine kinase (CK), CK-MB
isoenzyme and lactate dehydrogenase (LDH)]. The activity levels of
CK, CK-MB and LDH were detected using the creatine kinase assay kit
(cat. no. A032), creatine kinase MB isoenzyme assay kit (cat. no.
E006) and lactate dehydrogenase assay kit (cat. no. A020-1; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China), respectively,
in accordance with the manufacturer's protocols using an automatic
biochemical analyzer.
Western blotting
Heart tissues were lysed in radioimmunoprecipitation
assay lysis buffer (EMD Millipore, Billerica, MA, USA) containing
complete™ Protease Inhibitor Cocktail (CWBio, Beijing, China) and
homogenized with glass homogenizer until full lysis was achieved.
Cell lysates were obtained by centrifuging homogenates at 6,000 × g
for 10 min at 4°C. Protein concentration was determined using a
standard bicinchoninic acid assay. Total protein (10 µg/lane) was
separated using SDS-PAGE (10% gels) and then transferred onto
nitrocellulose membranes. The membranes were incubated with
anti-cleaved Caspase-8 (C-Caspase-8; cat. no. 66093-1-Ig),
anti-cleaved Caspase-3 (C-Caspase-3; cat. no. 19677-1-AP),
anti-B-cell lymphoma 2 (Bcl-2; cat. no. 12789-1-AP),
anti-Bcl2-associated X protein (Bax; cat. no. 50599-2-Ig),
anti-cleaved poly(ADP-ribose) polymerase (C-PARP; cat. no.
13371-1-AP; all 1:2,000) and anti-GAPDH (cat. no. 60004-1-Ig;
1:3,000; all Wuhan Sanying Biotechnology, Wuhan, China) primary
antibodies overnight at 4°C. Following this, membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
(cat. no. ab6721) and rabbit anti-mouse (cat. no. ab6728) secondary
antibodies (1:5,000; Abcam, Cambridge, MA, USA). Blots were
analyzed using an electrochemiluminescence detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Biochemical assays
To assess the extent of oxidative stress, the
activity of GPx and SOD, and levels of malondialdehyde (MDA) and
protein carbonyl (PC) in heart tissue were measured. Heart tissue
samples were homogenized in PBS, and the supernatant was obtained
by centrifuging at 6,000 × g for 10 min at 4°C and preserved at
−80°C until analysis. GPx and SOD activity levels were detected
using the methods described by Paglia and Valentine (19) and Snow-Lisy et al (20), respectively, and enzyme activity was
recorded as U/g of protein in heart tissue. MDA levels were
measured using a thiobarbituric acid reaction, which was described
by Ohkawa et al (21). The
assay used spectrophotometric measurements of the color produced
during the reaction of thiobarbituric acid with MDA at 535 nm,
which were then expressed as nmol/mg of protein. PC levels were
measured spectrophotometrically according to the reaction of the
carbonyl group with 2, 4-dinitrophenylhydrazine, which is used as a
reagent for proteins subjected to metal-catalyzed oxidation as
described in detail by Levine et al (22). The result was recorded as nmol
carbonyl/mg of protein.
Statistical analysis
Data were expressed as mean ± standard error of the
mean and analyzed using SPSS 18.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). Statistical analysis was performed using one-way
analysis of variance followed by a Student-Newman-Keuls post-hoc
test for multi-group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ebselen protects against myocardial
I/R injury and cardiac dysfunction
To detect whether ebselen protects against
myocardial I/R injury, rats were administered ebselen
intragastrically prior to the I/R-inducing operation. Rat hearts
were subjected to ischemia for 30 min, which was followed by
reperfusion for 2 h. Subsequently, hearts were harvested for
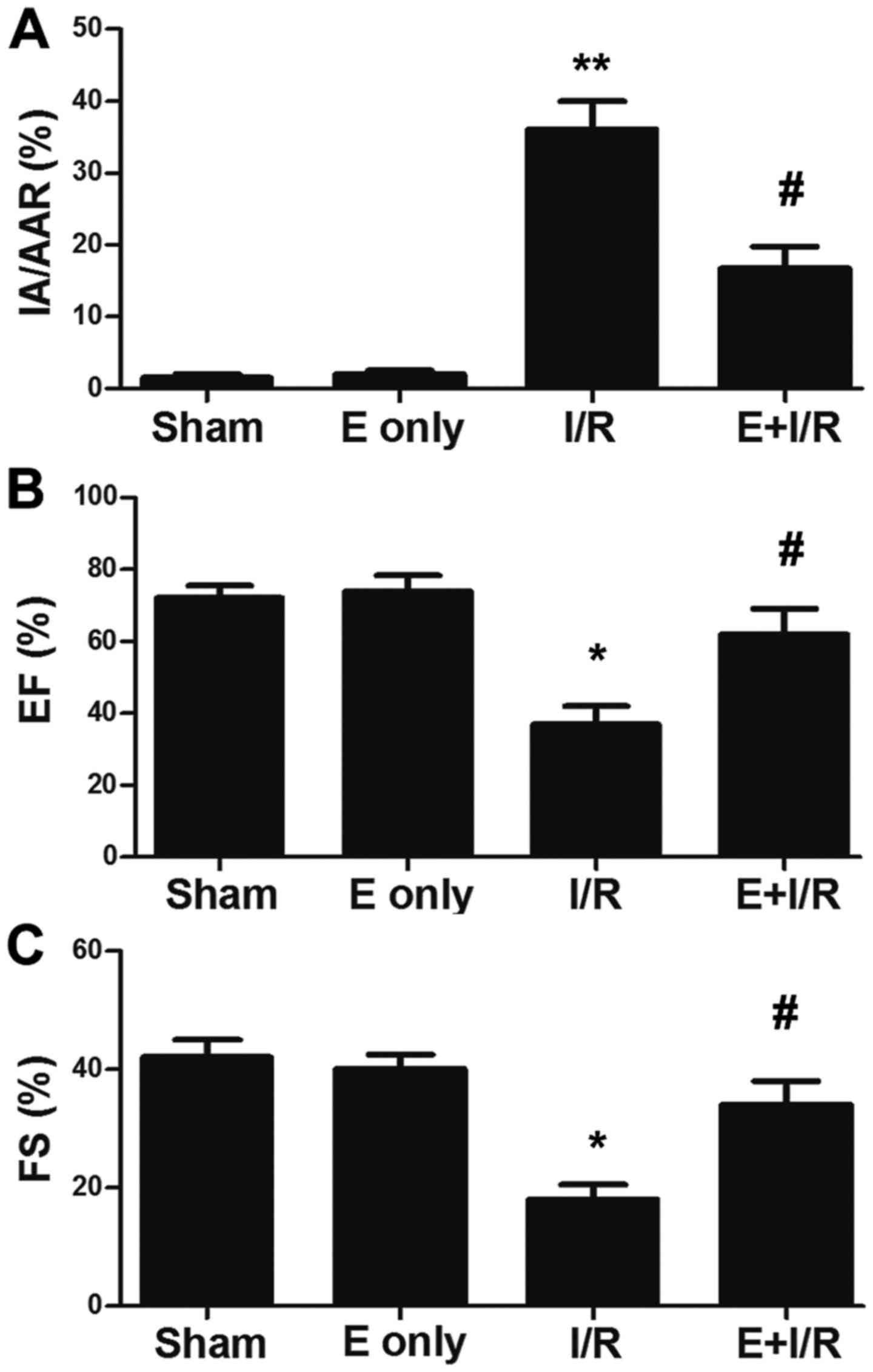
infarct size evaluation. As depicted in Fig. 1A, the infarct size was significantly
increased in the I/R group compared with in the sham group
(P<0.01), which was indicated by the ratio of IA/AAR. Meanwhile,
in the ebselen + I/R group, the infarct size was reduced compared
with in the I/R group (P<0.05). Additionally, the effect of
ebselen on cardiac function was evaluated following myocardial I/R
injury via echocardiography. As demonstrated in Fig. 1B and C, the EF% and %FS were
significantly decreased in the I/R group compared with in the sham
group at 7 days post I/R injury induction (P<0.05). EF% and %FS
were increased in the ebselen + I/R group compared with in the I/R
group (P<0.05), suggesting ebselen protected against the cardiac
dysfunction induced by myocardial I/R injury.
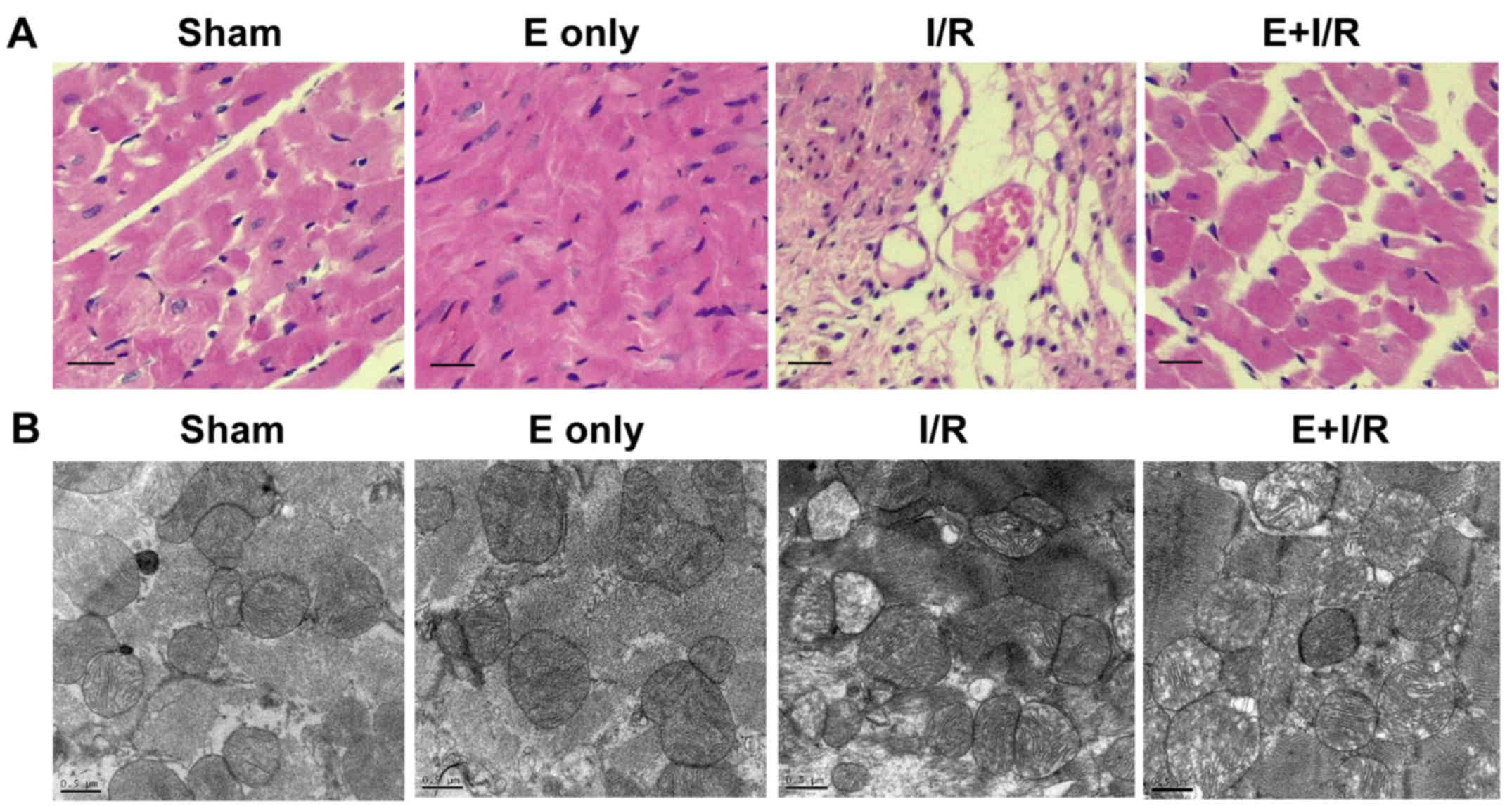
Histopathological and ultrastructural changes of the
myocardium were observed using light microscopy (H&E staining)
and electron microscopy. As shown in Fig. 2A, myocardial fibers exhibited an
orderly arrangement and no evidence of inflammatory cell
infiltration was evident in the sham and ebselen control groups.
Meanwhile, focal necrosis of the myocardium, myocardial cell
swelling, cell boundary and transverse stripe blur, and neutrophil
infiltration in the interstitial area and between cardiomyocytes
were observed in the I/R group. In the ebselen + I/R group, these
changes were markedly attenuated compared with in the I/R group.
However, the myocardial cells still exhibited some swelling and a
small amount of inflammatory cell infiltration was observed in the
ebselen + I/R group. Ultrastructural changes to the organelles in
the cytoplasm were observed using electron microscopy. As
demonstrated in Fig. 2B, in the sham
and ebselen control groups, closely and uniformly arranged
myocardial fibers, clear muscle segments, abundant mitochondria and
some vacuoles in the cytoplasm were observed. In the I/R group,
notable mitochondrial swelling induced by myocardial I/R injury was
observed along with abundant fatty and vacuolation degeneration in
cardiomyocytes compared with in the sham group. The arrangement of
myocardial fibers became disordered and damage of myocardial
fibrous membrane was also observed. In the ebselen + I/R group,
these changes were reduced and the cardiac cellular structure
remained clearly visible.
Ebselen attenuates I/R-induced
myocardial apoptosis and cardiac injury
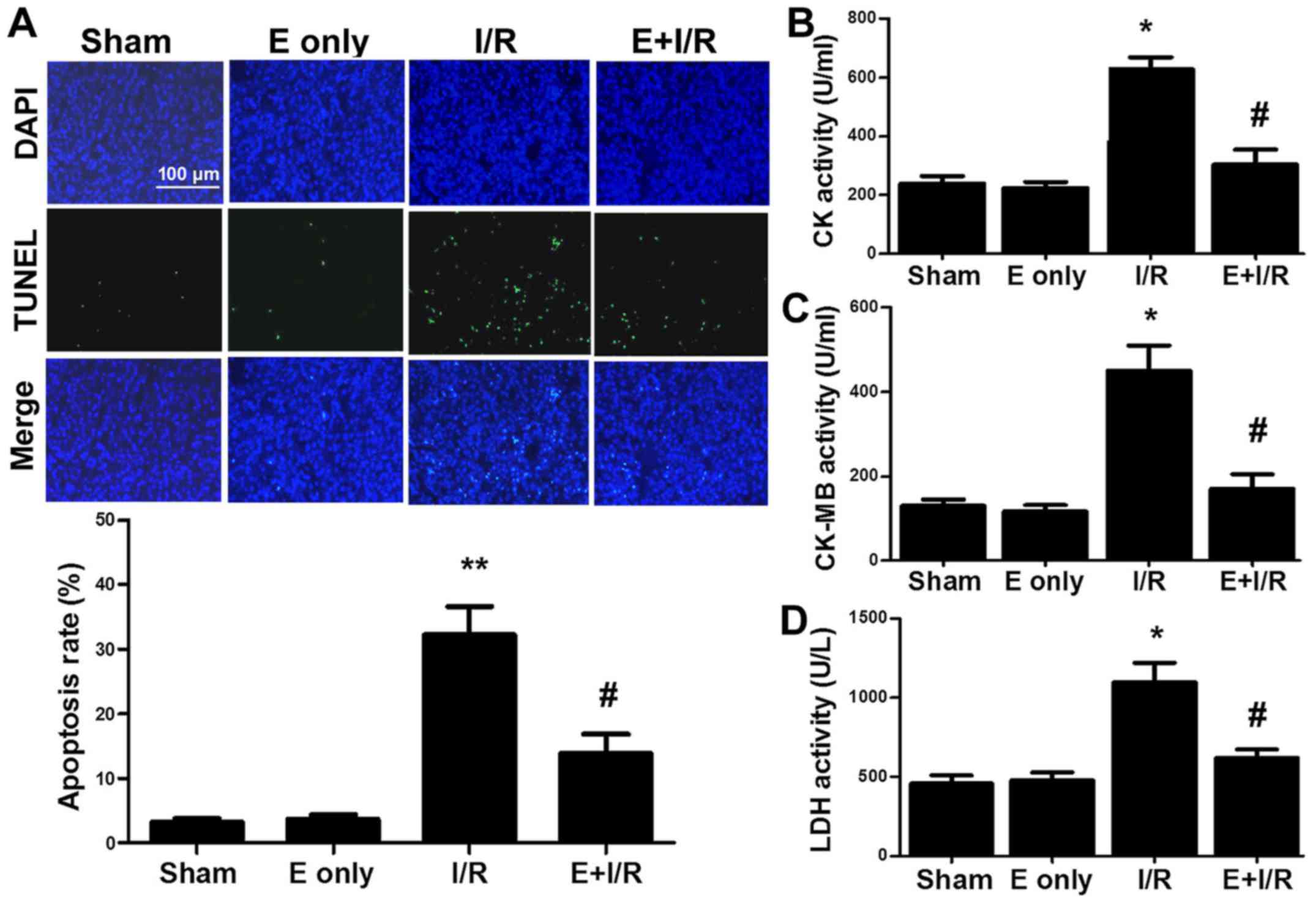
Narula et al (23) reported that cardiomyocyte apoptosis
contributes to myocardial I/R injury. In the current study, it was
determined whether ebselen serves an anti-apoptotic role during
myocardial I/R injury. TUNEL staining (Fig. 3A) indicated that I/R injury
significantly induced myocardial apoptosis by 29% compared with
sham treatment (P<0.01). Furthermore, the rate of myocardial
apoptosis in the ebselen + I/R group was reduced compared with in
the I/R group (P<0.05). The activity of the cardiac enzymes CK,
CK-MB and LDH in serum was also evaluated. As indicated in Fig. 3B-D, the activity levels of serum CK,
CK-MB and LDH were markedly increased in the I/R group compared
with in the sham group (all P<0.05). In turn, ebselen
significantly attenuated the activity levels of CK, CK-MB and LDH
compared with I/R alone (all P<0.05).
Ebselen influences the expression of
apoptotic proteins
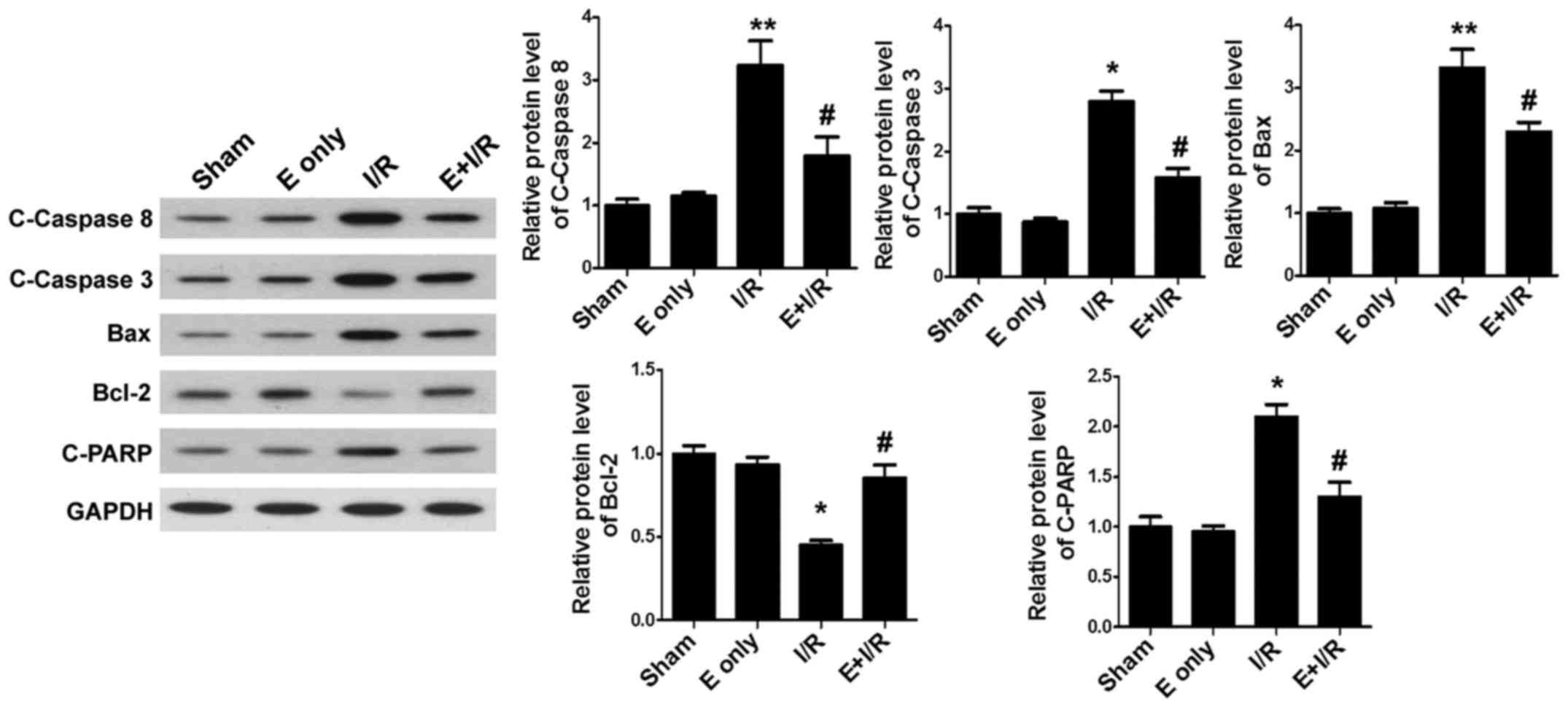
To clarify the mechanisms of ebselen in inhibiting
cardiomyocyte apoptosis, the expression of the apoptosis-related
proteins C-Caspase-8, C-Caspase-3, Bax, Bcl-2 and C-PARP was
assessed. Western blotting (Fig. 4)
demonstrated that I/R injury significantly increased the expression
of myocardial pro-apoptotic proteins C-Caspase-8, Bax (both
P<0.01), C-Caspase-3 and C-PARP, and decreased the expression of
the anti-apoptotic protein Bcl-2 (all P<0.05) when compared with
sham treatment. By contrast, treatment with ebselen suppressed the
expression of pro-apoptotic proteins in heart tissues and
significantly promoted the expression of Bcl-2 compared with I/R
alone (all P<0.05).
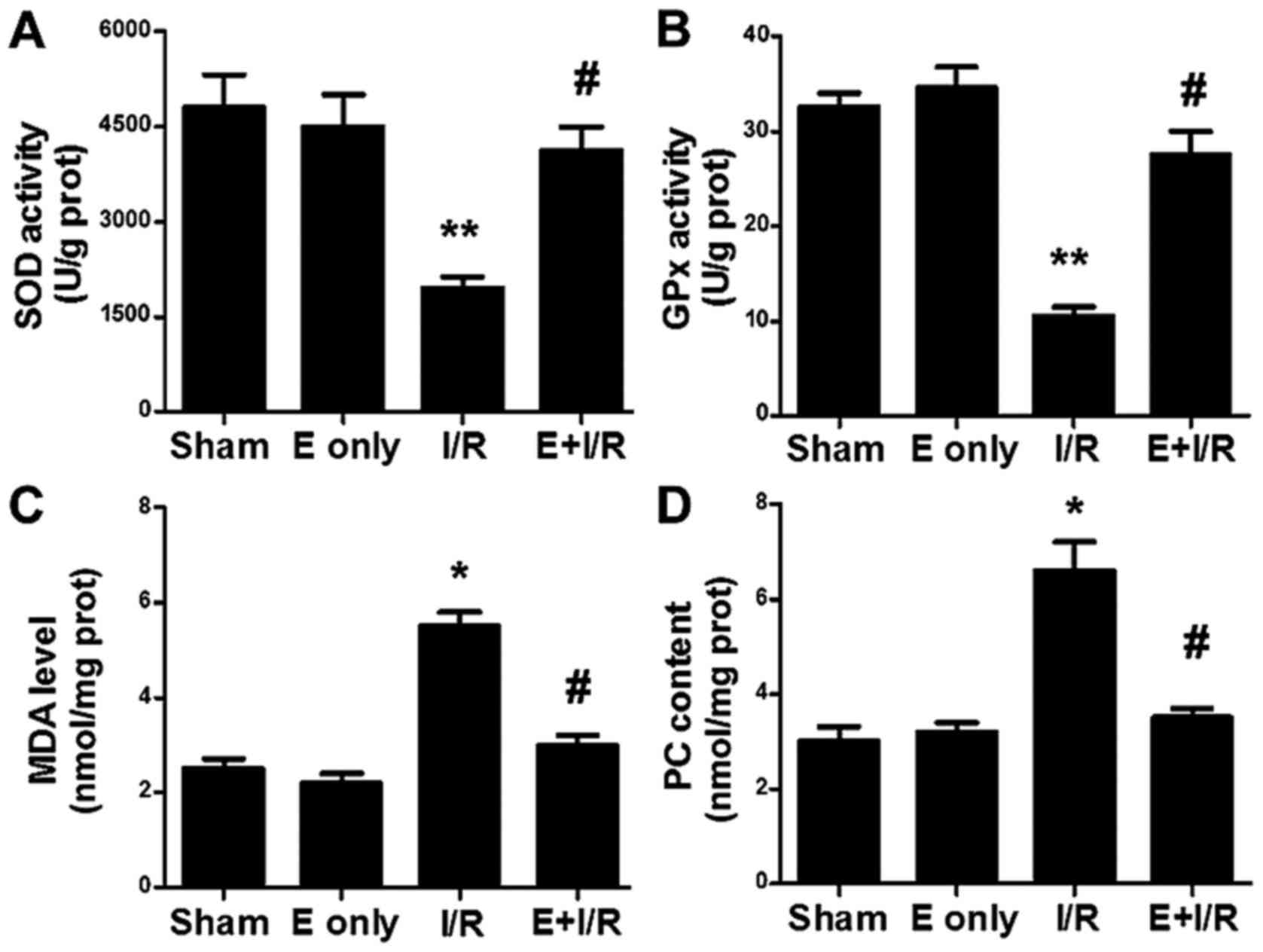
Effects of ebselen on the levels of
cardiac antioxidant enzymes, MDA and PC
The effect of ebselen on cardiac antioxidation
during I/R injury was evaluated. As indicated in Fig. 5A and B, SOD and GPx activity levels
were decreased in the I/R group compared with in the sham group
(P<0.01). In the ebselen + I/R group, the antioxidant enzyme
activities were significantly increased compared with in the I/R
group (P<0.05). MDA is the final product of lipid peroxidation.
MDA levels, as a marker of cardiac oxidative stress, were assayed
in heart tissues in the present study. As indicated in Fig. 5C, I/R injury significantly increased
the MDA levels in the heart tissues compared with in the sham group
(P<0.05). This I/R-induced increase in MDA levels was
significantly prevented by ebselen treatment (P<0.05). PC levels
in heart tissues were also determined (Fig. 5D). A marked increase in the tissue
levels of PC was observed in the I/R group (P<0.05 vs. sham),
suggesting increased protein oxidation. Meanwhile, PC levels were
significantly decreased in the ebselen + I/R group compared with in
the I/R group (P<0.05).
Discussion
Coronary reperfusion therapy is an established
approach for the management of acute myocardial infarction;
however, restoration of blood flow to previously ischemic
myocardium results in I/R injury (24,25).
Cumulative studies have uncovered that several primary factors,
including oxidative stress, intracellular calcium overload,
fluctuating physiological pH, the mitochondrial permeability
transition pore and inflammation mediate the detrimental effects of
myocardial I/R injury. These factors collectively induce apoptosis
and the death of cardiomyocytes during the I/R process (26–29).
Therefore, reducing cardiomyocyte apoptosis may protect the heart
against I/R-induced injury.
A number of studies have uncovered that ebselen has
beneficial effects in I/R-induced tissue injury, specifically in
intestinal (30), lung (31), kidney (32) and sciatic nerve (33) tissues. In the current study, the
effects of ebselen on heart injury induced by I/R in vivo
were examined. The major findings included the following: i)
Ebselen significantly attenuated I/R-induced myocardial infarction
and cardiac dysfunction; ii) ebselen attenuated the effects of I/R
in reducing SOD and GPx activities and increasing MDA and PC levels
in rat hearts, consistent with results reported by Baljinnyam et
al (34) in a rabbit model of
myocardial I/R injury; iii) ebselen prevented I/R-induced rat heart
injury, by attenuating histological and ultrastructural changes,
reducing serum CK, CK-MB and LDH activity levels, and decreasing
cell apoptosis, which to our knowledge is the first time this has
been systematically determined; and iv) ebselen pretreatment
markedly decreased the expression of C-Caspase-8, C-Caspase-3, Bax
and C-PARP, and increased the expression of Bcl-2.
In myocardial ischemia, hypoxia and reoxygenation,
as principal causes of reperfusion injury, induce an increase in
free radical production in cardiac tissues (35,36). ROS
and RNS produced through reoxygenation lead to direct oxidative
damage to cellular components; in particular, damage caused by
peroxidation of cellular membrane lipids and oxidation of cellular
proteins, which can be measured by assessing MDA and PC levels in
the heart tissue, respectively. The present results indicated that
I/R injury caused a significant increase in MDA and PC levels in
the heart tissues. However, this change was mostly restored in the
ebselen-pretreated I/R group. Additionally, it was examined whether
ebselen could enhance the antioxidant capacity of rat hearts in the
current study. It was identified that myocardial I/R injury
decreased SOD and GPx activities, and this effect was attenuated by
treatment with ebselen. The effects of ebselen on SOD and GPx
activities have been excessively studied in myocardial I/R injury
in several animal species including rabbit, in situ swine
and neonatal porcine models (34,37–39), and
the current results in a rat model are consistent with those of the
previous studies. However, there appears to be few reports that
have determined an increase in MDA and PC levels in myocardial I/R
injury, and that ebselen may decrease these levels. Additionally,
further investigation is needed to elucidate the role of SOD and
GPx in the ebselen-mediated cardioprotection during I/R injury and
the anti-oxidant effects of ebselen in myocardial I/R injury,
potentially occurring via nitric oxide signaling, in future
studies.
A key finding of the present study was that ebselen
exhibited various I/R-protective effects via antioxidant and
anti-apoptosis functions. Previous studies have indicated that
apoptosis induced by oxidative stress serves an important role in
the pathogenesis of heart dysfunctions induced by myocardial I/R
injury (40–42). Several critical factors, including
C-Caspase-8, C-Caspase-3, Bax, Bcl-2 and C-PARP, regulate the
process of apoptosis (43–47). In myocardial I/R injury, inhibition
of C-Caspase-8, C-Caspase-3 and C-PARP may reduce cardiomyocyte
apoptosis (43,44). Furthermore, previous results
indicated that overexpression of Bcl-2 and downregulation of Bax
inhibited myocyte apoptosis following reperfusion, and protected
the heart against I/R injury (45–47). In
the current study, the protein expression of genes associated with
cell apoptosis was detected using western blotting. According to
the results, C-Caspase-8, C-Caspase-3, Bax and C-PARP were
overexpressed in the I/R group compared with in the sham group; in
turn, the protein expression of these pro-apoptotic factors was
decreased in the ebselen-pretreated I/R group. By contrast, the
expression of Bcl-2 in rat hearts was decreased in the I/R group
compared with in the sham group, and subsequently increased by
ebselen pretreatment compared with the I/R group. To the best of
our knowledge, the present study is the first to indicate that
ebselen protects against myocardial I/R injury by upregulating
Bcl-2 and downregulating C-Caspase-8, C-Caspase-3, Bax and C-PARP,
thus inhibiting I/R-induced apoptosis.
In conclusion, the present study indicated that
ebselen could protect rat hearts against I/R injury. Ebselen
attenuated I/R-induced cardiomyocyte apoptosis seemingly by
increasing the expression of Bcl-2, decreasing the expression of
C-Caspase-8, C-caspase-3, Bax and C-PARP, and enhancing the
expression and function of antioxidant enzymes. Taken together, the
present results support the use of ebselen as an effective
therapeutic agent for the treatment of myocardial I/R injury.
Acknowledgements
The authors would like to thank Dr Jin Gong and Dr
Lin Han (Department of Pathology of Renmin Hospital of Wuhan
University, Wuhan, China) for technical assistance in
histopathological examination of myocardium.
Funding
The current study was supported by the Scientific
Research Project of Yichang City, Hubei in 2013 (grant no.
A13301-18).
Availability of data and materials
Please contact the corresponding author for data
requests.
Authors' contributions
BC and JPZ performed the animal and molecular
studies, collected experimental data and drafted the manuscript.
GLL, QXR and GL participated in the design of the study and
performed the histological examination of the hearts and the
statistical analysis. FXW and HJ conceived the study, participated
in its design and coordination and helped to draft the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Laboratory Animal Ethics Committee of Wuhan University (Wuhan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
I/R
|
ischemia reperfusion
|
|
TUNEL
|
terminal dUTP nick end-labelling
|
|
TTC
|
triphenyltetrazolium chloride
|
|
SOD
|
superoxide dismutase
|
|
GPx
|
glutathione peroxidase
|
|
MDA
|
malondialdehyde
|
|
PC
|
protein carbonyl
|
|
ROS
|
reactive oxygen species
|
|
RNS
|
reactive nitrogen species
|
|
CK[-MB]
|
creatine kinase [MB isoenzyme]
|
|
LDH
|
lactate dehydrogenase
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bax
|
B-cell lymphoma 2-associated X
protein
|
|
PARP
|
poly(ADP) ribose
|
|
C-
|
cleaved
|
|
EF%
|
ejection fraction
|
|
%FS
|
percent fractional shortening
|
|
IA
|
infarct area
|
|
AAR
|
area at risk
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Li C and Jackson RM: Reactive species
mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol
Cell Physiol. 282:C227–C241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mochizuki K, Ohno Y, Kanematsu T,
Sakurai-Yamashita Y, Niwa M, Hishikawa Y and Koji T: Possible
protection of sinusoidal endothelial cells by endothelin B receptor
during hepatic warm ischemia-reperfusion. Surg Today. 37:460–467.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fröhlich GM, Meier P, White SK, Yellon DM
and Hausenloy DJ: Myocardial reperfusion injury: Looking beyond
primary PCI. Eur Heart J. 34:1714–1722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diez ER, Altamirano LB, García IM, Mazzei
L, Prado NJ, Fornes MW, Carrión FD, Zumino AZ, Ferder L and Manucha
W: Heart remodeling and ischemia-reperfusion arrhythmias linked to
myocardial vitamin d receptors deficiency in obstructive
nephropathy are reversed by paricalcitol. J Cardiovasc Pharmacol
Ther. 20:211–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elahi MM, Kong YX and Matata BM: Oxidative
stress as a mediator of cardiovascular disease. Oxid Med Cell
Longev. 2:259–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tahara EB, Navarete FD and Kowaltowski AJ:
Tissue-, substrate-, and site-specific characteristics of
mitochondrial reactive oxygen species generation. Free Radic Biol
Med. 46:1283–1297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vassalle C, Pratali L, Boni C, Mercuri A
and Ndreu R: An oxidative stress score as a combined measure of the
pro-oxidant and anti-oxidant counterparts in patients with coronary
artery disease. Clin Biochem. 41:1162–1167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodrigo R, Libuy M, Feliú F and Hasson D:
Oxidative stress-related biomarkers in essential hypertension and
ischemia-reperfusion myocardial damage. Dis Markers. 35:773–790.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsumi H, Nishikawa M, Yasui H, Yamashita
F and Hashida M: Prevention of ischemia/reperfusion injury by
hepatic targeting of nitric oxide in mice. J Control Release.
140:12–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taki-Eldin A, Zhou L, Xie HY, Chen KJ, Yu
D, He Y and Zheng SS: Triiodothyronine attenuates hepatic
ischemia/reperfusion injury in a partial hepatectomy model through
inhibition of proinflammatory cytokines, transcription factors, and
adhesion molecules. J Surg Res. 178:646–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scott MD, Lubin BH, Zuo L and Kuypers FA:
Erythrocyte defense against hydrogen peroxide: Preeminent
importance of catalase. J Lab Clin Med. 118:7–16. 1991.PubMed/NCBI
|
|
12
|
Sabri A, Hughie HH and Lucchesi PA:
Regulation of hypertrophic and apoptotic signaling pathways by
reactive oxygen species in cardiac myocytes. Antioxid Redox Signal.
5:731–740. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Hu M, Wang Y, Lu H, Deng J and Yan
X: Hydrogen sulfide preconditioning protects against myocardial
ischemia/reperfusion injury in rats through inhibition of
endo/sarcoplasmic reticulum stress. Int J Clin Exp Pathol.
8:7740–7751. 2015.PubMed/NCBI
|
|
14
|
Wang Q, Lin P, Li P, Feng L, Ren Q, Xie X
and Xu J: Ghrelin protects the heart against ischemia/reperfusion
injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci.
186:50–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu SY, Zhang Y, Zhu PJ, Zhou H and Chen
YD: Liraglutide directly protects cardiomyocytes against
reperfusion injury possibly via modulation of intracellular calcium
homeostasis. J Geriatr Cardiol. 14:57–66. 2017.PubMed/NCBI
|
|
16
|
Ozaki M, Nakamura M, Teraoka S and Ota K:
Ebselen, a novel anti-oxidant compound, protects the rat liver from
ischemia-reperfusion injury. Transpl Int. 10:96–102. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denicola A and Radi R: Peroxynitrite and
drug-dependent toxicity. Toxicology. 208:273–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US); Washington, DC:
1996
|
|
19
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
20
|
Snow-Lisy DC, Sabanegh ES Jr, Samplaski
MK, Morris VB and Labhasetwar V: Superoxide dismutase-loaded
biodegradable nanoparticles targeted with a follicle-stimulating
hormone peptide protect Sertoli cells from oxidative stress. Fertil
Steril. 101:560–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levine RL, Garland D, Oliver CN, Amici A,
Climent I, Lenz AG, Ahn BW, Shaltiel S and Stadtman ER:
Determination of carbonyl content in oxidatively modified proteins.
Methods Enzymol. 186:464–478. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Narula J, Hajjar RJ and Dec GW: Apoptosis
in the failing heart. Cardiol Clin. 16:691–710. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piper HM, García-Dorado D and Ovize M: A
fresh look at reperfusion injury. Cardiovasc Res. 38:291–300. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zweier JL, Flaherty JT and Weisfeldt ML:
Direct measurement of free radical generation following reperfusion
of ischemic myocardium. Proc Natl Acad Sci USA. 84:1404–1407. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heusch G, Boengler K and Schulz R:
Inhibition of mitochondrial permeability transition pore opening:
The Holy Grail of cardioprotection. Basic Res Cardiol. 105:151–154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vinten-Johansen J: Involvement of
neutrophils in the pathogenesis of lethal myocardial reperfusion
injury. Cardiovasc Res. 61:481–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guven A, Tunc T, Topal T, Kul M, Korkmaz
A, Gundogdu G, Onguru O and Ozturk H: Alpha-lipoic acid and ebselen
prevent ischemia/reperfusion injury in the rat intestine. Surg
Today. 38:1029–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamacher J, Stammberger U, Weber E, Lucas
R and Wendel A: Ebselen improves ischemia-reperfusion injury after
rat lung transplantation. Lung. 187:98–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kizilgun M, Poyrazoglu Y, Oztas Y, Yaman
H, Cakir E, Cayci T, Akgul OE, Kurt YG, Yaren H, Kunak ZI, et al:
Beneficial effects of N-acetylcysteine and ebselen on renal
ischemia/reperfusion injury. Ren Fail. 33:512–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozyigit F, Kucuk A, Akcer S, Tosun M,
Kocak FE, Kocak C, Kocak A, Metineren H and Genc O: Different
dose-dependent effects of ebselen in sciatic nerve
ischemia-reperfusion injury in rats. Bosn J Basic Med Sci.
15:36–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baljinnyam E, Hasebe N, Morihira M,
Sumitomo K, Matsusaka T, Fujino T, Fukuzawa J, Ushikubi F and
Kikuchi K: Oral pretreatment with ebselen enhances heat shock
protein 72 expression and reduces myocardial infarct size.
Hypertens Res. 29:905–913. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hori M and Nishida K: Oxidative stress and
left ventricular remodelling after myocardial infarction.
Cardiovasc Res. 81:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou S, Sun W, Zhang Z and Zheng Y: The
role of Nrf2-mediated pathway in cardiac remodeling and heart
failure. Oxid Med Cell Longev. 2014:2604292014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maulik N and Yoshida T: Oxidative stress
developed during open heart surgery induces apoptosis: Reduction of
apoptotic cell death by ebselen, a glutathione peroxidase mimic. J
Cardiovasc Pharmacol. 36:601–608. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Liu J, Li S, Yan F, Xue Q, Wang H,
Sun P and Long C: Histidine-tryptophan-ketoglutarate solution with
added ebselen augments myocardial protection in neonatal porcine
hearts undergoing ischemia/reperfusion. Artif Organs. 39:126–133.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Babiker FA, Al-Jarallah A and Joseph S:
Understanding pacing postconditioning-mediated cardiac protection:
A role of oxidative stress and a synergistic effect of adenosine. J
Physiol Biochem. 73:175–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao ZQ: Oxidative stress-elicited
myocardial apoptosis during reperfusion. Curr Opin Pharmacol.
4:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pu J, Yuan A, Shan P, Gao E, Wang X, Wang
Y, Lau WB, Koch W, Ma XL and He B: Cardiomyocyte-expressed
farnesoid-X-receptor is a novel apoptosis mediator and contributes
to myocardial ischaemia/reperfusion injury. Eur Heart J.
34:1834–1845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu Z, Zhu J, Zhao X, Yang K, Lu L, Zhang
F, Shen W and Zhang R: All-trans retinoic acid ameliorates
myocardial ischemia/reperfusion injury by reducing cardiomyocyte
apoptosis. PLoS One. 10:e01334142015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie H, Zhang J, Zhu J, Liu LX, Rebecchi M,
Hu SM and Wang C: Sevoflurane post-conditioning protects isolated
rat hearts against ischemia-reperfusion injury via activation of
the ERK1/2 pathway. Acta Pharmacol Sin. 35:1504–1513. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Szobi A, Rajtik T, Carnicka S, Ravingerova
T and Adameova A: Mitigation of postischemic cardiac contractile
dysfunction by CaMKII inhibition: Effects on programmed necrotic
and apoptotic cell death. Mol Cell Biochem. 388:269–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma N, Bai J, Zhang W, Luo H, Zhang X, Liu
D and Qiao C: Trimetazidine protects against cardiac
ischemia/reperfusion injury via effects on cardiac miRNA-21
expression, Akt and the Bcl-2/Bax pathway. Mol Med Rep.
14:4216–4222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cui X, He Z, Liang Z, Chen Z, Wang H and
Zhang J: Exosomes from adipose-derived mesenchymal stem cells
protect the myocardium against ischemia/reperfusion injury through
Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol.
70:225–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu L, Cai N and Jia H: Pterostilbene
attenuates myocardial ischemia-reperfusion injury via the
phosphatidylinositol 3′-kinase-protein kinase B signaling pathway.
Exp Ther Med. 14:5509–5514. 2017.PubMed/NCBI
|