Introduction
In recent years, with the rapid development of
surgical procedures, the proportion of patients who undergo general
anesthesia has increased due to its diverse application, safety and
comfort (1,2). With this the increase in the number of
surgeries anesthetic complications, such as postoperative cognitive
dysfunction, have increased annually (3). The role of anesthetic drugs in the
central nervous system has been previously investigated, especially
the potential effect on the aptitude and memory of infants and
children (4–6).
Sevoflurane has been widely used due to its rapid
induction, early recovery, low impact on liver and kidney function,
and stable hemodynamics (7,8). Animal studies demonstrated that
sevoflurane can inhibit the proliferation of cortical progenitor
cells and promote central nervous system cortical neuron death,
thereby reducing the aptitude and memory of newborn animals
(9–11). Preclinical experiments have
demonstrated that the neurotoxic effect of sevoflurane on the
developing brain is associated with neuronal apoptosis and
subsequent cognitive dysfunction (12,13).
Therefore, a treatment method that can counteract neuronal
apoptosis may reduce sevoflurane-induced neurocognitive
impairment.
In the central nervous system, sphingomyelin,
especially nerve sphingomyelin, is an essential component of
oligodendrocytes and the myelin sheath (9). Sphingomyelin can be catalyzed by
Phospholipase C to form ceramide, which is further catalyzed by
ceramidase to form sphingosine (12). Subsequently, sphingosine is
phosphorylated by sphingosine kinases (SphK) to form
sphingosine-1-phosphate (S1P) (14).
The S1P signaling pathway can stimulate neuronal growth and
survival. Upregulation of S1P in cells has been shown to inhibit
the apoptosis of PC12 cells due to the addition of exogenous
neuraminic acid or lack of serous fluid (15,16).
Additionally, a study revealed that the inactivation of sphingosine
kinase 1/S1P signal transmission disrupted the growth and survival
of neuronal cells, and damaged the development of neural progenitor
cells in the sensory ganglion (17).
Metformin has been demonstrated to be a safe and
effective oral hypoglycemic agent that is widely used in the
treatment of diabetes (18,19). Studies have demonstrated that
metformin promotes the growth of newly-generated neurons in the
hippocampus and improves spatial learning and memory in mice
(20,21). In the present experimental study, the
effect of metformin on sevoflurane-induced neuronal apoptosis and
its potential mechanism were assessed.
Materials and methods
Preparation of hippocampal
neurons
A total of 120 male neonatal Sprague-Dawley rats
(age, 18 days; weight, 40–45 g) were obtained from Beijing Vital
River Laboratory Animal Technology (Beijing, China). Rats were
housed in environmentally controlled conditions (21±2°C, with a
12-h light/dark cycle and 30–40% humidity). All rats had free
access to food and water. Rats were sacrificed by decapitation and
the skin from the heads was removed. The bilateral cerebral
hemispheres were separated and placed in a petri dish on ice. The
cortex was isolated, and the hippocampus was exposed and removed.
Hippocampal tissues were repeatedly washed 3–5 times using
pre-cooled (4°C) Hank's D solution (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The hippocampal tissues were
crushed and the mixture was centrifuged at 100 × g for 5 min at
4°C. The supernatant was discarded and the centrifugation was
repeated. The supernatant was discarded and 10 µl membrane protease
(Tiangen Biotech Co., Ltd., Beijing, China) was added to digest the
hippocampal tissue for 15–20 min. The mixture was agitated once
every 5 min. Dulbecco's modified Eagle medium (DMEM) containing 10%
fetal bovine serum (FBS; both Gibco; Thermo Fisher Scientific,
Inc.) was added to terminate the digestion. The cells were filtered
through a 200-mesh copper filter screen. The filtered cells were
collected and centrifuged at 100 × g for 5 min at 4°C. The
supernatant was discarded and the single-cell suspension was
prepared by mixing with DMEM. The single-cell suspension was
transferred to a culture flask with DMEM and maintained at 37°C in
a 5% CO2-humidified incubator, allowing for differential
adhesion. Following 1-h incubation, non-adherent neuronal cells
were subsequently harvested and the adherent glial cells were
isolated. Three independent repeats were performed for each
experiment in the current study. The present study was approved by
the Animal Ethics Committee of Yan'an University Animal Center
(Yan'an, China; approval no. 20170125).
Hippocampal neuron treatment
Hippocampal neurons at logarithmic growth stage were
collected and seeded into 96-well plates at a density of
1×104 cells/well and cultured for 24 h. Cells were
subsequently incubated at 37°C with DMEM containing metformin (10
mmol/l; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), VPC23019
(0.5 µmol/l; Sigma-Aldrich; Merck KGaA) and/or U0126 (5 µmol/l;
Sigma-Aldrich; Merck KGaA) for 24 h. Five independent repeats were
performed for each experiment in the current study.
Exposure of cultured hippocampal
neurons to anesthetics
The anesthetic exposure box, a sealed, transparent
toughened glass box, was constructed in-house. An appropriate
amount of soda lime (~100 g; Highgreen Medical Technology, Co.,
Ltd., Weihai, China) was placed at the bottom of the glass box. The
lateral aperture on each side of the box was connected by threaded
pipe to the anesthesia machine (Dräger Fabius® GS
premium (Drägerwerk AG & Co., KGaA. Lübeck, Germany). The
threaded pipe was connected to a gas monitor (FI8000; Shenzhen Yice
Medical Test Co., Ltd., Shenzhen, China), so that the sevoflurane
concentration could be monitored. The primary hippocampal neurons
were seeded into 24-well plates pre-coated with Matrigel basement
membrane at a density of 1×106 cells/well. Cells were
cultured in Neurobasal medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 2% B27 and 1% N2 and maintained
at maintained at 37°C in a 5% CO2-humidified incubator.
The primary rat hippocampal neurons were exposed to sevoflurane for
3, 6, 9 and 12 h. The number of viable cells at each time point was
analyzed using an MTT assay.
The MTT assay
Cells in the logarithmic grow phase were collected
and seeded into the 96-well flat-bottomed plates at a density of
1×104 cells per well. Cells were maintained in a 5%
CO2 incubator at 37°C until the bottom of the wells were
covered with a cell monolayer. Following treatment with metformin
(10 mmol/l), VPC23019 (0.5 µmol/l) and/or U0126 (5 µmol/l), the
cells were cultured for a further 16–48 h and 20 µl MTT solution (5
mg/ml) was added to each well and incubated for 4 h. DMSO (150 µl)
was added to each well and the plates were placed on a rocking bed
for 10 min at a low speed to dissolve the crystals. The absorbance
value of each well was measured at a wavelength of 490 nm using an
enzyme-linked immunodetector. The apoptosis rate was calculated
using the following: Apoptosis rate=1-cell viability.
Lysis of cultured neurons for
extraction of total protein
The culture medium of the hippocampal neurons was
removed, PBS was added to each well and the plate was cooled to
4°C. The plate was gently agitated to perform cell washing.
Following the removal of the PBS, the plate was placed on ice for
temporary storage. Radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China) was added to each well,
and samples were lysed continuously on an ice bed and agitated
every 3–5 min for a total of 30 min. The lysed cells were
transferred to one side of the culture well with a small scraper,
and a pipette was used to transfer the lysate and cell debris to
the centrifuge tube. The cell mixture was centrifuged at 1,300 × g
for 5 min at 4°C and the supernatant was stored at −20°C for future
use.
Western blot analysis
Following lysis and protein extraction, total
protein was quantified using a bicinchoninic acid assay and 50 µg
protein/lane was separated via SDS-PAGE on a 10% gel. The separated
proteins were subsequently transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA) and blocked for 1 h
at room temperature with 5% non-fat milk powder. The membranes ere
incubated with primary antibodies against caspase-3 (1:1,000; cat.
no. ab13847), Bax (1:1,000; cat. no. ab3250), Bcl2 (1:1,000; cat.
no. ab196495), p-ERK (1:1,000; cat. no. ab201015), ERK (1:1,000;
cat. no. ab79853) and b-actin (1:1,000; cat. no ab8226; all Abcam,
Cambridge, MA, USA) overnight at 4°C. The membranes were washed
three times for 5 min with Tris-buffered saline with
Tween® 20 (TBST; Sigma-Aldrich; Merck KGaA). Following
the primary incubation, membranes were incubated with fluorescently
labeled goat anti-rabbit IgG secondary antibody (1:10,000;
ab150077; Abcam, Cambridge, MA, USA) for 1 h at 37°C. The membranes
were washed with TBST for 10 min three times, then with PBS for 5
min. Protein bands were visualized using an ECL reagent (cat. no.
P0019; Beyotime Institute of Biotechnology) using the Odyssey
far-infrared fluorescence scanning imaging system (LI-COR
Biosciences, Lincoln, NE, USA). Protein expression was quantified
using Image J software (version 1.38; National Institutes of
Health, Bethesda, MD, USA).
Statistical processing
SPSS19.0 statistical software package (IBM Corp.,
Armonk, NY, USA) was used for data analysis. Comparisons between
multiple groups were performed using one-way analysis of variance
followed by a post hoc test (Fisher's Least Significant
Difference). A Chi-squared test was used to analyze the
classification data. P<0.05 was considered to indicate a
statistically significant difference between groups.
Results
Sevoflurane increases neuronal
apoptosis
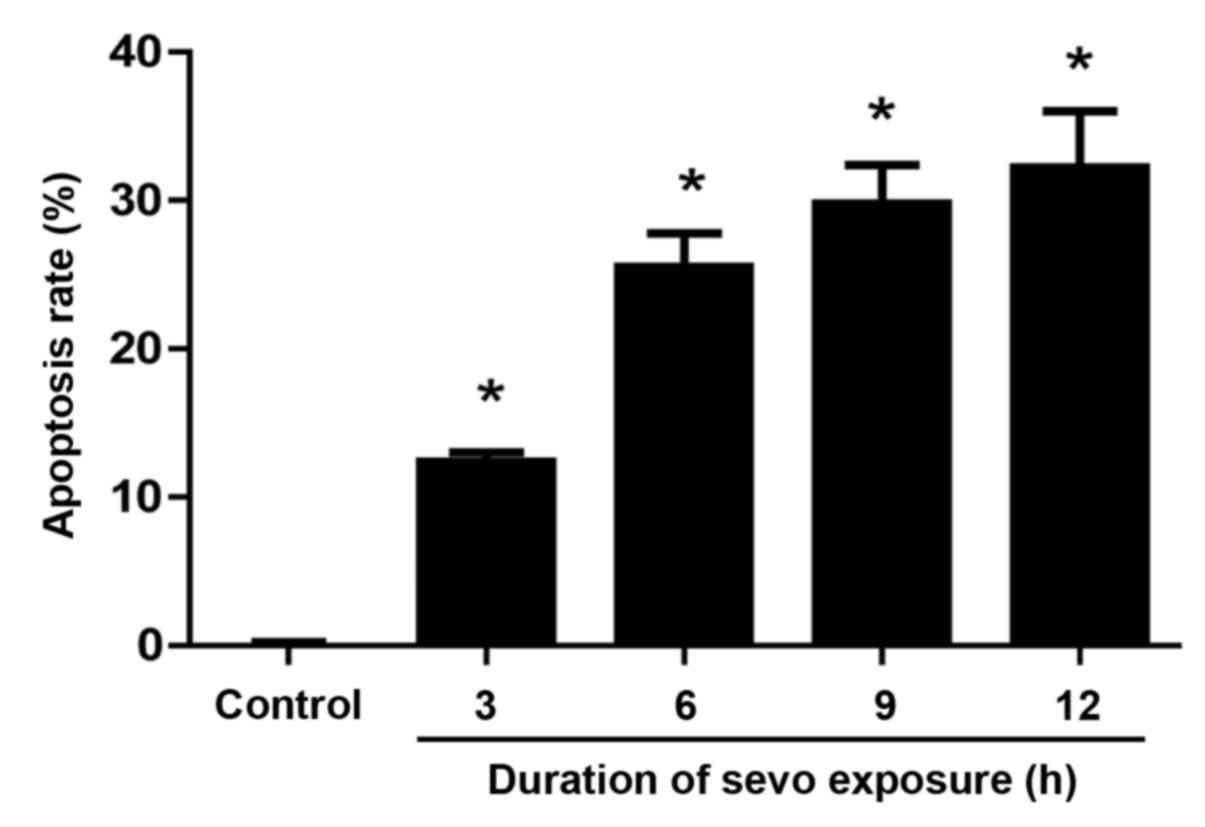
Neurons exposed to sevoflurane for 3, 6, 9 and 12 h
were assessed with a MTT assay to evaluate their apoptotic rate.
The results demonstrated that sevoflurane-induced neuronal
apoptosis was significantly increased in what appeared to be a
time-dependent manner when compared with that of the control group
(Fig. 1). The apoptotic rate after
sevoflurane treatment for 3, 6, 9 and 12 h was 12.4±0.6, 25.5±2.3,
29.8±2.6 and 32.2±3.8%, respectively. No significant changes in the
apoptotic rate were identified in the control group after neurons
were treated with culture medium without sevoflurane for 9 h.
Metformin reduces the apoptosis rate
and pro-apoptosis proteins of sevoflurane-treated cells
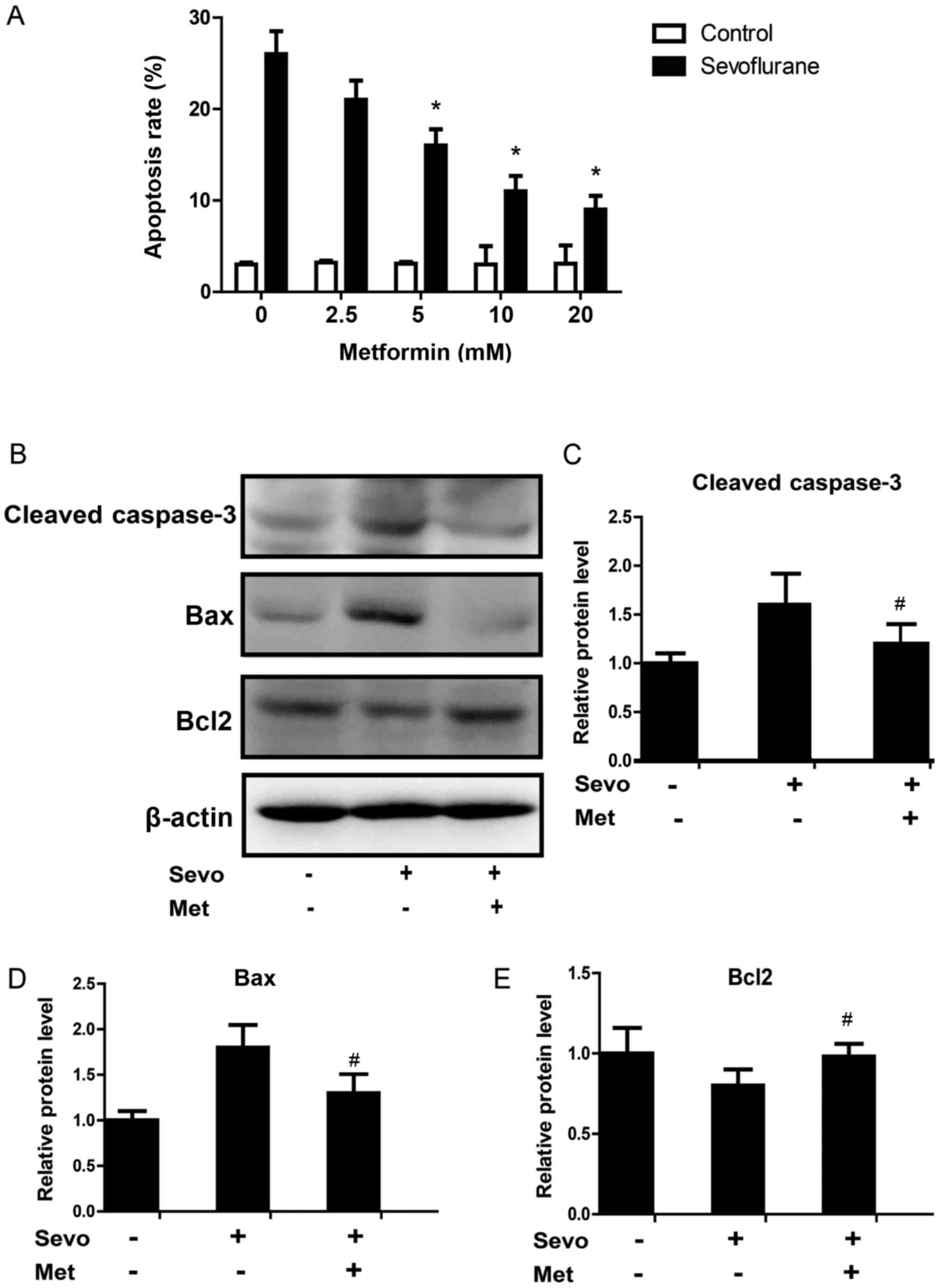
In order to verify the protective effect of
metformin on sevoflurane-induced neuronal apoptosis, different
concentrations of metformin (2.5, 5, 10 and 20 mM) were incubated
with neurons that were previously exposed to 3% sevoflurane
(Fig. 2). The results revealed that
5 mM metformin could effectively protect neurons from the
pro-apoptotic effects of sevoflurane by significantly decreasing
the apoptotic rate compared with control cells (Fig. 2A). Metformin appeared to have a
dose-dependent protective effect on sevoflurane-induced neuronal
apoptosis. Western blotting results also revealed that the protein
expression levels of cleaved-caspase-3 and apoptosis regulator BAX
(Bax) significantly decreased, and apoptosis regulator Bcl-2 (Bcl2)
significantly increased in neurons treated with sevoflurane and 10
mM metformin compared with sevoflurane-treated cells (Fig. 2B-E).
Sphingosine 1-phosphate receptor 1
(S1P1) antagonism increases the apoptosis rate and pro-apoptosis
proteins of sevoflurane- and metformin-treated cells in vitro
S1P1 is associated with signal transduction that
promotes cell survival functions in neurons (15), thus the authors of the current study
speculated that metformin exerted its anti-apoptosis effect on
neurons via S1P1. Sevoflurane- and metformin-treated cells
incubated with VPC23019, a selective S1P1 antagonist, significantly
increased the apoptosis rate compared with sevoflurane- and
metformin-treated cells (Fig. 3A).
VPC23019 also significantly increased the protein expression levels
of cleaved-caspase-3 and Bax, and significantly decreased Bcl2
compared with sevoflurane- and metformin-treated cells (Fig. 3B-E). These results indicate that
metformin could reduce sevoflurane-induced neuronal apoptosis
through binding to S1P1.
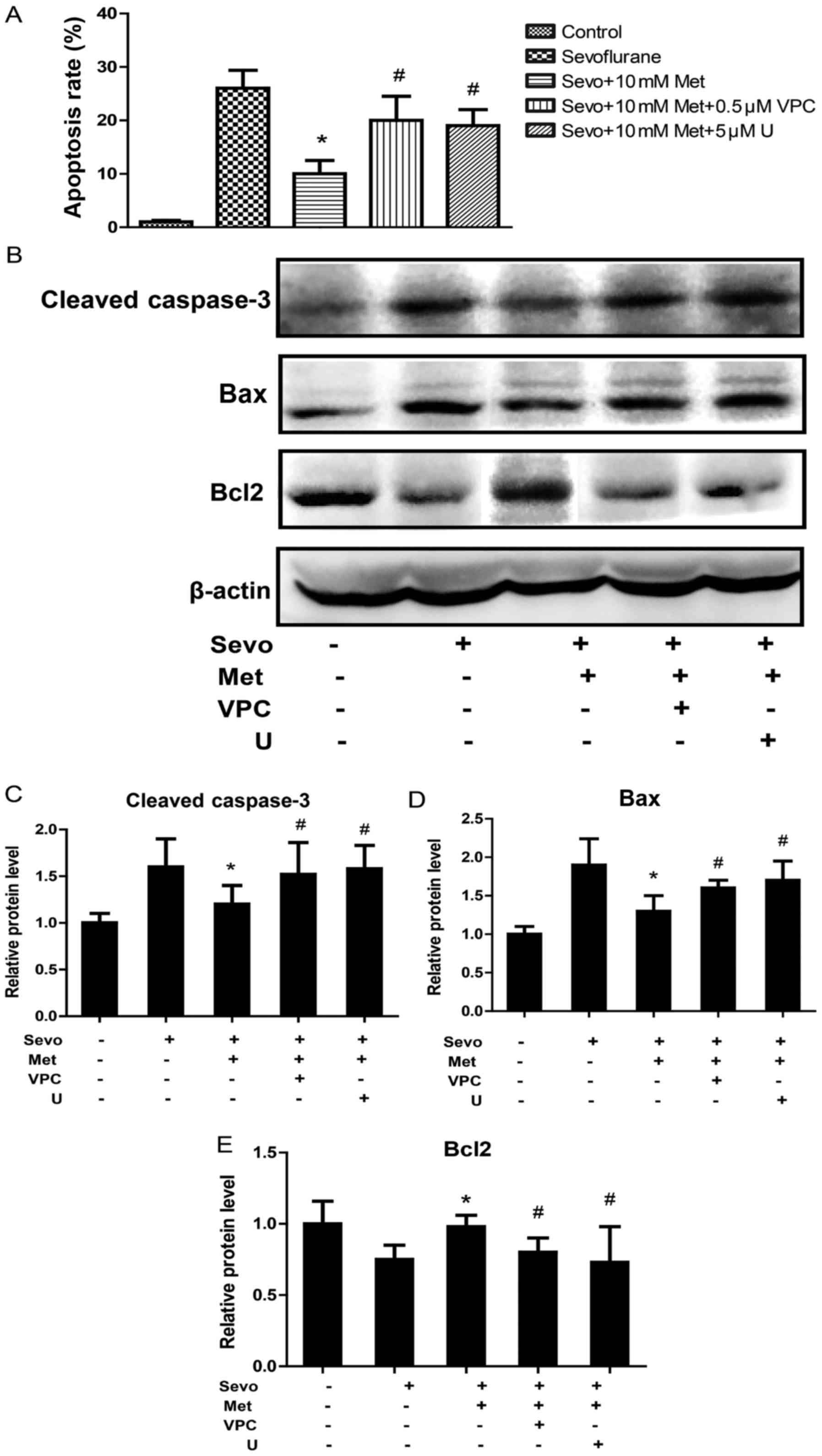 | Figure 3.Sphingosine 1-phosphate receptor 1
antagonism increases the apoptosis rate and pro-apoptosis proteins
of sevo- and metformin-treated cells in vitro. Neurons were
untreated, or treated with sevo, sevo and met, sevo and met and VPC
or sevo and met and U. (A) The apoptotic rate of neurons was
assessed by an MTT assay. (B) Protein expression of
cleaved-caspase-3, Bax and Bcl2 were assessed using western
blotting. Quantification of the protein expression of (C)
cleaved-caspase-3, (D) Bax and (E) Bcl2. *P<0.05 vs. Sevo.
#P<0.05 vs. Sevo+10 mM Met. Sevo, sevoflurane; Met,
metformin; VPC, VPC23019 (a sphingosine 1-phosphate receptor 1
antagonist); U, U0126 (a mitogen-activated protein kinase kinase
inhibitor); Bax, apoptosis regulator BAX; Bcl2, apoptosis regulator
Bcl-2. |
Metformin prevents neuronal apoptosis
by phosphorylation of mitogen-activated protein kinase
(ERK)1/2
Studies have suggested that S1P1 activation promotes
cell survival functions associated with ERK1/2 (22,23). The
current study examined whether metformin could exert anti-apoptotic
effects through the activation of ERK1/2. To clarify the role of
ERK1/2 in metformin neuroprotection, metformin and U0126, a
mitogen-activated protein kinase kinase (MEK) inhibitor, were used
to examine sevoflurane-treated neurons. The anti-apoptotic effect
of metformin was eliminated by U0126. U0126 significantly increased
the apoptosis rate, and cleaved-caspase-3 and Bax protein
expression levels, and significantly decreased Bcl2 compared with
sevoflurane- and metformin-treated cells (Fig. 3).
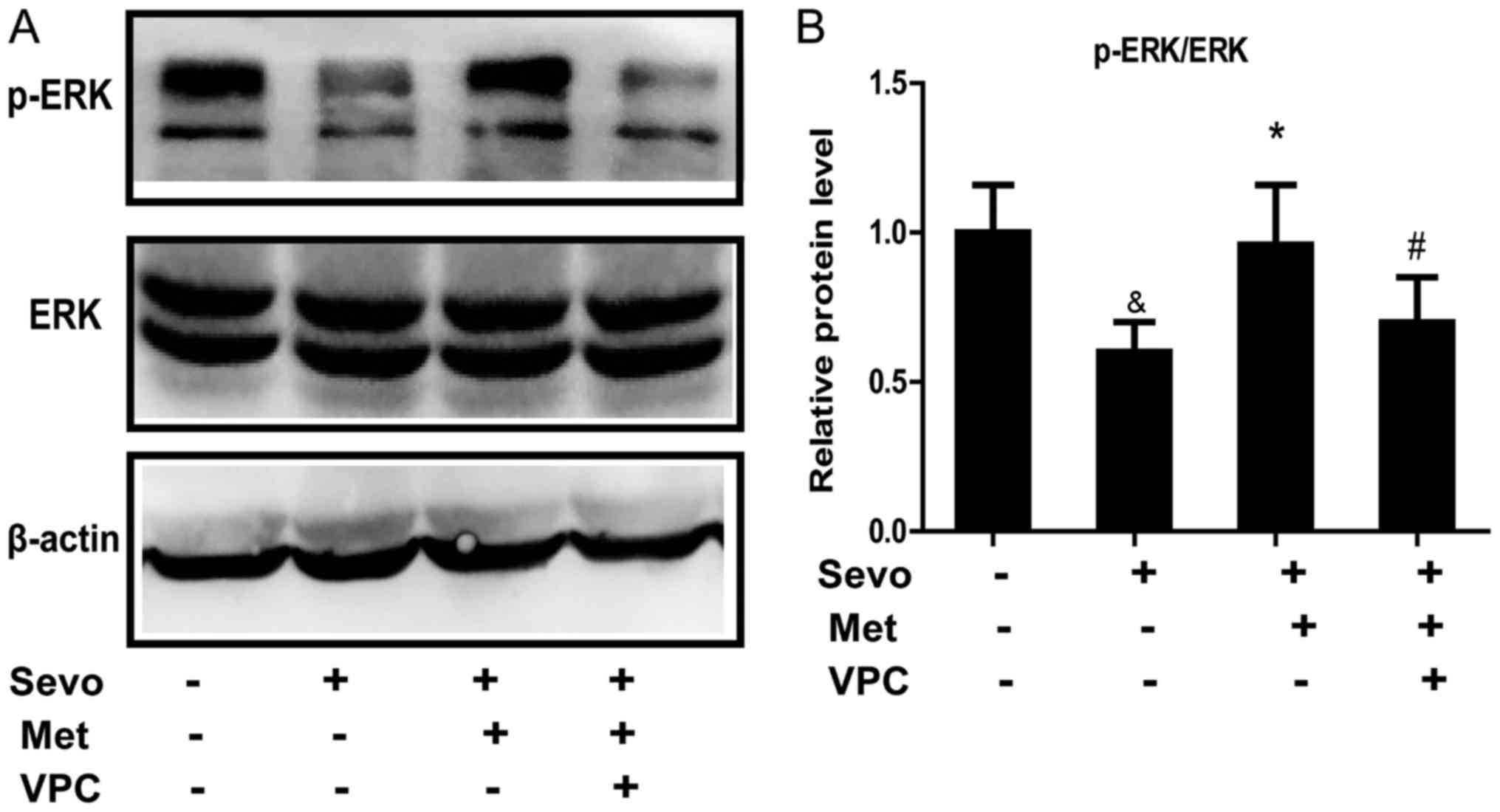
When the cultured neurons were exposed to
sevoflurane, phosphorylated (p-)ERK1/2 protein levels were
significantly decreased compared with that of the control group
(Fig. 4). However, the level of
p-ERK1/2 protein expression in neurons treated with both
sevoflurane and metformin was significantly increased compared with
cells treated with sevoflurane only. VPC23019 incubation
significantly decreased p-ERK1/2 in sevoflurane- and
metformin-treated cells compared with those without VPC23019. Since
VPC23019-treated neurons could counteract the metformin-induced
increase in p-ERK1/2 levels, it could be further demonstrated that
metformin activated ERK1/2 signal transduction by binding to S1P1.
These data indicate that metformin protected neurons against
sevoflurane-induced apoptosis through the activation of the
S1P1-dependent ERK1/2 signaling pathway.
Discussion
Sevoflurane is a commonly used inhaled anesthetic
gas (7,8). It remains controversial whether the use
of sevoflurane for anesthesia negatively affects the nervous system
(10). A number of preclinical
studies have revealed that general inhaled anesthetics, such as
sevoflurane, can induce the apoptosis of a wide range of cerebral
neurons, resulting in specific long-term cognitive and memory
impairments (24,25). A number of studies have demonstrated
that sevoflurane application not only causes neurotoxicity in
normal people, but also leads to neurological damage and neuronal
apoptosis in patients with Alzheimer's disease (26,27). Its
mechanism of action may be achieved through a variety of signal
transduction pathways, including nuclear factor NF-κ-B and S1P
(16,28).
In the present study, significant hippocampal
neuronal apoptosis was induced by 3% sevoflurane treatment for 3 h
and it induced neuronal apoptosis in what appeared to be a
time-dependent manner. Metformin was first clinically used as a
hypoglycemic agent (18,19). Subsequent studies demonstrated that
metformin had different effects on various tissues and systems
(29,30). Metformin was revealed to prevent the
sevoflurane-induced apoptosis of neurons, which was reversed by a
specific S1P1 antagonist (31).
However, the mechanism by which metformin produces this protective
effect on the nervous system remains unclear. Currently, it is
hypothesized that metformin can reduce neuronal apoptosis, and
protect against lymphocyte aggregation, inflammatory responses and
blood vessel dysfunction (32–34).
Previous studies have determined that the S1P
signaling pathway can stimulate neuronal growth and survival. It
has been reported that S1P can promote the survival of PC12 cells
and cultured midbrain neurons (16,35).
Studies have suggested that the downregulation of
ERK is correlated with anesthetics-induced neuronal apoptosis
(36,37). The current study demonstrated that
p-ERK1/2 levels significantly decreased in the sevoflurane-exposed
group when compared with that of the control group. However,
p-ERK1/2 levels in sevoflurane- and metformin-treated neurons were
almost normal. VPC23019 treatment decreased the metformin-induced
increase in p-ERK1/2 levels, therefore metformin may activate
ERK1/2 signal transduction by binding to S1P1. Additionally, the
anti-apoptotic effect of metformin was eliminated by U0126,
indicating that metformin protected neurons from
sevoflurane-induced apoptosis via activating the S1P1-dependent
ERK1/2 signaling pathway.
The present study indicated that the ERK1/2 signal
transduction pathway serves an important role in
anesthetics-induced neuronal apoptosis. Maintaining the level of
p-ERK1/2 during anesthesia helps to maintain the viability of
neurons (38). The results of the
current study also indicated that metformin regulated
sevoflurane-induced neuronal apoptosis through the regulation of
Bcl-2 and Bax. Metformin was demonstrated to protect neurons
against sevoflurane-induced apoptosis through the activation of the
S1P1-dependent ERK1/2 signaling pathway. The present study
demonstrated that metformin is able to protect against
sevoflurane-induced neurotoxicity possibly through activation of
the S1P1-dependent ERK1/2 signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was funded by Shaanxi Self-heating
Science Foundation Project Fund (grant no. 2014jm2-8176).
Availability of data and materials
All datasets used and/or generated during the
present study are available from the corresponding author in
reasonable request.
Authors' contributions
HY and ML designed the study, performed the
experiments and prepared the manuscript. HY and BH collected the
data, and HY and ZL analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Yan'an University Animal Center (Yan'an, Shaanxi).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wan Hassan WMN, Tan HS and Mohamed Zaini
RH: Comparison of the effects of dexmedetomidine on the induction
of anaesthesia using marsh and schnider pharmacokinetic models of
propofol Target-Controlled infusion. Malays J Med Sci. 25:24–31.
2018.PubMed/NCBI
|
|
2
|
Afolayan JM, Areo PO, Adegun PT, Ogundipe
KO and Filani AB: Comparison of ease of induction of spinal
anaesthesia in sitting with legs parallel on the table versus
traditional sitting position. Pan Afr Med J. 28:2232017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson BJ: Drug error in paediatric
anaesthesia: Current status and where to go now. Curr Opin
Anaesthesiol. 31:333–341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gautam B, Niroula S, Sharma M and Lama SM:
Effects of intrathecal dexmedetomidine as an adjuvant to hyperbaric
bupivacaine for spinal anaesthesia in adults undergoing elective
infra-umbilical surgery. JNMA J Nepal Med Assoc. 56:379–387. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwartz C: Enhanced recovery after
posterior minimally invasive total hip arthroplasty with continuous
intraarticular anaesthesia. Eur J Orthop Surg Traumatol.
28:761–769. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moran PJ, Fennessy P and Johnson MZ:
Establishing a new national standard for the documentation of
regional anaesthesia in Ireland. BMJ Open Qual. 6:e0002102017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Juodzente D, Macas A, Karveliene B,
Petkevicius S and Riskeviciene V: Comparison of the cardiovascular
and respiratory effects and sevoflurane requirement in dogs
premedicated with two doses of medetomidine and butorphanol
undergoing surgical sterilization. Pol J Vet Sci. 21:101–110.
2018.PubMed/NCBI
|
|
8
|
Suzuki T, Kurazumi T, Ueda T, Nagata H,
Yamada T, Kosugi S, Hashiguchi S, Ito K and Morisaki H: Desflurane
anesthesia worsens emergence agitation in adult patients undergoing
thyroid surgery compared to sevoflurane anesthesia. JA Clin Rep.
3:362017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang LM, Zhang DX, Zhao XC and Sun W:
Erythropoietin rescues primary rat cortical neurons by altering the
nrf2:Bach1 ratio: Roles of extracellular Signal-Regulated kinase
1/2. Neurochem Res. Jan 12–2017.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Li R, Zhang LM and Sun WB: Erythropoietin
rescues primary rat cortical neurons from pyroptosis and apoptosis
via Erk1/2-Nrf2/Bach1 signal pathway. Brain Res Bull. 130:236–244.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang DX, Zhang LM, Zhao XC and Sun W:
Neuroprotective effects of erythropoietin against
sevoflurane-induced neuronal apoptosis in primary rat cortical
neurons involving the EPOR-Erk1/2-Nrf2/Bach1 signal pathway. Biomed
Pharmacother. 87:332–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang YE, Jeong SA, Kim SY, Song IK, Lee
JH, Kim JT and Kim HS: The efficacy of intraoperative EEG to
predict the occurrence of emergence agitation in the postanesthetic
room after sevoflurane anesthesia in children. J Perianesth Nurs.
33:45–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu G, Xu H, Zhao W, Zhang J, Rao D and Xu
S: Upregulation of long noncoding RNA Gadd45a is associated with
sevoflurane-induced neurotoxicity in rat neural stem cells.
Neuroreport. 29:605–614. 2018.PubMed/NCBI
|
|
14
|
Szepanowski F, Derksen A, Steiner I, Meyer
Zu Hörste G, Daldrup T, Hartung HP and Kieseier BC: Fingolimod
promotes peripheral nerve regeneration via modulation of
lysophospholipid signaling. J Neuroinflammation. 13:1432016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho MC, Park K, Chai JS, Lee SH, Kim SW
and Paick JS: Involvement of
sphingosine-1-phosphate/RhoA/Rho-kinase signaling pathway in
corporal fibrosis following cavernous nerve injury in male rats. J
Sex Med. 8:712–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joly S and Pernet V: Sphingosine
1-phosphate receptor 1 is required for retinal ganglion cell
survival after optic nerve trauma. J Neurochem. 138:571–586. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costello RW, Maloney M, Atiyeh M, Gleich G
and Walsh MT: Mechanism of sphingosine 1-phosphate- and
lysophosphatidic acid-induced up-regulation of adhesion molecules
and eosinophil chemoattractant in nerve cells. Int J Mol Sci.
12:3237–3249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan X, Wei W, Bao Q, Chen H, Jin P and
Jiang W: Metformin inhibits glioma cells stemness and
epithelial-mesenchymal transition via regulating YAP activity.
Biomed Pharmacother. 102:263–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu X, Pan Y, Cao Q, Li B, Wang S, Du H,
Duan N and Li X: Metformin restores electrophysiology of small
conductance calcium-activated potassium channels in the atrium of
GK diabetic rats. BMC Cardiovasc Disord. 18:632018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ge XH, Zhu GJ, Geng DQ, Zhang HZ, He JM,
Guo AZ, Ma LL and Yu DH: Metformin protects the brain against
ischemia/reperfusion injury through PI3K/Akt1/JNK3 signaling
pathways in rats. Physiol Behav. 170:115–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Deng J, Sheng W and Zuo Z: Metformin
attenuates Alzheimer's disease-like neuropathology in obese,
leptin-resistant mice. Pharmacol Biochem Behav. 101:564–574. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rutherford C, Childs S, Ohotski J, McGlynn
L, Riddick M, MacFarlane S, Tasker D, Pyne S, Pyne NJ, Edwards J
and Palmer TM: Regulation of cell survival by
sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent
suppression of Bim and PI-3-kinase/protein kinase C-mediated
upregulation of Mcl-1. Cell Death Dis. 4:e9272013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brizuela L, Rábano M, Gangoiti P, Narbona
N, Macarulla JM, Trueba M and Gómez-Muñoz A:
Sphingosine-1-phosphate stimulates aldosterone secretion through a
mechanism involving the PI3K/PKB and MEK/ERK 1/2 pathways. J Lipid
Res. 48:2264–2274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
SÖbbeler FJ, Carrera I, Pasloske K,
Ranasinghe MG, Kircher P and Kästner SBR: Effects of isoflurane,
sevoflurane, propofol and alfaxalone on brain metabolism in dogs
assessed by proton magnetic resonance spectroscopy ((1)H MRS). BMC
Vet Res. 14:692018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wen T, Wang L, Sun XJ, Zhao X, Zhang GW
and Li-Ling J: Sevoflurane preconditioning promotes activation of
resident CSCs by transplanted BMSCs via miR-210 in a rat model for
myocardial infarction. Oncotarget. 8:114637–114647. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Epstein RH, Maga JM, Mahla ME, Schwenk ES
and Bloom MJ: Prevalence of discordant elevations of state entropy
and bispectral index in patients at amnestic sevoflurane
concentrations: A historical cohort study. Can J Anaesth.
65:512–521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He H, Liu W, Zhou Y, Liu Y, Weng P, Li Y
and Fu H: Sevoflurane post-conditioning attenuates traumatic brain
injury-induced neuronal apoptosis by promoting autophagy via the
PI3K/AKT signaling pathway. Drug Des Devel Ther. 12:629–638. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang YH, Vasko MR and Nicol GD:
Intracellular sphingosine 1-phosphate mediates the increased
excitability produced by nerve growth factor in rat sensory
neurons. J Physiol. 575:101–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pisani A, Riccio E, Bruzzese D and
Sabbatini M: Metformin in autosomal dominant polycystic kidney
disease: Experimental hypothesis or clinical fact? BMC Nephrol.
19:2822018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmed FW, Bakhashab S, Bastaman IT,
Crossland RE, Glanville M and Weaver JU: Anti-angiogenic miR-222,
miR-195, and miR-21a plasma levels in T1DM are improved by
metformin therapy, thus elucidating its cardioprotective effect:
The MERIT study. Int J Mol Sci. 19(pii): E32422018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayes M, Rabasseda X and Prous JR:
Gateways to clinical trials. Methods Find Exp Clin Pharmacol.
24:217–248. 2002.PubMed/NCBI
|
|
32
|
Ma J, Liu J, Yu H, Chen Y, Wang Q and
Xiang L: Beneficial effect of metformin on nerve regeneration and
functional recovery after sciatic nerve crush injury in diabetic
rats. Neurochem Res. 41:1130–1137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanaka Y, Uchino H, Shimizu T, Yoshii H,
Niwa M, Ohmura C, Mitsuhashi N, Onuma T and Kawamori R: Effect of
metformin on advanced glycation endproduct formation and peripheral
nerve function in streptozotocin-induced diabetic rats. Eur J
Pharmacol. 376:17–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gudbjornsdottir S, Friberg P, Elam M,
Attvall S, Lönnroth P and Wallin BG: The effect of metformin and
insulin on sympathetic nerve activity, norepinephrine spillover and
blood pressure in obese, insulin resistant, normoglycemic,
hypertensive men. Blood Press. 3:394–403. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nicol GD: Nerve growth factor,
sphingomyelins, and sensitization in sensory neurons. Sheng Li Xue
Bao. 60:603–604. 2008.PubMed/NCBI
|
|
36
|
Wang S and Zhou Y: Baicalein inhibits
neuroapoptosis via pathways in sevoflurane induced rats. Transl
Neurosci. 9:88–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Osinde M, Mullershausen F and Dev KK:
Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes
via S1P receptors. Neuropharmacology. 52:1210–1218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Zhang X, Zhang W, Gu G and Wang P:
Effects of sevoflurane on young male adult C57BL/6 mice spatial
cognition. PLoS One. 10:e1342172015.
|