Introduction
Spinal cord injury (SCI) is one of the most common
injuries that requires spinal surgery (1). SCI is often caused by traffic
accidents, falls, construction accidents and sports injuries
(1). The incidence of SCI has
increased with urban and transportation development (1). Recent epidemiological studies have
suggested that 11,000 new cases of SCI are reported each year in
the USA (1,2). SCI is often accompanied by serious
complications, including respiratory dysfunction and failure,
pneumonia, pulmonary edema and embolism (1).
High mobility group box-1 (HMGB-1) is a non-histone
protein that is located in eukaryotic nuclei and comprises 215
amino acid residues (1,3). It has a highly conserved structure and
is primarily synthesized in damaged cells or peripheral mononuclear
macrophages (1,4). HMGB-1 activates the downstream
inflammatory signaling pathway. As such, it serves a role in a
variety of tumors, inflammatory reactions and organ damage
(1,4). HMGB-1 receptors include toll-like
receptor (TLR)2, TLR4 and the receptor for advanced glycation end
products, which is also a member of the TLR family (5). The HMGB-1/TLR4 pathway has previously
been demonstrated to serve a vital role in the pathogenesis of SCI
caused by burn, trauma and shock (6). Furthermore, HMGB-1 is reported to be
involved in a number of central nervous system injuries (6). A recent study indicated that HMGB-1
mediates SCI-related localized neuronal apoptosis (6).
Number of microRNAs (miRs or miRNAs) are associated
with and serve important roles in the progression from SCI to ASCI
(7). A microarray study of ASCI in
rats suggested that many miRNAs are differentially expressed
following ASCI (7). Bioinformatics
analysis revealed that changes in miRNA expression serve a role in
the pathogenesis of ASCI in rats (7). Furthermore, changes in miRNA expression
are crucial for cell apoptosis, oxidative stress, angiogenesis,
inflammatory response and other mechanisms (7). However, the specific functions and
underlying mechanisms of differentially expressed miRNAs are widely
unknown. Yuan et al (8)
demonstrated that miR-34a modulates endothelial inflammation after
fetal cardiac bypass in the goat placenta. The aim of the present
study was to investigate the effect of miR-34a on SCI-induced
inflammation and the possible underlying mechanism.
Materials and methods
Animals and spinal cord surgery
A total of 12 Male Wistar rats weighing 220–250 g
and aged 10–12 weeks old were purchased from The Animal Centre of
Nanchang University (Nanchang, China) and housed in our laboratory
at 22–23°C in 55–60% humidity, with a 12 h light/dark cycle, and
free access to food and water. The rats underwent urinary bladder
massage at least twice per day until the recovery of spontaneous
micturition (9). The present study
was approved by the Research Council and Animal Care and Use
Committee of Shangrao People's Hospital (Shangrao, China), and
performed according to the National Institutes of Health Guide for
the Care and Use of Laboratory Animals (9). Rats were randomly divided into two
groups: Control (n=6) and SCI model (n=6). Rats in the SCI model
group were anaesthetized using 35 mg/kg pentobarbital sodium
(intravenous injection; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), following which an incision was made on the back
posterior to the lower thoracic region. Back muscles were separated
and the dorsal surface of the spinal cord was exposed at T10. The
lower thoracic cord was transected using fine scissors and the
surgical wound was closed in two layers. Rats in the control group
were anaesthetized using 35 mg/kg pentobarbital sodium and did not
undergo surgery. At 12 h following spinal surgery, rats in all
groups were anaesthetized using 35 mg/kg pentobarbital sodium and
sacrificed by decollation. The back muscles were then separated at
T10 and the spinal cord was harvested. Spinal cord tissues was
collected from spinal surgery and washed with PBS. Tissue samples
were then fixed with 4% paraformaldehyde for 24 h at room
temperature.
MicroRNA quantification
Total RNA was extracted from the spinal cord using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). First-strand cDNA was synthesized using a Takara RNA PCR kit
(Takara Bio, Inc., Otsu, Japan) at 37°C for 30 min and 84°C for 10
sec. miR-34a expression was measured using SYBR Select Master Mix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and a CFX 96TM
Connect Real-Time system (Bio-Rad Laboratories, Inc.). The PCR
conditions were as follows: 95°C for 10 min; 40 cycles of 95°C for
30 sec, 60°C for 30 sec and 72°C for 30 sec. Primers used were as
follows: miR-34a forward, 5′-TCTGTCTCTCTTGGCAGTGTCTT-3′ and
reverse, 5′-CTCGCTTCGGCAGCACA-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The thermocycling conditions were as
follows: 95°C for 10 min; 40 cycles of 95°C for 30 sec, 60°C for 30
sec and 72°C for 30 sec. Relative mRNA expression was quantified
using the 2−ΔΔCq method (10).
Cell culture and transfection
PC12 cells were purchased from Shanghai Cell Bank of
the Chinese Academy of Sciences (Shanghai, China) and cultured in
Dulbecco's Modified Eagle's Medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin at
37°C in an atmosphere containing 5% CO2. MiR-34a mimics
(miR-34a overexpression), anti-miR-34a (miR-34a knockdown) and
negative control miRNA (control) were purchased from Sangon Biotech
Co., Ltd. (Shanghai, China). Cells were transfected with 100 ng of
miR-34a mimics (miR-34a overexpression), anti-miR-34a (miR-34a
knockdown) and negative control miRNAs (control) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 4 h post-transfection, PC12 cells were
treated with lipopolysaccharide (50 ng/ml; Invitrogen; Thermo
Fisher Scientific, Inc.) and TAK-242 (1 nM; MedChemExpress,
Shanghai, China) for 24, 48 and 72 h at 37°C for cell proliferation
assays and for 48 h for all other assays.
Cell proliferation assay
MTT (10 ml; 5 mg/ml; Beyotime Institute of
Biotechnology, Haimen, China) was added to cells after transfecting
the cells for 24, 48 or 72 h; MTT was incubated with the cells at
37°C for 4 h in the dark. Dimethyl sulfoxide was added for 20 min
at 37°C after the culture medium was removed. The absorbance was
measured using a microplate reader (FluoDia T70; Photon Technology
International, Lawrenceville, NJ, USA) at a wavelength of 490
nm.
Flow cytometry
At 48 h post-transfection, cells were washed three
times with PBS and resuspended with 5 µl annexin V-fluorescein
isothiocyanate and 5 µl propidium iodide (BD Biosciences, Franklin
Lakes, NJ, USA) at room temperature for 15 min. Apoptosis was
measured using a CyAn™ ADP cytometer (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA).
Western blotting
Proteins were extracted from cells at 48 h after
transfection using radioimmunoprecipitation assay buffer (Kaiji,
Shanghai, China) and quantified using a BCA assay. Proteins (50
µg/lane) were separated by 8–10% SDS-PAGE and blotted onto
polyvinylidene fluoride membranes. Membranes were subsequently
blocked using 5% non-fat dry milk in 0.1% TBS/Tween at 37°C for 1
h, following which they were incubated with antibodies against TLR4
(sc-8694; 1:1,000), HMGB-1 (sc-26351; 1:1,000) and GAPDH
(sc-293335; 1:2,000; all Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at 4°C overnight. The membranes were next washed with 0.1%
TBS/Tween and incubated with a horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (sc-2004; 1:2,000; Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h. Proteins were visualized
using an enhanced chemiluminescent detection reagent (Pierce;
Thermo Fisher Scientific, Inc.). Protein bands were measured using
Image Lab 3.0 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance was used for comparisons between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-34a expression in an in vitro
model of SCI
In order to investigate the mechanism of miR-34a in
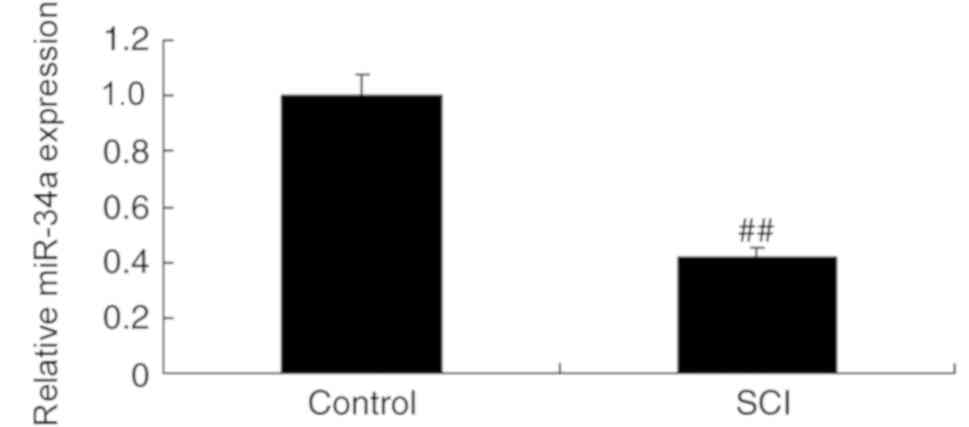
SCI, miR-34a expression was measured in SCI model rats and control
rats. The results indicate that miR-34a was downregulated in the
SCI model group compared with the control group (Fig. 1). This suggests that miR-34a
expression may be associated with the pathogenesis of SCI.
Effects of miR-34a on inflammation in
an in vitro model of SCI
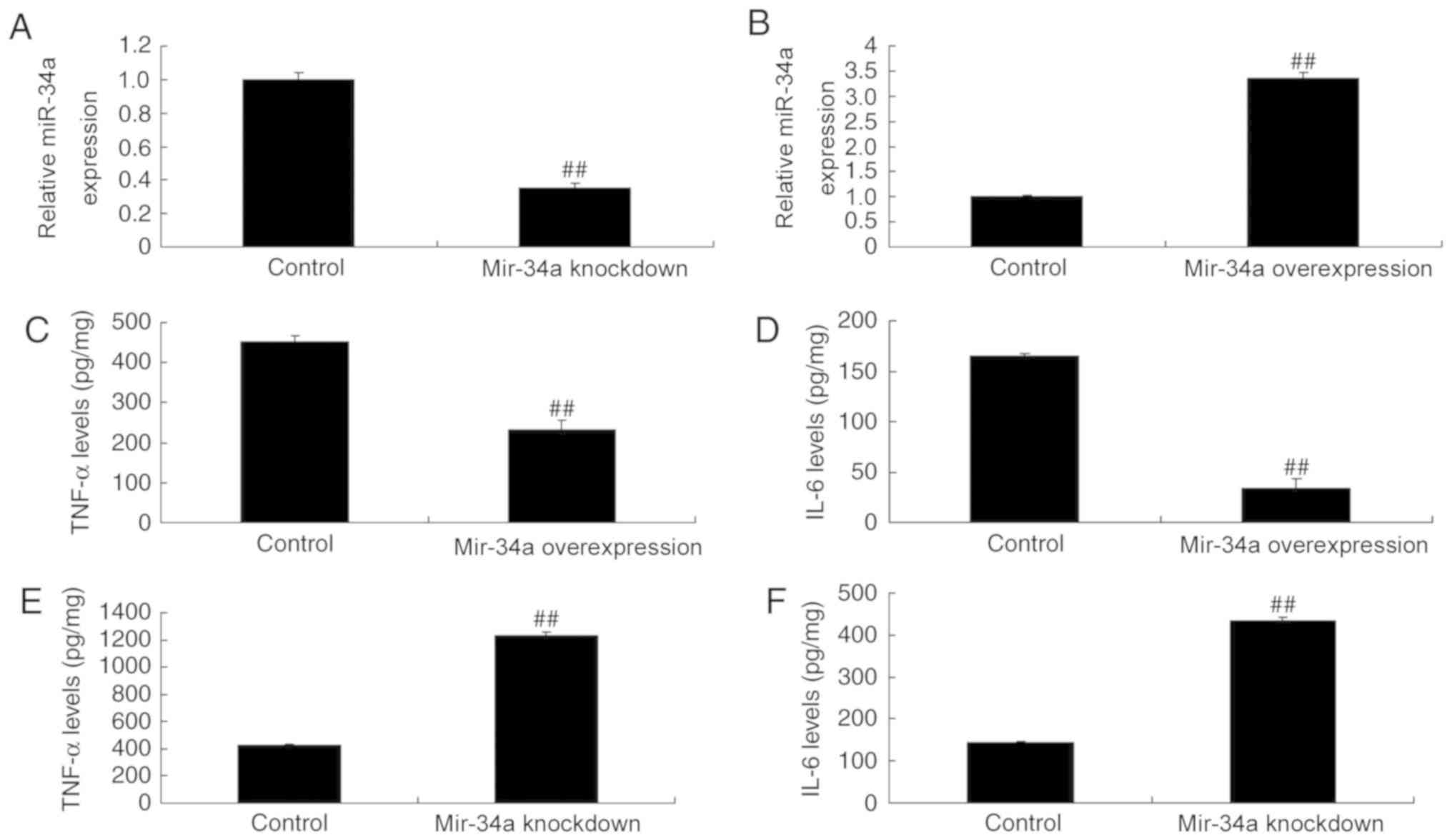
MiR-34a mimics and anti-miR-34a mimics were used to
induce miR-34a overexpression and knockdown, respectively, in
vitro (Fig. 2A and B). MiR-34a
overexpression downregulated TNF-α and IL-6 in SCI expression
compared with control cells (Fig. 2C and
D), while miR-34a knockdown upregulated TNF-α and IL-6
(Fig. 2E and F).
Effects of miR-34a on cell growth in
an in vitro model of SCI
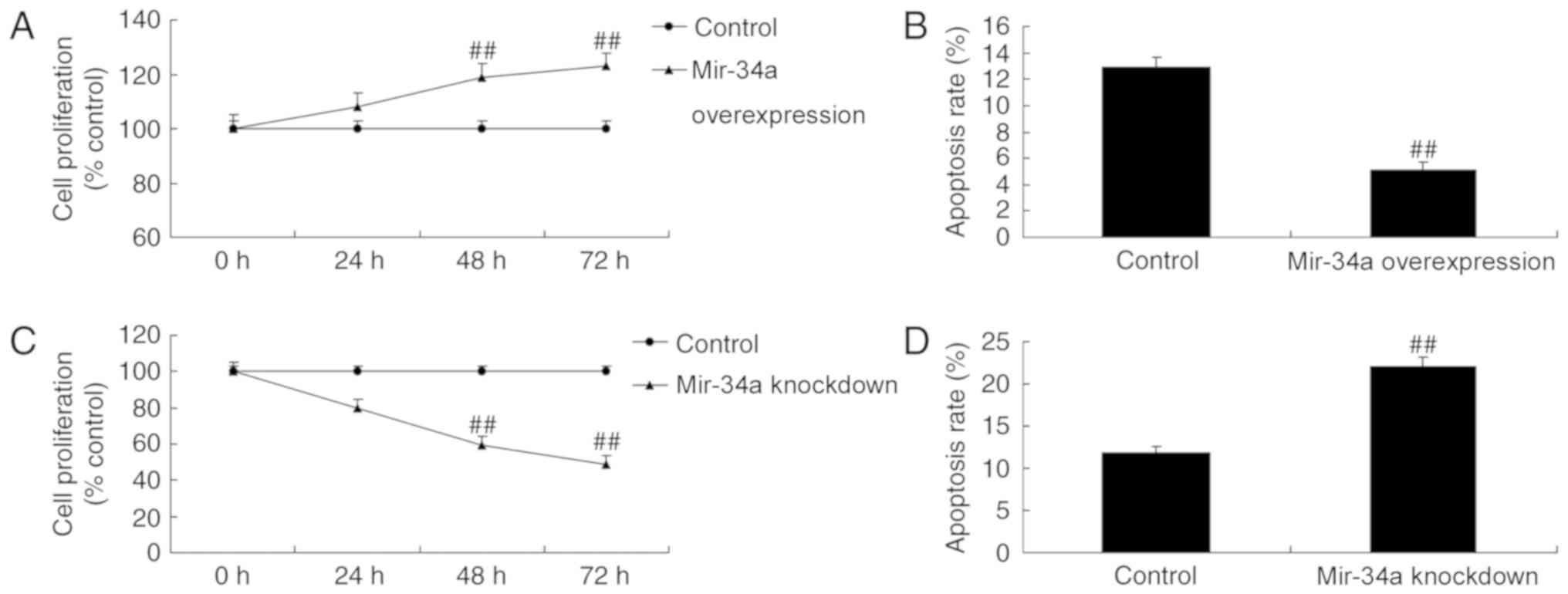
MiR-34a overexpression increased cell proliferation
and reduced apoptosis in an in vitro SCI model compared with
control cells (Fig. 3A-B). In cell
proliferation was inhibited and apoptosis was increased in miR-34a
knockdown cells compared with the control (Fig. 3C-D).
Effects of miR-34a on COX-2 and NF-κB
protein expression in an in vitro model of SCI
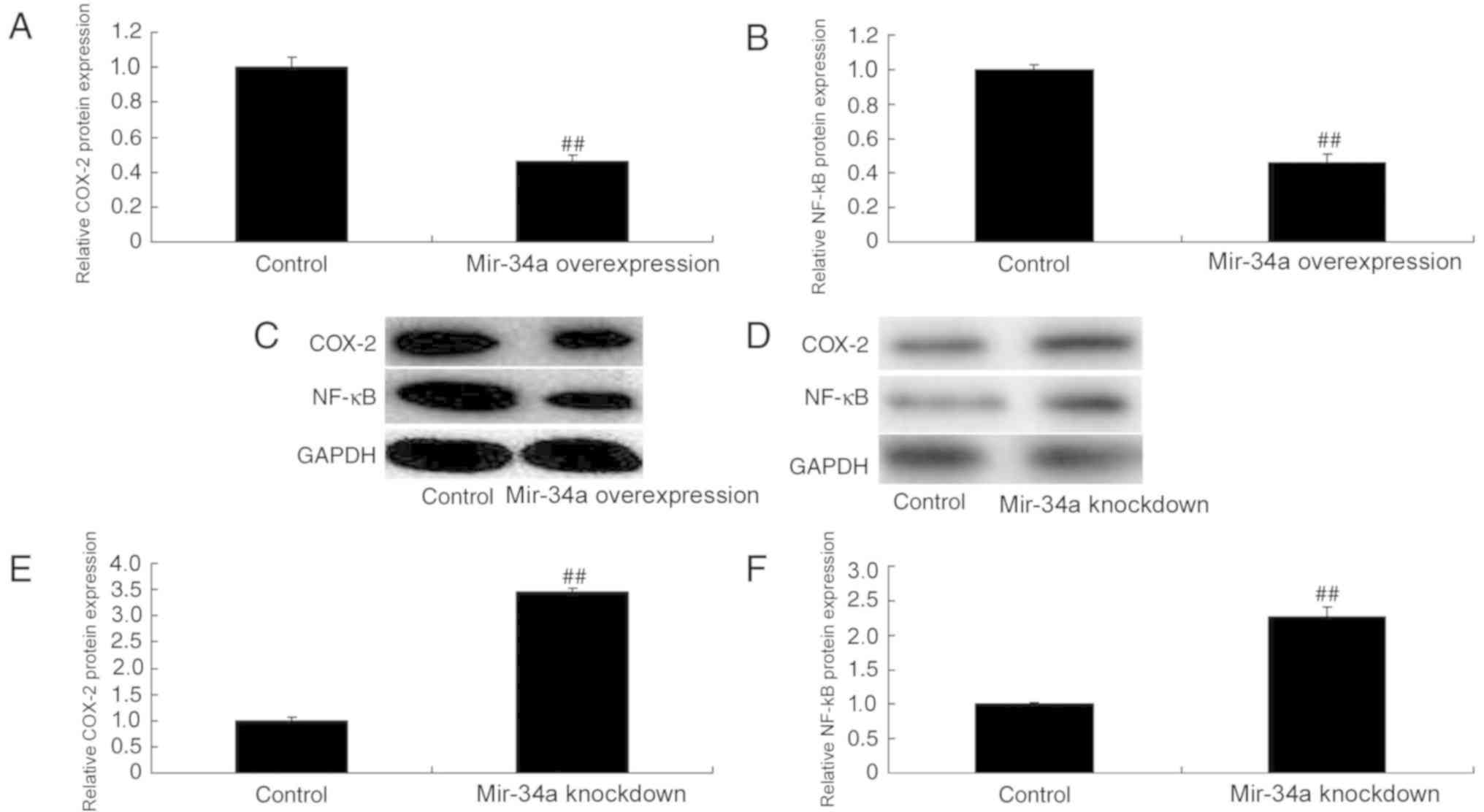
COX-2 and NF-κB were downregulated in miR-34a
overexpression cells compared with the control, whereas they were
upregulated in miR-34a knockdown cells (Fig. 4).
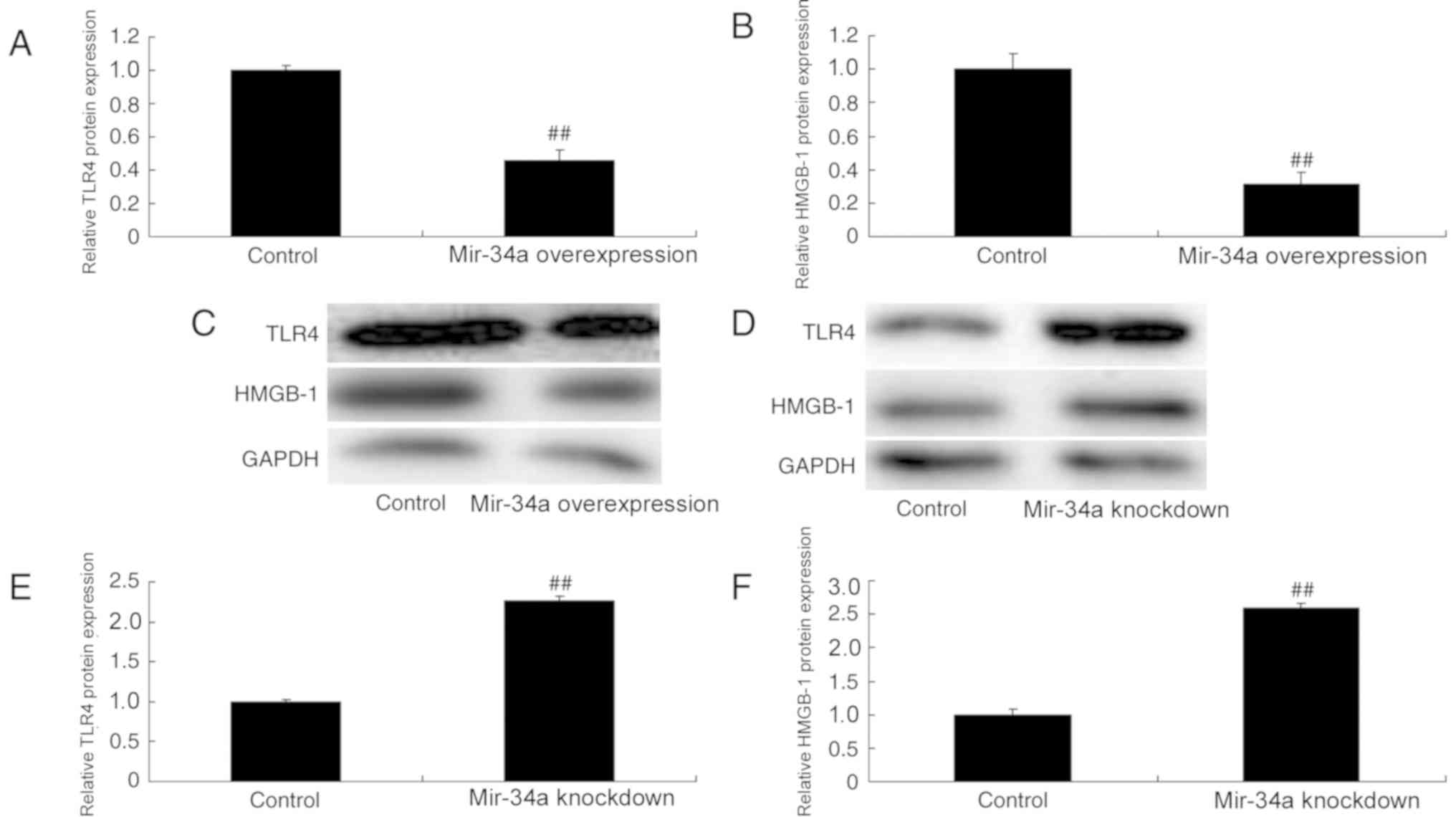
Effects of miR-34a on TLR4 and HMGB-1
protein expression in an in vitro model of SCI
TLR4 and HMGB-1 expression was assessed using
western blotting. The results revealed that TLR4 and HMGB-1 were
downregulated in miR-34a overexpression cells compared with the
control; however, they were upregulated in miR-34a knockdown cells
(Fig. 5).
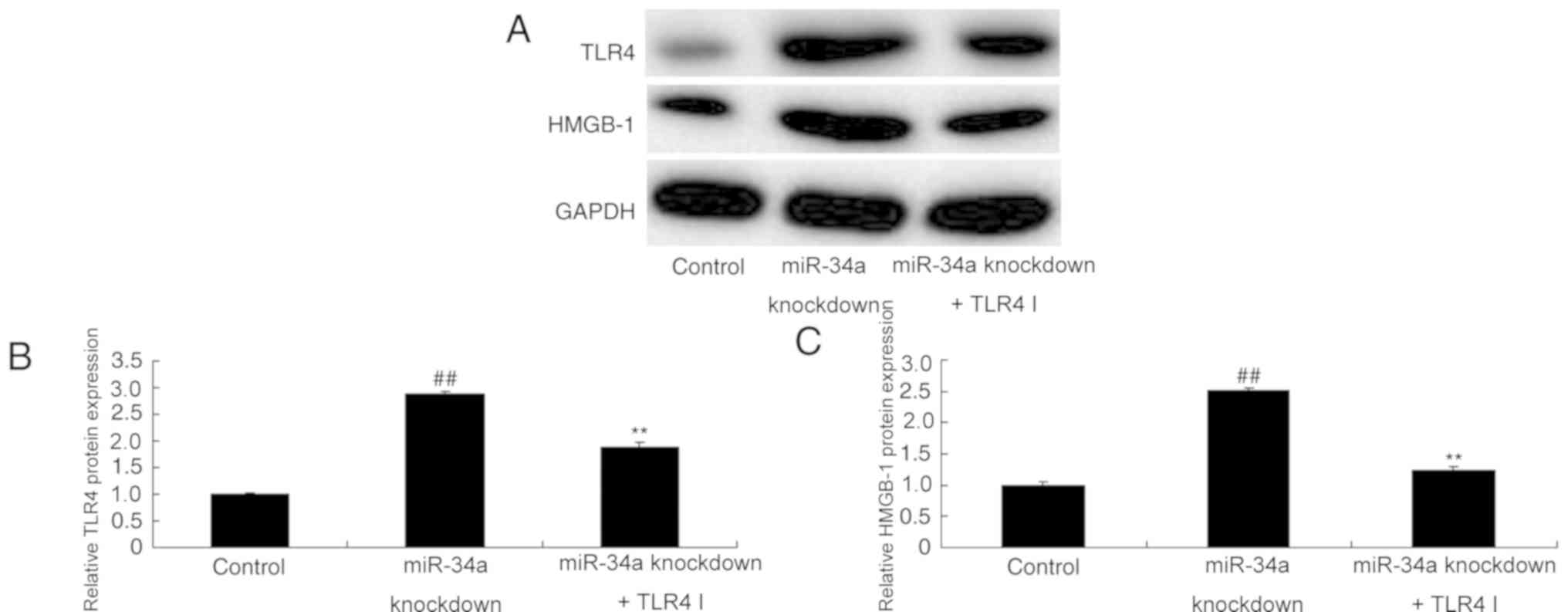
TLR4 inhibitor attenuates the effect
of miR-34a downregulation and reduces TLR4 and HMGB-1 protein
expression in SCI
The role of TLR4 in miR-34a downregulation in SCI
was further assessed using a TLR4 inhibitor. The results revealed
that TLR4 inhibitor ameliorated the miR-34a knockdown-induced
overexpression of TLR4 and HMGB-1 in an in vitro model of
SCI (Fig. 6).
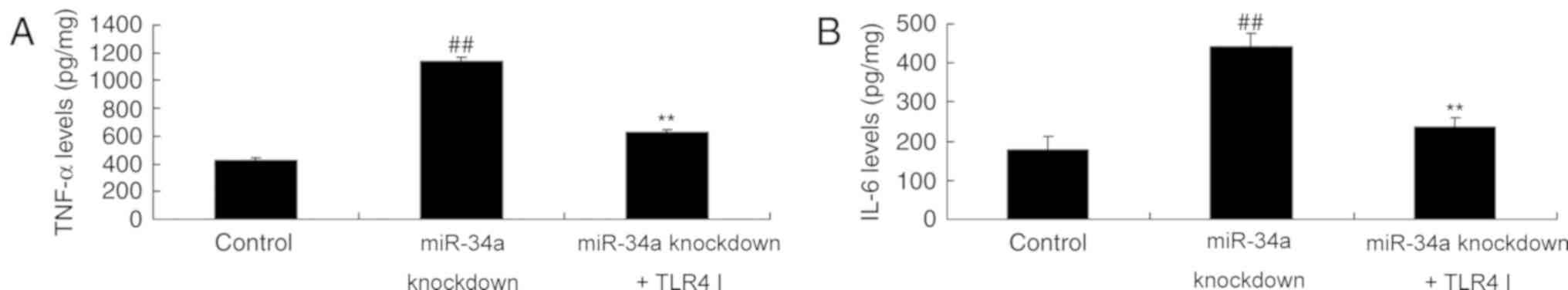
TLR4 inhibitor decreases the effect of
miR-34a downregulation on inflammation and cell growth in SCI
Treatment with the TLR4 inhibitor was demonstrated
to ameliorate the miR-34a knockdown-induced overexpression of TNF-α
and IL-6 levels and reverse the effects of miR-34a knockdown on
cell proliferation and apoptosis (Fig.
7).
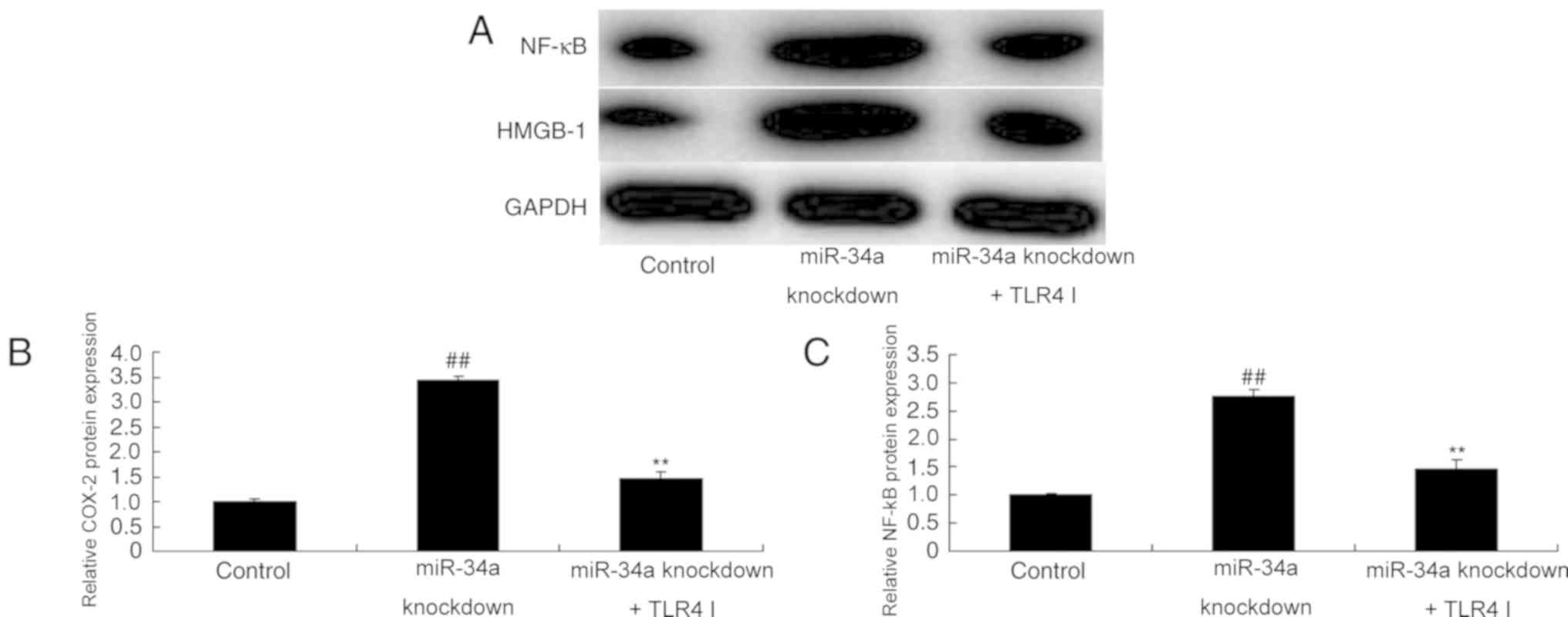
TLR4 inhibitor decreases the effect of
miR-34a downregulation on COX-2 and NF-κB protein expression in
SCI
TLR4 inhibitor was demonstrated to suppress the
expression of COX-2 and NF-κB proteins in an in vivo model
of SCI model following miR-34a knockdown, compared with untreated
miR-34a knockdown cells (Fig.
8).
Discussion
SCI can be classified as primary or secondary
according to its mechanism (1,2). Primary
SCI occurs as a result of direct or indirect external force on the
spinal cord, while secondary SCI results from destructive lesions
in the integrated tissues that occur due to primary SCI through a
series of physiological and biochemical mechanisms (11). These mechanisms include oxidative
stress, increased inflammatory response and overexpression of
excitatory amino acids (1). As such,
secondary injury may further aggravate SCI and expand the scope of
injury. The inflammatory response following SCI is complicated,
involving the nervous system, immune system and various other
dynamic factors (12). A number of
studies have reported that the SCI-induced inflammatory response
has a dual-effect of nerve injury and neuroprotection (1). In the present study, miR-34a expression
was demonstrated to be reduced in a rat model of SCI compared with
the control group.
The oxidative activity of serum inflammatory cells,
including neutrophils, has been reported to be increased in SCI
patients (13). Furthermore, levels
of free radicals are elevated, NF-κB is upregulated and
myeloperoxidase activity is increased (13). Damaged nerve cells have been
demonstrated to generate and release certain inflammatory factors
and other stimulating proteins during the pathogenesis of SCI
(14). They enter the circulation
through the injured blood-brain barrier, thus mediating the
systemic inflammatory response and causing lung injury (14). The results of the present study
suggest that suppressing miR-34a expression aggravates
inflammation, inhibits cell proliferation, enhances apoptosis and
upregulates iNOS protein expression and NO levels in an in
vitro model of SCI. Yuan et al (8), demonstrated that miR-34a is able to
modulate endothelial inflammation after fetal cardiac bypass in the
goat placenta, which is consistent with the present study.
Necrotic nerve cells release a variety of
inflammatory proteins during the progression of SCI (15). They are able to mediate SCI and may
participate in systemic organ damage (15). HMGB-1 has been demonstrated to be
closely associated with SCI (15),
while TLR4 can mediate multiple inflammation and injury processes
(1,16). Injured nerve cells release HMGB-1
during early SCI and is highly expressed in spinal cord tissues
(1,17). In the present study, miR-34a
knockdown induced TLR4 and HMGB-1 protein expression in an in
vitro model of SCI.
HMGB-1 released by necrotic nerve cells is able to
bind with TLRs receptor as an endogenous ligand to activate the
downstream inflammatory pathway and mediate secondary SCI (18). Importantly, HMGB-1 has been reported
to be of great significance during the occurrence of lung injury
(19). Consequently, it is able to
initiate the corresponding signaling pathway to activate NF-κB and
other transcription factors through the MyD88-dependent or
MyD88-independent pathway (17).
This in turn leads to the transcription of inflammatory factors and
other immunomodulatory molecules and contributes to SCI (17). TLR4 inhibitor was revealed to
ameliorate the effects of miR-34a downregulation on inflammation
and cell growth in SCI. Jiang et al (20), suggested that the miR-34a/TLR4 axis
serves an important role in the development of hepatocellular
carcinoma (1), which was consistent
with the present study.
In conclusion, the results of the present study
suggest that miR-34a expression is associated with SCI. However,
the significance of these findings needs to be confirmed in studies
with a larger sample size.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ designed the experiment, analyzed the data and
wrote the manuscript. OS, JL, ZC, CW and WW performed the
experiments.
Ethics approval and consent to
participate
The present study was approved by the Research
Council and Animal Care and Use Committee of Shangrao People's
Hospital (Shangrao, China), and performed according to the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (9).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Flueck JL, Schlaepfer MW and Perret C:
Effect of 12-week vitamin D supplementation on 25[OH]D status and
performance in athletes with a spinal cord injury. Nutrients.
8(pii): E5862016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laubacher M, Perret C and Hunt KJ:
Work-rate-guided exercise testing in patients with incomplete
spinal cord injury using a robotics-assisted tilt-table. Disabil
Rehabil Assist Technol. 10:433–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Böhm MR, Schallenberg M, Brockhaus K,
Melkonyan H and Thanos S: The pro-inflammatory role of
high-mobility group box 1 protein (HMGB-1) in photoreceptors and
retinal explants exposed to elevated pressure. Lab Invest.
96:409–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang FC, Pei JX, Zhu J, Zhou NJ, Liu DS,
Xiong HF, Liu XQ, Lin DJ and Xie Y: Overexpression of HMGB1 A-box
reduced lipopolysaccharide-induced intestinal inflammation via
HMGB1/TLR4 signaling in vitro. World J Gastroenterol. 21:7764–7776.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun J, Shi S, Wang Q, Yu K and Wang R:
Continuous hemodiafiltration therapy reduces damage of multi-organs
by ameliorating of HMGB1/TLR4/NFκB in a dog sepsis model. Int J
Clin Exp Pathol. 8:1555–1564. 2015.PubMed/NCBI
|
|
6
|
Wang H, Cui Z, Sun F and Ding H: Glucan
phosphate inhibits HMGB-1 release from rat myocardial H9C2 cells in
sepsis via TLR4/NF-кB signal pathway. Clin Invest Med. 40:E66–E72.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai X, Zhou Y, Chen P, Yang M and Xu J:
MicroRNA-142-5p induces cancer stem cell-like properties of
cutaneous squamous cell carcinoma via inhibiting PTEN. J Cell
Biochem. 119:2179–2188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan HY, Zhou CB, Chen JM, Liu XB, Wen SS,
Xu G and Zhuang J: MicroRNA-34a targets regulator of calcineurin 1
to modulate endothelial inflammation after fetal cardiac bypass in
goat placenta. Placenta. 51:49–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia B, Xia L and Cao F: The role of
miR-766-5p in cell migration and invasion in colorectal cancer. Exp
Ther Med. 15:2569–2574. 2018.PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fenton JJ, Warner ML, Lammertse D,
Charlifue S, Martinez L, Dannels-McClure A, Kreider S and Pretz C:
A comparison of high vs standard tidal volumes in ventilator
weaning for individuals with sub-acute spinal cord injuries: A
site-specific randomized clinical trial. Spinal Cord. 54:234–238.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoekstra F, van Nunen MP, Gerrits KH,
Stolwijk-Swüste JM, Crins MH and Janssen TW: Effect of robotic gait
training on cardiorespiratory system in incomplete spinal cord
injury. J Rehabil Res Dev. 50:1411–1422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Qiang QH, Ling Q, Yu CX, Li X, Liu
S and Yang S: Effects of Danggui Sini decoction on neuropathic
pain: experimental studies and clinical pharmacological
significance of inhibiting glial activation and proinflammatory
cytokines in the spinal cord. Int J Clin Pharmacol Ther.
55:453–464. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pei JP, Fan LH, Nan K, Li J, Dang XQ and
Wang KZ: HSYA alleviates secondary neuronal death through
attenuating oxidative stress, inflammatory response, and neural
apoptosis in SD rat spinal cord compression injury. J
Neuroinflammation. 14:972017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge L, Wei LH, Du CQ, Song GH, Xue YZ, Shi
HS, Yang M, Yin XX, Li RT, Wang XE, et al: Hydrogen-rich saline
attenuates spinal cord hemisection-induced testicular injury in
rats. Oncotarget. 8:42314–42331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ni B, Cao Z and Liu Y: Glycyrrhizin
protects spinal cord and reduces inflammation in spinal cord
ischemia-reperfusion injury. Int J Neurosci. 123:745–751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen KB, Uchida K, Nakajima H, Yayama T,
Hirai T, Rodriguez Guerrero A, Kobayashi S, Ma WY, Liu SY, Zhu P
and Baba H: High-mobility group box-1 and its receptors contribute
to proinflammatory response in the acute phase of spinal cord
injury in rats. Spine (Phila Pa 1976). 36:2122–2129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Zhang J, Yu P, Chen M, Peng Q,
Wang Z and Dong N: Remote ischaemic preconditioning and sevoflurane
postconditioning synergistically protect rats from myocardial
injury induced by ischemia and reperfusion partly via inhibition
TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem. 41:22–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tao X, Sun X, Yin L, Han X, Xu L, Qi Y, Xu
Y, Li H, Lin Y, Liu K and Peng J: Dioscin ameliorates cerebral
ischemia/reperfusion injury through the downregulation of TLR4
signaling via HMGB-1 inhibition. Free Radic Biol Med. 84:103–115.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang ZC, Tang XM, Zhao YR and Zheng L: A
functional variant at miR-34a binding site in toll-like receptor 4
gene alters susceptibility to hepatocellular carcinoma in a Chinese
Han population. Tumour Biol. 35:12345–12352. 2014. View Article : Google Scholar : PubMed/NCBI
|