Introduction
Pancreatic cancer (PC) is a common aggressive
malignancy, and one of the leading causes of cancer-related
mortality (1). Based on previous
reports, >227,000 deaths are caused by PC worldwide each year
(2). At present, the pathogenesis of
PC has remained unclear, and the prognosis continues to be poor
(5-year survival rate, <2%) (3).
Gemcitabine and 5-fluorouracil are the most commonly used anti-PC
agents; however, the efficacy of current anti-PC therapies remains
unsatisfactory, in part due to the chemoresistance of cancer cells
(4). Previous studies have indicated
that genetics may serve important roles in the tumorigenesis of PC,
through the activation of oncogenes and the silencing of
tumor-suppressor genes (5–7). Therefore, it is essential to identify
key molecules associated with the pathogenesis of PC that may serve
as potential therapeutic targets.
In recent years, the roles of microRNAs (miRNA or
miRs) in cancer have been investigated intensively. miRNAs are
small, non-coding RNAs (~22 nucleotides) that can bind to the
3′-untranslated region (UTR) of one or more target genes and
negatively regulate the expression of those genes (8). Aberrant expression of miRNAs has been
observed in tissue and serum samples from PC patients, as well as
PC cell lines, suggesting that miRNAs may participate in the
pathogenesis of PC (9–13).
miR-183 has been reported to have important roles in
many types of cancer. Previous studies demonstrated that miR-183
may participate in various cellular and molecular events during the
process of tumorigenesis and development (14–16).
miR-183 has been demonstrated to be aberrantly expressed in human
breast cancer, colorectal cancer and esophageal cancer, and the
role of miR-183 either as a tumor-suppressor or an oncomiR has been
discussed previously (17–20). A previous study demonstrated that
miR-183 was overexpressed in breast cancer, and that it
participated in the processes of cancer development through
promoting the proliferation and metastasis of cancer cells
(17); conversely, in cervical
cancer, miR-183 has been reported as a tumor-suppressor that may
inhibit the invasion and metastasis of cancer cells (21).
It has been reported that the expression of miR-183
is upregulated in PC (22); however,
the biological characteristics and targets of miR-183 are not well
understood. In the present study, the role of miR-183 in PC was
explored, paying special attention to the effects of miR-183 on
cancer cell proliferation, apoptosis, and chemosensitivity to
5-fluorouracil and gemcitabine in the human pancreatic carcinoma
cell line PANC-1.
Materials and methods
Cell lines
The human normal pancreatic cell line H6C7 was
purchased from GuangZhou Jennio Biological Technology Co., Ltd.
(Guangzhou, China), and cultured in keratinocyte serum-free medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS). The human
pancreatic carcinoma cell line PANC-1 was purchased from Shanghai
Meixuan Biological Technology Ltd. (Shanghai, China), and cultured
in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS. Cultures were
maintained in a humidified atmosphere at 37°C with 5%
CO2. H6C7 cells were used to determine the miR-183
expression in normal pancreatic cells and PANC-1 cells were used in
all other experiments.
Cell transfection
The miR-183 inhibitor (5′-AGUGAAUUCUACCAGUCCCAUA-3′)
and inhibitor control (5′-CAGUACUUUUGUGUAGUACAA-3′) (22) were purchased from Shanghai GenePharma
Co., Ltd. (Shanghai, China). Transfections were performed using
Lipofectamine® RNAiMAX transfection reagent (Invitrogen;
Thermo Fisher scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. Further analyses were performed
following a 48-h incubation at 37°C with 5% CO2. PANC-1
cells were divided into the following three groups: Untransfected
(NC), cells transfected with inhibitor control, and cells
transfected with miR-183 inhibitor.
Cell proliferation assay
Cell proliferation was determined using an MTT Cell
Proliferation and Cytotoxicity Assay kit (Beyotime Institute of
Biotechnology, Haimen, China) 48 h following transfection,
according to the manufacturer's protocol. DMSO was used to dissolve
the purple formazan in cells, which was measured at a wavelength of
490 nm using a SynergyTM 2 Multi-function Microplate
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
Representative images of the MTT assay were captured using an
inverted microscope (CKX31; Olympus Corporation, Tokyo, Japan;
magnification, ×100).
Cell apoptosis
Cells were stained using an Annexin V/Propidium
Iodide (PI) Apoptosis Detection Kit (BD Biosciences, Franklin
Lakes, NJ, USA) 48 h following transfection. The cells were seeded
into 6-well plates (4×105 cells/well). Following
transfection, the cells were collected, washed with PBS, and
resuspended in 500 µl HEPES buffer solution (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Subsequently, 5 µl Annexin V-FITC and 5
µl PI were added to the buffer and incubated at room temperature
for 15 min in the dark. The apoptosis rate was analyzed using a
FACSVerse™ flow cytometer with the FACSCanto II FACP
Array™ software (version 3.0; BD Biosciences).
Cell cycle assay
The cell cycle assay was performed using the Cell
Cycle and Apoptosis Analysis kit (Beyotime Institute of
Biotechnology). Cells were seeded into 6-well plates
(4×105 cells/well). Following transfection, the cells
were collected and washed with PBS. Subsequently, 100 µl RNase A
solution was added, then the cells were incubated at 37°C for 30
min. Finally, 400 µl PI was added and incubated at room temperature
for 30 min. The DNA content was detected with a FACSVerse™ flow
cytometer with FACSCanto II; FACP Array™ software
(version 3.0; BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA samples from the cells were isolated using
TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
expression of miR-183 was quantified using a Maxima®
SYBR Green/ROX qPCR Master Mix (2X; Thermo Fisher Scientific,
Inc.), with U6 as the internal control. The expression levels of
phosphoinositide 3-kinase (PI3K), serine/threonine-protein kinase B
(Akt), phosphatase and tensin homolog deleted on chromosome ten
(PTEN), B cell lymphoma-2 (Bcl-2) and Bcl-2 associated X protein
(Bax) were determined by performing RT with a Revert Aid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) and qPCR
analysis using a StepOne™ Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. GAPDH was applied as the internal control. The sequences
of the primers used were as follows: miR-183, forward
5′-CGGTATGGCACTGGTAGAATTCACT-3′ and reverse,
5′-GCTTTCCAATGCACTGACCATT-3′; U6, forward 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; PI3K forward,
5′-TATTTGGACTTTGCGACAAGACT-3′ and reverse,
5′-TCGAACGTACTGGTCTGGATAG-3′; Akt forward,
5′-TCCTCCTCAAGAATGATGGCA-3′ and, reverse
5′-GTGCGTTCGATGACAGTGGT-3′; PTEN forward,
5′-TTTGAAGACCATAACCCACCAC-3′ and reverse,
5′-ATTACACCAGTTCGTCCCTTTC-3′; Bcl-2 forward,
5′-GGTGGGGTCATGTGTGTGG-3′ and reverse,
5′-CGGTTCAGGTACTCAGTCATCC-3′; Bax forward,
5′-CCCGAGAGGTCTTTTTCCGAG-3′ and reverse,
5′-CCAGCCCATGATGGTTCTGAT-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 2 min; denaturation at
95°C for 30 sec; annealing at 59°C for 45 sec and elongation at
72°C for 60 sec for 30 cycles, followed by elongation at 72°C for 7
min.
Western blot analysis
Total cell protein was extracted using a
radioimmunoprecipitation assay lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) according to the manufacturer's
protocol. The concentration of protein was determined using a BCA
Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal
amounts of protein (30 µg/lane) were separated using 10% SDS-PAGE
and transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then blocked
with 5% non-fat milk at 4°C for 2 h, and incubated overnight at 4°C
with primary antibodies (anti-PI3K, 1:1,000, ab151549; anti-Akt,
1:10,000, ab179463; anti-PTEN, 1:10,000, ab32199; anti-Bcl-2
1:1,000, ab32124; anti-Bax, 1:5,000, ab32503; and anti-β-actin,
1:200, ab115777; all purchased from Abcam, Cambridge, MA, USA). The
following day, the membranes were washed and incubated at room
temperature with the horseradish peroxidase-conjugated secondary
antibody (1:1,000; A0208; Beyotime Institute of Biotechnology) for
45 min. Finally, membranes were incubated with BeyoECL Plus
(Beyotime Institute of Biotechnology), and detected using a
ChemiDoc™ XRS+imaging system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). -actin was applied as the internal control.
Gemcitabine and 5-fluorouracil
chemosensitivity assay
PANC-1 cells were treated with various
concentrations of gemcitabine (0.01, 0.1, 1, 10 and 100 µM; cat.
no. 95058-81-4; Shanghai Xinyu Biotechnology Pharmaceutical Co.,
Ltd., Shanghai, China) or 5-fluorouracil (0.01, 0.1, 1, 10 and 100
µM; cat. no. 51-21-8; WuHan Dong Kang Yuan Technology Co., Ltd.,
Wuhan, China) at 37°C with 5% CO2 for 1.5 h, and the
percentage of viable cells was determined using an MTT assay, as
aforementioned.
In vivo tumor xenograft model
In vivo tumor xenograft assays were conducted
using 4–6-week-old male BALB/c nude mice (weight, 15–20 g)
purchased from the Animal Center of Nanjing Medical University
(Nanjing, China). All animal research protocols were approved by
the Institutional Animal Care and Use Committee at Shanghai Pudong
Hospital (Pudong, China). The mice were randomly divided into three
groups (5 per group) and maintained in a pathogen-free
environmentally controlled room (temperature, 22°C±2°C; humidity,
50–65%; 12 h light/dark cycle) with free access to standard animal
feed and filtered tap water for 7 days to acclimatize to their new
environment. Subsequently, PANC-1 cells (untransfected or
transfected with the miR-183 inhibitor or inhibitor control) were
subcutaneously injected into separate nude mice (5×106
cells per mouse), who still received free access to food and water
during the experiment. All mice were euthanized at 30 days
following injection and tumor sizes were measured. Tumor size was
calculated using the following equation: Tumor volume=tumor length
× tumor width × tumor height/2. Tumor weight was measured using a
Sartorius Electronic Balance (Suzhou Science Instrument Co., Ltd.,
Suzhou, China).
Dual-luciferase reporter assay
To assess potential miR-183-related target genes,
the miRanda database (www.microrna.org) was used to predict possible
targets. The results revealed that PTEN was a potential target of
miR-183. To confirm this prediction, the luciferase activity assay
was performed in 96-well plates. The pmirGLO plasmid was obtained
from Promega Corporation (Madison WI, USA). PANC-1 cells were
co-transfected with PTEN 3′UTR pmirGLO plasmid (containing
wild-type or mutant PTEN 3′UTRs; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) and a miR-183 inhibitor or inhibitor control (NC)
vector using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The 3′UTR-wild type (WT) comprised PANC-1 cells
containing the wild-type PTEN 3′UTR pmirGLO plasmid. The
3′UTR-mutant (MUT) group comprised PANC-1 cells containing the
mutant PTEN 3′UTR pmirGLO plasmid. As a control, the vector
containing Renilla luciferase was co-transfected for
normalization. At 48 h following transfection, firefly and
Renilla luciferase were assayed using the Dual-Luciferase
Reporter System (Promega Corporation) according to the
manufacturer's instructions. The relative luciferase activity was
reported following the normalization of firefly luminescence to
that of Renilla.
If the miR-183 seed sequence was matched to the PTEN
3′UTR, then the miR-183 seed sequence had activated the PTEN 3′UTR
to decrease the expression of the luciferase gene. The decreased
luciferase reacted with the substrate to produce a weak
fluorescence, which was detected by the Dual-Luciferase Reporter
System to demonstrate that the miR-183 seed sequence could matched
the PTEN 3′UTR.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM Corp., Armonk, NY, USA). Data are presented as the mean ±
standard deviation. Statistical significance was determined using a
Student's t-test between two groups of data sets. Comparisons among
multiple groups were evaluated using one-way analysis of variance
followed by a post-hoc LSD test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-183 is upregulated in PANC-1
cells
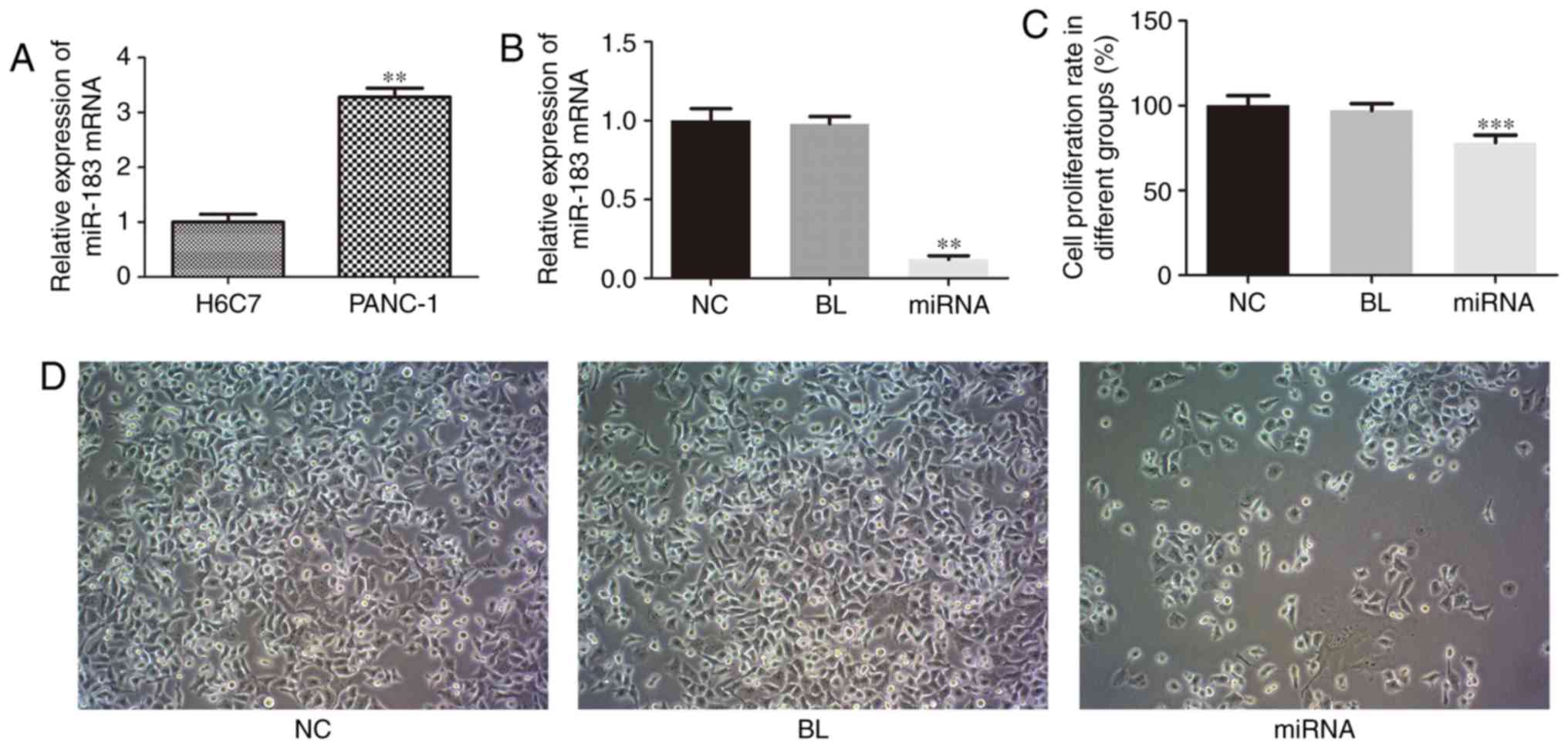
RT-qPCR was performed to examine the expression
level of miR-183 in PC cells. As presented in Fig. 1A, the expression of miR-183 in PANC-1
cells was significantly upregulated compared with that in H6C7
cells (P<0.01). These results indicated that miR-183 may be a
potential marker associated with PC.
miR-183 knockdown inhibits cell
proliferation in PANC-1 cells
To investigate the biological functions of miR-183
in PANC-1 cell proliferation, apoptosis and cell cycle, which are
associated with tumorigenesis, PANC-1 cells were transfected with a
miR-183 inhibitor, and the effect on cell proliferation was
evaluated using an MTT assay. As presented in Fig. 1B, miR-183 expression was markedly
decreased in the miR-183 inhibitor group compared with
untransfected cells (P<0.01). As presented in Fig. 1C, compared with the untransfected
cells, knockdown of miR-183 induced a significant decrease in cell
proliferation (P<0.001), thus suggesting that miR-183 is a
positive regulator of PANC-1 cell proliferation. As presented in
Fig. 1D, the number of PANC-1 cells
was markedly decreased and the morphology was markedly altered,
with the demolishment of membrane-cytoskeleton, when compared with
untransfected cells.
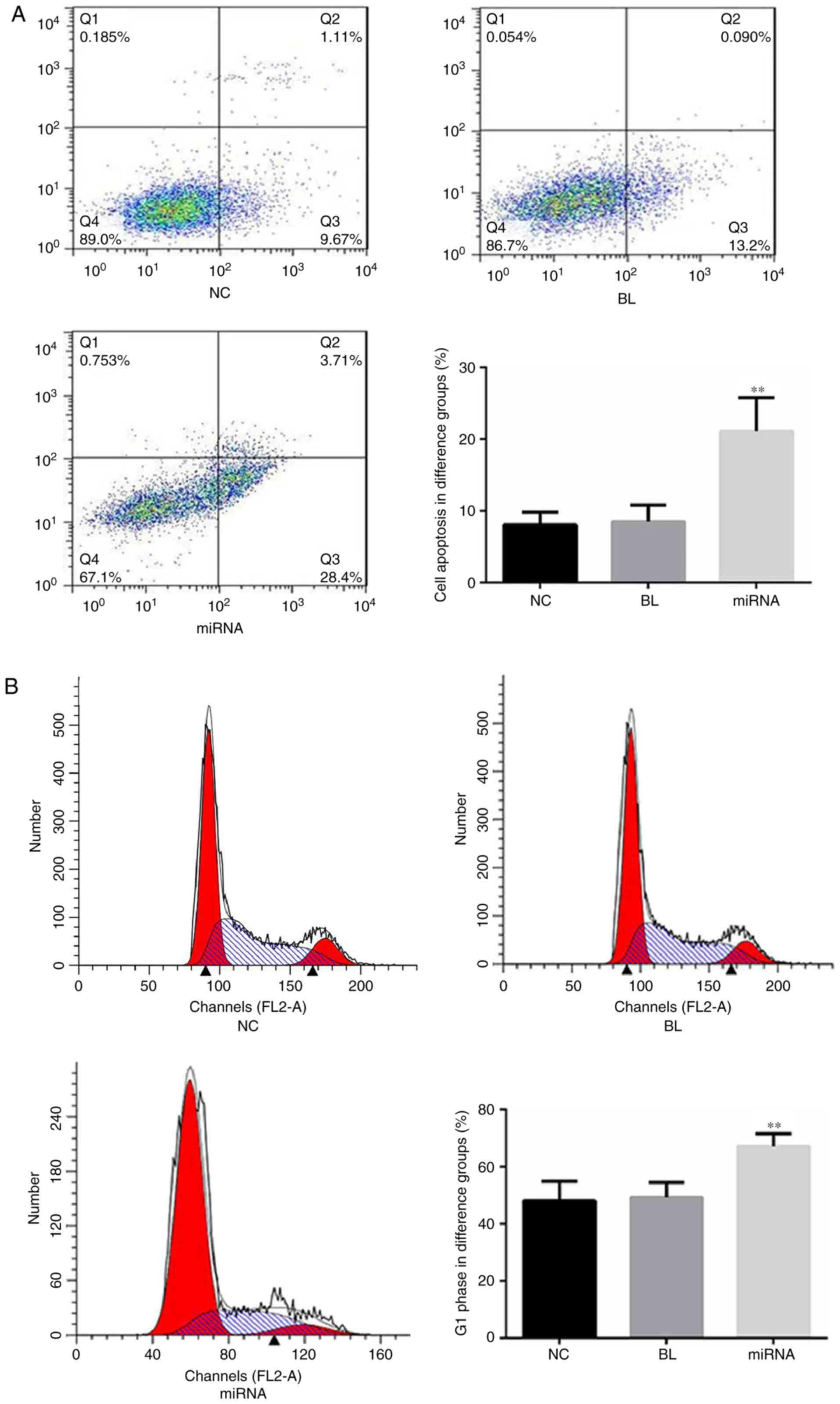
Knockdown of miR-183 promotes
apoptosis and affects the cell cycle of PANC-1 cells in vitro
The role of miR-183 in apoptosis and the cell cycle
of PANC-1 cells was evaluated using flow cytometry. As presented in
Fig. 2A, cells transfected with
miR-183 inhibitors demonstrated a significant increase in cell
apoptosis (P<0.01) compared with the untransfected group.
Furthermore, compared with the untransfected cells, the percentage
of cells in the G1 phase was significantly increased in
the miR-183 inhibitor-transfected group (P<0.01; Fig. 2B).
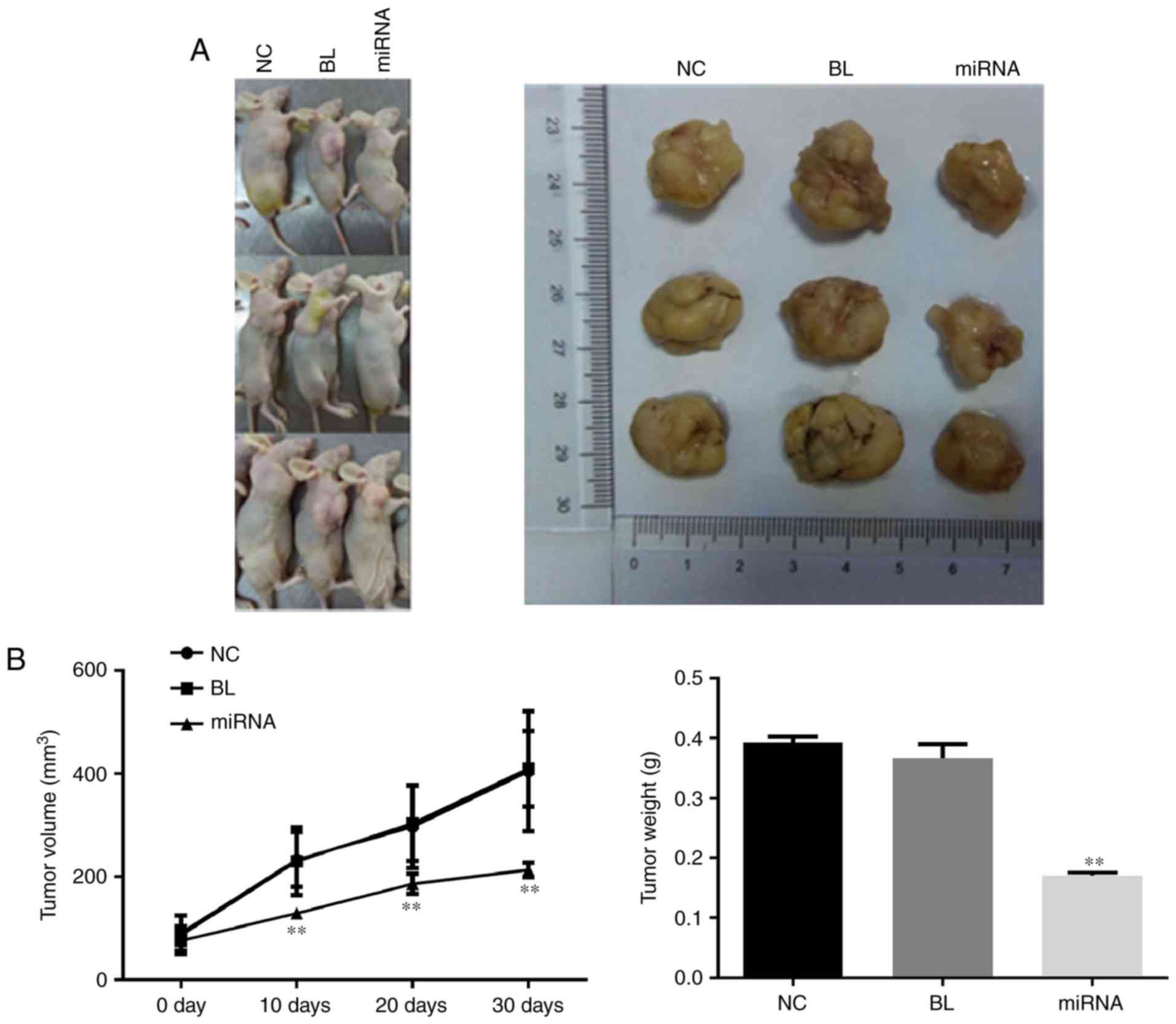
Knockdown of miR-183 delays tumor
growth
To confirm the tumor growth inhibitory effects of
miR-183 downregulation, in vivo tumor xenograft models were
generated by implanting PANC-1 cells into nude mice. As presented
in Fig. 3A, the tumor size in the
miR-183 inhibitor-transfected group was markedly smaller than that
in the untransfected group, which was consistent with the results
of the cell proliferation assay. The tumor growth curve and results
of tumor weight measurement indicated that knockdown of miR-183
significantly delays tumor growth (P<0.01; Fig. 3B).
miR-183 may affect the PTEN/PI3K/Akt
signaling pathway in PANC-1 cells
The PI3K pathway is known to regulate metabolism,
cell growth and cell survival (23).
Therefore, to further explore the underlying mechanisms of the
effects of miR-183 on PANC-1 cells, RT-qPCR and western blot
analyses were performed to investigate the effect of miR-183 on the
PTEN/PI3K/Akt signaling pathway. As presented in Fig. 4, compared with untransfected cells,
knockdown of miR-183 induced significant increases in the mRNA
expression levels of PTEN and Bax, and significant decreases in the
mRNA expression of PI3K, Akt and Bcl-2 in PANC-1 cells (P<0.001)
(Fig. 4A-E). Similar marked
differences were observed at the protein level (Fig. 4F).
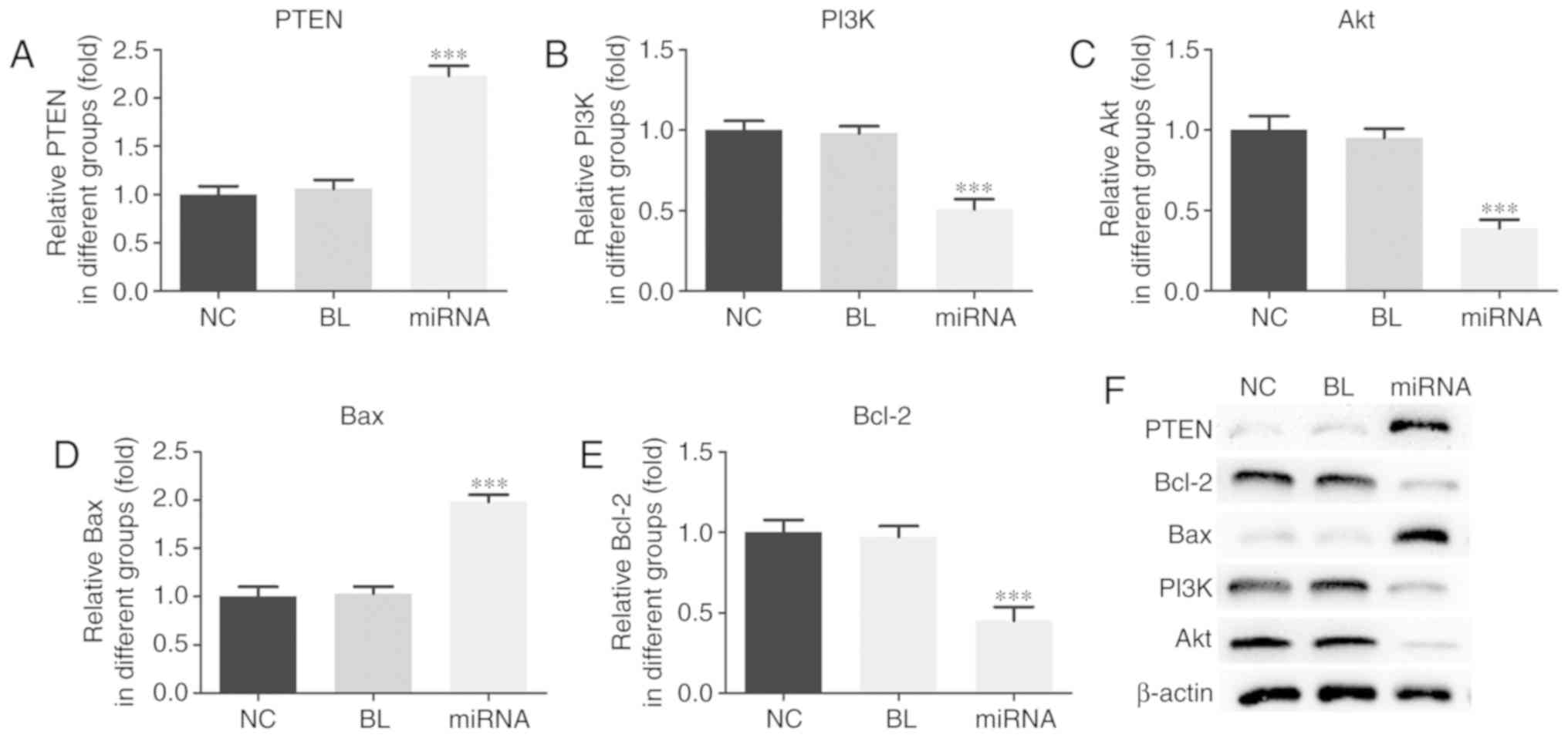 | Figure 4.miR-183 affects the PTEN/PI3K/Akt
signaling pathway in PANC-1 cells. mRNA levels of (A) PTEN, (B)
PI3K, (C) Akt, (D) Bax and (E) Bcl-2 were assessed by reverse
transcription-quantitative polymerase chain reaction. (F) Protein
levels were assessed by western blotting. ***P<0.001 vs. NC.
miR, microRNA; PTEN, phosphatase and tensin homolog deleted on
chromosome ten; PI3K, phosphoinositide 3-kinase; Akt, protein
kinase B; Bcl-2, B cell lymphoma-2; Bax, Bcl-2 associated X
protein; NC, untransfected group; BL, inhibitor control group;
miRNA, miR-183 inhibitor group. |
PTEN is a direct target of
miR-183
As presented in Fig.
5A, the miR-183 seed sequence matched the PTEN 3′-UTR. A
dual-luciferase reporter assay was performed to verify this. The
results demonstrated that miR-183 significantly inhibited the
luciferase activity of the reporter containing the wild-type PTEN
3′-UTR compared with the control group (P<0.05) (Fig. 5B). No significant effect was observed
in the cells transfected with the reporter containing the
mutant-type 3′-UTR of PTEN. These results indicated that PTEN is a
direct target of miR-183 in PANC-1 cells.
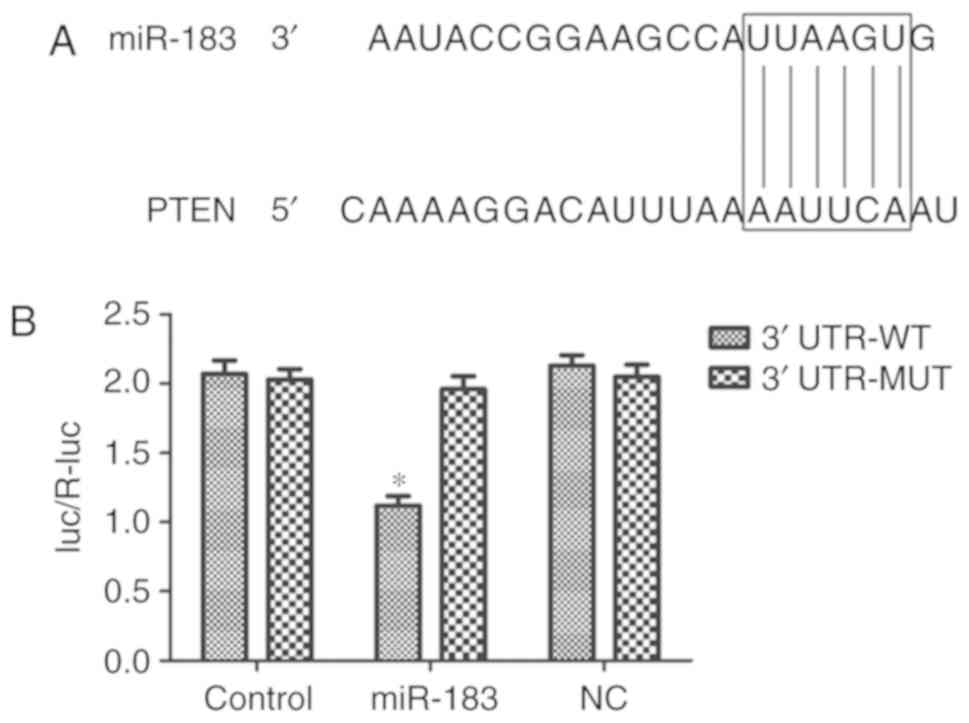 | Figure 5.PTEN is a direct target of miR-183.
(A) The 3′-UTR of PTEN mRNA was observed to contain a sequence
complementary to miR-183, using miRanda. (B) Overexpression of
miR-183 significantly suppressed the luciferase activity of
reporters containing the WT 3′-UTR of PTEN, but not of the
reporters containing the mutant-type MT 3′-UTR of PTEN. *P<0.05
vs. NC. PTEN, phosphatase and tensin homolog deleted on chromosome
ten; miR, microRNA; UTR, untranslated region; WT, wild type; MT,
mutant type; luc/R-luc, luciferase/Renilla luciferase; NC,
inhibitor control transfected group, Control, untransfected
group. |
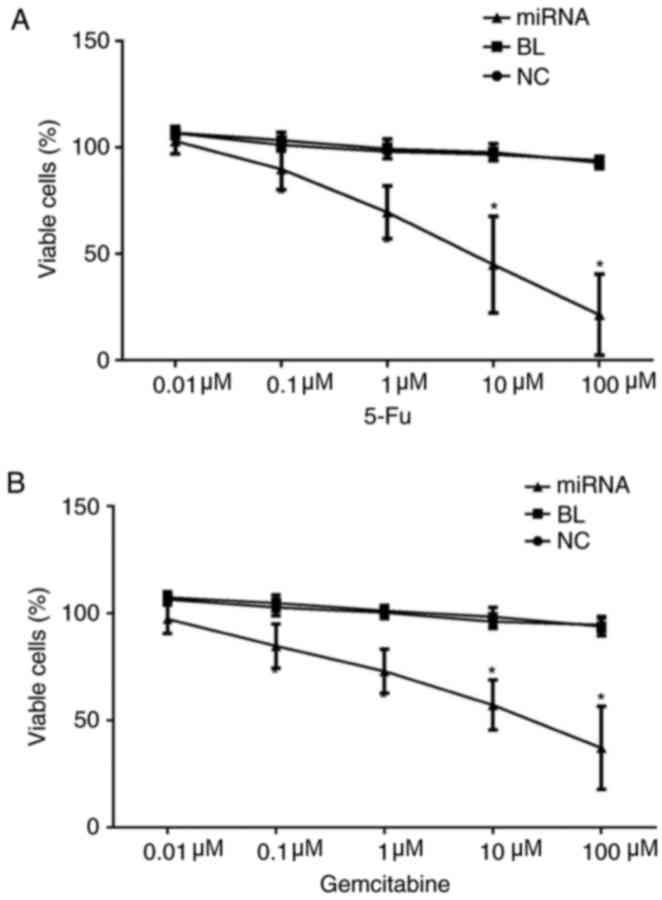
Knockdown of miR-183 increases the
chemosensitivity PANC-1 cells to 5-fluorouracil and gemcitabine in
vitro
Finally, the effect of miR-183 on chemosensitivity
to 5-fluorouracil and gemcitabine in PANC-1 cells was examined
using an MTT assay. The findings indicated indicated that knockdown
of miR-183 significantly increased the chemosensitivity of PANC-1
cells to both 5-fluorouracil and gemcitabine (P<0.05; Fig. 6).
Discussion
The roles of miR-183 in PC have been discussed
previously. Using a microarray assay, Yu et al (24) demonstrated that miR-183 was
upregulated in tissue samples from patients with pancreatic
intraepithelial neoplasms, suggesting that miR-183 may serve an
important role in the pathogenesis of PC. Zhou et al
(25) observed low expression of
miR-183 in pancreatic ductal adenocarcinoma (PDAC) tissue samples
and cell lines, and the results indicated that miR-183 levels were
associated with the severity of the disease, as well as with the
poor survival and prognosis of the patients. Furthermore, it was
demonstrated that miR-183 could affect the proliferation and
apoptosis of PDAC cells through targeting B cell-specific Moloney
murine leukemia virus insertion site 1. Lu et al (26) reported that miR-183 could regulate
the proliferation, apoptosis and invasion of the PC cell line
SW1990 through targeting PDCD4, which indicates that miR-183 has
the potential for use as a therapeutic target for PC treatment. In
a recent study, Miao et al (27) demonstrated that miR-183-5p could not
only promote proliferation, invasion and metastasis, but also
inhibited the apoptosis of human pancreatic adenocarcinoma cells by
downregulating the expression of suppressor of cytokine signaling
6. In the present study, PANC-1 cells were transfected with miR-183
inhibitors, and it was discovered that silencing miR-183 induced a
significant decrease in the proliferation, and a marked increase in
the apoptosis of PANC-1 cells; furthermore, transfection with
miR-183 inhibitors also resulted in a significant increase in the
percentage of cells at the G1 phase. These results are
consistent with previous findings, suggesting that miR-183 can
regulate the proliferation, apoptosis and cell cycle of PANC-1
cells in vitro.
The role of PTEN as a tumor-suppressor has been well
investigated. Previous findings suggested that PTEN is
downregulated in pancreatic cancer, colon cancer and hepatocellular
carcinoma, and PTEN has been presented to regulate the
proliferation, apoptosis, migration and invasion of cancer cells,
including PC cells (28–31). PI3K and Akt are downstream kinases of
PTEN. In cancer, the downregulation of PTEN leads to activation of
the PI3K/Akt signaling pathway, which can partially contribute to
the genesis and development of tumors (32–34).
Results from previous studies suggested that miRNAs can regulate
the proliferation and apoptosis of cancer cells through targeting
PTEN/PI3K/Akt signaling (32–34). In
the present study, it was initially demonstrated that knockdown of
miR-183 induced increases in the expression of PTEN and Bax, and
decreases in the expression of PI3K, Akt and Bcl-2 in PANC-1 cells
at both the mRNA and protein levels, as compared with untransfected
cells. These results indicated that miR-183 can affect the
PTEN/PI3K/Akt signaling pathway in PANC-1 cells.
At present, chemotherapeutic agents for treating PC
are limited. The most common anti-PC drugs are the DNA-damaging
compounds 5-fluorouracil and gemcitabine. However, patients may
develop drug resistance over the course of such therapies, leading
to unsatisfactory efficacy (35,36). In
recent years, the roles of miRNAs in regulating the drug
sensitivity of PC cells have been investigated. Ma et al
(37) recently reported that miR-223
can regulate the epithelial-mesenchymal transition in
gemcitabine-resistant PC cells; Liang et al (38) demonstrated that miR-33a can enhance
the gemcitabine sensitivity of human PC cells through suppressing
the nuclear translocation of β-catenin; Wei et al (39) reported that overexpression of miR-21
in human PC cells may increase 5-fluorouracil resistance by
inhibiting the expression of PTEN and PDCD4; Wang et al
(40) discovered that miR-320a can
increase chemoresistance by targeting PDCD4 in human PC cells. In
the present study, an MTT assay was performed to investigate the
role of miR-183 in the chemosensitivity to 5-fluorouracil and
gemcitabine of human PC cells. The present findings indicated that
silencing miR-183 could increase chemosensitivity to both
5-fluorouracil and gemcitabine in PANC-1 cells.
In conclusion, the results of the present study
demonstrated that inhibition of miR-183 decreased proliferation and
promoted apoptosis in PANC-1 cells, as well as enhanced the
chemosensitivity of the cells to 5-fluorouracil and gemcitabine,
via regulating the PTEN/PI3K/Akt signaling pathway. These findings
suggest that the miR-183 has potential to become a therapeutic
target for the treatment of PC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Academic
Leaders Training Program of Pudong Health Bureau of Shanghai (grant
no. PWRd2016-04), Zhejiang Natural Science Foundation (grant no.
LY16H160045) and the Hangzhou Science and Technology Development
Project (grant no. 20150733Q16).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and BY conceptualized and designed the current
study, and XY performed the majority of the experiments. XY, WW and
XZ constructed the in vivo tumor xenograft model and
performed the follow-up anatomical experiment. QZ and LC cultured
the cells and performed cell transfections. XY, QZ and LC acquired
and interpreted the data and wrote the manuscript. XY and BY made
comments, suggested appropriate modifications and made corrections.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal research protocols were approved by the
Institutional Animal Care and Use Committee at Shanghai Pudong
Hospital (Pudong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basha R, Connelly SF, Sankpal UT, Nagaraju
GP, Patel H, Vishwanatha JK, Shelake S, Tabor-Simecka L, Shoji M,
Simecka JW and El-Rayes B: Small molecule tolfenamic acid and
dietary spice curcumin treatment enhances antiproliferative effect
in pancreatic cancer cells via suppressing Sp1, disrupting NF-κB
translocation to nucleus and cell cycle phase distribution. J Nutr
Biochem. 31:77–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louvet C and Philip PA: Accomplishments in
2007 in the treatment of metastatic pancreatic cancer. Gastrointest
Cancer Res. 2:S37–S41. 2008.PubMed/NCBI
|
|
3
|
Lee C, He H, Jiang Y, Di Y, Yang F, Li J,
Jin C and Fu D: Elevated expression of tumor miR-222 in pancreatic
cancer is associated with Ki67 and poor prognosis. Med Oncol.
30:7002013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi X, Liu S, Kleeff J, Friess H and
Büchler MW: Acquired resistance of pancreatic cancer cells towards
5-Fluorouracil and gemcitabine is associated with altered
expression of apoptosis-regulating genes. Oncology. 62:354–362.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang W, Zhao S, Jiang X, Zhang E, Hu G,
Hu B, Zheng P, Xiao J, Lu Z, Lu Y, et al: The circadian clock gene
Bmal1 acts as a potential anti-oncogene in pancreatic cancer by
activating the p53 tumor suppressor pathway. Cancer Lett.
371:314–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li HL, Li JL, Shi BL and Chen F:
MicroRNA-296 targets AKT2 in pancreatic cancer and functions as a
potential tumor suppressor. Mol Med Rep. 16:466–472. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu JH, Wu J, Pei XC, Tan ZJ, Shi JQ and
Lubman DM: Annexin A10 is a candidate marker associated with the
progression of pancreatic precursor lesions to adenocarcinoma. PLoS
One. 12:e01750392017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berindan-Neagoe I and Calin GA: Molecular
pathways: microRNAs, cancer cells, and microenvironment. Clin
Cancer Res. 20:6247–6253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen WY, Liu WJ, Zhao YP, Zhou L, Zhang
TP, Chen G and Shu H: Induction, modulation and potential targets
of miR-210 in pancreatic cancer cells. Hepatobiliary Pancreat Dis
Int. 11:319–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Y, Ou Y, Wu K, Chen Y and Sun W:
miR-143 inhibits the metastasis of pancreatic cancer and an
associated signaling pathway. Tumour Biol. 33:1863–1870. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawaguchi T, Komatsu S, Ichikawa D,
Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T,
Hirajima S, et al: Clinical impact of circulating miR-221 in plasma
of patients with pancreatic cancer. Br J Cancer. 108:361–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin Y, Dang X, Li W and Ma Q: miR-133a
functions as a tumor suppressor and directly targets FSCN1 in
pancreatic cancer. Oncol Res. 21:353–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao G, Zhang JG, Liu Y, Qin Q, Wang B,
Tian K, Liu L, Li X, Niu Y, Deng SC and Wang CY: miR-148b functions
as a tumor suppressor in pancreatic cancer by targeting AMPKα1. Mol
Cancer Ther. 12:83–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen D, Li SG, Chen JY and Xiao M: MiR-183
maintains canonical Wnt signaling activity and regulates growth and
apoptosis in bladder cancer via targeting AXIN2. Eur Rev Med
Pharmacol Sci. 22:4828–4836. 2018.PubMed/NCBI
|
|
15
|
Sun X, Xu Y, Zhang S, Li X and Wang Y,
Zhang Y, Zhao X, Li Y and Wang Y: MicroRNA-183 suppresses the
vitality, invasion and migration of human osteosarcoma cells by
targeting metastasis-associated protein 1. Exp Ther Med.
15:5058–5064. 2018.PubMed/NCBI
|
|
16
|
Zhang XL, Xu G, Zhou Y and YAN JJ:
MicroRNA-183 promotes the proliferation and metastasis of renal
cell carcinoma through targeting Dickkopf-related protein 3. Oncol
Lett. 15:6003–6008. 2018.PubMed/NCBI
|
|
17
|
Cheng Y, Xiang G, Meng Y and Dong R:
MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in
human breast cancer by targeting the PDCD4. Reprod Biol.
16:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huangfu L, Liang H, Wang G, Su X, Li L, Du
Z, Hu M, Dong Y, Bai X, Liu T, et al: miR-183 regulates autophagy
and apoptosis in colorectal cancer through targeting of UVRAG.
Oncotarget. 7:4735–4745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang M, Liu R, Li X, Liao J, Pu Y, Pan E,
Yin L and Wang Y: miRNA-183 suppresses apoptosis and promotes
proliferation in esophageal cancer by targeting PDCD4. Mol Cells.
37:873–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Quan H, Wang S, Li X and Che X:
MiR-183 promotes growth of non-small cell lung cancer cells through
FoxO1 inhibition. Tumour Biol. 36:8121–8126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X,
Jin X and Tian X: MicroRNA-183 functions as the tumor suppressor
via inhibiting cellular invasion and metastasis by targeting MMP-9
in cervical cancer. Gynecol Oncol. 141:166–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanci V, Yurdakan G, Yurtlu S, Turan IÖ
and Sipahi EY: Protective effect of dexmedetomidine in a rat model
of α-naphthylthiourea-induced acute lung injury. J Surg Res.
178:424–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han B, Jiang P, Li ZX, Yu Y, Huang T, Ye X
and Li X: Coptisine-induced apoptosis in human colon cancer cells
(HCT-116) is mediated by PI3K/Akt and mitochondrial-associated
apoptotic pathway. Phytomedicine. 48:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, Li A, Hong SM, Hruban RH and Goggins
M: MicroRNA alterations of pancreatic intraepithelial neoplasias.
Clin Cancer Res. 18:981–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Zhang WG, Wang DS, Tao KS, Song WJ
and Dou KF: MicroRNA-183 is involved in cell proliferation,
survival and poor prognosis in pancreatic ductal adenocarcinoma by
regulating Bmi-1. Oncol Rep. 32:1734–1740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu YY, Zheng JY, Liu J, Huang CL, Zhang W
and Zeng Y: miR-183 induces cell proliferation, migration, and
invasion by regulating PDCD4 expression in the SW1990 pancreatic
cancer cell line. Biomed Pharmacother. 70:151–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao F, Zhu J, Chen Y, Tang N, Wang X and
Li X: MicroRNA-183-5p promotes the proliferation, invasion and
metastasis of human pancreatic adenocarcinoma cells. Oncol Lett.
11:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni S, Wang H, Zhu X, Wan C, Xu J, Lu C,
Xiao L, He J, Jiang C, Wang W and He Z: CBX7 suppresses cell
proliferation, migration, and invasion through the inhibition of
PTEN/Akt signaling in pancreatic cancer. Oncotarget. 8:8010–8021.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi X, Gu HT, Lin SB, Zhang Y, Yang J and
Qian CJ: Abnormal expression of PTEN and PIK3CA in
pemetrexed-resistant human pancreatic cancer cell line Patu8988.
Genet Mol Res. 15:2016. View Article : Google Scholar
|
|
30
|
Wang L, Yu ZR, Ren SY, Song JK, Wang JH
and Du GH: Metabolic reprogramming in colon cancer reversed by DHTS
through regulating PTEN/AKT/HIF1α mediated signal pathway. Biochim
Biophys Acta Gen Subj. 1862:2281–2292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Long H, Wu Y, Li H, Dong L, Zhong
JL, Liu Z, Yang X, Dai X, Shi L, et al: HRD1-mediated PTEN
degradation promotes cell proliferation and hepatocellular
carcinoma progression. Cell Signal. 50:90–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim HJ, Wang X, Crowe P, Goldstein D and
Yang JL: Targeting the PI3K/PTEN/AKT/mTOR pathway in treatment of
sarcoma cell lines. Anticancer Res. 36:5765–5771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu XX, Cao LY, Chen X, Xiao J, Zou Y and
Chen Q: PTEN inhibits cell proliferation, promotes cell apoptosis,
and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT
pathway in lung adenocarcinoma A549 cells. Biomed Res Int.
2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang LL, Hao S, Zhang S, Guo LJ, Hu CY,
Zhang G, Gao B, Zhao JJ, Jiang Y, Tian WG, et al: PTEN/PI3K/AKT
protein expression is related to clinicopathologic features and
prognosis in breast cancer with axillary lymph node metastases. Hum
Pathol. 61:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mynhardt C, Damelin LH, Jivan R, Peres J,
Prince S, Veale RB and Mavri-Damelin D: Metformin-induced
alterations in nucleotide metabolism cause 5-fluorouracil
resistance but gemcitabine susceptibility in oesophageal squamous
cell carcinoma. J Cell Biochem. 119:1193–1203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amponsah PS, Fan P, Bauer N, Zhao Z,
Gladkich J, Fellenberg J and Herr I: microRNA-210 overexpression
inhibits tumor growth and potentially reverses gemcitabine
resistance in pancreatic cancer. Cancer Lett. 388:107–117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng
L, Shi Y, Wang H, Yin B, Xia J and Wang Z: Down-regulation of
miR-223 reverses epithelial-mesenchymal transition in
gemcitabine-resistant pancreatic cancer cells. Oncotarget.
6:1740–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang C, Wang Z, Li YY, Yu BH, Zhang F and
Li HY: miR-33a suppresses the nuclear translocation of beta-catenin
to enhance gemcitabine sensitivity in human pancreatic cancer
cells. Tumour Biol. 36:9395–9403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei X, Wang W, Wang L, Zhang Y, Zhang X,
Chen M, Wang F, Yu J, Ma Y and Sun G: MicroRNA-21 induces
5-fluorouracil resistance in human pancreatic cancer cells by
regulating PTEN and PDCD4. Cancer Med. 5:693–702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Zhao L, Wei X, Wang L, Liu S, Yang
Y, Wang F, Sun G, Zhang J, Ma Y, et al: MicroRNA-320a promotes 5-FU
resistance in human pancreatic cancer cells. Sci Rep. 6:276412016.
View Article : Google Scholar : PubMed/NCBI
|