Introduction
Childbirth is a natural physiological process that
every parturient has to go through. Because of the physiological
pain of labor, labor analgesia has been applied in clinic for many
years, and is increasingly used by parturients (1). The labor analgesia technique of
epidural anesthesia has been widely used in clinic in recent years
because of its obvious analgesic effect, good safety and convenient
operation, and has created good conditions for parturient labor
(2). Studies have shown that in
clinical anaesthesia, the lower the concentration of local
anesthetic, the less likely patients are to develop adverse
reactions, but at the same dosage, the duration of drug action is
relatively shorter. Therefore, finding a suitable anesthetic
concentration is of great significance in clinical application
(3). Ropivacaine, as a long-acting
amide local anesthetic with a single enantiomer, blocked the
excitation and transmission of nerves by inhibiting the sodium
channel of nerve cells. Ropivacaine can bind to proteins to form
macromolecules, so it cannot pass through the placental barrier for
the central nervous system, and has fewer toxic side effects on the
central nervous system. In addition, it has little adverse effects
on the fetus and it is a common anesthetic in clinical delivery
(4). There have been few studies on
the effects of labor analgesia on the physical environment of
parturient. However, there are also reports that one of the effects
of epidural analgesia on parturient is to increase the febrile rate
of parturient, which has certain adverse effects on the delivery of
parturient and the safety of mothers and infants (5), and intrapartum fever caused by epidural
labor analgesia has become a hot topic in obstetrics (6,7). At
present, the mechanism of fever in parturient during epidural
analgesia is not very clear, but some studies have reported that
fever is related to the thermotaxic center of parturient, but the
most likely mechanism is inflammation (8).
Interleukin-6 (IL-6) and tumor necrosis factor-α
(TNF-α) are very important cytokines and cellular activity factors
in parturition, which are closely related to the delivery process.
It has been reported that IL-6 is not only closely related to
pregnancy, but also plays a very important role in labor (9). IL-6 can increase the synthesis of
prostaglandins and induce uterine contraction to initiate labor
process. It is also a very important cell factor during the process
of immune response and involved in inflammation (10). As an important inflammatory
transmitter and immunomodulatory factor, TNF-α is produced by
mononuclear macrophages, and its mechanism of labor facilitation
during labor is similar to that of IL-6 (11). According to relevant reports, if
epidural analgesia is used in labor, the level of IL-6 and TNF-α in
maternal serum will increase significantly as analgesia time
increases, and the IL-6 level in serum of febrile parturient is
significantly higher than that of the non-febrile parturient
(12). Therefore, some scholars
believe that the increase in febrile rate during parturition may be
due to the release of inflammatory factors caused by the use of
extracellular analgesia (13).
In order to make a further demonstration of the
above conclusion, this study explored the effects of ropivacaine at
different concentrations on intrapartum fever, IL-6 and TNF-α in
the parturient with epidural labor analgesia.
Materials and methods
General materials
Medical records of 198 cases of primiparas admitted
to Obstetrics and Gynecology Department in Shanghai First Maternity
and Infant Hospital, Tongji University School of Medicine
(Shanghai, China) from January 2017 to January 2018 were analyzed
retrospectively and divided into 2 groups. A total of 105 patients
were treated with 0.075% ropivacaine injection 10 ml and 0.5 µg/ml
sulfentanyl injection 100 ml in parturition as the experimental
group. The average age of the patients in the experimental group
was 25.9±1.7 years, the gestational age was 37–41 weeks, the
average gestational age was 39.3±0.5 weeks, the body weight was
60.3±2.9 kg, and the dilatation degree of cervix before analgesia
was 1.9±0.4 cm. A total of 93 patients were treated with 0.1%
ropivacaine injection 10 ml and 0.5 µg/ml sulfentanyl injection 100
ml in parturition as the control group. The average age of the
patients in the control group was 26.1±1.5 years, the gestational
age was 38–42 weeks, the average gestational age was 38.9±0.7
weeks, the body weight was 60.1±3.1 kg, and the dilatation degree
of cervix before analgesia was 2.0±0.6 cm.
Inclusion and exclusion criteria
Inclusion criteria: all parturients were primiparas,
full-term singletons and transvaginal pregnancies. Regular uterine
contraction was <10 h, and labor analgesia by epidural
anaesthesia was requested by primiparas themselves. Exclusion
criteria: parturients with cardiopulmonary dysfunction, endocrine
disease history, contraindications for epidural puncture, and body
temperature higher than 37.5°C before analgesia were excluded. All
parturients and their families signed an informed consent, and
cooperated with the medical staff to complete the diagnosis and
treatment. The study was approved by the Ethics Committee of
Shanghai First Maternity and Infant Hospital, Tongji University
School of Medicine.
Experimental reagents and
instruments
Sodium lactate was purchased from Shanghai Baxter
Medical Supplies Co., Ltd. (SFDA Approval no. H19993749; Shanghai,
China). Ropivacaine was purchased from Jiangsu Hengrui Medicine
Co., Ltd. (SFDA Approval no. H20060137; Jiangsu, China).
Sulfentanyl was purchased from Yichang Renfu Pharmaceutical Co.,
Ltd. (SFDA Approval no. H20054256; Yichang, China). IL-6 (ELISA)
kit and TNF-α (ELISA) kit were purchased from Shanghai
Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China).
Methods
Sodium lactate 8 ml/(kg/h) was injected after the
establishment of upper limb intravenous access, upper fetal heart
monitoring and ECG monitoring were conducted when two groups of
parturient entered the labor room, and the temperature was adjusted
to 23–25°C. Epidural puncture was performed from the L2-3 space and
the catheter was inserted into side of head, and the depth was
about 3–4 cm. 1.5% lidocaine 3 ml was injected into the body, if
there was no adverse reaction after 5 min, the catheter was fixed
and connected to the patient-controlled epidural analgesia pump.
0.075% ropivacaine injection 10 ml, 0.5 µg/ml sulfentanyl injection
10 ml in the experimental group, and 0.1% ropivacaine injection 10
ml, and 0.5 µg/ml sulfentanyl injection 100 ml in the control group
were injected into the epidural analgesia pump for anaesthesia. In
both groups, the loading dose was 3 ml, the background infusion
dose was 4 ml/h, the single supplementary PCA dose was 2 ml/time,
and the locking time was 15 min. Anaesthesia infusion was stopped
at full opening of the uterus and epidural catheter was removed
after the perineal stitch. Venous blood 2 ml was taken at T1
(cervix open to 2 cm), T2 (cervix fully open) and T3 (24 h
postpartum) and the concentration of IL-6 TNF-α was detected by
ELISA. All the operations were carried out strictly according to
the instructions of the kit.
Observation index
Visual analogue score (VAS) of parturient pain,
labor duration, analgesic time, febrile rate of parturient after
administration at cervix open to 2 cm, cervix open to 4 cm, cervix
fully open, and the concentration changes of IL-6 and TNF-α in
serum of parturient at T1, T2 and T3 were observed and compared.
During epidural labor analgesia, the temperature of the parturient
was measured and recorded once an hour until the end of labor. If
body temperature was higher than 37.5°C, it was considered febrile.
VAS judging criteria (14): painless
is 0 point, mild pain is 1–3 points, moderate pain is 4–7 points,
and severe pain is 8–10 points.
Statistical analysis
The statistical analysis was conducted by SPSS 15.0
[AsiaAnalytics (formerly SPSS China)] statistical software, and the
measurement data were represented by mean ± SD. Student's t-test
was used for comparison between the two groups. Repeated analysis
of variance was used at different time-points. Chi-square test was
used for enumeration data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of normal information
between the two groups
There was no significant difference in age, body
weight, gestational age, blood loss and blood sugar between the two
groups (Table I).
 | Table I.Basic information. |
Table I.
Basic information.
| Factors | Experimental group
n=105 | Control group
n=93 | t | P-value |
|---|
| Age | 25.9±1.7 | 26.1±1.5 | 0.873 | 0.384 |
| Body weight (kg) | 60.3±2.9 | 60.1±3.1 | 0.469 | 0.640 |
| Height (cm) | 160.2±4.2 | 159.7±6.3 | 0.664 | 0.508 |
| Gestational age
(week) | 39.0±0.5 | 38.9±0.6 | 1.279 | 0.203 |
| Cervix dilatation
degree (cm) | 1.9±0.4 | 2.0±0.6 | 1.394 | 0.165 |
| Blood loss (ml) | 249.35±78.14 | 261.47±80.71 | 1.073 | 0.285 |
| Complete blood
count |
|
Hemoglobin (g/l) | 98±22 | 102±17 | 1.418 | 0.158 |
| RBC
(×1012/l) | 3.09±0.69 | 3.11±1.04 | 0.161 | 0.872 |
| Leucocyte
count (×10/l) | 11.04±1.21 | 10.91±1.26 | 0.740 | 0.460 |
| Blood
sugar (mmol/l) | 5.97±1.01 | 6.22±0.98 | 1.763 | 0.080 |
Comparison of VAS scores between the
two groups at different time-points
The VAS scores were 7.2±1.3, 4.1±1.5 and 1.9±0.4,
respectively at cervix open to 2 cm, cervix open to 4 cm, cervix
fully open in the experimental group, and the VAS scores were
6.9±1.2, 4.2±1.1 and 1.8±0.4 in the control group. There was no
significant difference in VAS score between the two groups
(P>0.05; Table II).
 | Table II.Comparison of VAS scores between the
two groups at different time-points. |
Table II.
Comparison of VAS scores between the
two groups at different time-points.
| Time | Experimental group
n=105 | Control group
n=93 | t | P-value |
|---|
| Cervix open to 2
cm | 7.2±1.3 | 6.9±1.2 | 1.680 | 0.095 |
| Cervix open to 4
cm | 4.1±1.5 | 4.2±1.1 | 0.529 | 0.597 |
| Cervix fully
open | 1.9±0.4 | 1.8±0.4 | 1.756 | 0.081 |
Comparison of body temperature and
febrile rate between two groups at different time points
Before analgesia and 1, 2, 3 h after analgesia, the
temperature of parturients in both groups did not increase
significantly and there was no significant difference in body
temperature between the two groups (P<0.05). While the body
temperature was significantly higher at 4 and 5 h after analgesia,
parturition and 1 h postpartum than that before analgesia, and the
body temperature in the control group was significantly higher than
that in the experimental group at 4 and 5 h after analgesia
(P<0.05). There were 21 febrile parturients in the experimental
group, and the febrile rate was 20.00%. There were 39 febrile
parturients in the control group, and the febrile rate was 41.94%.
The febrile rate in the experimental group was significantly lower
than that in the control group (P<0.05; Table III).
 | Table III.Comparison of body temperature and
febrile rate between the two groups at different time-points. |
Table III.
Comparison of body temperature and
febrile rate between the two groups at different time-points.
| Time | Experimental group
n=105 | Control group
n=93 | t/χ2 | P-value |
|---|
| Before analgesia | 36.3±0.3 | 36.4±0.3 |
2.341 |
0.020 |
| 1 h after
analgesia | 36.6±0.3 | 36.7±0.4 |
2.004 |
0.047 |
| 2 h after
analgesia | 36.8±0.4 | 36.7±0.5 |
1.728 |
0.086 |
| 3 h after
analgesia | 36.8±0.6 | 36.7±0.5 |
1.265 |
0.208 |
| 4 h after
analgesia |
36.9±0.3a |
37.1±0.5a |
3.457 | <0.001 |
| 5 h after
analgesia |
37.0±0.5a |
37.3±0.6a |
3.836 | <0.001 |
| At parturition |
37.0±0.6a |
37.1±0.6a |
1.170 |
0.243 |
| 1 h postpartum |
36.9±0.7a |
37.0±0.6a |
1.072 |
0.285 |
| Febrile rate [n,
(%)] | 21 (20.00) | 39 (41.94) | 11.24 | <0.001 |
Comparison of labor duration at
different stages and analgesia time between the groups
There was no significant difference in the first and
third stage of labor between the two groups (P>0.05). However,
the second stage of labor and analgesic time in the experimental
group were significantly shorter than those in the control group
(P<0.05; Table IV).
 | Table IV.Comparison of labor duration at
different stages and analgesia time between two groups (min). |
Table IV.
Comparison of labor duration at
different stages and analgesia time between two groups (min).
| Factors | Experimental group
n=105 | Control group
n=93 | t | P-value |
|---|
| First stage | 639.18±45.27 | 634.31±43.41 | 0.770 | 0.442 |
| Second stage | 52.75±1.66 | 74.74±3.42 | 58.57 | <0.001 |
| Third stage | 8.76±0.69 | 8.51±0.85 | 2.282 | 0.024 |
| Analgesic time | 169.72±14.98 | 257.32±25.09 | 30.21 | <0.001 |
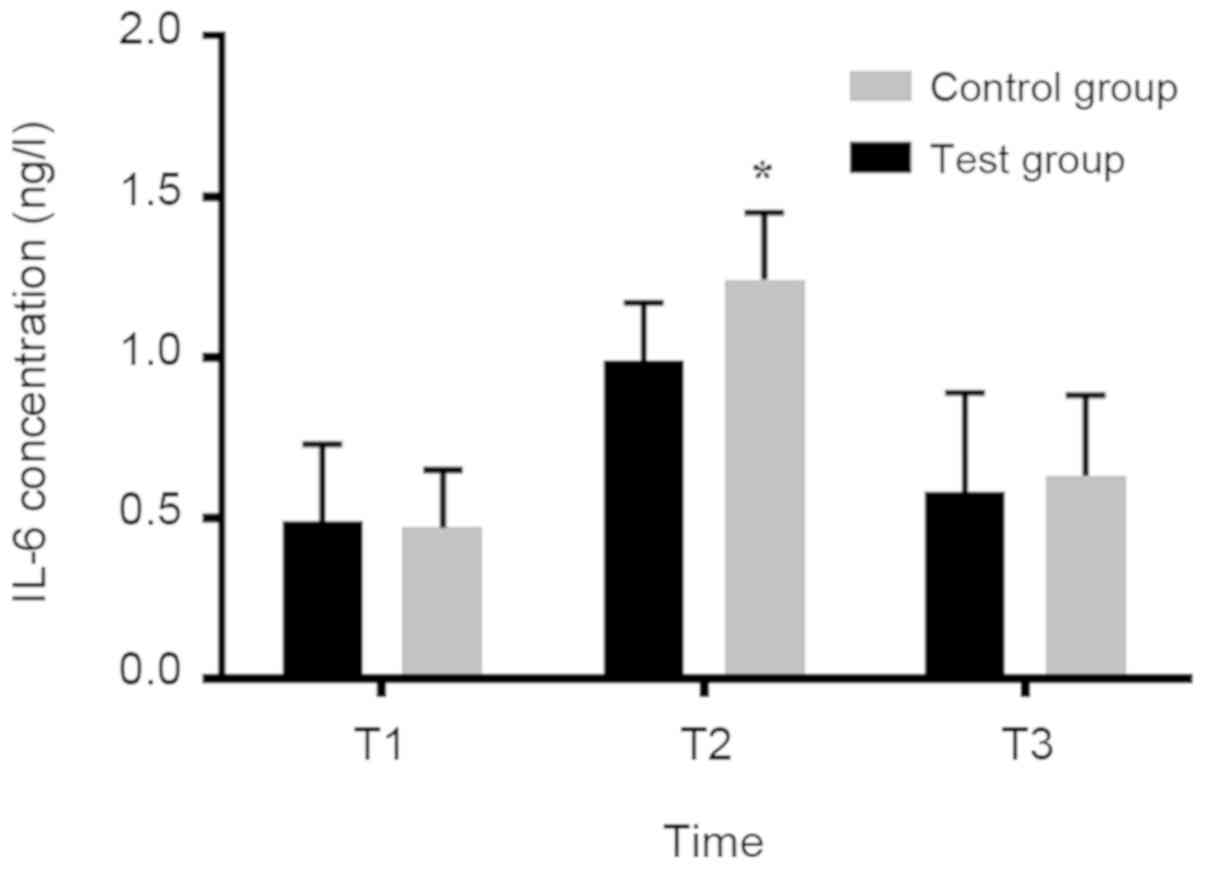
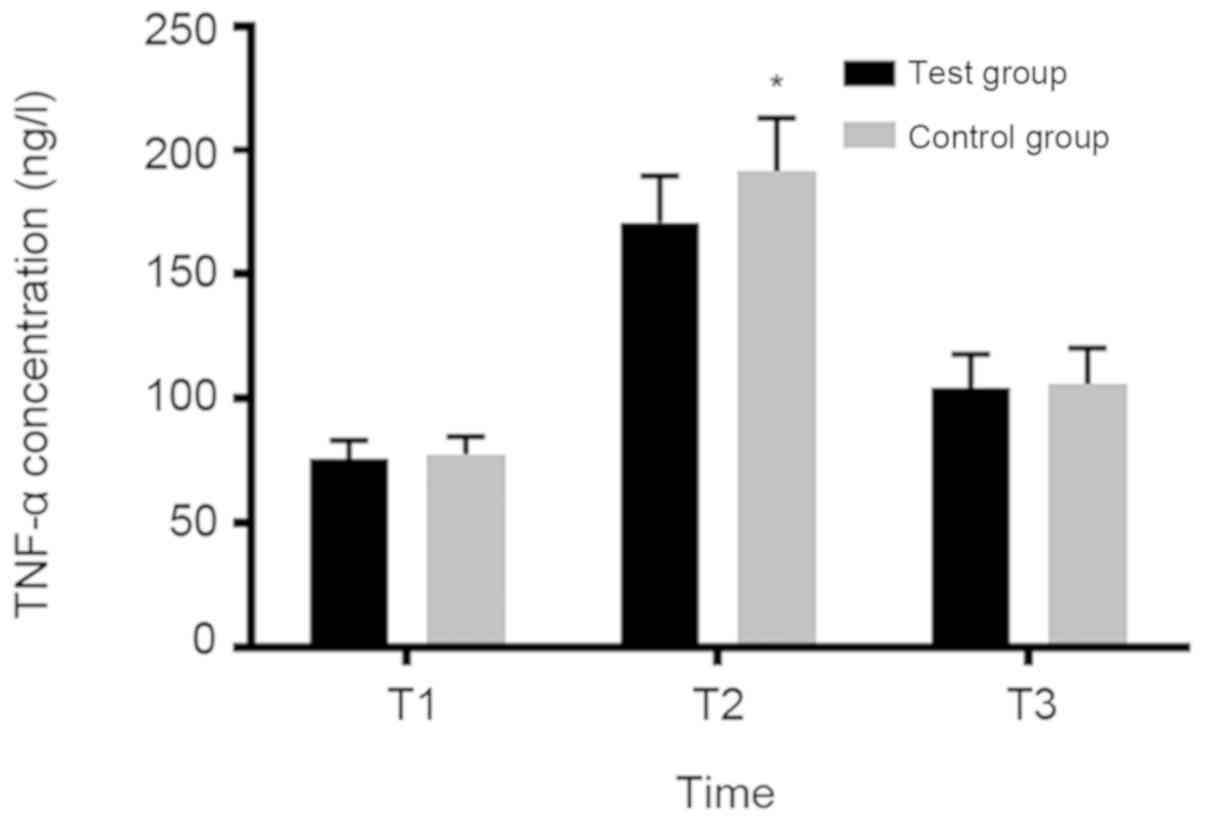
Concentration changes of IL-6 and
TNF-α in serum of parturient at T1, T2 and T3
There was no significant diffe-rence in IL-6 and
TNF-α between the two groups at T1 and T3 (P>0.05). However,
IL-6 and TNF-α concentrations in the experimental group were
significantly lower than those in the control group at T2
(P<0.05; Figs. 1 and 2).
Discussion
Labor pain is a complex, subjective,
multidimensional response to sensory nerve stimulation during labor
(15). For many parturients, labor
analgesia not only alleviates the pain during delivery, but also
reduces the stress and cesarean section rate of parturient.
Therefore, an increasing number of parturients choose labor
analgesia during delivery (16,17).
Epidural labor analgesia creates a good labor condition for
parturients, but there are also some problems, among which fever in
parturient due to epidural anaesthesia is a subject of great
concern at present (18). Fever or
even ardent fever during delivery may lead to intrauterine fetal
death, neonatal septicemia and other problems (19). Therefore, clarifying the mechanism of
epidural anesthesia in increasing maternal temperature and
controlling the temperature rise of parturient by adjusting the way
of administration and metering of anesthesia has become a hot
research topic in recent years. IL-6 and TNF-α are important
factors during parturition. The concentration of IL-6 and TNF-α
changes with the concentration change of epidural anesthetic, which
affects maternal body temperature in a different degree (20). Observing the effect of ropivacaine at
different concentrations on cell factor level in parturient with
epidural labor analgesia is helpful to provide reference for
clinical use of epidural labor analgesia.
In this study, it was found that there was no
significant difference in VAS score between the experimental and
control groups (P>0.05) at cervix open to 2 cm, cervix open to 4
cm and cervix fully open, which indicated that there was no
significant difference in the analgesic effect of parturients when
ropivacaine concentration was 0.1 and 0.075%, respectively. When
comparing the body temperature and febrile rate of the groups at
different time-points, it was found that before analgesia and at 1,
2, 3 h after analgesia, the temperature of parturients in both
groups did not increase significantly and there was no significant
difference in body temperature between the two groups (P>0.05).
The body temperature significantly increased at 4 and 5 h after
analgesia, parturition and 1 h postpartum, and the body temperature
in the control group was significantly higher than that in the
experimental group at 4 and 5 h after analgesia (P<0.05). There
were 21 febrile parturients in the experimental group, and the
febrile rate was 20.00%. There were 39 febrile parturients in the
control group, and the febrile rate was 41.94%. The febrile rate in
the experimental group was significantly lower than that in the
control group (P<0.05). The results indicated that the increase
in body temperature and febrile rate of epidural labor analgesia
with 0.075% ropivacaine combined with sulfentanyl were smaller than
that with 0.1% ropivacaine, which was consistent with the results
of Gogarten et al (21). The
comparison of labor duration at different stages and analgesia time
showed that the duration of the first and third stages of labor was
the same, and there was no significant difference between the two
groups (P>0.05). However, the second stage of labor and
analgesic time in the experimental group were significantly shorter
than those in the control group (P<0.05). Studies have also
shown that epidural anesthesia with 0.075% ropivacaine combined
with sulfentanyl could not only achieve a good analgesic effect,
but also result in a shorter second stage of labor (22,23). The
reason is that the concentration of ropivacaine is positively
correlated with the time of analgesia, and the effect of low dose
of anaesthesia on uterine contraction is small. IL-6, as the main
pro-inflammatory cytokine, has been used as a marker of
perioperative inflammatory response in many studies. TNF-α, as an
inflammatory cytokine closely related to the pain acceleration, can
cause late immunologic injury (4,24). The
concentration changes of IL-6 and TNF-α levels in serum of
parturients at T1, T2 and T3 were evaluated in this study. The
result showed that there was no significant difference in IL-6 and
TNF-α concentration between the two groups at T1 and T3
(P>0.05). However, the IL-6 and TNF-α concentrations in the
experimental group were significantly lower than those in the
control group at T2 (P<0.05). This suggested that the use of
0.075% ropivacaine contributed to inflammation <0.1%
ropivacaine.
In conclusion, the effect of patient-controlled
epidural administration with 0.075% ropivacaine injection combined
with 0.5 mg/ml sulfentanyl injection on labor analgesia is shorter
than that with 0.1% ropivacaine combined with sulfentanyl. It can
also result in a shorter second stage of labor and analgesia time,
lower intrapartum febrile rate, and contributed to inflammation
<0.1% ropivacaine combined with sulfentanyl. However, labor
itself is a complex process, and is affected by many factors. We
mainly studied the influence of the preliminary test of anesthesia
record on labor, so we did not record the auxiliary anesthesia
dosage used by the labor in the experiment, which is also a
negligence of this study. Therefore, the way and dosage of labor
analgesia in clinical application need to be further explored and
studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and JL recorded and analyzed the general
information of patients. SD was responsible for upper fetal heart
monitoring and ECG monitoring. ZX and ZL contributed to observation
index analysis. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shanghai First Maternity and Infant Hospital, Tongji University
School of Medicine (Shanghai, China) and written informed consents
were signed by the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He ZY, Jiao QL, Miao Y and Sun Y: Clinical
observation of ropivacaine compuled with sufentanil for painless
childbirth. Pak J Pharm Sci. 29 Suppl 2:707–709. 2016.PubMed/NCBI
|
|
2
|
Palm S, Gertzen W, Ledowski T, Gleim M and
Wulf H: Minimum local analgesic dose of plain ropivacaine vs.
ropivacaine combined with sufentanil during epidural analgesia for
labour. Anaesthesia. 56:526–529. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kampe S, Tausch B, Paul M, Kasper SM,
Bauer K, Diefenbach C and Kiencke P: Epidural block with
ropivacaine and bupivacaine for elective caesarean section:
maternal cardiovascular parameters, comfort and neonatal
well-being. Curr Med Res Opin. 20:7–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purdy M, Kokki M, Anttila M, Aspinen S,
Juvonen P, Korhonen R, Selander T, Kokki H and Eskelinen M: Does
the rectus sheath block analgesia reduce the inflammatory response
biomarkers' IL-1ra, IL-6, IL-8, IL-10 and IL-1β concentrations
following surgery? A randomized clinical trial of patients with
cancer and benign disease. Anticancer Res. 36:3005–3011.
2016.PubMed/NCBI
|
|
5
|
Camorcia M and Capogna G: Epidural
levobupivacaine, ropivacaine and bupivacaine in combination with
sufentanil in early labour: a randomized trial. Eur J Anaesthesiol.
20:636–639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng SW, Xu SQ, Ma L, Li CJ, Wang X, Yuan
HM, Wang FZ, Shen XF and Ding ZN: Regular intermittent bolus
provides similar incidence of maternal fever compared with
continuous infusion during epidural labor analgesia. Saudi Med J.
35:1237–1242. 2014.PubMed/NCBI
|
|
7
|
Saito M, Okutomi T, Kanai Y, Mochizuki J,
Tani A, Amano K and Hoka S: Patient-controlled epidural analgesia
during labor using ropivacaine and fentanyl provides better
maternal satisfaction with less local anesthetic requirement. J
Anesth. 19:208–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Xu S, Qin X, Li X, Feng SW, Liu Y,
Wang W, Guo X, Shen R, Shen X, et al: Comparison between the use of
ropivacaine alone and ropivacaine with sufentanil in epidural labor
analgesia. Medicine (Baltimore). 94:e18822015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schulpis KH, Vlachos GD, Karikas GA,
Papakonstantinou ED, Vlachos DG, Papassotiriou I, Antsaklis A and
Tsakiris S: The effect of the mode of delivery on maternal-neonatal
interleukin-6, biogenic amine and their precursor amino acid
concentrations. Clin Chem Lab Med. 46:1624–1630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dunn AB, Paul S, Ware LZ and Corwin EJ:
Perineal injury during childbirth increases risk of postpartum
depressive symptoms and inflammatory markers. J Midwifery Womens
Health. 60:428–436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prusty BK, Hedau S, Singh A, Kar P and Das
BC: Selective suppression of NF-kBp65 in hepatitis virus-infected
pregnant women manifesting severe liver damage and high mortality.
Mol Med. 13:518–526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams M: The combined spinal-epidural
technique for the provision of analgesia in obstetric anaesthesia.
Anaesthesia. 56:5002001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goetzl L: Epidural analgesia and maternal
fever: A clinical and research update. Curr Opin Anaesthesiol.
25:292–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu D, He X, Zheng W, Zhang Y, Li D, Wang
W, Li J and Xu W: Translation and validation of the simplified
Chinese new Knee Society Scoring System. BMC Musculoskelet Disord.
16:3912015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abushaikha LA: Methods of coping with
labor pain used by Jordanian women. J Transcult Nurs. 18:35–40.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuczkowski KM: Labor analgesia for the
parturient with herbal medicines use: What does an obstetrician
need to know? Arch Gynecol Obstet. 274:233–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma SK, McIntire DD, Wiley J and Leveno
KJ: Labor analgesia and cesarean delivery: an individual patient
meta-analysis of nulliparous women. Anesthesiology. 100:142–148;
discussion 6A. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon HJ, Do SH and Yun YJ: Comparing
epidural surgical anesthesia and spinal anesthesia following
epidural labor analgesia for intrapartum cesarean section: A
prospective randomized controlled trial. Korean J Anesthesiol.
70:412–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kokki H, Ruuskanen A and Karvinen M:
Comparison of epidural pain treatment with sufentanil-ropivacaine
infusion with and without epinephrine in children. Acta
Anaesthesiol Scand. 46:647–653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mantha VR, Vallejo MC, Ramesh V, Jones BL
and Ramanathan S: Maternal and cord serum cytokine changes with
continuous and intermittent labor epidural analgesia: a randomized
study. ScientificWorldJournal. 2012:6079382012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gogarten W, Van de Velde M, Soetens F, Van
Aken H, Brodner G, Gramke HF, Soetens M and Marcus MA: A
multicentre trial comparing different concentrations of ropivacaine
plus sufentanil with bupivacaine plus sufentanil for
patient-controlled epidural analgesia in labour. Eur J
Anaesthesiol. 21:38–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boulier V, Gomis P, Lautner C, Visseaux H,
Palot M and Malinovsky JM: Minimum local analgesic concentrations
of ropivacaine and levobupivacaine with sufentanil for epidural
analgesia in labour. Int J Obstet Anesth. 18:226–230. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yue HL, Shao LJ, Li J, Wang YN, Wang L and
Han RQ: Effect of epidural analgesia with 0.075% ropivacaine versus
0.1% ropivacaine on the maternal temperature during labor: A
randomized controlled study. Chin Med J (Engl). 126:4301–4305.
2013.PubMed/NCBI
|
|
24
|
Zhao Y, Wang W, Wu X, Ma X, Qu R, Chen X,
Liu C, Liu Y, Wang X, Yan P, et al: Mangiferin antagonizes
TNF-α-mediated inflammatory reaction and protects against
dermatitis in a mice model. Int Immunopharmacol. 45:174–179. 2017.
View Article : Google Scholar : PubMed/NCBI
|