Introduction
Chronic eczema, which is an allergic inflammatory
dermatosis with high incidence in dermatology, belongs to type IV
allergic dermatosis, and severe itching, symmetrical location,
multiple lesions and recurrent attacks are four main symptoms of
eczema (1,2). In recent years, due to global warming,
excessive use of chemical industry and food additives, and the
acceleration of social development, the incidence of chronic eczema
is increasing year by year and is prevalent worldwide (3,4). Many
studies on the specific causes of chronic eczema have indicated
that immune dysfunction, genetic and environmental factors play an
important role (5,6).
Currently, H1 receptor antagonists such as
phenhydramine, chlorpheniramine and glucocorticoids are the main
drugs for the treatment of chronic eczema, but the clinical staff
and patients are not satisfied with the therapeutic effect because
of adverse reactions such as dizziness, nausea, lethargy, full moon
face and buffalo back, and the application is also greatly limited
(7,8). Therefore, it is very important to find
new safe and effective drugs. Total glucoside of paeony is the
first anti-inflammatory and immunomodulatory drug to be approved
for sale and extracted from root of Radix Paeoniae Paeoniae Alba,
showing a good prospect in the treatment of inflammatory and immune
diseases such as rheumatoid arthritis, effectively improving the
immune balance and having good safety (9,10).
Inflammatory reactions and immune damage also occurs in the
pathogenesis of chronic eczema, including red swelling and allergic
reactions (11). Therefore, total
glucosides of paeony may also be effective in the treatment of
chronic eczema, IL-4 may respond to the immune reaction and ICAM
may respond to inflammatory reaction (12,13). We
speculate that the regulation of IL-4 and ICAM is one of the
mechanisms of the action of total glucoside of paeony.
In this study, a pathological model of KM mice with
chronic eczema was established to explore the therapeutic effect
and mechanism of total glucosides of paeony in chronic eczema.
Materials and methods
Forty mature KM mice of SPF grade were purchased
from the Animal Experimental Center of Gansu College of Traditional
Chinese Medicine (Lanzhou, China) [production license SCXK (Gan)
2004-0006-0000911] and all were fed with full price nutrient pellet
feed (Jiangsu Medison Biopharmaceutical Co., Ltd., Jiansgsu, China;
website: http://www.medison.co.il/). KM mice
were aged 45–55 days with an average age of 47.5±2.5 days and body
weight 30–35 g with an average body weight of 33.4±2.7 g. The
feeding temperature was 16–26°C with relative humidity 55.6±8.3%
and the mice were kept separately in the feeding box. The nest
changed 1–2 times a week, ambient noise was <85 dB, ammonia
concentration was not more than 20 ppm with ventilation 8–12 times
per hour. The fluorescent lamp interval was a 12 h light/dark
cycle. At last, the mice could feed and drink freely, with the
feeding box changing 3–4 times a week and the water bottle changing
2–3 times a week. Ten mice were selected as the control group using
random number table and the remaining 30 mice were used to
establish chronic eczema model and randomly divided into the model
and treatment groups after the model was successfully
established.
The study was approved by the Ethics Committee of
The People's Hospital of Danyang (Zhenjiang, China).
Establishment of rat model
Hair of KM mice was cut off ~3 × 3 cm from the
abdomen using electric clipper one day before sensitization, and 5%
dinitrochlorobenzene acetone solution (DNCB) (a product of Chemical
Reagent Co., Ltd., Shanghai, China) 100 µl was smeared to the
abdomen of mice for sensitization on the 1st day of the experiment.
On the 6th day, the mice were stimulated with 1% DNCB 5 µl on the
medial side of the right ear, while the left ear was treated with
acetone once three times for four times.
Treatment methods
After grinding total glucosides of paeony (guo yao
zhun zi H20055058; Liwah Pharma, Hangzhou, China; website:
http://www.nbliwah.com/) into powder form, the
suspension of 10 mg/ml was prepared with 0.5% sodium
carboxymethyl-cellulose, and each medication group was treated with
administration of the drug on the same day as sensitization. The
treatment group was treated with total glucoside of paeony
suspension of 60 mg/kg/day, while the control and non-intervention
model groups were given equivoluminal 0.5% sodium
carboxymethylcellulose as control until the last stimulation of
mice on the 18th day.
ELISA
The peripheral blood of mice was collected using
caudal vein blood collection before modeling, at day 1, 6, 9, 12
and 15. IL-4 and ICAM-1 in serum were tested using ELISA. The
methods were carried out according to the kit instructions. IL-4
and ICAM-1 test kits were purchased from Shanghai Crystal Pure
Reagent Co., Ltd. (Shanghai, China).
Observation indicators
The ear thickness and weight before and after
modeling were observed. The thickness difference of the middle
right ear of mice was measured using vernier caliper. The rats were
sacrificed 24 h after the last stimulation. The same position of
the two ears was obtained using 6 mm perforator and the quality of
it was measured using electronic balance. The changes of IL-4 and
ICAM-1 levels in mouse serum were measured before and after
administration of the drug. The correlation of IL-4 and ICAM-1
levels with ear thickness and treatment time was observed.
Statistical analysis
SPSS19.0 [AsiaAnalytics (formerly SPSS China),
Shanghai, China] was used for statistical analysis. The enumeration
data were expressed by rate and the comparison of rate was tested
using χ2 test. The measurement data were expressed as
mean ± standard deviation, the comparison among multiple groups was
analyzed using ANOVA, the comparison between two groups was tested
using LSD, and repeated variance measurement experiment was used to
compare different time within the group. The correlation of IL-4
and ICAM-1 levels with ear thickness and treatment time was
analyzed using Spearmans correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Result of modeling
The chronic eczema model was established in 30 KM
mice, 28 mice were successfully established, and the success rate
of modeling was 93.3%. Two mice in the treatment group died midway
because of the drug, but the data were not included in the data of
mice in the treatment group. The abdominal texture of mice in the
control group was soft, the color was bright red, and there was no
red swelling, scab skin and keratosis of the epidermis. After
sensitizing mice in the model group, slight red swelling occurred
in the abdominal skin, and scratches were seen in individual mouse
skin. After the last stimulation, a variety of bright red patches
occurred in the abdominal skin of the mice, the texture was
relatively hard, and there were also scratches and edema in varying
degrees.
Changes of mouse ear thickness
There was no significant difference in ear thickness
between the three groups of mice before modeling and at day 1
(P>0.05), but there were differences at day 6, 9, 12, 15 and 18
(all P<0.05). Mouse ear thickness in the model group was higher
than that in the treatment and control groups (all P<0.05), and
that in the treatment group was higher than that in the control
group (P<0.05). There was no significant change in mouse ear
thickness in the control group during the experiment period
(P>0.05), and the mouse ear thickness in the model and treatment
groups increased compared to that before modeling and continued to
significantly increase from day 6 (P<0.05) (Table I).
 | Table I.Changes of mouse ear thickness
(mm). |
Table I.
Changes of mouse ear thickness
(mm).
| Variables | Control group | Model group | Treatment group | Statistical
value | P-value |
|---|
| Number (a) | 10 | 15 | 13 |
|
|
| Before modeling | 0.173±0.012 | 0.176±0.017 | 0.174±0.014 | 0.135 | 0.894 |
| day 1 | 0.177±0.015 | 0.179±0.018 | 0.180±0.021 | 0.076 | 0.927 |
| day 6 | 0.183±0.024 |
0.252±0.032a,b,g |
0.201±0.022a,b,h | 22.580 | <0.001 |
| day 9 | 0.186±0.023 |
0.315±0.035a–c,g |
0.244±0.027a–c,h | 58.829 | <0.001 |
| day 12 | 0.192±0.027 |
0.341±0.041a–c,g |
0.298±0.031a–d,h | 56.991 | <0.001 |
| day 15 | 0.202±0.031 |
0.391±0.046a–e,g |
0.312±0.032a–d,h | 74.216 | <0.001 |
| day 18 | 0.221±0.032 |
0.416±0.043a–e,g |
0.345±0.032a–f,h | 84.504 | <0.001 |
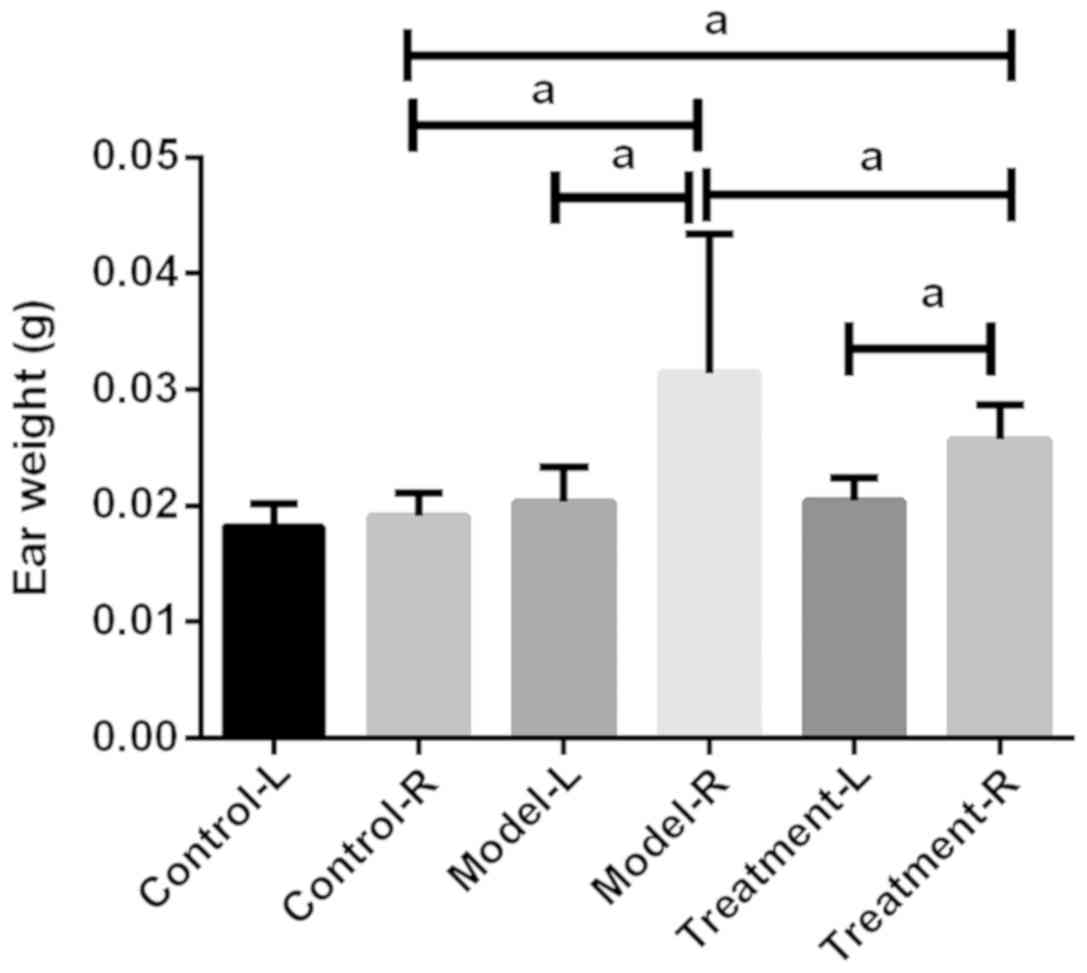
Measurement results of mouse ear
weight
There was no significant difference in mouse
binaural weight in the control group (P>0.05), and the weight of
mouse right ear in the model and treatment groups was significantly
higher than that of left ear (P<0.05). There was no significant
difference in the weight of mouse left ear between the three groups
(P>0.05), but that of right ear was significantly different
(P<0.05), and the model group was higher than the treatment and
control groups (all P<0.05), and the treatment group was higher
than the control group (P<0.05) (Fig.
1).
Changes of IL-4 level in mouse
serum
There was no significant difference in IL-4 level
between the three groups of mice before modeling (P>0.05), but
there were significant diffe-rences at day 1, 6, 9, 12, 15 and 18
(all P<0.05). The IL-4 level of mice in the model group was
higher than that in the treatment and control groups (all
P<0.05), and that in the treatment group was higher than that in
the control group (all P<0.05). There was no significant change
in IL-4 level of mice in the control group during the experiment
period (P<0.05). Finally, the IL-4 level in mice in the model
group increased compared to that before modeling and continued to
significantly increase from day 1 (all P<0.05) (Table II).
 | Table II.Changes of IL-4 level in mice serum
(ng/ml). |
Table II.
Changes of IL-4 level in mice serum
(ng/ml).
| Variables | Control group | Model group | Treatment
group | Statistical
value | P-value |
|---|
| Number (a) | 10 | 15 | 13 |
|
|
| Before
modeling | 0.362±0.032 | 0.363±0.031 | 0.362±0.032 | 0.005 | 0.996 |
| day 1 | 0.369±0.031 |
0.432±0.032a,g |
0.397±0.033a,h | 11.958 | 0.001 |
| day 6 | 0.365±0.032 |
0.501±0.035a,b,g |
0.425±0.032a,b,h | 52.049 | <0.001 |
| day 9 | 0.364±0.033 |
0.585±0.036a–c,g |
0.458±0.034a–c,h | 64.970 | <0.001 |
| day 12 | 0.367±0.032 |
0.622±0.039a–d,g |
0.472±0.035a–d,h | 70.648 | <0.001 |
| day 15 | 0.362±0.033 |
0.694±0.041a–e,g |
0.492±0.037a–e,h | 76.976 | <0.001 |
| day 18 | 0.366±0.031 |
0.734±0.052a–g |
0.512±0.036a–f,h | 81.631 | <0.001 |
Changes of ICAM-1 level in mouse
serum
There was no significant difference in ICAM-1 level
between the three groups of mice before modeling (P>0.05), but
there were significant differences at day 1, 6, 9, 12, 15 and 18
(all P<0.05). The ICAM-1 level of mice in the model group was
higher than that in the treatment and control groups (all
P<0.05), and that in the treatment group was higher than that in
the control group (all P<0.05). There was no significant change
in ICAM-1 level in mice in the control group during the experiment
period (P<0.05). The ICAM-1 level in mice in the model group
increased compared to that before modeling and continued to
significantly increase from day 1 (P<0.05), the ICAM-1 level in
mice in the treatment group increased compared to that before
modeling and continued to significantly increase from day 6
(P<0.05) (Table III).
 | Table III.Changes of ICAM-1 level in mouse
serum (ng/ml). |
Table III.
Changes of ICAM-1 level in mouse
serum (ng/ml).
| Variables | Control group | Model group | Treatment
group | Statistical
value | P-value |
|---|
| Number (a) | 10 | 15 | 13 |
|
|
| Before
modeling | 2.25±0.17 | 2.23±0.18 | 2.24±0.16 | 0.042 | 0.959 |
| day 1 | 2.27±0.12 |
2.45±0.19a,g |
2.33±0.15h | 4.140 | 0.024 |
| day 6 | 2.26±0.14 |
3.12±0.21a,b,g |
2.89±0.16a,b,h | 22.301 | <0.001 |
| day 9 | 2.28±0.15 |
4.57±0.22a–c,g |
3.42±0.18a–c,h | 44.402 | <0.001 |
| day 12 | 2.25±0.17 |
6.33±0.25a–d,g |
4.01±0.17a–d,h | 68.754 | <0.001 |
| day 15 | 2.24±0.16 |
8.12±0.29a–e,g |
4.78±0.21a–e,h | 71.251 | <0.001 |
| day 18 | 2.28±0.18 |
8.65±0.31a–g |
5.12±0.19a–f,h | 82.336 | <0.001 |
Correlation analysis
The results of Spearmans correlation analysis showed
that the IL-4 and ICAM-1 levels in mice were positively correlated
with ear thickness in the model group (r=0.865, P=0.002; r=0.833,
P=0.009). The IL-4 level in mice was positively correlated with and
the ICAM-1 level in the model group (r=0.812, P=0.014). IL-4 and
ICAM-1 levels in mice were positively correlated with the treatment
time in the treatment group (r=0.712, P=0.024; r=0.734, P=0.021)
(Table IV).
 | Table IV.Correlation analysis. |
Table IV.
Correlation analysis.
| Groups | Ear thickness | IL-4 | Treatment time |
|---|
| Model group |
|
IL-4 | r=0.865 |
|
|
|
| P=0.002 |
|
|
|
ICAM-1 | r=0.833 | r=0.812 |
|
|
| P=0.009 | P=0.014 |
|
| Treatment
group |
|
IL-4 |
|
| r=0.712 |
|
|
|
| P=0.024 |
|
ICAM-1 |
|
| r=0.734 |
|
|
|
| P=0.021 |
Discussion
Chronic eczema is a global public health problem
(14), and the immune imbalance of
Th2/Th1 plays a crucial role in the occurrence and development of
chronic eczema, contributing to abnormal secretion of related
cytokines such as IL-4 and ICAM-1 (15,16).
Mouse IL-4, which is mainly produced by Th2 subgroup and
participates in humoral immunity, is also an important indicator
reflecting the degree of immune reaction of Th2 and Th1 cells
(12) ICAM-1, which belongs to the
immunoglobulin superfamily of adhesion molecules, is an important
adhesion molecule in mediating adhesion reaction, playing an
important role in the promotion of the adhesion of inflammatory
sites and the regulation of the immune reaction of the body
(13). Total glucoside of paeony is
an anti-inflammatory and immunomodulatory drug (9), but there are few reports on the related
treatment of chronic eczema, and the mechanism is not clear. In
this study, we explored the curative effect and the mechanism of
total glucosides of paeony through dynamically monitoring the
changes of IL-4 and ICAM-1 levels in mice with chronic eczema
during the treatment with total glucoside of paeony.
In this study, DNCB was used to sensitize mice to
induce inflammatory reaction and to replicate the pathological
model of chronic eczema, which is a classic animal model (17). This study showed that the mice
developed related pathological manifestations such as red swelling,
plaque and scratches after the last stimulation of DNCB, suggesting
that the model was successfully established, similar to the
manifestations of mice model with chronic eczema in related reports
(18,19). However, two mice in the treatment
group died in this experiment. The maximum dose of administration
was 0.21 ml in this experiment, while the maximum dose of tolerance
of mice was 0.5 ml (20), so we
speculated that the death of mice might be caused by the drug. In
this study, from the measurement results of mouse ear thickness, it
could be seen that with the increase of stimulation times, the
mouse ear thickness in the model and treatment groups increased,
but that of the treatment group was significantly lower than that
of the model group. The curative effect of anti-dermatitis drug
could be judged according to the degree of ear swelling (21). The results of this study also showed
that the mouse ear quality in the treatment group was lower than
that in the model group, suggesting that total glucoside of paeony
can obviously improve the mouse ear thickness and quality. It
indicated that total glucosides of paeony had a good therapeutic
effect on mice with chronic eczema, which might be related to the
improvement of mice with inflammatory and immune injury with total
glucosides of paeony. In this study, the changes of IL-4 and ICAM-1
levels were dynamically monitored during the sensitization and
stimulation of mice, and the IL-4 and ICAM-1 levels in mice in the
model group also increased with the increase of stimulation times.
Spearmans correlation analysis also showed that IL-4 and ICAM-1
levels were positively correlated with ear thickness. Song et
al (22) showed that the IL-4
level in eczema patients increased significantly, and it was higher
as the severity of eczema increased. Studies reported by them
indicated that the IL-4 level in eczema patient serum increased
significantly (23,24). We speculated that this may be related
to the immune imbalance of Th2/Th1 in mice with chronic eczema, and
there was an immune imbalance of Th2/Th1 in chronic eczema mice,
which was dominated by the activation of Th2 cells, thus leading to
the increase of IL-4 expression level in mice (25,26).
Huang et al (27) and Han
et al (28) also reported
that ICAM-1 level in chronic eczema patients were significantly
higher than those in normal controls, similar to our results.
ICAM-1 can be expressed in keratinocytes under the stimulation of
sensitization, while scales, as one of the special diagnosis of
chronic eczema, can further develop keratinocytes (29,30),
which may also be the reason for the increase of ICAM-1 level in
chronic eczema patients. Although the IL-4 and ICAM-1 levels in
mice in the treatment group also increased with the increase of
stimulation times, and Spearmans correlation analysis also showed
that the IL-4 and ICAM-1 levels in mice in the treatment group were
positively correlated with the treatment time and the level in the
treatment group was significantly lower than that in the model
group, suggesting that total glucosides of paeony may improve the
inflammatory immune injury of mice by improving IL-4 and ICAM-1
levels. In recent years, there have been few reports on the
treatment of chronic eczema with total glucosides of paeony. In the
study of Wang et al (9), it
was indicated that total glucosides of paeony could improve the
ICAM-1 level in mice with allergic contact dermatitis. In a
previous study, it was shown that total glucosides of paeony may
improve the ICAM-1 level in diabetic rats (31). Both allergic contact dermatitis and
diabetes are immune diseases, so we can believe that total
glucosides of paeony have a function in the improvement of ICAM-1
level in chronic eczema mice. However, mice and humans have more
similarities, but the conclusion cannot represent the results of
clinical trials, so we still need more clinical data to prove our
conclusions.
In conclusion, IL-4 and ICAM-1 may be involved in
the pathologic process of chronic eczema and total glucosides of
paeony may play a role in the treatment of chronic eczema by
regulating the IL-4 and ICAM-1 levels.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ drafted the manuscript. HS established the rat
model. MZ and HS were responsible for ELISA and observation
indicators. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The People's Hospital of Danyang (Zhenjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Galli E, Rocchi L, Carello R, Giampietro
PG, Panei P and Meglio P: Serum Vitamin D levels and Vitamin D
supplementation do not correlate with the severity of chronic
eczema in children. Eur Ann Allergy Clin Immunol. 47:41–47.
2015.PubMed/NCBI
|
|
2
|
Walling HW and Swick BL: Update on the
management of chronic eczema: New approaches and emerging treatment
options. Clin Cosmet Investig Dermatol. 3:99–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoo J and Jue MS: Intractable pruritus
with chronic eczema in an elderly patient caused by long-term
intake of calcium channel blocker. Contact Dermat. 77:339–340.
2017. View Article : Google Scholar
|
|
4
|
Fowler J: Chronic hand eczema: A prevalent
and challenging skin condition. Cutis. 82 Suppl:4–8.
2008.PubMed/NCBI
|
|
5
|
Williams HC and Grindlay DJ: What's new in
atopic eczema? An analysis of systematic reviews published in 2007
and 2008. Part 1. Definitions, causes and consequences of eczema.
Clin Exp Dermatol. 35:12–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grimbacher B, Peters T and Peter HH:
Lactose-intolerance may induce severe chronic eczema. Int Arch
Allergy Immunol. 113:516–518. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wollenberg A, Oranje A, Deleuran M, Simon
D, Szalai Z, Kunz B, Svensson A, Barbarot S, von Kobyletzki L,
Taieb A, et al: European Task Force on Atopic Dermatitis/EADV
Eczema Task Force: ETFAD/EADV Eczema task force 2015 position paper
on diagnosis and treatment of atopic dermatitis in adult and
paediatric patients. J Eur Acad Dermatol Venereol. 30:729–747.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan KS: Efficacy of leukotriene receptor
antagonist with an anti-H1 receptor antagonist for treatment of
chronic idiopathic urticaria. J Dermatolog Treat. 20:194–197. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Yuan J, Wu HX, Chang Y, Wang QT,
Wu YJ, Zhou P, Yang XD, Yu J and Wei W: Total glucosides of paeony
inhibit the inflammatory responses of mice with allergic contact
dermatitis by restoring the balanced secretion of
pro-/anti-inflammatory cytokines. Int Immunopharmacol. 24:325–334.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng YQ, Wei W, Zhu L and Liu JX: Effects
and mechanisms of Paeoniflorin, a bioactive glucoside from paeony
root, on adjuvant arthritis in rats. Inflamm Res. 56:182–188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiang N, Li XM, Zhang MJ, Zhao DB, Zhu P,
Zuo XX, Yang M, Su Y, Li ZG, Chen Z, et al: Total glucosides of
paeony can reduce the hepatotoxicity caused by Methotrexate and
Leflunomide combination treatment of active rheumatoid arthritis.
Int Immunopharmacol. 28:802–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
N'diaye M, Warnecke A, Flytzani S,
Abdelmagid N, Ruhrmann S, Olsson T, Jagodic M, Harris RA and
Guerreiro-Cacais AO: Rat bone marrow-derived dendritic cells
generated with GM-CSF/IL-4 or FLT3L exhibit distinct phenotypical
and functional characteristics. J Leukoc Biol. 99:437–446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liou GY, Döppler H, Necela B, Edenfield B,
Zhang L, Dawson DW and Storz P: Mutant KRAS-induced expression of
ICAM-1 in pancreatic acinar cells causes attraction of macrophages
to expedite the formation of precancerous lesions. Cancer Discov.
5:52–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Check JH and Chan S: Complete eradication
of chronic long standing eczema and keratosis pilaris following
treatment with dextroamphetamine sulfate. Clin Exp Obstet Gynecol.
41:202–204. 2014.PubMed/NCBI
|
|
15
|
Sio TT, Pittelkow MR, Nagle MA and
Martenson JA: External beam radiation therapy for recalcitrant
dermatitis. Acta Derm Venereol. 94:717–719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marth E, Jelovcan S, Kleinhappl B, Gutschi
A and Barth S: The effect of heavy metals on the immune system at
low concentrations. Int J Occup Med Environ Health. 14:375–386.
2001.PubMed/NCBI
|
|
17
|
Wan HL, Chen HZ and Shi XQ: Study on
effect of Traditional Chinese Medicine Jianpi Chushi decoction and
ointment on chronic eczema. Asian Pac J Trop Med. 9:920–923. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li YM and Li XR: Effects of Qingshi Cream
on chronic dermatitis-eczema in mice. J Chin Integr Med.
7:1164–1166. 2009.(In Chinese). View Article : Google Scholar
|
|
19
|
Zöller M, Gupta P, Marhaba R, Vitacolonna
M and Freyschmidt-Paul P: Anti-CD44-mediated blockade of leukocyte
migration in skin-associated immune diseases. J Leukoc Biol.
82:57–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hafizur RM, Hameed A, Shukrana M, Raza SA,
Chishti S, Kabir N and Siddiqui RA: Cinnamic acid exerts
anti-diabetic activity by improving glucose tolerance in vivo and
by stimulating insulin secretion in vitro. Phytomedicine.
22:297–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsang MS, Jiao D, Chan BC, Hon KL, Leung
PC, Lau CB, Wong EC, Cheng L, Chan CK, Lam CW, et al:
Anti-inflammatory activities of pentaherbs formula, berberine,
gallic acid and chlorogenic acid in atopic dermatitis-like skin
inflammation. Molecules. 21:5192016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song HS, Jung SE, Kim YC and Lee ES:
Nipple eczema, an indicative manifestation of atopic dermatitis? A
clinical, histological, and immunohistochemical study. Am J
Dermatopathol. 37:284–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simon D, Kozlowski E and Simon H: Natural
killer T cells expressing IFN-gamma and IL-4 in lesional skin of
atopic eczema. Allergy. 64:1681–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Callard RE, Hamvas R, Chatterton C, Blanco
C, Pembrey M, Jones R, Sherriff A and Henderson J: ALSPAC Study
Team. Avon Longitudinal Study of Parents and Children: An
interaction between the IL-4Ralpha gene and infection is associated
with atopic eczema in young children. Clin Exp Allergy. 32:990–993.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Czarnowicki T, Esaki H, Gonzalez J,
Malajian D, Shemer A, Noda S, Talasila S, Berry A, Gray J, Becker
L, et al: Early pediatric atopic dermatitis shows only a cutaneous
lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults
acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol.
136:941–951.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heath VL, Murphy EE, Crain C, Tomlinson MG
and O'Garra A: TGF-beta1 down-regulates Th2 development and results
in decreased IL-4-induced STAT6 activation and GATA-3 expression.
Eur J Immunol. 30:2639–2649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang WC, Dai YW, Peng HL, Kang CW, Kuo CY
and Liou CJ: Phloretin ameliorates chemokines and ICAM-1 expression
via blocking of the NF-κB pathway in the TNF-α-induced HaCaT human
keratinocytes. Int Immunopharmacol. 27:32–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han HY, Ryu MH, Lee G, Cheon WJ, Lee C, An
WG, Kim H and Cho SI: Effects of Dictamnus dasycarpus Turcz., root
bark on ICAM-1 expression and chemokine productions in vivo and
vitro study. J Ethnopharmacol. 159:245–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quraishi BM, Zhang H, Everson TM, Ray M,
Lockett GA, Holloway JW, Tetali SR, Arshad SH, Kaushal A, Rezwan
FI, et al: Identifying CpG sites associated with eczema via random
forest screening of epigenome-scale DNA methylation. Clin
Epigenetics. 7:682015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang B, Yang W, Sherman VR and Meyers MA:
Pangolin armor: Overlapping, structure, and mechanical properties
of the keratinous scales. Acta Biomater. 41:60–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu L, Wei P, Cao Y, Zhang Q, Liu M, Liu
XD, Wang ZL and Zhang PY: Effect of total peony glucoside
pretreatment on NF-κB and ICAM-1 expression in myocardial tissue of
rat with myocardial ischemia-reperfusion injury. Genet Mol Res.
15:152016. View Article : Google Scholar
|