Introduction
Humeral fractures are common and account for 5% of
total body fractures (1). Of the
total cases of humeral fractures, ~80% are non-displaced or
slightly displaced stable fractures that do not require surgical
treatment (2). Displaced unstable
proximal humeral fractures are usually treated surgically (3). Heterotopic ossification refers to the
occurrence of ossification outside the bone tissue, and
surgery-complicated or secondary heterotopic ossification is
clinically known as traumatic ossifying myositis (4).
Currently, more than a dozen bone morphogenetic
proteins (BMPs) have been identified (5,6). BMP-2
was originally recognized as an inducer of bone formation belonging
to the transforming growth factor-β family (7), and it serves an important role in bone
formation (8,9) and fracture healing (10). BMP-2 also has an important role in
the ossification process, as it stimulates the differentiation of
pluripotent stem cells into osteoblasts and enhances the function
of osteoblasts (6,11,12).
There are various ways to regulate the expression of BMP-2, in
which microRNA (miRNA) have been extensively studied. For example,
miR-98 (13), miR-203 and miR-320
(14) have been reported to regulate
the expression of BMP-2.
MiRNAs are a class of small RNA molecules 18–22 nt
in length. They regulate mRNA translation through specific binding
to the 3′-untranslated region (UTR) of the target mRNA and are
important post-transcriptional regulators (15). Previous studies have confirmed that
miRNA serve important roles in various diseases, including cancer,
cardiovascular and endocrine disease (16,17).
MiR-421 is cell-growth associated and was discovered in recent
years (18). It serves an important
role in proliferation, invasion and metastasis of multiple tumors
(18,19).
In the present study, the expression levels of BMP-2
in bone tissues and blood of patients with humeral fractures at
different periods and patients with/without heterotopic
ossification following humeral fractures were detected.
Bioinformatics analysis was performed to identify miRNA sequences
that may regulate BMP-2. To the best of our knowledge, this is the
first report to discuss the regulatory effect of miR-421 on
BMP-2.
Patients and methods
Clinical data of patients
In order to investigate the role of BMP-2 and
miR-421 in bone fracture, 38 patients who received humeral fracture
surgery at the Weifang People's Hospital (Weifang, China) between
June 2013 and January 2017 were enrolled in the present study.
Among them, 16 patients (5 male and 11 female patients) received
surgery within 1–7 days of fracture. Their age range was 60–72
years. There were 22 patients who received the surgery within 8–14
days of fracture, including 9 males and 13 females. Their ages
ranged from 61–73 years. Reconstructive and fixation surgery was
performed according to the fracture site, and complications,
including swelling, dislocation and congestion, were treated with
respective drugs (Table I). To
investigate the role of BMP-2 and miR-421 in the heterotopic
ossification process, 18 patients who were diagnosed with
heterotopic ossification in the post-surgical follow-up (14–16
months) and required surgery were also enrolled. Among them, there
were 5 males and 13 females. Their age ranged from 60–71 years.
Presurgical treatment for these patients was the same as in
previous treatments of patients with humeral fractures. Another 26
patients with humeral fractures without heterotopic ossification
who had received surgery to remove intramedullary nails due to good
healing conditions during the same time period were also included.
Among them, there were 10 males and 16 females. Their age range was
60–73 years old. These patients were treated as aforementioned
(Table II). Patients with
rheumatoid arthritis and long-term hormone treatment were excluded
from the present study. Bone tissues and blood were collected from
all patients. All of the above patients were clearly diagnosed with
humeral fractures or heterotopic ossification by pathology
analysis. Prior written informed consent was obtained from all
patients and the present study was approved by the ethics review
board of Weifang People's Hospital.
 | Table I.Characteristics of patients with
humeral fractures. |
Table I.
Characteristics of patients with
humeral fractures.
|
| No. of days after
which surgery was performed following fracture |
|---|
|
|
|
|---|
| Clinical
characteristics | 1–7 | 8–14 |
|---|
| Case number | 16 | 22 |
| Age (years) | 65.5±6.7 | 66.7±6.2 |
| Sex |
|
|
|
Male | 5 | 9 |
|
Female | 11 | 13 |
| Fracture cause |
|
|
|
Fall | 6 | 7 |
| Car
accident | 12 | 8 |
|
Bruise | 3 | 2 |
| Treatment | Mannitol
dehydration, traction, promoting blood circulation with safflower
injection | Mannitol
dehydration, traction, promoting blood circulation with safflower
injection |
 | Table II.Characteristics of patients with and
without HO. |
Table II.
Characteristics of patients with and
without HO.
|
| HO |
|---|
|
|
|
|---|
| Clinical
characteristics | Yes | No |
|---|
| Case number | 18 | 26 |
| Age (years) | 64.5±7.2 | 65.1±7.8 |
| Sex |
|
|
|
Male | 5 | 10 |
|
Female | 13 | 16 |
| Cause of
surgery | HO | Intramedullary nail
removal |
| Treatment | Untreated prior to
surgery | Untreated prior to
surgery |
| Time of
sampling | 2–12 weeks after
surgery | 2–12 weeks after
surgery |
Sample collection
Fracture site bone tissues and heterotopic
ossification bone tissues were obtained from surgery. The
non-heterotopic ossification bone tissues were obtained from the
fracture tissues of the fixed plate when it was removed. The bone
tissues were immediately stored in liquid nitrogen (−196°C) and
ground into powder for further use. Peripheral blood (30 ml) was
collected from the patients following fasting on the day of
surgery. Serum was isolated from peripheral blood by centrifugation
(200 × g, 4°C, 10 min).
Reagents
miRcute miRNA isolation kit, miRcute miRNA
first-strand cDNA synthesis kit, miRNA miRcute fluorescent
quantitative detection kit (FP401), SuperReal PreMix (SYBR Green)
and miRNA first-strand cDNA synthesis kit were all purchased from
Tiangen Biotech Co., Ltd., (Beijing, China). Rabbit anti-human
BMP-2 primary antibody (ab14933), β-actin primary antibody
(ab8227), horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (ab6721) and enhanced chemiluminescence (ECL)
liquid (ab65623) were all purchased from Abcam (Cambridge, MA,
USA). TRIzol agent (10606ES60) was purchased from Yisheng
Biological Co., Ltd., (Shanghai, China). A bicinchoninic acid assay
(BCA) kit (RTP7102) was purchased from Real-Times Beijing
Biotechnology Co., Ltd., (Beijing, China). An miRNeasy Serum/Plasma
kit (JL217184) was purchased from Guangzhou Jianlun Biological
Technology Co., Ltd., (Guangzhou, China). A Human BMP-2 ELISA kit
(ab119581) was purchased from Abcam. Image Lab software (v. 3.0)
was free from Bio-Rad Laboratories, Inc., (Hercules, CA, US). All
plasmids/agomiR were synthesized by Sangon Biotech Co., Ltd.,
(Shanghai, China). The pMIR-REPORT™ miRNA Expression Reporter
Vector System (AM5795) was purchased from Ambion (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissues or blood
samples using TRIzol reagent. The integrity of RNA bands was
examined by 1% agarose gel electrophoresis, and the RNA purity was
measured by a 260/280 ratio using a spectrophotometer. Following
this, RNA was reverse transcribed into cDNA using a miRNA
first-strand cDNA synthesis kit. The qPCR reaction solution
consisted of 10 µl SuperReal PreMix Plus (SYBR-Green), 0.5 µl
upstream primers, 0.5 µl downstream primers, 2 µl cDNA and 7 µl
ddH2O. The following primer sequences were used: BMP-2
forward, 5′-CCTATATGCTCGACCTGTAC-3′ and reverse,
5′-CCCACTCATTTCTGAAAGTTC-3′; and GAPDH forward,
5′-GCACAGTCAAGGCTGAGAAT-3′ and reverse, 5′-TGAAGACGCCAGTAGACTCC-3′.
The qPCR reaction conditions for BMP-2 were as follows:
Pre-denaturation at 95°C for 30 sec, followed by 39 cycles of 5 sec
at 95°C and 20 sec at 60°C. miRNAs were extracted using a miRcute
miRNA isolation kit and reverse transcribed with miRcute miRNA
first-strand cDNA synthesis kit. For the measurement of miR-421,
qPCR was performed using a miRNA miRcute fluorescent quantitative
detection kit and the primers listed in Table III. The reaction conditions were as
follows: Pre-denaturation at 95°C for 5 min, followed by 40 cycles
of 15 sec at 95°C, 15 sec at 60°C and 10 sec at 72°C. GAPDH and U6
were used as internal controls. The results were calculated using
the 2−ΔΔCq method (20).
 | Table III.The primers used in this study. |
Table III.
The primers used in this study.
| Name | Sequence
(5′-3′) |
|---|
| U6 forward |
GCAAATTCGTGAAGCGTTCCAT |
| U6 reverse |
GGGATCAACAGACATTAATT |
| miR-421
forward |
TGGAAAACTTCCCGAAGAAC |
| miR-421
reverse |
GGGATCAACAGACATTAATT |
Western blot analysis
Total proteins were extracted from the bone tissues
and heterotopic ossification tissues using an
E.Z.N.A®Total DNA/RNA/Protein kit (Omega Bio-Tek, Inc.,
Norcross, GA, USA) and the protein concentrations were determined
using a BCA protein assay kit. Following this, proteins (50 µg)
were separated by 10% SDS-PAGE and transferred to a polyvinylidene
difluoride membrane. The membrane was blocked with 5% skim milk at
room temperature for 1 h. Subsequently, rabbit anti-human BMP-2
primary antibody (1:1,000) and anti-β-actin primary antibody
(1:5,000) were added and incubated at 4°C overnight. Following
washing with TBST, the secondary antibody (1:3,000) was added and
incubated at room temperature for 1 h. The membrane was developed
with ECL solution. Image Lab v. 3.0 software was used to analyze
the protein bands. The ratio of the gray value of the target
protein band to that of the β-actin band was determined as the
relative content of the target protein.
ELISA
ELISA was conducted with a Human BMP-2 ELISA kit,
according to the manufacturer's protocol. Briefly, 10 µl serum and
40 µl diluent were added. Except for the blank wells, 100 µl
horseradish peroxidase-labeled antibody was added to the wells. The
plate was sealed with film and incubated at 37°C for 1 h. Following
five washes with the provided washing reagent, 50 µl of substrate A
and B were added to each well and incubated at 37°C for 15 min.
Then, 50 µl termination solution was added to each well, and the
optical density value at 450 nm was measured within 15 min.
Prediction of the upstream regulatory
miRNA of BMP-2
The target gene prediction software of DIANAmT
(Ensemble v84;
diana.imis.athena-innovation.gr/DianaTools/index.php?r=mrmicrot/index)
(21), miRanda (last modified,
August 2010; http://34.236.212.39/microrna/home.do) (22), miRDB (last modified, May 2016;
mirdb.org/) (23),
miRWalk (v2.0; zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/index.html)
(24), RNAhybrid (last modified,
September 2017; bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
(25), PICTAR4 and PICTAR5 (v4.5;
pictar.mdc-berlin.de/) (26), PITA (v6; genie.weizmann.ac.il/pubs/mir07/mir07_data.html)
(27), RNA22 (v2.0; cm.jefferson.edu/rna22/) (28) and TargetScan (v6.2; targetscan.org) (29)
were used to predict the upstream regulatory miRNA of BMP-2.
Dual-luciferase reporter assay
The mutant binding sequence was designed as
described previously by Guo et al (30). The wild-type and mutant seed regions
of the 3′-UTR of the BMP-2 gene containing the miR-421 binding site
were chemically synthesized in vitro. Following the addition
of the cleavage sites of SpeI and HindIII at both
ends, they were cloned into the pMIR-REPORT luciferase reporter
plasmid. The mutated 3′-UTR seed region was used as a control. The
seed region sequence for the double luciferase reporter plasmid was
constructed as follows:
5′-TAATCAAAAGAAGTATCGGGTTTGTACATAATTTTCCAAAAATTGTAGTTGTTTTCAGTTGTGTGTATTTAAGATGAAAAGTCTACATGGAAGGTTACTCTGGCAAAGTGCTTAGCACGTTTGCTTTTTTGCAGTGCTACTGTTGAGTTCACAAGTTCAAGTCCAGAAAAAAAAAGTGGATAATCCACTCTGCTGACTTTCAAGATTATTATATTATTCAATTCTCAGGAATGTTGCAGAGTGATTGTCC-3′.
The bold letters represent the target region, and the mutant
sequence was GACAACT in the experiments. The plasmid containing the
wild-type or mutant 3′-UTR sequences were transfected into 293T
cells (Type Culture Collection of the Chinese Academy of Sciences,
Shanghai, China) using liposomes (Lipofectamine® 3000;
Thermo Fisher Scientific Inc.) and then the agomiR-421-containing
plasmid was transfected (100 nM) into the cells. After 24 h of
incubation at 37°C, each group of cells was lysed according to the
manufacturer's instruction of the Dual-Luciferase Reporter Assay
system (Promega Corporation, Fitchburg, WI, USA). The luciferase
reporter activity was recorded by a GloMax 20/20 luminometer
(Promega Corporation), using Renilla luciferase activity as
the internal standard.
Statistical analysis
All experiments were performed in triplicate
independently. All reported data were processed by SPSS 18.0 (SPSS,
Inc., Chicago, IL, USA), and the data was presented as the mean ±
standard deviation. Normality tests were performed for the data.
Student's t-tests were used for the comparison between two groups.
Multiple groups of measurement data were analyzed by one-way
analysis of variance. If the variance was homogeneous, the least
significant differences and Student-Newman-Keuls post hoc methods
were used; if the variance was heterogeneous, Tamhane's T2 or
Dunnett's T3 post hoc methods were used. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression difference of each indicator
at different time periods following fracture
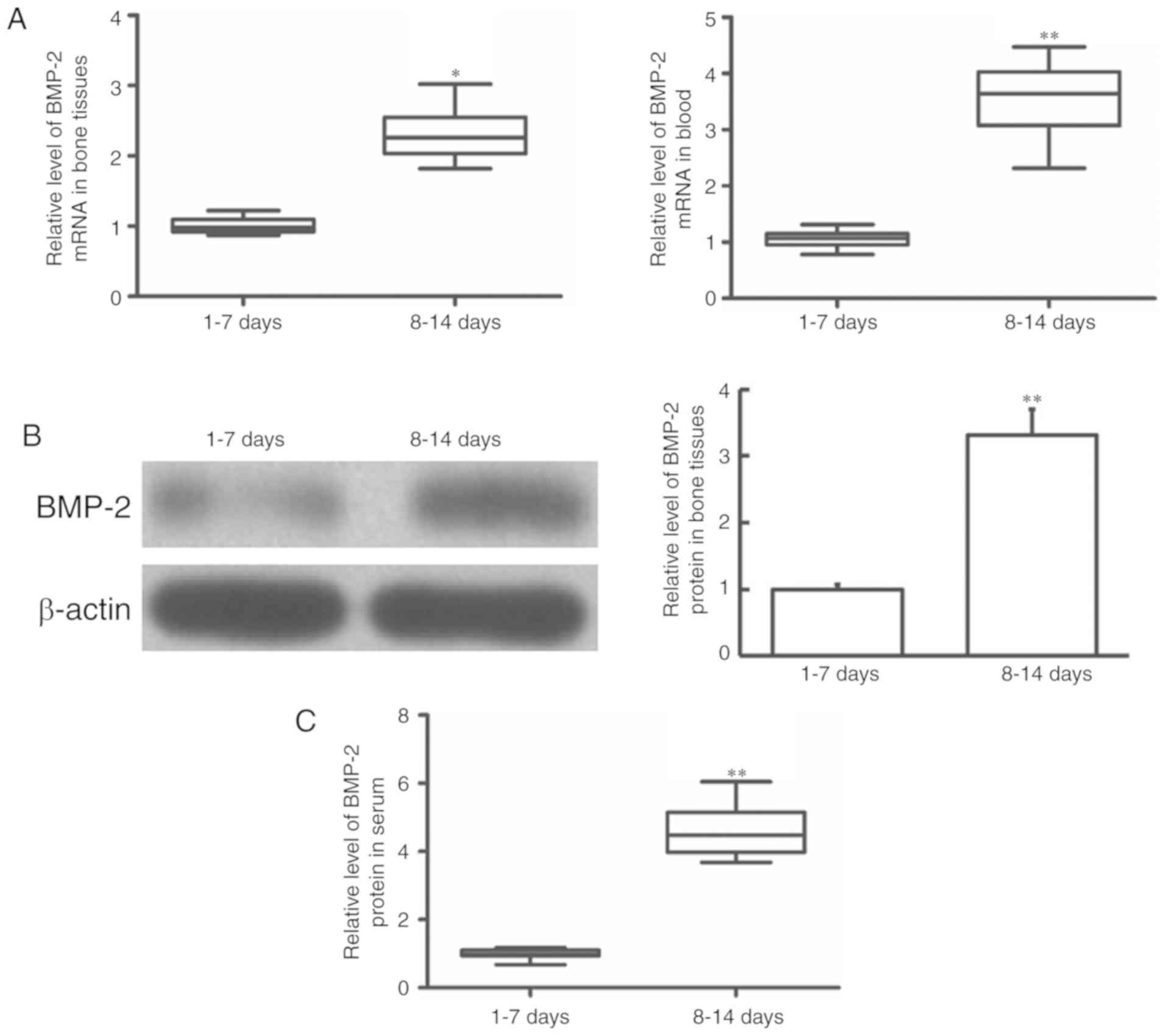
Changes of BMP-2 mRNA expression in
bone tissue and blood samples in patients with humeral
fractures
To determine the changes of BMP-2 mRNA expression in
each sample, RT-qPCR was performed. Compared with the patients who
received surgery 1–7 days after fracture, the patients who accepted
surgery 8–14 days after fracture demonstrated significantly
increased BMP-2 mRNA expression levels in their bone tissues
(P<0.05) and blood (P<0.01) (Fig.
1A). The different levels of BMP-2 mRNA expression suggests
that BMP-2 is upregulated over time within the two weeks after
humeral fracture, which may serve a particular regulatory role in
humeral fractures.
Changes of BMP-2 protein expression in
bone tissue and blood samples in patients with humeral
fractures
To determine the expression of BMP-2 protein in the
bone tissue of humeral fractures, western blotting was performed.
The patients who accepted surgery 8–14 days after fracture had
significantly upregulated BMP-2 protein levels in their bone
tissues compared to those observed in patients who received surgery
1–7 days after fracture (P<0.01) (Fig. 1B).
To determine the expression of BMP-2 protein in the
serum of patients with humeral fractures, ELISA was performed.
Compared with the patients who received surgery 1–7 days after
fracture, the patients who accepted surgery 8–14 days after
fracture had significantly elevated BMP-2 protein levels in their
serum (P<0.01) (Fig. 1C), which
was consistent with the upregulation trend of mRNA expression. The
upregulated BMP-2 protein expression level in the blood may
indicate that BMP-2 is released from the injured bone tissues.
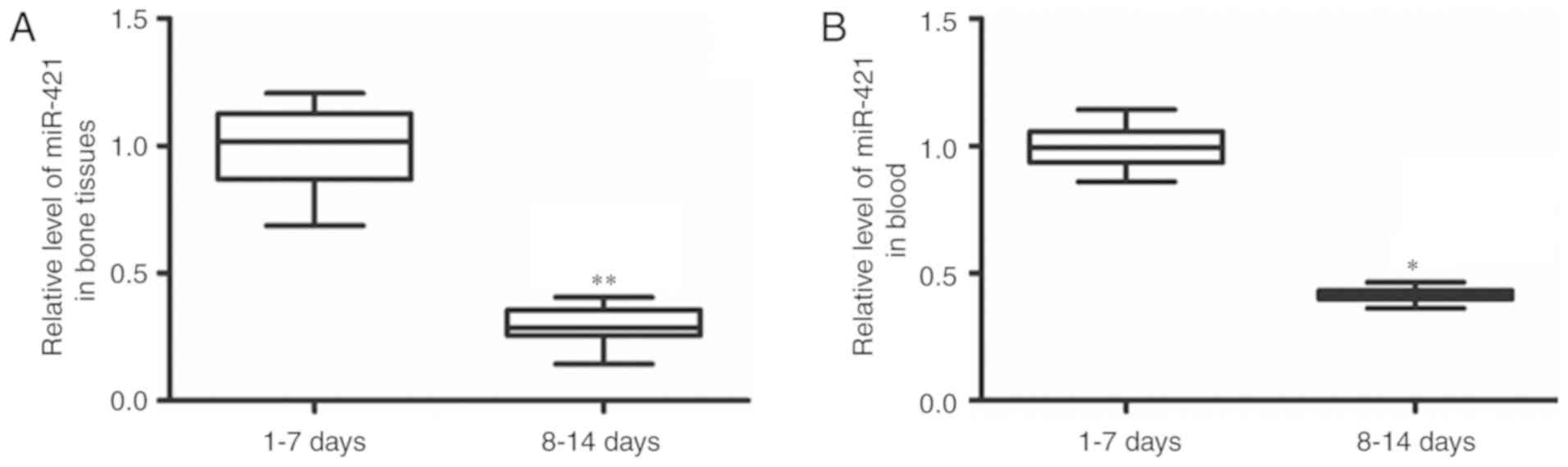
Changes of miR-421 expression in bone
tissue and blood samples in patients with humeral fractures
To determine the changes of miR-421 expression in
each sample, RT-qPCR was performed. The patients who accepted
surgery 8–14 days after fracture had significantly decreased
miR-421 expression levels in their bone tissue samples (P<0.01)
and blood samples (P<0.05) compared to those in the patients who
received surgery 1–7 days after fracture (Fig. 2). This result suggests that miR-421
may serve a regulatory role in the recovery of the humeral
fracture.
Expression difference of each indicator
between heterotopic and non-heterotopic ossification
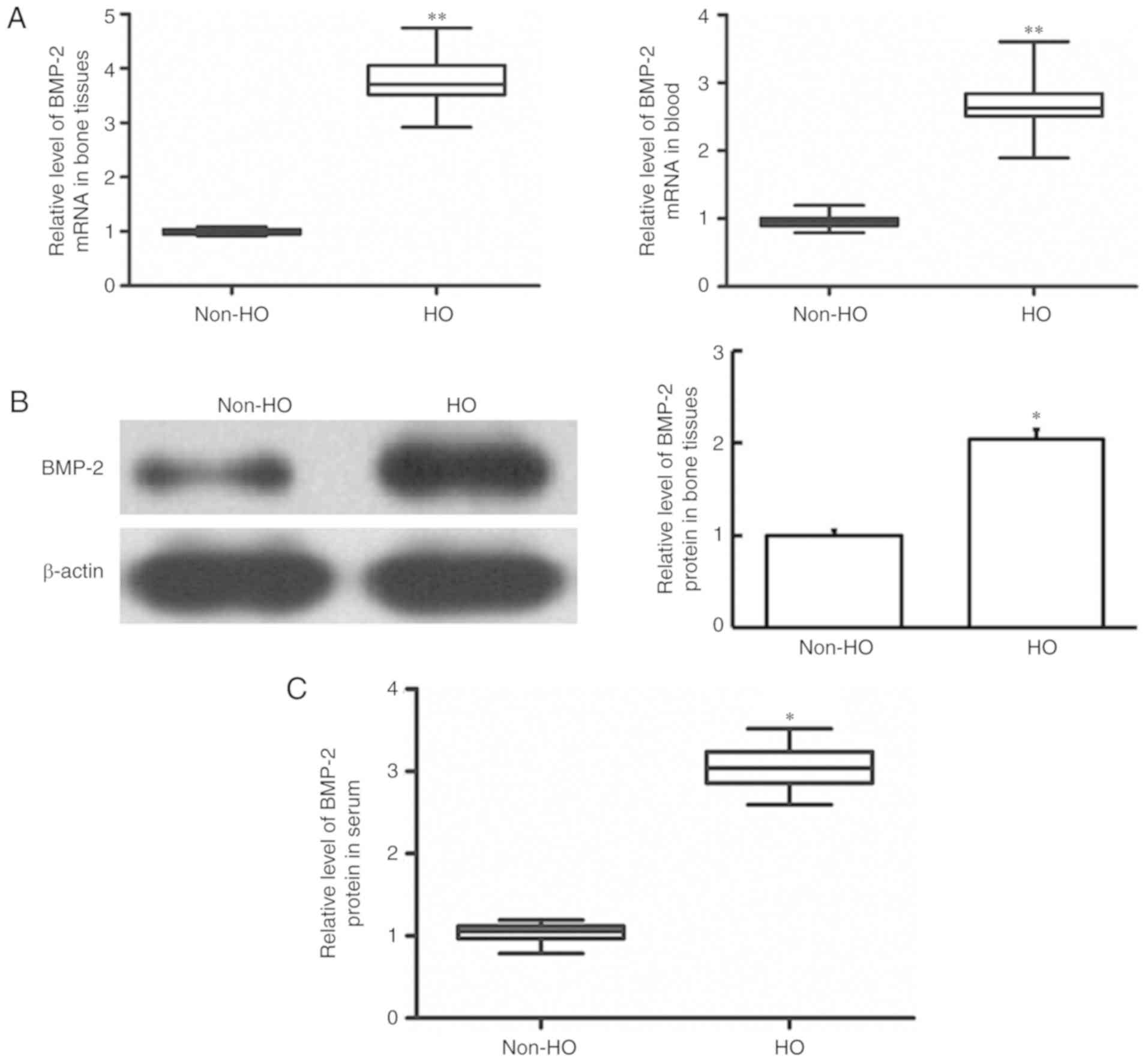
Changes of BMP-2 mRNA expression in
bone tissue and blood samples in patients with heterotopic
ossification
To determine the BMP-2 mRNA level in each sample,
RT-qPCR was performed. Compared with the patients without
heterotopic ossification, the patients with heterotopic
ossification demonstrated significantly upregulated BMP-2 mRNA
expression levels in their bone tissues and blood (P<0.01)
(Fig. 3A). This suggests that BMP-2
is upregulated in heterotopic tissues following humeral fracture,
and may serve a particular regulatory role in the disease.
Changes of BMP-2 protein expression in
bone tissue and blood samples in patients with heterotopic
ossification
To determine the expression of BMP-2 protein
expression in heterotopic ossification bone tissue following
humeral fracture, western blotting was performed. Patients with
heterotopic ossification demonstrated significantly upregulated
BMP-2 protein expression levels in their bone tissues, compared
with those observed in patients without heterotopic ossification
(P<0.05) (Fig. 3B), which was
consistent with the upregulation trend of mRNA.
To determine the expression level of BMP-2 protein
in the serum of patients with heterotopic ossification, ELISA was
performed. As demonstrated in Fig.
3C, compared with patients that did not have heterotopic
ossification, the BMP-2 protein levels were significantly higher in
the serum of the patients with heterotopic ossification
(P<0.05). These results suggest that BMP-2 may serve a
regulatory role in the recovery of heterotopic ossification at the
transcription and protein function levels.
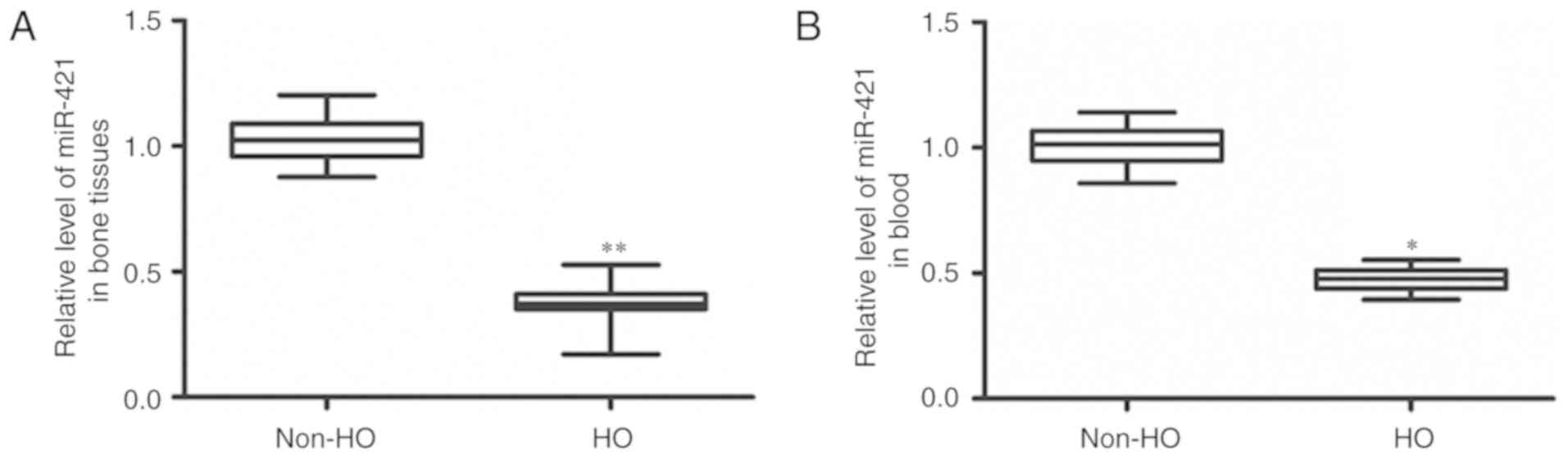
Changes of miR-421 expression in bone
tissue and blood samples of patients with heterotopic
ossification
To determine the expression of miR-421, RT-qPCR was
utilized. As indicated in Fig. 4,
the patients with heterotopic ossification demonstrated
significantly downregulated miR-421 expression levels in their bone
tissue samples (P<0.01) and blood (P<0.05) compared with
those in the patients without heterotopic ossification. This result
indicates that miR-421 may serve a regulatory role in the
heterotopic ossification process.
Prediction of miRNA sequences that
target BMP-2
To identify miRNA sequences that may target BMP-2,
10 databases were screened. There were 99 sequences predicted from
DIANAmT, 303 sequences from miRanda, 11 sequences from miRDB, 117
sequences from miRWalk, 0 sequence from RNAhybrid, 0 sequences from
PICTAR4, 241 sequences from PICTAR5, 0 sequences from PITA, 62
sequences from RNA22 and 128 sequences from TargetScan, with a
total of 961 sequences predicted as the target genes of miR-421.
Apart from the three databases that demonstrated no results, the
remaining seven databases predicted BMP-2 as the target gene of
miR-421. This high predicting proportion suggests that miR-421 may
be the upstream gene of BMP-2. In addition, CUGUUGA was a common
sequence of the seed region of miR-421 in the positive databases,
indicating that this sequence is highly likely to be the seed
region.
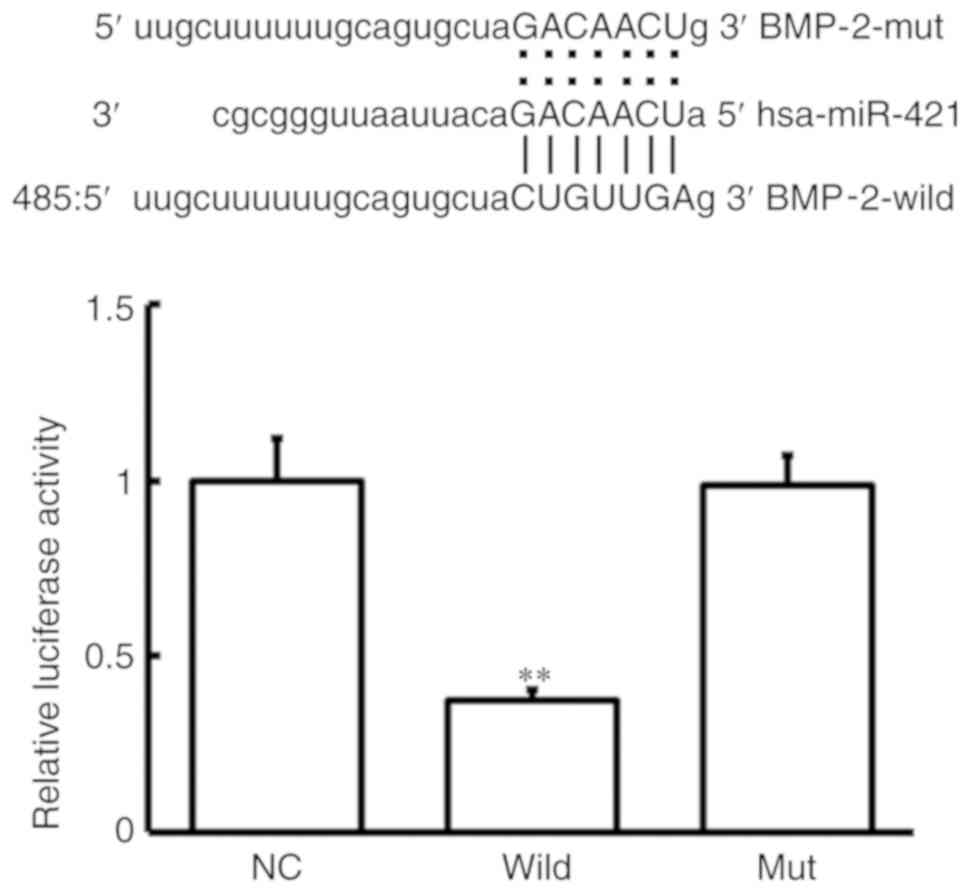
miR-421 targets BMP-2
To determine whether the BMP-2 gene was the target
gene of miR-421, a dual-luciferase reporter assay was conducted.
The wild and mutanttypes ofthe binding sequences between BMP-2 and
miR-421 are demonstrated in Fig. 5.
The mutant type of binding sequence was designed as described
previously by Guo et al (30). Compared with the negative control,
the relative luciferase activity was significantly decreased
following co-transfection with agomiR-421 and the wild-type 3′-UTR
of BMP-2 (P<0.05), while there was no significant difference
observed in the mutant group (P>0.05). These results indicate
that miR-421 may directly bind to the 3′-UTR of BMP-2 and regulate
its expression.
Discussion
Humeral fractures can be complex, and the fracture
may combine with fracture-peripheral nerves or blood vessels and
cause soft tissue damage, which may lead to impeded blood
circulation and neurotrophic disorders, increasing the difficulty
of fracture healing (31). Excessive
surgical treatment in the case of complex injuries not only
destroys the local blood supply of the fracture, but also fails to
achieve effective fracture fixation, eventually resulting in the
failure of humeral fracture healing (31).
It is well accepted that BMPs are the strongest
osteogenic factor, which promotes mesenchymal cells to
differentiate into bone, cartilage, ligamentous, tendon and nerve
tissues (32). BMP-2 is able to
specifically convert mouse myoblast cell lines into osteoblast cell
lines (33,34). It is believed that the level of BMP-2
in osteoblasts may reflect its osteogenesis ability (35,36). In
the present study, BMP-2 mRNA and protein expression levels in the
bone fracture tissue and blood of the patients who received surgery
1–7 days after fracture were significantly lower than those in the
patients who received surgery after 8–14 days. This suggests that
the body may respond to fracture damage within 2 weeks and then
upregulate BMP-2 expression in blood and fracture tissues to induce
osteogenesis and repair fracture damages.
Heterotopic ossification is a very important
secondary disease following fracture, in which bone structure forms
outside the skeletal system (37).
It is characterized by rapid formation of calcified bone in the
soft tissue, causing swelling and pain around the joints and
disorders in joint movement (38). A
study by Qu et al (39)
suggested that heterotopic ossification required certain
incentives, including osteoblasts and an appropriate
microenvironment. A study by Mukai et al (40) demonstrated that a decrease of BMP-2
alkaline phosphatase activity served a role in the bone
differentiation microenvironment and reduced the occurrence of
heterotopic ossification (41). This
suggests that heterotopic ossification following fracture may be
caused by the fracture-induced overregulation of BMP-2. To verify
the role of BMP-2 in heterotopic ossification following humeral
fracture, the present study measured the expression of BMP-2 in the
ossified tissues and blood of patients with heterotopic
ossification. A significantly increased expression level of BMP-2
in the ossified tissues and blood of patients with heterotopic
ossification following humeral fractures was observed compared with
the levels in patients without heterotopic ossification. This
suggests that heterotopic ossification may be related to the
upregulation of BMP-2.
Many miRNA have become biomarkers of various
diseases (41–43), thus the upstream regulatory miRNA of
BMP-2 were also investigated in the present study. The upstream
regulatory genes of BMP-2 were predicted by bioinformatics, and
miR-421 was identified to be one of them. The expression level of
miR-421 in gastric cancer has been previously studied (44). In addition, it has been demonstrated
that miR-421 was upregulated in neuroblastoma, pancreatic cancer,
prostate cancer and other malignant tumors (45–47).
miR-421 is closely related to cell growth. A study by Jiang et
al (18) determined that miR-421
expression in gastric cancer tissue was higher than that in normal
tissues, indicating that miR-421 may serve an important role in the
early growth of gastric cancer. A study by Zhou et al
(19) detected the expression of
miR-421 in peripheral blood mononuclear cell lines by RT-PCR and
transfected miR-421 inhibitors into animals, and found that tumor
growth was inhibited. Significant downregulation of miR-421
expression was observed in the humeral fracture and the heterotopic
ossification tissue samples in the present study. As
aforementioned, BMP-2 mRNA and protein were upregulated in these
samples. This suggests that the upregulation of BMP-2 may be
regulated by the downregulation of its upstream regulatory gene,
miR-421. In the present study, the BMP-2 dual-luciferase gene
reporter plasmid was constructed and transfected into 293T cells
together with agomiR-421. It was revealed that overexpression of
miR-421 could significantly reduce the luminance of the BMP-2
plasmid, demonstrating that BMP-2 is the direct target gene of
miR-421.
In conclusion, BMP-2 levels were increased and
miR-421 levels were decreased in bone tissue samples and blood of
patients with humeral fractures and heterotopic ossification. BMP-2
was a direct target of miR-421. These results indicate that miR-421
may serve an important role in humeral fracture healing through
regulating BMP-2. This study may provide a new therapeutic target
for fracture and heterotopic ossification.
Acknowledgments
We would like to thank Dr Weizhi Wang and Dr Xuefang
Zhao of the Department of Anesthesiology at the Weifang People's
Hospital for guidance and assistance with the experiments.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CJ, ZL and YJ collaborated to design the study. CJ,
ZL and CZ were responsible for performing experiments. CJ and ZL
analyzed the data. All authors collaborated to interpret results
and develop the manuscript. The final version of the manuscript has
been read and approved by all authors, and each author believes
that the manuscript represents honest work.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Weifang People's Hospital.
Written informed consent was obtained from all patients or their
families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Court-Brown CM and Caesar B: Epidemiology
of adult fractures: A review. Injury. 37:691–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Court-Brown CM, Garg A and McQueen MM: The
epidemiology of proximal humeral fractures. Acta Orthop Scand.
72:365–371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russo R, Cautiero F, Ciccarelli M and
Vernaglia Lombardi L: Reconstruction of unstable, complex proximal
humeral fractures with the da Vinci cage: Surgical technique and
outcome at 2 to 6 years. J Shoulder Elbow Surg. 22:422–431. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vanden Bossche L and Vanderstraeten G:
Heterotopic ossification: A review. J Rehabil Med. 37:129–136.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barcak EA and Beebe MJ: Bone morphogenetic
protein: Is there still a role in orthopedic trauma in 2017? Orthop
Clin North Am. 48:301–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krishnakumar GS, Roffi A, Reale D, Kon E
and Filardo G: Clinical application of bone morphogenetic proteins
for bone healing: A systematic review. Int Orthop. 41:1073–1083.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dahlin C, Linde A, Gottlow J and Nyman S:
Healing of bone defects by guided tissue regeneration. Plast
Reconstr Surg. 81:672–676. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yilgor P, Hasirci N and Hasirci V:
Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J
Biomed Mater Res A. 93:528–536. 2010.PubMed/NCBI
|
|
9
|
Ishibe T, Goto T, Kodama T, Miyazaki T,
Kobayashi S and Takahashi T: Bone formation on apatite-coated
titanium with incorporated BMP-2/heparin in vivo. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 108:867–875. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murata M, Maki F, Sato D, Shibata T and
Arisue M: Bone augmentation by onlay implant using recombinant
human BMP-2 and collagen on adult rat skull without periosteum.
Clin Oral Implants Res. 11:289–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bae SJ, Kim HJ, Won HY, Min YK and Hwang
ES: Acceleration of osteoblast differentiation by a novel
osteogenic compound, DMP-PYT, through activation of both the BMP
and Wnt pathways. Sci Rep. 7:84552017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song R, Wang D, Zeng R and Wang J:
Synergistic effects of fibroblast growth factor-2 and bone
morphogenetic protein-2 on bone induction. Mol Med Rep.
16:4483–4492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang GP, Zhang J, Zhu CH, Lin L, Wang J,
Zhang HJ, Li J, Yu XG, Zhao ZS, Dong W and Liu GB: MicroRNA-98
regulates osteogenic differentiation of human bone mesenchymal
stromal cells by targeting BMP2. J Cell Mol Med. 21:254–264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laxman N, Mallmin H, Nilsson O and
Kindmark A: miR-203 and miR-320 regulate bone morphogenetic
protein-2-induced osteoblast differentiation by targeting
distal-less homeobox 5 (Dlx5). Genes (Basel). 8(pii): E42016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia W, Wu Y, Zhang Q, Gao GE, Zhang C and
Xiang Y: Expression profile of circulating microRNAs as a promising
fingerprint for cervical cancer diagnosis and monitoring. Mol Clin
Oncol. 3:851–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang XI, Luo Y, Zhao S, Chen Q, Jiang C,
Dai Y, Chen Y and Cao Z: Clinical significance and expression of
microRNA in diabetic patients with erectile dysfunction. Exp Ther
Med. 10:213–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Juzėnas S, Saltenienė V, Kupcinskas J,
Link A, Kiudelis G, Jonaitis L, Jarmalaite S, Kupcinskas L,
Malfertheiner P and Skieceviciene J: Analysis of deregulated
microRNAs and their target genes in gastric cancer. PLoS One.
10:e01323272015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Z, Guo J, Xiao B, Miao Y, Huang R,
Li D and Zhang Y: Increased expression of miR-421 in human gastric
carcinoma and its clinical association. J Gastroenterol. 45:17–23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Xiao B, Zhou F, Deng H, Zhang X,
Lou Y, Gong Z, Du C and Guo J: MiR-421 is a functional marker of
circulating tumor cells in gastric cancer patients. Biomarkers.
17:104–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C,
Dalamagas T and Hatzigeorgiou AG: DIANA-microT web server v5.0:
Service integration into miRNA functional analysis workflows.
Nucleic Acids Res. 41:(Web Server Issue). W169–W173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36:(Database Issue). D149–D153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:(Database Issue). D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Liang SH and Lu X: Potential role of
ezrin and its related microRNA in ovarian cancer invasion and
metastasis. Zhonghua Fu Chan Ke Za Zhi. 45:787–792. 2010.(In
Chinese). PubMed/NCBI
|
|
27
|
Oliveira AC, Bovolenta LA, Nachtigall PG,
Herkenhoff ME, Lemke N and Pinhal D: Combining results from
distinct MicroRNA target prediction tools enhances the performance
of analyses. Front Genet. 8:592017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miranda KC, Huynh T, Tay Y, Ang YS, Tam
WL, Thomson AM, Lim B and Rigoutsos I: A pattern-based method for
the identification of MicroRNA binding sites and their
corresponding heteroduplexes. Cell. 126:1203–1217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
30
|
Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen
T, Chen Z, Huang S, Gu J, Li J, et al: MiR-199a-5p is negatively
associated with malignancies and regulates glycolysis and lactate
production by targeting hexokinase 2 in liver cancer. Hepatology.
62:1132–1144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z and Li W: Surgical treatment of
upper humerus fracture nonunion. Zhongguo Xiu Fu Chong Jian Wai Ke
Za Zhi. 24:673–676. 2010.(In Chinese). PubMed/NCBI
|
|
32
|
Sykaras N and Opperman LA: Bone
morphogenetic proteins (BMPs): How do they function and what can
they offer the clinician? J Oral Sci. 45:57–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gutierrez J, Osses N and Brandan E:
Changes in secreted and cell associated proteoglycan synthesis
during conversion of myoblasts to osteoblasts in response to bone
morphogenetic protein-2: Role of decorin in cell response to BMP-2.
J Cell Physiol. 206:58–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mundy G, Garrett R, Harris S, Chan J, Chen
D, Rossini G, Boyce B, Zhao M and Gutierrez G: Stimulation of bone
formation in vitro and in rodents by statins. Science.
286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Su JL, Chiou J, Tang CH, Zhao M, Tsai CH,
Chen PS, Chang YW, Chien MH, Peng CY, Hsiao M, et al: CYR61
regulates BMP-2-dependent osteoblast differentiation through the
{alpha}v{beta}3 integrin/integrin-linked kinase/ERK pathway. J Biol
Chem. 285:31325–31336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dey D, Wheatley BM, Cholok D, Agarwal S,
Yu PB, Levi B and Davis TA: The traumatic bone: Trauma-induced
heterotopic ossification. Transl Res. 186:95–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muthukumar N: Dural ossification in
ossification of the ligamentum flavum: A preliminary report. Spine
(Phila Pa 1976). 34:2654–2661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mania VM, Kallivokas AG, Malavaki C,
Asimakopoulou AP, Kanakis J, Theocharis AD, Klironomos G, Gatzounis
G, Mouzaki A, Panagiotopoulos E and Karamanos NK: A comparative
biochemical analysis of glycosaminoglycans and proteoglycans in
human orthotopic and heterotopic bone. IUBMB Life. 61:447–452.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qu X, Chen Z, Fan D, Xiang S, Sun C, Zeng
Y, Li W, Guo Z, Qi Q, Zhong W and Jiang Y: Two novel BMP-2 variants
identified in patients with thoracic ossification of the ligamentum
flavum. Eur J Hum Genet. 25:565–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mukai T, Otsuka F, Otani H, Yamashita M,
Takasugi K, Inagaki K, Yamamura M and Makino H: TNF-alpha inhibits
BMP-induced osteoblast differentiation through activating SAPK/JNK
signaling. Biochem Biophys Res Commun. 356:1004–1010. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gerlach CV and Vaidya VS: MicroRNAs in
injury and repair. Arch Toxicol. 91:2781–2797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Garnero P: The utility of biomarkers in
osteoporosis management. Mol Diagn Ther. 21:401–418. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsuzaki J and Ochiya T: Circulating
microRNAs and extracellular vesicles as potential cancer
biomarkers: A systematic review. Int J Clin Oncol. 22:413–420.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chureau C, Chantalat S, Romito A, Galvani
A, Duret L, Avner P and Rougeulle C: Ftx is a non-coding RNA which
affects Xist expression and chromatin structure within the
X-inactivation center region. Hum Mol Genet. 20:705–718. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hao J, Zhang S, Zhou Y, Liu C, Hu X and
Shao C: MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer.
Biochem Biophys Res Commun. 406:552–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Östling P, Leivonen SK, Aakula A, Kohonen
P, Mäkelä R, Hagman Z, Edsjö A, Kangaspeska S, Edgren H, Nicorici
D, et al: Systematic analysis of microRNAs targeting the androgen
receptor in prostate cancer cells. Cancer Res. 71:1956–1967. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu H, Du L, Nagabayashi G, Seeger RC and
Gatti RA: ATM is down-regulated by N-Myc-regulated microRNA-421.
Proc Natl Acad Sci USA. 107:1506–1511. 2010. View Article : Google Scholar : PubMed/NCBI
|