Introduction
The ‘gold standard’ for treatment of myocardial
infarction is to immediately reperfuse the occluded coronary artery
(1). However, this treatment leads
to myocardial ischemia/reperfusion (I/R) injury (MIR), in which
blood perfusion has been restored following a long period of
ischemia and results in such dysfunctions as systolic function
decline, coronary flow and vascular reactivity variations (2). As MIR serves an important role in
developing heart diseases, the mechanism of MIR has been widely
investigated (3–5). It is a complex phenomenon and maybe
caused by calcium overload, endothelial cell activation,
mitochondrial damage, the formation of reactive oxygen species
(ROS), dysregulated vascular relaxation and white cell plug
formation (6). The treatment for MIR
is limited due to the lack of comprehensive understanding of the
pathological process during ischemia/reperfusion (I/R) (7,8).
Therefore, there is a pressing need to elucidate the underlying
mechanisms of MIR.
microRNAs (miRNAs or miRs) are small (19–24
nucleotides), endogenous, non-coding RNAs that have an effect on
gene expression at the transcriptional or post-transcriptional
level through binding to the complementary 3′-untranslated region
(3′-UTR) of their target mRNAs (9–11).
miRNAs have been demonstrated to affect processes
such as cell apoptosis, glucose and lipid metabolism, signal
transduction (12). For instance,
miR-146b prevents cardiomyocyte injury in myocardial I/R by
targeting mothers against decapentaplegic homolog 4 (13). miR-93 inhibits I/R-induced
cardiomyocyte apoptosis by targeting phosphatase and tensin homolog
(PTEN) (14). E2F1-dependent miR-421
regulates mitochondrial fragmentation and myocardial infarction by
targeting PTEN-induced putative kinase 1 (15).
miR-144 has been demonstrated to be dysregulated
during tumorigenesis, development, and metastasis of various
cancers including lung cancer, oral squamous cell carcinoma and
breast cancer (16–18), suggesting its potential role in tumor
diagnosisand treatment. However, the effect of miR-144 on ischemic
injury hasrarely been reported.
In the present study, the alteration of miR-144
levels was studied in a rat I/R model and a cardiomyocyte
hypoxia/reoxygenation (H/R) model, and it was observed that miR-144
was notably downregulated in I/R and H/R. Overexpression of miR-144
constrained the infarction size and myocardial apoptosis that
results from I/R. In addition, miR-144 inhibited apoptosis in
cardiomyocytes under H/R treatment. Furthermore, miR-144 exerted
this protective effect through regulation of apoptotic gene
expression.
Materials and methods
Cell culture and transfection
H9c2 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, 100
µg/ml streptomycin and 110 mg/ml sodium pyruvate under a humidified
air condition of 5% CO2 at 37°C. A total of
~3×105 cells were seeded into each well of a 12-well
plate, incubated overnight, and subsequently transfected with 200
nM miR-144 mimics (Forward, TACAGTATAGATGATGTACT; Reverse,
AGTACATCATCTATACTGTA) and matched scrambled control (Forward,
ATCATGCGTAGCTGACGTGA; Reverse, TCACGTCAGCTACGCATGAT) (Shanghai
GenePharma Co., Ltd., Shanghai, China) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer'sprotocol. In the
rescue experiment part, cells were transfected with 200 nM miR-144
mimic or scramble together with 300 nM FOXO1 overexpression vector
or negative control vector.
In vivo gene transfer in the rat model
of I/R injury
A total of 24 Sprague-Dawley male rats (weight:
250–300 g; age, 8–10 weeks) were obtained from the Animal Center of
Central South University (Changsha, China) and housed in an animal
holding facility under standard light (12-h light/dark cycle),
temperature (22.0±0.5°C) and humidity (55–60%). Rats received
standard chow and water ad libitum. The present study was
approved by the Renmin Hospital of Wuhan University Animal Care and
Use Committee (Wuhan, China). Rats were randomly divided into four
different groups (each, n=6): Sham (served as controls), I/R,
I/R+miR-144 and miR-144 treatment groups. Rats were anesthetized
with 40 mg/kg pentobarbital (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) intraperitoneally and placed on a temperature-controlled
heating pad and a left thoracotomy was performed in the fourth
intercostal space. A 100-µl solution of adenovirus (Ad)-miR-144
with DMEM (1×109; plaque forming unit, PFU) or
Ad-Scramble (1×109 PFU) were injected into six sites on
the left ventricular anterior wall. The chest cavity was closed
following this administration, and the rats were allowed to
recover. Three days later, all rats were anesthetized again with 40
mg/kg pentobarbital and ventilated artificially. Subsequently, the
left anterior descending coronary artery (LAD) was exposed, and 6-0
silk sutures were used to ligate the LAD. Cyanosis in the anterior
ventricular wall was used to confirm the ligation. Ischemia was
induced using a small vinyl tube that was threaded through the
ligature. Following 30 min of ischemia, tubes were translocated,
and coronary circulation was restored for 2 h. Subsequently, the
infarct area of myocardium tissues and the blood samples were
collected for the following analysis. Sham control animals were
subjected to the entire surgical procedure without LAD
ligation.
H/R model
At 48 h following transfection with miR-144 mimic or
scramble, H9c2 cell hypoxia was induced by treating the cells in a
modular incubator (Forma; Thermo Fisher Scientific, Inc.) in an
atmosphere containing 1% O2, 94% N2 and 5%
CO2 for 24 h at 37°C. Subsequently, the cells were
incubated for 3 h in DMEM with 10% FBS at 37°C in an atmosphere
containing 5% CO2.
Infarct size determination
Evans blue/triphenyltetrazolium chloride (TTC) was
used to stain and measure the myocardial infarct size. Immediately
after reperfusion ended, Evans blue dye solution (3 ml, 2% wt/vol)
was injected into the left ventricle to identify areas at risk
(AARs) at room temperature for 2 h. Immediately following
reperfusion, rats were sacrificed via exsanguination which were
anesthetized as aforementioned. The hearts were harvested and were
frozen for 30 min at −20°C. Subsequently, the left ventricle was
sliced transversely at a thickness of 1 mm. These slices were
immediately injected with 1% TTC (Sigma-Aldrich; Merck KGaA) to
detect ischemic and infarcted tissue at 37°C. The infarcted areas
are those that were not stained and were regarded as non-viable,
whereas the non-infarcted areas that were stained were regarded as
viable. The AAR and infarct size were measured using imageJ 1.48
software (National Institutes of Health, Bethesda, MD, USA).
LDH (lactate dehydrogenase) and CK
(creatine kinase) activity assay
Following reperfusion, blood was obtained from the
carotid artery of rats, and stored at room temperature for 30 min.
Serum was subsequently collected by centrifugation at 5,000 × g for
10 min at 4°C. CK (cat. no. A032) and (cat. no. A020-1) LDH assay
kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
were used to measure activity of CK and LDH according to the
manufacturer's protocols.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
TUNEL staining was used to evaluate cardiomyocyte
apoptosis. Heart tissue fixed in 4% formaldehyde at room
temperature for 24 h and was cut into 4-µm slices following
washing, dehydrating, and immersing in paraffin. The apoptotic
cardiomyocytes were detected using an In situ cell death
detection kit (Roche Diagnostics, Basel, Switzerland). Cells with
brown stained nuclei indicated apoptotic cells and were counted in
5 microscopic fields of view under fluorescence microscopy. The
ratio of TUNEL positive cells to total cardiomyocytes was
calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from H9c2 cells and rat
myocardium using TriZOL reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer'sprotocol. The cDNA
was synthesized using 1 µl reverse transcriptase, 2 µl dNTPs, 5 µl
buffer (TAKARA BIO INC, Dalian, China), a stem-loop RT primer
(Invitrogen; Thermo Fisher Scientific, Inc.) and 2 µg RNA, the
temperature protocol was as follows: 42°C for 30 min and 85°C for 5
min. The relative amount of miRNA was detected using a SYBR Green
Realtime PCR Master Mix (Takara Biotechnology Co., Ltd., Dalian,
China). The primers utilized were as follows: MiR-144, forward:
CCTCGCACCTGGAGGCTGGCTG reverse: TTATCAGTTGGGAAAATAGTTA; U6,
forward: CTCGCTTCGGCAGCACA reverse: AACGCTTCACGAATTTGCGT. The
thermocycling conditions consisted of an initial, single cycle of 2
min at 94°C, followed by 40 cycles of 15 sec at 94°C, 20 sec at
63°C and 30 sec at 68°C. The 2−ΔΔCq method (19) was utilized for measuring expression
levels, and the U6 small nuclear RNA was used as an internal
reference.
Western blot analysis
Liquid nitrogen was used to freeze tissue samples,
and protein was extracted following lysis with RIPA lysis buffer
(BioTeke Corporation, Beijing, China) containing 1 mM
phenylmethylsulfonyl fluoride. A bicinchoninic acid assay kit
(Beyotime Institute of Biotechnology, Haimen, China) was used to
evaluate the concentration of protein. Subsequently, 30 µg protein
was separated by 10% SDS-PAGE and electrophoretically transferred
to polyvinylidene fluoride membranes. The membranes were blocked in
5% non-fat milk in TBSTween-20 buffer (100 mM NaCl, 10 mM Tris-HCl;
pH 7.4; 0.1% Tween-20) for 2 h at room temperature. Subsequently,
the membranes were incubated at 4°C overnight with primary
antibodies (all 1:200; Abcam, Cambridge, UK) against forkhead box
protein O1 (FOXO1; cat. no. ab39670), Bcl-2 associated X protein
(Bax; cat. no. ab182733), B-cell lymphoma 2 (bcl-2; cat. no.
ab196495), cytochrome c (cat. no. ab18738), caspase9 (cat.
no. ab184786), caspase3 (cat. no. ab32042) or GAPDH (cat. no.
ab181602). After washing with PBS, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibody for 2 h
at room temperature. Protein bands were detected with an enhanced
chemiluminescence detection kit (cat. no. 631701; Pierce; Thermo
Fisher Scientific, Inc.) and ImageJ software 1.48 (National
Institutes of Health).
Flow cytometry
Cell apoptosis was evaluated using a flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA) and Annexin V-FITC
Apoptosis Detection kit to determine the percentage of apoptotic
cells. A total of ~106 cells were suspended in 200 µl
binding buffer, and 10 µl Annexin V-fluorescein isothiocyanate and
5 µl propidium iodide (all BD Biosciences) were added to the cells
and subsequently incubated in the dark for 30 min at room
temperature. Cell apoptosis was evaluated using flowjo 7.6.1
software (BD Biosciences).
Bioinformatics analysis
To elucidate the mechanism by which miR-144 inhibits
I/R injury, two publicly available bioinformatics tools, miRanda
(www.microrna.org/) and TargetScan (http://www.targetscan.org/vert_71/), were
searched for genes containing potential binding sites for miR-144
in their 3′-UTRs.
Dual-luciferase reporter assay
The putative wild and mutant miR-144 binding
sequence from the 3′UTR segment of FOXO1 mRNA was cloned into the
p-GL3-basic luciferase reporter plasmid (Hanbio Biotechnology Co.,
Ltd., Shanghai, China). H9c2 cells were transfected with 200 nM
miR-144 ASO (inhibitor of miR-144; 5′-AGTACATCATCTATACTGTA-3′) or
mimic with a reporter gene p-GL3-basic containing wild-type or
mutated 3′UTR sequence of FOXO1 as aforementioned. At 48 h
following transfection, the cells were harvested, and the
luciferase activity was measured using a Dual Luciferase Reporter
Gene Assay kit (Promega Corporation, Madison, WI, USA).
Renilla luciferase activity was used to normalize the
firefly luciferase intensity.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One-way analysis of variance with a post-hoc Tukey's test
and two-tailed Student's t-test were utilized to analyze the
differences between groups, and P<0.05 was considered to
indicate a statistically significant difference. Analysis was
performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
All experiments were performed in triplicate.
Results
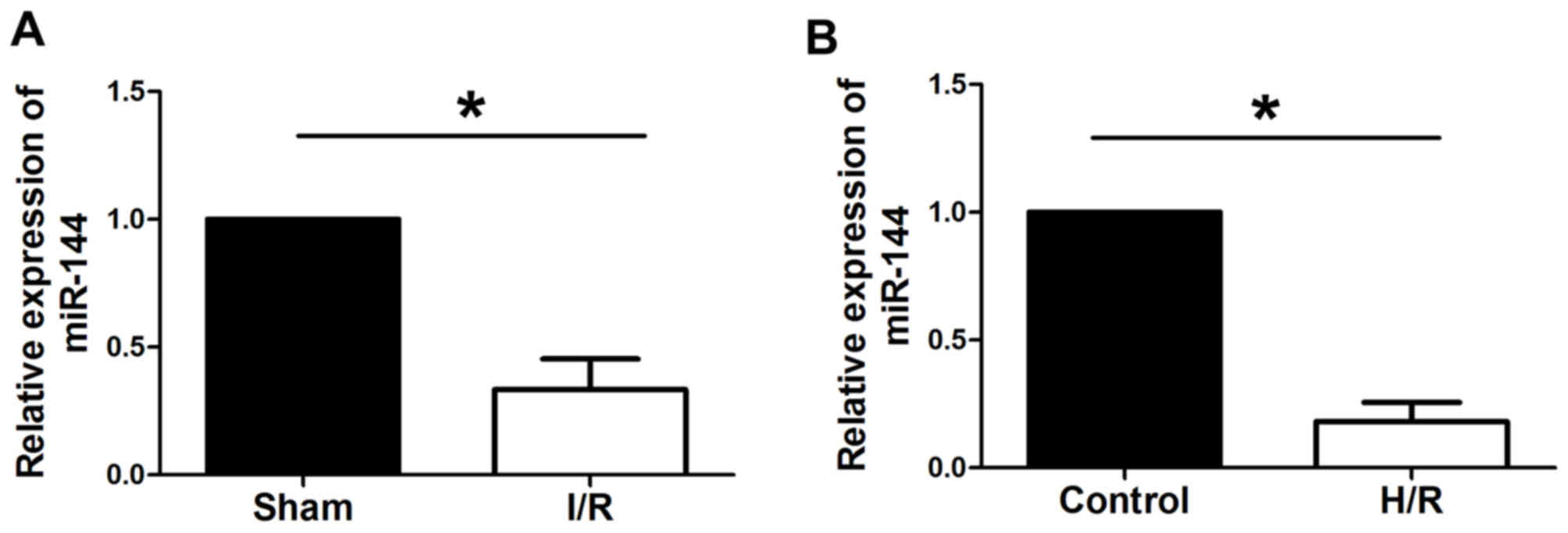
miR-144 is downregulated in I/R and
H/R
RT-qPCR was used to evaluate the expression levels
of miR-144 in I/R myocardium and in H9c2 cells subjected to H/R. As
presented in Fig. 1, miR-144 was
significantly downregulated in myocardial I/R rats comparedwith
sham rats and in H9c2 cells subjected to H/R compared with control
group cells.
miR-144 reduces myocardial infarct
size and apoptosis in I/R rats
To explore the importance of miR-144 in myocardial
I/R injury, the adenovirus-mediated miR-144 transfer was performed
via injection into six sites in the left ventricular anterior wall
of the rats. The infarct area of hearts was measured using Evans
blue and TTC staining. The ischemic area (IA)/AAR ratio was
markedlyincreased in the I/R group compared with the sham group,
demonstrating the success of I/R establishment (Fig. 2A). Overexpression of miR-144
following ischemia treatment significantly reduced the IA/AAR
comparedwith the sham group (Fig. 2A and
B). TUNEL staining was performed in cardiac isografts from each
experimental group. Compared with the I/R group, Ad-miR-144
administration significantly reduced the number of TUNEL-positive
cardiomyocytes (Fig. 2C and D). In
addition, CK and LDH activity were measured, which indicates
oxidative stress-induced injury. Overexpression of miR-144 resulted
in significantly decreased activity of CK and LDH compared with the
I/R group (Fig. 2E and F).
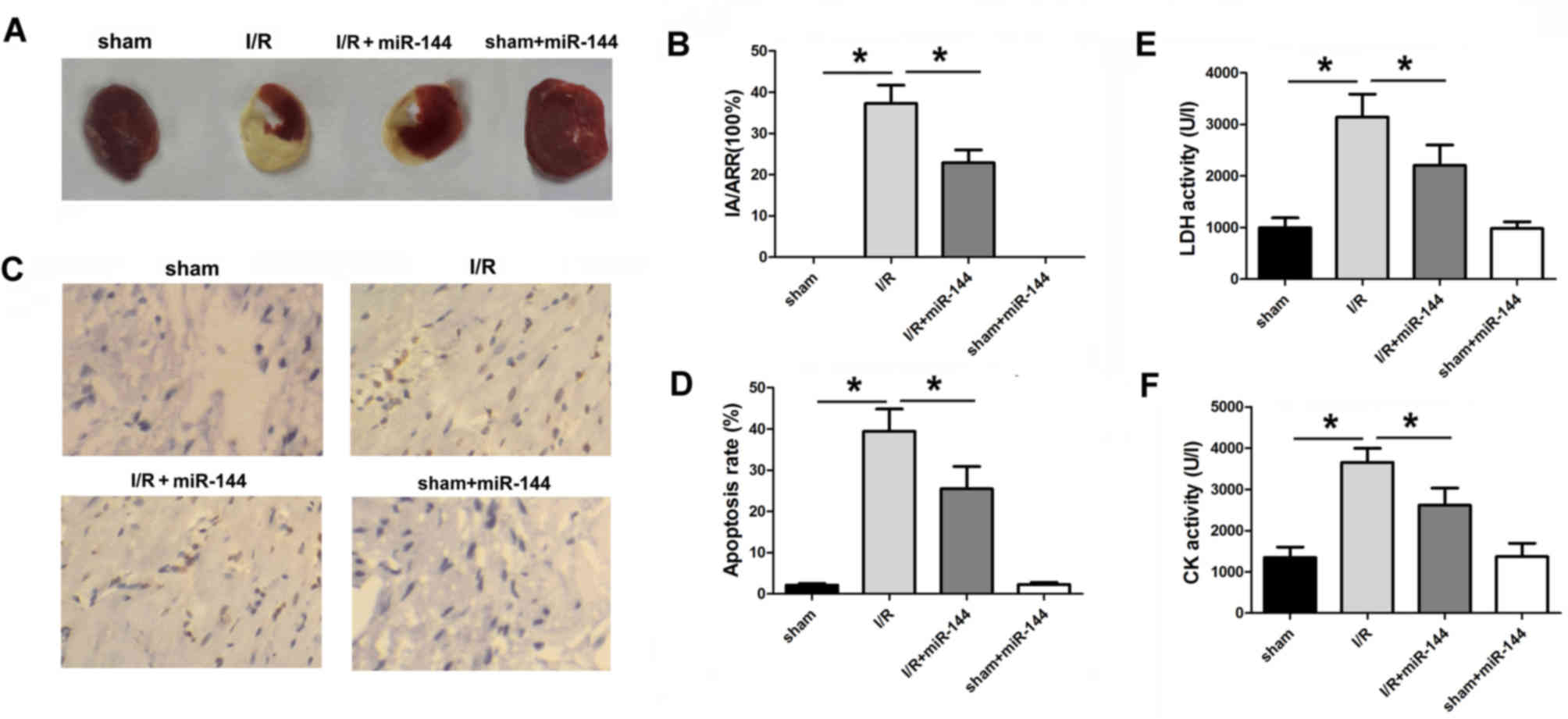 | Figure 2.miR-144 induces a cardioprotective
effect in vivo. (A) TTC and Evans blue-stained heart slices
from the sample. (B) Infarct size was measured by TTC and Evans
blue staining (F=167.992, df=3). (C) The TUNEL assay was applied to
evaluate the apoptosis in the myocardium (magnification, ×200). (D)
Percentage of TUNEL-positive-stained cardiomyocytes was calculated
to determine apoptosis rate (F=92.326, df=3). (E) LDH activity in
serum was measured (F=148.563, df=3). (F) CK activity in serum was
measured (F=65.462, df=3). Data are presented as the mean ±
standard error of the mean (n=6). *P<0.01. miR, microRNA; TTC,
triphenyltetrazolium chloride; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling; LDH,
lactate dehydrogenase; CK, creatine kinase; df, degrees of freedom;
I/R, ischemia/reperfusion. |
miR-144 regulates apoptotic protein
expression
The expression of apoptosis-related proteins was
subsequently assessed. Compared with the sham group, the
anti-apoptotic protein Bcl-2 was downregulated in the I/R group and
apoptotic proteins, Bax, cytochrome c, caspase9 and
caspase3, were all significantly upregulated in the I/R group
(Fig. 3). To explore whether miR-144
administration without I/R treatment would lead to the changes
mentioned above, Ad-miR-144 was administered following sham
treatment. The results revealed that miR-144 administration in the
absence of I/R did not have any significant effect on the IA/AAR,
myocardial apoptosis, CK and LDH activity or apoptotic proteins
expression, in comparison with the sham group (Figs. 1–3).
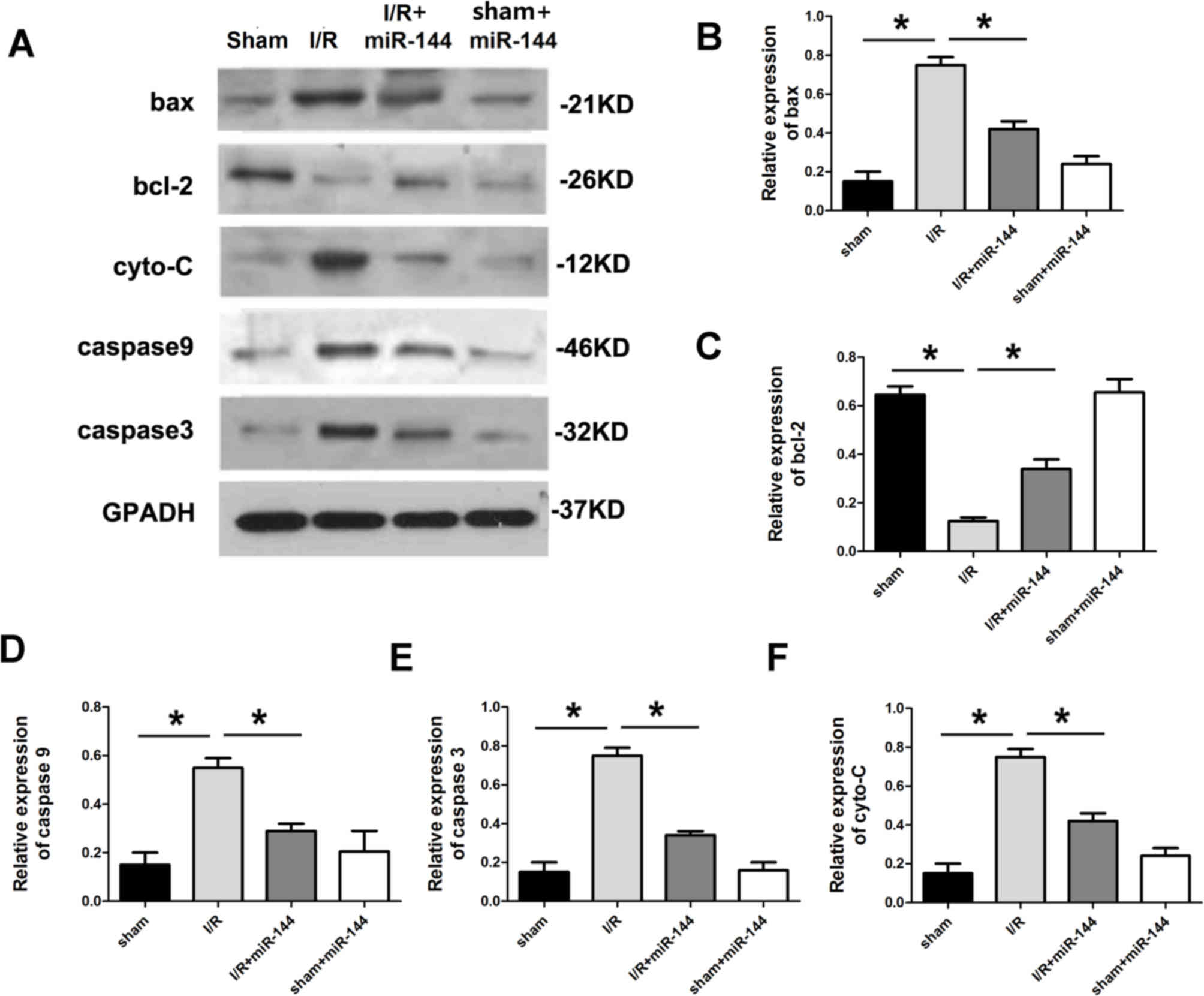 | Figure 3.miR-144 regulated apoptotic protein
expression. (A) Western blot analysis showing the relative
apoptotic protein levels including (B) Bax (F=32.352, df=3), (C)
Bcl-2 (F=22.374, df=3), (D) caspase-9 (F=28.542, df=3), (E)
caspase-3 (F=20.347, df=3) and (F) cyto-C (F=39.458, df=3) in the
myocardium. Data are presented as the mean ± standard error of the
mean (n=6). *P<0.01. miR, microRNA; Bcl-2, B cell lymphoma-2;
Bax, Bcl-2 associated X protein; cyto-C, cytochrome c; df,
degrees of freedom, I/R, ischemia reperfusion. |
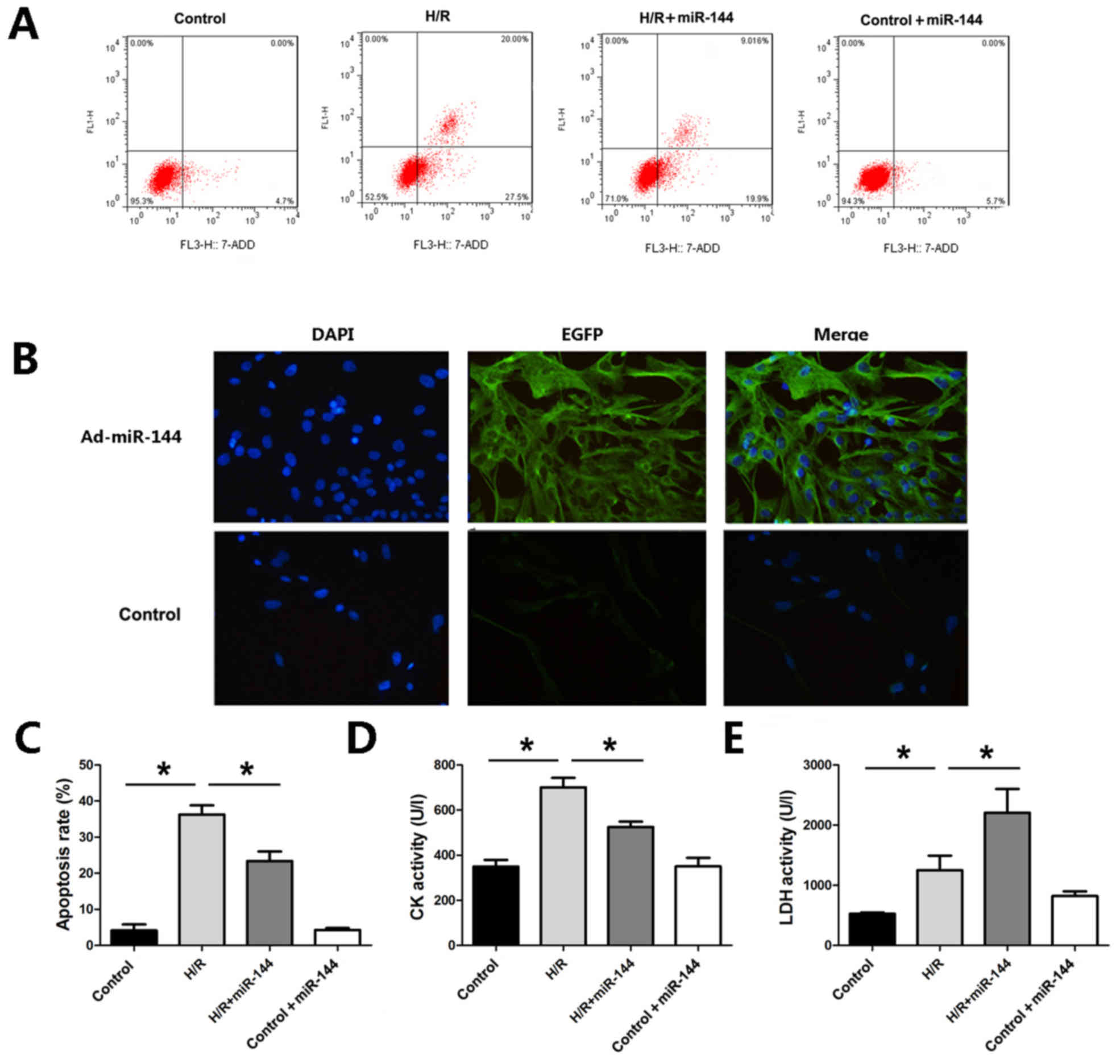
miR-144 attenuates H9c2 cell
apoptosis
To further evaluate the effect of miR-144 on
myocardial infarction protection, an H/R model was established in
H9c2 cells. Flow cytometry was used to assess apoptosis in H9c2
cells. As presented in Fig. 4A,
induction of H/R resulted in a marked increase in apoptosis in H9c2
cells, whereas Ad-miR-144 treatment decreased the apoptosis rates
in comparison with the H/R group (Fig.
4A and B). Furthermore, miR-144 overexpression significantly
attenuated CK and LDH activities, which were significantly elevated
by the H/R treatment, as compared to the control group (Fig. 4C-E). To explore whether miR-144
administration without H/R treatment would lead to any of these
findings, Ad-miR-144 was administered in the control H9c2 cells.
The results revealed that miR-144 administration did not result in
any significant changes in the cell apoptosis, CK or LDH activity
in the absence of H/R, compared with the control group.
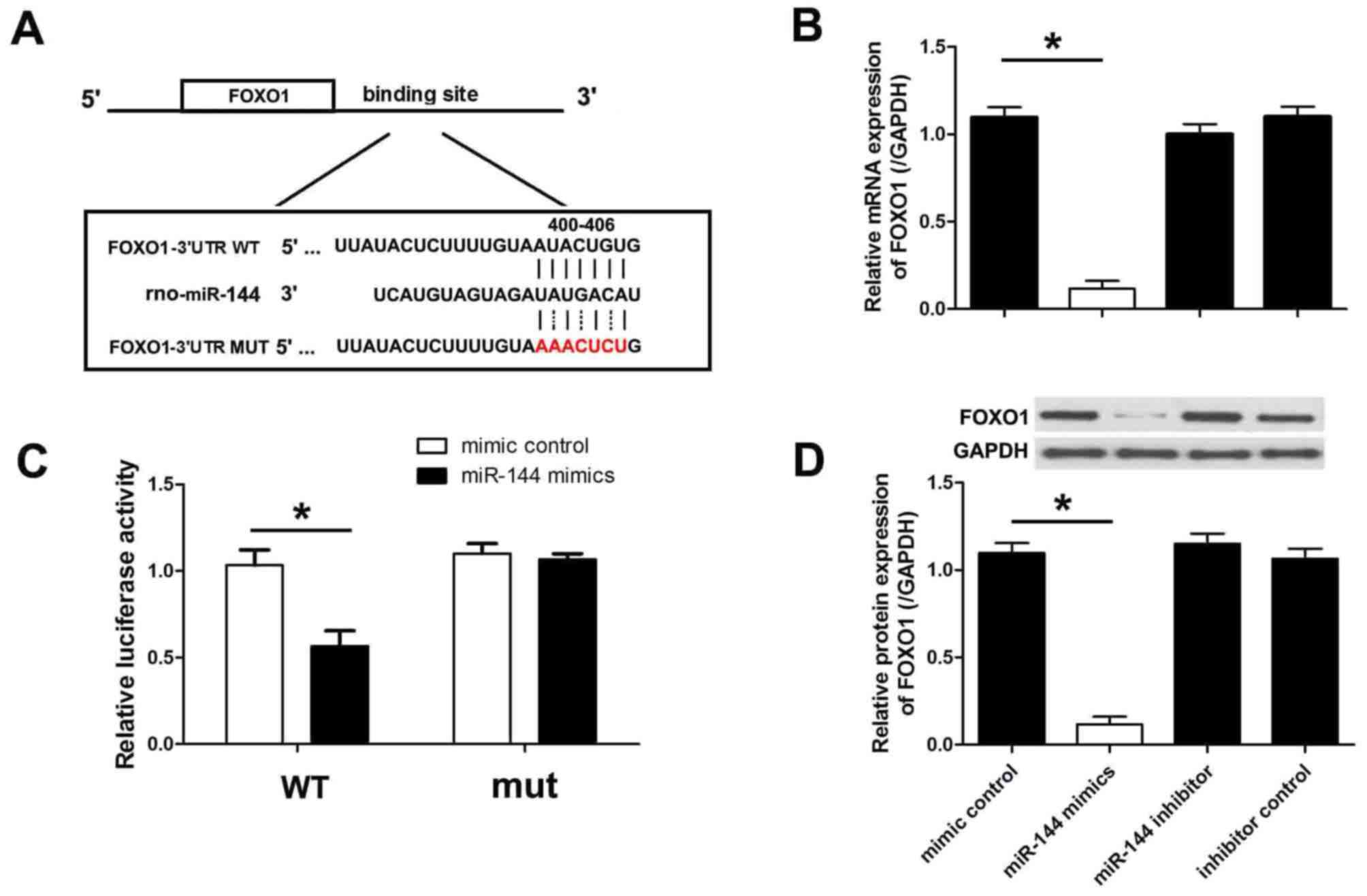
miR-144 directly targets FOXO1
The levels of FOXO1 were analyzed by MiRanda and
TargetScan, and the results indicated that FOXO1 is targeted by
miR-144. The 3′-UTR of the FOXO1 mRNA has a binding site for
miR-144. To verify this binding, the putative miR-144 binding
sequence from the 3′UTR segment of FOXO1 mRNA was cloned into the
luciferase reporter plasmid. An additional luciferase reporter
vector was constructed with a mutation in the miR-144 binding site
(Fig. 5A). H9c2 cells were
co-transfected with miR-144 mimics or negative control miRNA for 48
h, and luciferase activity was subsequently measured. It was
observed that miR-144 mimics significantly inhibited luciferase
activity whereas the negative control miRNA did not (Fig. 5B). Both miR-144 and negative control
did not change the luciferase activity when co-transfected with
mutant FOXO1. To demonstrate that miR-144 targets FOXO1 and
inhibits endogenous FOXO1 expression, the protein levels of FOXO1
were measured in H9c2 cells transfected with miR-144 mimics or the
negative control. As presented in Fig.
5C and D, transfection with miR-144 mimics significantly
downregulated the mRNA and protein levels of FOXO1.
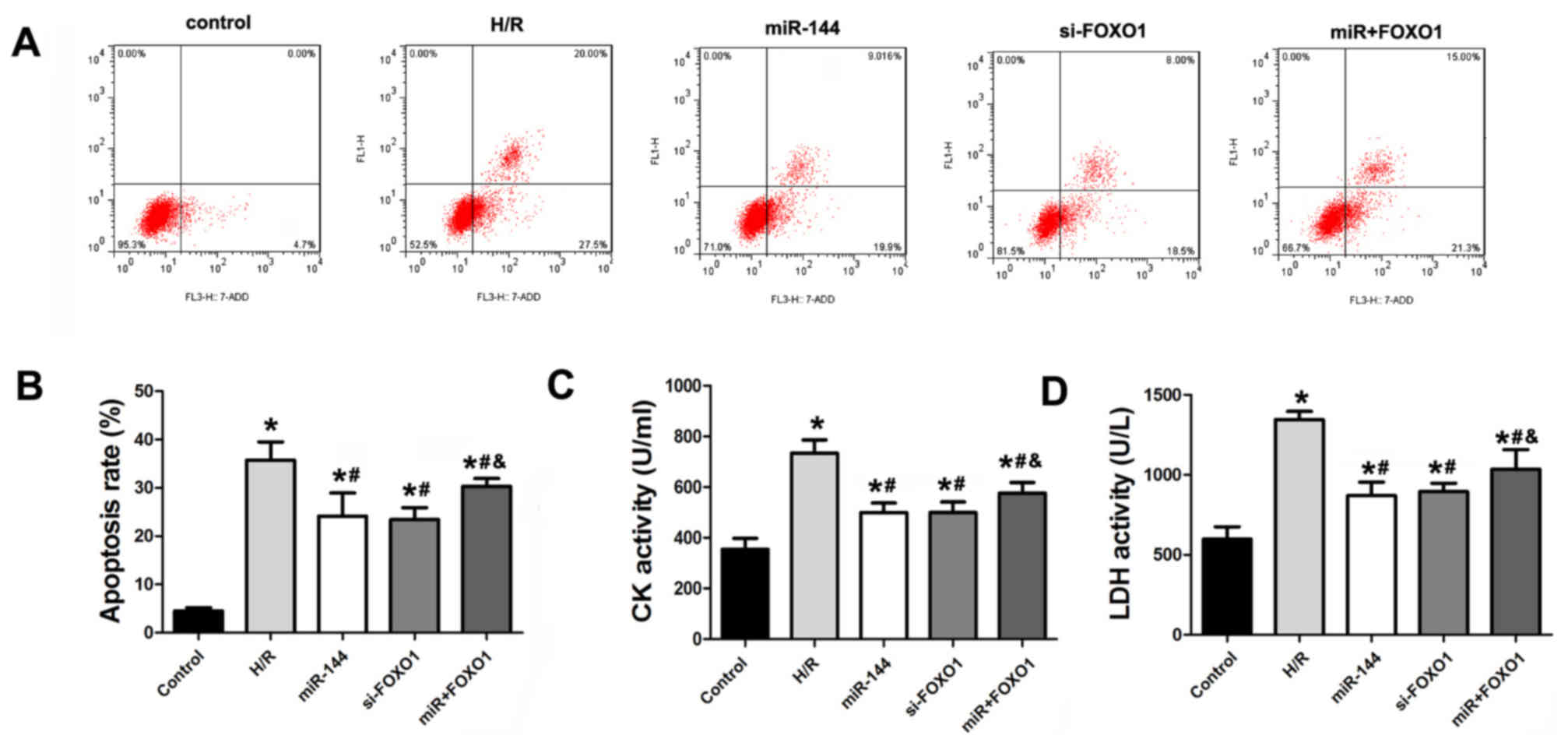
FOXO1 overexpression reverses the
effect of miR-144 on cardiomyocyte injury
To demonstrate the assumption that miR-144 targets
FOXO1, cardiomyocytes were transfected with 100 nM miR-144 mimics
and a FOXO1 overexpression vector to ectopically increase miR-144
and FOXO1 levels. Overexpression of FOXO1 significantly reversed
the apoptosis-inhibiting effect of miR-144 (Fig. 6A and B). In addition, the activity of
CK and LDH were measured in these cells. Consistently, FOXO1
overexpression reversed the significantly increased activities of
CK and LDH when compared with the miR-144 treatment group, which
indicates that expression was decreased by miR-144 (Fig. 6C and D).
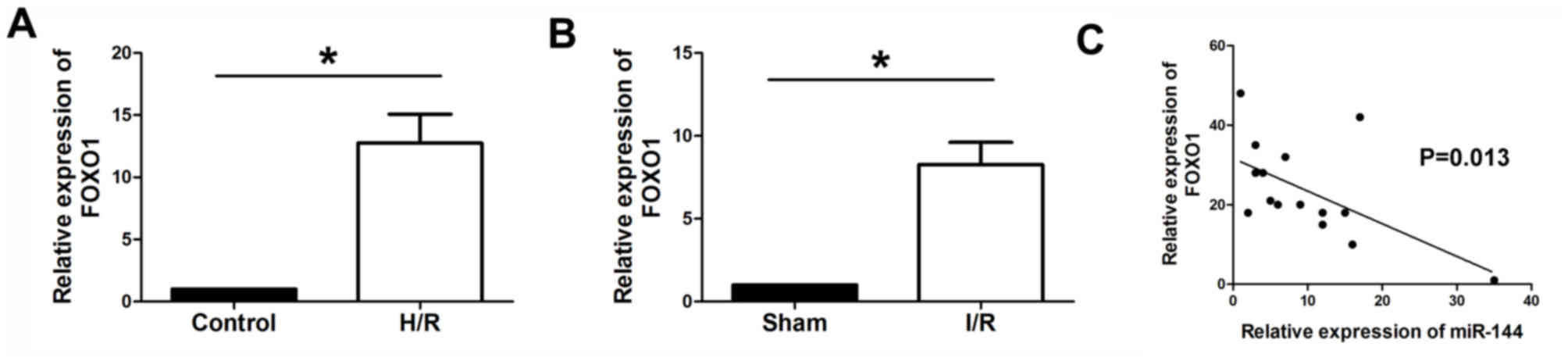
FOXO1 is upregulated in myocardium and
H9c2 cells, and is inversely correlated with miR-144
expression
RT-qPCR was performed to determine the expression
levels of FOXO1 in myocardium and H9c2 cells. The results
demonstrated that FOXO1 was significantly upregulated in the
H9c2 cells subjected to H/Rand I/R myocardium, compared with the
control and sham groups, respectively (Fig. 7A and B). In addition, Pearson
analysis was performed to evaluate the correlation between miR-144
and FOXO1. It was observed that the expression level of
FOXO1 was inversely correlated with the expression level of
miR-144 in myocardium tissues (Fig.
7C).
Discussion
Previous studies have identified miR-144 as a
circulating effector of rIPC-induced cardioprotection (20). Upregulation of the miR-144/451
cluster is correlated with cardioprotection against hypoxic stress
through the CUGBP, Elav-like family member 2-cyclooxygenase-2
signal pathway (21). These findings
have indicated the potential role of miR-144 in
cardioprotection.
In the present study, miR-144 expression levels were
measured in ischemic hearts using a well-established I/R model
in vivo and in vitro. It was observed that miR-144
was dysregulated. In addition, overexpression of miR-144 reduced
the infarct size and cardiomyocyte apoptosis, whereas depletion of
miR-144 increased the sensitivity to I/R in cell apoptosis. These
results confirmed that miR-144 was a positive regulator of
cardioprotection following I/R injury.
Apoptosis is a type of cell death that is associated
with morphological problems and is significantly associated with
nuclear pyknosis and karyorhexis (22). Cardiomyocyte apoptosis serves a key
role in ischemic injuries. Increasing evidence has indicated that
apoptosis caused by I/R results in myocyte injury and that
suppressing cardiomyocyte apoptosis leads to cardioprotection
against I/R injury (23,24). miR-144 has primarily been reported to
affect the apoptosis process in human cancers. For instance,
miR-144-3p has been reported to lead to apoptosis inhuman salivary
adenoid carcinoma cells by targeting mechanistic target of
rapamycin (14). In addition,
miR-144 is able to induce apoptosis and autophagy in lung cancer
cells by targeting TP53-inducible glycolysis and apoptosis
regulator, and zinc finger X-linked, and in glioblastoma by
targeting c-Met (15–17).
miRNAs elicit their function via
post-transcriptional regulation of target mRNAs. Computational
analysis was utilized to predict the potential gene targets.
miR-144 was predicted to target various genes in many other kinds
of cells (25,26). However, these predicted targets must
be experimentally verified as gene expression, as well as cellular
functions, have specificity in different cell types. In the present
study, FOXO1 was predicted to be a target of miR-144 in
cardiomyocytes. As predicted, a luciferase activity assay verified
that miR-144 directly targeted FOXO1.
FOXO1 is an important forkhead transcription factor,
which is influential in the regulation of cellular metabolism,
proliferation, and cell death (27).
Hosaka et al (28) previously
reported that the FOXO1 gene affects the development of the
cardiovascular system. It was also reported that FOXO1 mediates
cardiomyocyte apoptosis by activating the expression of inducible
nitric oxide synthase (29,30). In the cardiovascular system, sirtuin
1 may protect the ischemia-stressed cardiomyocytes from apoptosis
by regulating FOXO1 (31).
Similarly, any defect of FOXO1 that exists in mouse cardiomyocytes
may contribute to decreased myocardial function following acute I/R
by increasing oxidative damage (26). Furthermore, apelin improves
cardiomyocyte viability and compensates excessive
mitochondria-derived ROS formation via the FOXO1 pathway in obese
mice (18). FOXO1 was reported to be
targeted by numerous miRNAs, such as miR-181a, miR-3188, miR-9 and
miR-155, particularly in human cancers (32–35). The
present study assessed the effect of miR-144 and FOXO1 on
ischemia/reperfusion injury; however, the precise mechanism of this
effect was not elucidated. Thus, it may be necessary to perform
transgenic animals studies in the future.
It was demonstrated in the present study that
miR-144, a significantly upregulated miRNA in H/R myocardial cells,
serves a crucial role in H/R apoptosis in myocardial cells by
regulating FOXO1. Based on these findings, it appears that miR-144
may serve as a promising target for the prevention of myocardial
I/R injury, although further study of the in vivo effect is
required to fully elucidate the functional consequences of this
miRNA.
Acknowledgements
Not applicable.
Funding
Funding was provided by the Natural Science
Foundation of Inner Mongolia autonomous region (funding no.
2016MS0867).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LE contributed to the completement of the
experiments and data analysis. HJ was a contributor in analysing
the data and writting the manuscript. ZL contributed to the
experimental design and manuscript revision.
Ethics approval and consent to
participate
The present study was approved by the Renmin
Hospital of Wuhan University Animal Care and Use Committee (Wuhan,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dorweiler B, Pruefer D, Andrasi TB, Maksan
SM, Schmiedt W, Neufang A and Vahl CF: Ischemia-reperfusion injury:
Pathophysiology and clinical implications. Eur J Trauma Emerg Surg.
33:600–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang JX, Zhang XJ, Li Q, Wang K, Wang Y,
Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, et al: MicroRNA-103/107
regulate programmed necrosis and myocardial ischemia/reperfusion
injury through targeting FADD. Circ Res. 117:352–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Wen S, Yao X, Liu W, Shen J, Deng W,
Tang J, Li C and Liu K: MicroRNA-378 protects against intestinal
ischemia/reperfusion injury via a mechanism involving the
inhibition of intestinal mucosal cell apoptosis. Cell Death Dis.
8:e31272017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang B, Li W, Xi W, Yi Y, Ciren Y, Shen H,
Zhang Y, Jiang H, Xiao J and Wang Z: Hydrogen sulfide protects
cardiomyocytes against apoptosis in ischemia/reperfusion through
MiR-1-regulated histone deacetylase 4 pathway. Cell Physiol
Biochem. 41:10–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rakotovao A, Tanguy S, Toufektsian MC,
Berthonneche C, Ducros V, Tosaki A, de Leiris J and Boucher F:
Selenium status as determinant of connexin-43 dephosphorylation in
ex vivo ischemic/reperfused rat myocardium. J Trace Elem Med Biol.
19:43–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barandier C, Tanguy S, Pucheu S, Boucher F
and De Leiris J: Effect of antioxidant trace elements on the
response of cardiac tissue to oxidative stress. Ann N Y Acad Sci.
874:138–155. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valinezhad Orang A, Safaralizadeh R and
Kazemzadeh-Bavili M: Mechanisms of miRNA-mediated gene regulation
from common downregulation to mRNA-specific upregulation. Int J
Genomics. 2014:9706072014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bier A, Giladi N, Kronfeld N, Lee HK,
Cazacu S, Finniss S, Xiang C, Poisson L, deCarvalho AC, Slavin S,
et al: MicroRNA-137 is downregulated in glioblastoma and inhibits
the stemness of glioma stem cells by targeting RTVP-1. Oncotarget.
4:665–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang WB, Chen PH, Hsu T I, Fu TF, Su WC,
Liaw H, Chang WC and Hung JJ: Sp1-mediated microRNA-182 expression
regulates lung cancer progression. Oncotarget. 5:740–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holik AK, Lieder B, Kretschy N, Somoza MM,
Held S and Somoza V: N(ε)-Carboxymethyllysine increases the
expression of miR-103/143 and enhances lipid accumulation in 3T3-L1
cells. J Cell Biochem. 117:2413–2422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di YF, Li DC, Shen YQ, Wang CL, Zhang DY,
Shang AQ and Hu T: MiR-146b protects cardiomyocytes injury in
myocardial ischemia/reperfusion by targeting Smad4. Am J Transl
Res. 9:656–663. 2017.PubMed/NCBI
|
|
14
|
Ke ZP, Xu P, Shi Y and Gao AM: MicroRNA-93
inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by
targeting PTEN. Oncotarget. 7:28796–28805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Zhou LY, Wang JX, Wang Y, Sun T,
Zhao B, Yang YJ, An T, Long B, Li N, et al: E2F1-dependent miR-421
regulates mitochondrial fragmentation and myocardial infarction by
targeting Pink1. Nat Commun. 6:76192015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Xu Z and Wang L: Shuanghuang
Shengbai granule cures myelosuppression and suppresses lung cancer
progression: Mechanism and therapeutic targets from the aspect of
microRNAs. Oncotarget. 8:62154–62166. 2017.PubMed/NCBI
|
|
17
|
Manikandan M, Deva Magendhra Rao AK,
Arunkumar G, Manickavasagam M, Rajkumar KS, Rajaraman R and
Munirajan AK: Oral squamous cell carcinoma: microRNA expression
profiling and integrative analyses for elucidation of
tumourigenesis mechanism. Mol Cancer. 15:282016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madhavan D, Peng C, Wallwiener M, Zucknick
M, Nees J, Schott S, Rudolph A, Riethdorf S, Trumpp A, Pantel K, et
al: Circulating miRNAs with prognostic value in metastatic breast
cancer and for early detection of metastasis. Carcinogenesis.
37:461–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Rohailla S, Gelber N, Rutka J, Sabah
N, Gladstone RA, Wei C, Hu P, Kharbanda RK and Redington AN:
MicroRNA-144 is a circulating effector of remote ischemic
preconditioning. Basic Res Cardiol. 109:4232014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu
WT, Jegga AG and Fan GC: Synergistic effects of the GATA-4-mediated
miR-144/451 cluster in protection against simulated
ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell
Cardiol. 49:841–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Z, Ye B, Dai Z, Wu X, Lu Z, Shan P
and Huang W: Curcumin inhibits autophagy and apoptosis in
hypoxia/reoxygenation-induced myocytes. Mol Med Rep. 11:4678–4684.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin Y, Guan Y, Duan J, Wei G, Zhu Y, Quan
W, Guo C, Zhou D, Wang Y, Xi M and Wen A: Cardioprotective effect
of Danshensu against myocardial ischemia/reperfusion injury and
inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2
phosphorylation. Eur J Pharmacol. 699:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hadi NR, Yusif FG, Yousif M and Jaen KK:
Both castration and goserelin acetate ameliorate myocardial
ischemia reperfusion injury and apoptosis in male rats. ISRN
Pharmacol. 2014:2069512014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pase L, Layton JE, Kloosterman WP,
Carradice D, Waterhouse PM and Lieschke GJ: miR-451 regulates
zebrafish erythroid maturation in vivo via its target gata2. Blood.
113:1794–1804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sangokoya C, Telen MJ and Chi JT: microRNA
miR-144 modulates oxidative stress tolerance and associates with
anemia severity in sickle cell disease. Blood. 116:4338–4348. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Puthanveetil P, Wan A and Rodrigues B:
FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell
survival. Cardiovasc Res. 97:393–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hosaka T, Biggs WR III, Tieu D, Boyer AD,
Varki NM, Cavenee WK and Arden KC: Disruption of forkhead
transcription factor (FOXO) family members in mice reveals their
functional diversification. Proc Natl Acad Sci USA. 101:2975–2980.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puthanveetil P, Wang Y, Zhang D, Wang F,
Kim MS, Innis S, Pulinilkunnil T, Abrahani A and Rodrigues B:
Cardiac triglyceride accumulation following acute lipid excess
occurs through activation of a FoxO1-iNOS-CD36 pathway. Free Radic
Biol Med. 51:352–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puthanveetil P, Zhang D, Wang Y, Wang F,
Wan A, Abrahani A and Rodrigues B: Diabetes triggers a PARP1
mediated death pathway in the heart through participation of FoxO1.
J Mol Cell Cardiol. 53:677–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen CJ, Yu W, Fu YC, Wang X, Li JL and
Wang W: Resveratrol protects cardiomyocytes from hypoxia-induced
apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res
Commun. 378:389–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu H, Zhu J, Hu C, Song H and Li Y:
Inhibition of microRNA-181a may suppress proliferation and invasion
and promote apoptosis of cervical cancer cells through the
PTEN/Akt/FOXO1 pathway. J Physiol Biochem. 72:721–732. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q,
Luo X, Chen Y, Deng X, Liang Z, et al: miR-3188 regulates
nasopharyngeal carcinoma proliferation and chemosensitivity through
a FOXO1-modulated positive feedback loop with
mTOR-p-PI3K/AKT-c-JUN. Nat Commun. 7:113092016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan C, Chen J, Li M, Xuan W, Su D, You H,
Huang Y, Chen N and Liang X: A decrease in hepatic microRNA-9
expression impairs gluconeogenesis by targeting FOXO1 in obese
mice. Diabetologia. 59:1524–1532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hou L, Chen J, Zheng Y and Wu C: Critical
role of miR-155/FoxO1/ROS axis in the regulation of non-small cell
lung carcinomas. Tumour Biol. 37:5185–5192. 2016. View Article : Google Scholar : PubMed/NCBI
|