Introduction
Intracerebral hemorrhage (ICH) is a common
cardiovascular and cerebrovascular disease, one of the diseases
that cause human death and disability, seriously threatening
people's health. The main factors leading to ICH are hypertensive
fine arteriosclerosis, atherosclerotic plaque formation, diabetic
microangiopathy, increased blood viscosity, and long-term
hypertension (1). Together with the
increase of people's living standard and the aging trend, ICH
incidence and mortality also increase year by year, ranking only
second to tumors, and first in many cities (2). According to the bleeding site, ICH can
be divided into several types: lobar hemorrhage, deep hemorrhage,
brain stem hemorrhage and cerebellar hemorrhage. Only 20% of the
patients have self-care ability 6 months after onset (3). Antiplatelet drugs can effectively
reduce the incidence of ischemic diseases, but excessive inhibition
of platelets increases the occurrence of ICH and the risk of
bleeding during treatment (4).
Compared with intracranial hemorrhage caused by other reasons, the
annual incidence caused by oral antiplatelet drugs has increased by
5.9–12.0 times with mortality >52% (5). At present, the antiplatelet drug
therapy for ICH treatment is mainly focused on intraoperative
bleeding and postoperative re-bleeding (6).
A retrospective study on antiplatelet drug-related
ICH patients treated in the First Affiliated Hospital of Bengbu
Medical College (Bengbu, China) was performed, and the effect of
platelet transfusion on the intraoperative bleeding volume,
difficulty of hemostasis, and postoperative re-bleeding in
antiplatelet drug-related ICH patients was investigated to provide
a theoretical basis for clinical treatment.
Patients and methods
Patient information
A retrospective study on 82 ICH patients admitted to
the First Affiliated Hospital of Bengbu Medical College from
February 2014 to February 2016 was performed. Among them, 51
patients treated with platelet transfusion served as observation
group, including 30 males and 21 females, with an average age of
57±11.2 years. Thirty-one patients without platelet transfusion
served as control group, including 17 males and 14 females, with an
average age of 58±9.2 years.
Inclusion and exclusion criteria
Inclusion criteria: patients >18 years of age;
with definite cardiovascular or cerebrovascular disease; with no
history of intracranial hemorrhage; no recent blood donation or
blood transfusion; no other organ infection; treated with
antiplatelet drugs; with complete clinical data. Exclusion
criteria: patients with coagulation disorders; with severe organ
dysfunction; not actively involved; with intracranial hemorrhage
due to trauma; that had recently used anticoagulant drugs. The
study was approved by the Ethics Committee of The First Affiliated
Hospital of Bengbu Medical College. All patients had complete
clinical data. Signed informed consents were obtained from the
patients or their guardians.
Thromboelastography (TEG) detection
method
TEG instrument was purchased from Beijing Tailin
Dongfang Trading Co., Ltd. (Beijing, China). The detection
parameters were: i) clot formation time; ii) reaction time; iii)
Angle angle; iv) maximum amplitude (MA); v) EPL value; vi)
fibrinolysis indicator (LY30); and vii) arachidonic acid
(AA)-induced platelet inhibition rate, and adenosine diphosphate
(ADP) also induced it. When AA inhibition rate was >90%, ADP
inhibition rate was >90%, and maximum blood clot diameter
induced by adenosine diphosphate (MAADP) was <3 mm, the
reactivity increased the risk of bleeding with a high drug content.
When AA inhibition rate was 50–90%, ADP inhibition rate was 30–90%,
and MAADP ranged from 31–47 mm, the therapeutic effect was
satisfactory.
Platelet transfusion
According to the specific condition of the patient's
bleeding, 1–3 units of platelets were transfused. A certain amount
of apheresis platelets was transfused in strict accordance with the
transfusion standard, and TEG was reviewed after 1 h.
Surgical treatment
All patients underwent hematoma removal under
general anesthesia, and most of the hematomas were removed under
direct microscopy. Bipolar electrocoagulation, hemostatic gauze,
and gelatin sponge were used for hemostasis, and the subcutaneous
drainage tube was removed 2–3 days after surgery.
Evaluation indicators
Evaluation indicators were intraoperative bleeding
volume, blood transfusion volume, postoperative hematoma residual
volume, drainage volume and conditions of secondary surgery. The
normal value of platelet was 100–300×109/l.
Statistical methods
SPSS 17.0 software (Shanghai Cabit Information
Technology Co., Ltd., Shanghai, China) was used for the analysis of
all data of this study. Measurement data are presented as mean ±
standard deviation, and count data as rate (%). Paired t-test was
used for the comparison of data before and after treatment
(Table II). ANOVA was used for
comparisons between groups, and Dunnett's test was used as a post
hoc test. χ2 test was used for count data.
Intraoperative total blood transfusion volume, as a kind of skewed
distribution measurement data, was measured using rank sum test and
expressed as median (interquartile range) [M(Q)]. P<0.05 was
considered to indicate a statistically significant difference.
 | Table II.Comparison of platelet number between
two groups of patients before and after treatment. |
Table II.
Comparison of platelet number between
two groups of patients before and after treatment.
|
|
| Platelet number |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | Before treatment
(×109/l) | After treatment
(×109/l) | t | P-value |
|---|
| Observation | 51 | 59.6±20.1 | 186.3±42.3 | 4.553 | 0.001 |
| Control | 31 | 58.3±26.5 | 132.5±12.6 | 2.093 | 0.040 |
| t |
| 0.251 | 3.324 |
|
|
| P-value |
| 0.802 | 0.001 |
|
|
Results
Basic information
The difference was not statistically significant in
sex, age, smoking history, diabetes history, history of heart
disease, bleeding site, preoperative bleeding volume, diastolic
blood pressure, systolic blood pressure, prothrombin time (PT),
activated partial thromboplastin time (APTT), and fibrinogen (Fib)
between the two groups (P>0.05) (Table I).
 | Table I.Comparison of clinical characteristics
between two groups of patients (n, %). |
Table I.
Comparison of clinical characteristics
between two groups of patients (n, %).
| Characteristics | Observation group
(n=51) | Control group
(n=31) | χ2/t | P-value |
|---|
| Age (years) |
|
| 0.017 | 0.982 |
| ≥55 | 32 (62.75) | 19 (61.29) |
|
|
|
<55 | 19 (37.25) | 12 (38.71) |
|
|
| Sex |
|
| 0.226 | 0.496 |
| Male | 30 (58.82) | 17 (54.84) |
|
|
|
Female | 21 (41.18) | 14 (45.16) |
|
|
| Smoking history |
|
| 0.059 | 0.821 |
| Yes | 20 (39.22) | 13 (41.94) |
|
|
| No | 31 (60.78) | 18 (58.06) |
|
|
| Diabetes history |
|
| 1.786 | 0.125 |
| No | 10 (19.61) | 11 (35.48) |
|
|
| Yes | 41 (80.39) | 20 (64.52) |
|
|
| History of heart
disease |
|
| 0.370 | 0.445 |
| No | 12 (23.53) | 10 (32.26) |
|
|
| Yes | 39 (76.47) | 21 (67.74) |
|
|
| Bleeding site |
|
| 0.606 | 0.315 |
| Basal
ganglia | 20 (39.22) | 10 (32.26) |
|
|
| Cerebral
ganglion | 10 (19.61) | 8 (25.81) |
|
|
|
Cerebellum | 10 (19.61) | 6 (19.35) |
|
|
| Cerebral
ventricle | 8 (15.69) | 5 (16.13) |
|
|
| Lobe | 3 (5.88) | 2 (6.45) |
|
|
| Preoperative bleeding
volume (ml) | 64.29±4.35 | 63.45±5.33 | 0.740 | 0.462 |
| Diastolic blood
pressure (mmHg) | 83.64±7.92 | 86.29±5.67 | 1.760 | 0.080 |
| Systolic blood
pressure (mmHg) | 145.33±6.23 | 146.95±5.13 | 1.277 | 0.206 |
| PT (sec) | 11.25±0.61 | 10.50±0.85 | 1.429 | 0.160 |
| APTT (sec) | 26.32±2.66 | 25.41±1.65 | 1.912 | 0.060 |
| Fib (g/l) | 2.71±0.52 | 2.83±0.42 | 1.145 | 0.256 |
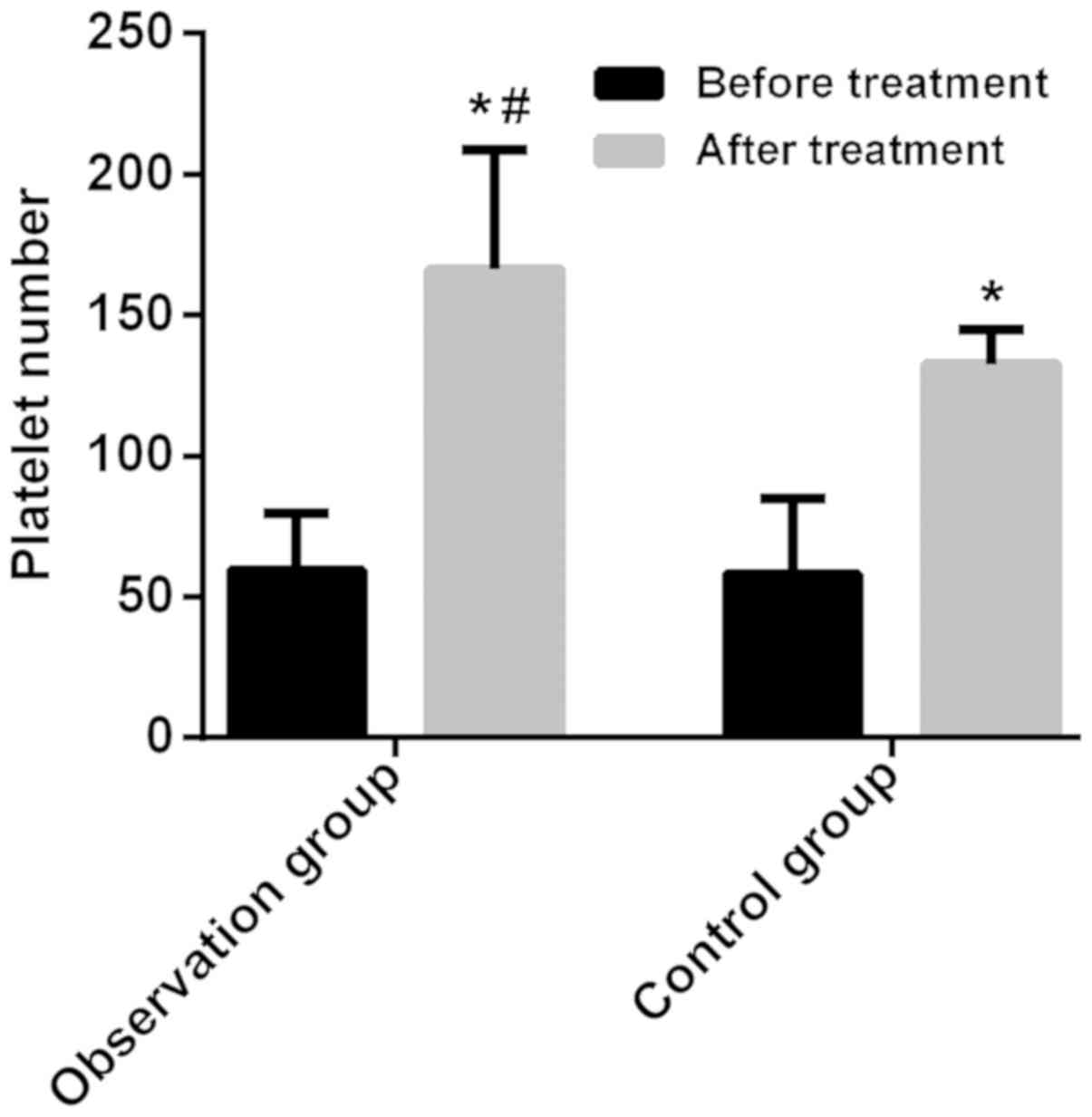
Platelet number of two groups of
patients before and after treatment
The difference in the platelet number before
treatment was not statistically significant between the two groups
(P>0.05). After treatment, the platelet number in the two groups
of patients significantly increased, and the platelet number in the
observation group was higher than that in control group, with a
statistically significant difference (P<0.05) (Table II; Fig.
1).
Treatment effect
The intraoperative bleeding volume, blood
transfusion volume, postoperative hematoma residual volume and
drainage volume in observation group were lower than those in
control group, and the differences were statistically significant
(P<0.05). There were 54 cases with MAADP <20 mm and 28 cases
with MAADP >20 mm (Table
III).
 | Table III.Comparison of treatment status between
two groups of patients. |
Table III.
Comparison of treatment status between
two groups of patients.
| Groups | Intraoperative
bleeding volume (ml) | Blood transfusion
volume (ml) | Postoperative
hematoma residual volume (ml) | Drainage volume
(ml) |
|---|
| Observation
(n=51) | 506.00±111.32 | 200.00
(0–600.00) | 12.35±2.62 | 105.68±20.62 |
| Control (n=31) | 860.00±135.22 | 652.00
(0–1,200.00) | 23.53±5.12 | 211.44±45.31 |
| t/Z | 1.994 | 2.681 | 2.145 | 2.401 |
| P-value | 0.050 | 0.036 | 0.035 | 0.019 |
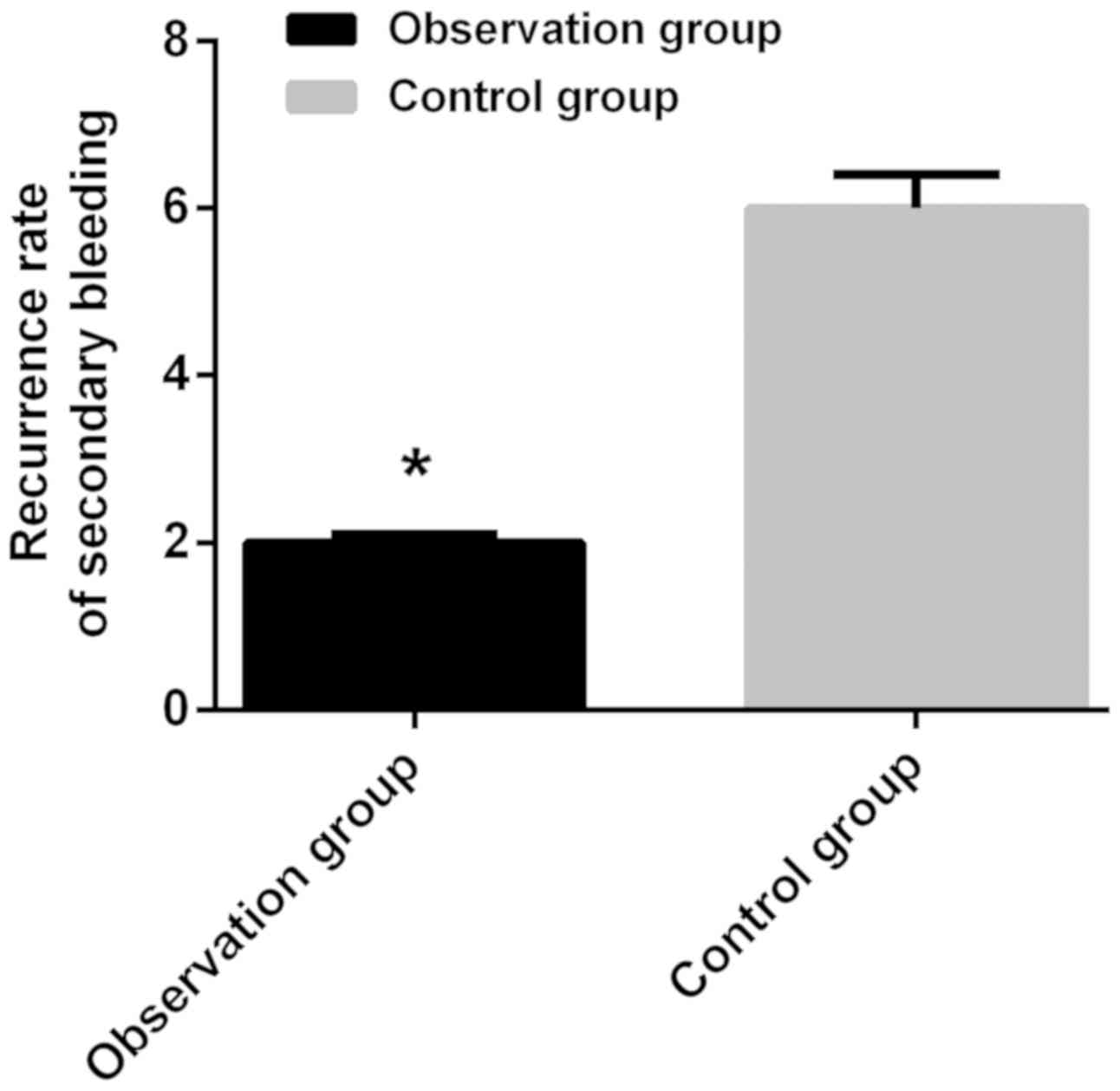
Postoperative bleeding in two groups
of patients
Only 3.92% of the patients in observation group and
19.35% in control group had secondary surgery due to postoperative
re-bleeding, and the comparison of the rate of secondary bleeding
between the two groups was statistically significant
(χ2=3.610, P=0.048) (P<0.05) (Fig. 2).
Discussion
ICH is a severe cardiovascular and cerebrovascular
disease. In Europe and the United States, 50,000–110,000 patients
experience ICH every year, and its incidence is on the rise due to
the increase in aging population (7). In China, the incidence of ICH ranks
second only to that of ischemic cerebral death, and mortality rate
at 30 days after ICH is 21–39%, with approximately half of the
patients dying within 48 h from the onset of the disease (8). Antiplatelet drugs have been widely used
in primary and secondary prevention of cardiovascular and
cerebrovascular diseases to reduce the incidence of thromboembolic
events (6). In recent years,
antiplatelet therapeutic drugs have been increasingly used in the
clinic. Correspondingly, the incidence of concurrent ICH has also
increased year by year. Once a significant increase in intracranial
pressure is caused, an urgent surgical intervention is needed
(9). Coagulation dysfunction is a
taboo for surgery, so the treatment of such patients is more
difficult.
The results of this study revealed that the platelet
number after treatment significantly increased in both groups, and
in observation group it was significantly higher than that in
control group (P<0.05). Platelet transfusion could improve the
coagulation function of patients and reduce the amount of ICH
(10). In the early stages of the
disease, exogenously supplemented platelets can participate in the
process of thrombosis and coagulation, thereby forming thrombus as
soon as possible through the ruptured blood vessel wall, rapidly
stopping bleeding, and increasing platelet count (11). TEG can quickly and accurately respond
to platelet function, and can detect coagulation abnormalities that
cannot be detected by traditional coagulation (12). Patients' platelet function was
evaluated before surgery, and patients with oral antiplatelet drugs
had excessive inhibition of platelet function. Platelet transfusion
was used in patients with platelet inhibition rates >87% prior
to enrollment. The intraoperative bleeding volume, blood
transfusion volume, postoperative hematoma residual volume and
drainage volume were compared between the two groups of patients,
and observation group was superior to control group, with a
statistically significant difference (P<0.05). After treatment
with platelet transfusion, the difficulty of intraoperative
hemostasis was reduced, and the intraoperative bleeding volume,
blood transfusion volume and probability of postoperative
re-bleeding were reduced, indicating that targeted platelet
transfusion before surgery could improve the intraoperative
bleeding in ICH patients and reduce the occurrence of major
bleeding (13). TEG detection can
accurately assess the patient's platelet function and provides the
possibility of individualized platelet transfusion (14). This study showed that the incidence
of secondary surgery in control group was significantly higher than
that in observation group, and the difference between the two
groups was statistically significant (P<0.05). The concentration
of antiplatelet drugs decreases in ICH patients treated with
platelet transfusion, and it cannot inhibit the new platelet
transfusion function, so the efficacy is optimal (15). TEG detection can reduce the waste of
platelet transfusion as much as possible, and can promptly correct
the abnormal platelet function of ICH patients during and after
surgery. It also assists with improving the safety and timeliness
of postoperative antiplatelet therapy (16).
This study attempted to overcome the bias and
disadvantages caused by the uncertainty in the detection of
platelet function of previous studies. Since the patients in this
study were all surgical patients, whether the TEG detection method
under the guidance of platelet transfusion therapy can reduce the
risk of conservative treatment of re-bleeding in ICH patients needs
to be further studied.
In conclusion, TEG detection indicators can more
accurately evaluate the preoperative coagulation function of
patients. Targeted platelet transfusion before surgery can improve
the intraoperative and postoperative bleeding and reduce blood
transfusion volume in ICH patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ was responsible for the thrombelastographic
detection method. LC and HH contributed to platelet transfusion and
surgical treatment. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Bengbu Medical College (Bengbu,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
An SJ, Kim TJ and Yoon BW: Epidemiology,
risk factors, and clinical features of intracerebral hemorrhage: An
update. J Stroke. 19:3–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inagawa T, Ohbayashi N, Takechi A,
Shibukawa M and Yahara K: Primary intracerebral hemorrhage in Izumo
City, Japan: incidence rates and outcome in relation to the site of
hemorrhage. Neurosurgery. 53:1283–1297; discussion 1297–1288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosand J, Eskey C, Chang Y, Gonzalez RG,
Greenberg SM and Koroshetz WJ: Dynamic single-section CT
demonstrates reduced cerebral blood flow in acute intracerebral
hemorrhage. Cerebrovasc Dis. 14:214–220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsanos K, Spiliopoulos S, Saha P,
Diamantopoulos A, Karunanithy N, Krokidis M, Modarai B and
Karnabatidis D: Comparative efficacy and safety of different
antiplatelet agents for prevention of major cardiovascular events
and leg amputations in patients with peripheral arterial disease: A
systematic review and network meta-analysis. PLoS One.
10:e01356922015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugiyama N, Matsuda S, Shimizu M, Obara S,
Ikegami M, Yokoyama J, Miyashita Y, Takizawa S and Takagi S:
Recurrent idiopathic cerebral infarction in a 5-year-old boy, with
emphasis on the importance of platelet aggregation analysis for
appropriate selection of anti-platelet drugs. No To Hattatsu.
41:47–51. 2009.(In Japanese). PubMed/NCBI
|
|
6
|
Gouya G, Arrich J, Wolzt M, Huber K,
Verheugt FW, Gurbel PA, Pirker-Kees A and Siller-Matula JM:
Antiplatelet treatment for prevention of cerebrovascular events in
patients with vascular diseases: A systematic review and
meta-analysis. Stroke. 45:492–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing Y, An Z, Zhang X, Yu N, Zhao W, Ning
X and Wang J: Sex differences in the clinical features, risk
factors, and outcomes of intracerebral hemorrhage: A large
hospital-based stroke registry in China. Sci Rep. 7:2862017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han JH, Lee JM, Koh EJ and Choi HY: The
spot sign predicts hematoma expansion, outcome, and mortality in
patients with primary intracerebral hemorrhage. J Korean Neurosurg
Soc. 56:303–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shih FY, Chang HH, Wang HC, Lee TH, Lin
YJ, Lin WC, Chen WF, Ho JT and Lu CH: Risk factors for delayed
neuro-surgical intervention in patients with acute mild traumatic
brain injury and intracranial hemorrhage. World J Emerg Surg.
11:132016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baschin M, Selleng S, Zeden JP, Westphal
A, Kohlmann T, Schroeder HW, Greinacher A and Thiele T: Platelet
transfusion to reverse antiplatelet therapy before decompressive
surgery in patients with intracranial haemorrhage. Vox Sang.
112:535–541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue M, Luo D, Yu S, Liu P, Zhou Q, Hu M,
Liu Y, Wang S, Huang Q, Niu Y, et al: Misshapen/NIK-related kinase
(MINK1) is involved in platelet function, hemostasis, and thrombus
formation. Blood. 127:927–937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lisman T and Porte RJ: The role of
platelets in liver inflammation and regeneration. Semin Thromb
Hemost. 36:170–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Estcourt LJ, Stanworth SJ, Doree C,
Hopewell S, Trivella M and Murphy MF: Comparison of different
platelet count thresholds to guide administration of prophylactic
platelet transfusion for preventing bleeding in people with
haematological disorders after myelosuppressive chemotherapy or
stem cell transplantation. Cochrane Database Syst Rev.
11:CD0109832015.
|
|
14
|
Mishra PK, Thekkudan J, Sahajanandan R,
Gravenor M, Lakshmanan S, Fayaz KM and Luckraz H: The role of
point-of-care assessment of platelet function in predicting
postoperative bleeding and transfusion requirements after coronary
artery bypass grafting. Ann Card Anaesth. 18:45–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spiess BD, Royston D, Levy JH, Fitch J,
Dietrich W, Body S, Murkin J and Nadel A: Platelet transfusions
during coronary artery bypass graft surgery are associated with
serious adverse outcomes. Transfusion. 44:1143–1148. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joseph B, Pandit V, Sadoun M, Larkins CG,
Kulvatunyou N, Tang A, Mino M, Friese RS and Rhee P: A prospective
evaluation of platelet function in patients on antiplatelet therapy
with traumatic intracranial hemorrhage. J Trauma Acute Care Surg.
75:990–994. 2013. View Article : Google Scholar : PubMed/NCBI
|