Introduction
Systemic-onset juvenile idiopathic arthritis (SOJIA)
is a severe and distinctive type of juvenile arthritis (1). The pathophysiology of systemic
manifestations of SOJIA appears to be associated with continuous
activation of innate immune pathways, resulting in dysregulated
production of proinflammatory cytokines (2). For example, interleukin (IL)-1β
(3,4)
and IL-6 (5) have been indicated to
be important cytokines in the pathogenesis of SOJIA. Therefore,
targeting IL-1 and IL-6 has become a treatment strategy for SOJIA
(6,7).
Tacrolimus (TAC) is a relatively novel
immunosuppressive drug, which inhibits the transcription of the
early activation genes of IL-2 and suppresses the production of
tumor necrosis factor (TNF)-α, IL-1β and IL-6 through T cell
activation (8,9). In addition to being used during
transplantation, TAC has also been used without serious adverse
effects for treating refractory nephrotic syndrome (10,11),
lupus nephritis (12), and
rheumatoid arthritis (8,9). However, few studies have reported the
use of treatment with TAC in patients with SOJIA. The aim of the
current study was to investigate the effect of TAC on SOJIA.
Patients and methods
Patients
The current data were obtained retrospectively from
medical records of patients diagnosed with SOJIA. All patients were
under 18 years old and received TAC treatment at the Children's
Hospital of Fudan University (Shanghai, China) between January 2015
and January 2018.
Patient characteristics and
outcomes
Data were collected from the beginning of TAC
treatment for 12 months, with the 12-month endpoint defined as the
last follow-up. Clinical characteristics including sex, age,
duration of the disease, TAC dose, erythrocyte sedimentation rate
(ESR), C reactive protein (CRP), hemoglobin (Hb), platelet (PLT)
and white blood cell (WB) levels, prednisolone (PDN) dose and
interleukin-6 (IL-6) expression at the beginning of TAC treatment
(0 months) and following treatment with TAC for 6 months and 12
months were obtained from medical records retrospectively.
Statistical analysis
Data are presented as the mean ± standard error.
Statistical analyses were performed using the General Linear Model
for repeated measurement. P<0.05 was considered to indicate a
statistically significant difference. The statistical analysis was
performed using SPSS software (version 13.0; SPSS, Inc., Chicago,
IL, USA).
Results
Summary of clinical
characteristics
Clinical characteristics are summarized in Table I. A total of 6 Chinese patients with
SOJIA, including 3 males and 3 females from Children's Hospital of
Fudan University, were included for studying the effect of TAC on
SOJIA. Patient ages ranged from 5.5 to 14.0 years old (mean,
10.8±3.0 years), and the duration of disease ranged from 1.1 to 5.0
years. During follow-up time, TAC doses were 1.0–3.0 mg/day. The
baseline characteristics of the patients were: ESR, 67.8±18.7 mm/h;
CRP, 128.0±38.9 mg/l; Hb, 108.7±13.7 g/l; PLT,
416.8±90.1×109/l; WB, 26.0±10.2×109/l; PDN
dose, 49.0±17.1 mg/day.
 | Table I.Summary of clinical
characteristics. |
Table I.
Summary of clinical
characteristics.
|
|
|
|
| ESR, mm/h | CRP, mg/l | Hb, g/l | PLT,
109/l | WB,
109/l | PDN, mg/day |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no | Sex | Age, years | TAC, mg/day | Baseline | Last
visit | Baseline | Last
visit | Baseline | Last
visit | Baseline | Last
visit | Baseline | Last
visit | Baseline | Last
visit |
|---|
| 1 | M | 13.0 | 2.0–3.0 | 68.0 | 12.0 | 160.0 | 8.0 | 117.0 | 125.0 | 433.0 | 242.0 | 37.1 | 7.3 | 60.0 | 2.5 |
| 2 | F | 11.5 | 2.0–3.0 | 72.0 | 5.0 | 119.0 | 8.0 | 102.0 | 104.2 | 460.0 | 249.0 | 37.0 | 8.3 | 60.0 | 5.0 |
| 3 | M | 11.0 | 2.0–3.0 | 78.0 | 4.0 | 160.0 | 8.0 | 117.0 | 124.0 | 319.0 | 316.0 | 28.1 | 6.9 | 60.0 | 0.0 |
| 4 | F | 5.5 | 1.0–2.0 | 58.0 | 9.0 | 67.0 | 8.0 | 91.2 | 102.2 | 326.0 | 233.0 | 10.7 | 6.2 | 24.0 | 0.0 |
| 5 | F | 9.5 | 1.0–2.0 | 38.0 | 3.0 | 102.0 | 8.0 | 127.0 | 138.0 | 560.0 | 364.0 | 20.5 | 7.3 | 30.0 | 0.0 |
| 6 | M | 14.0 | 1.0–2.0 | 93.0 | 1.0 | 160.0 | 8.0 | 98.2 | 116.0 | 403.0 | 117.0 | 22.5 | 5.5 | 60.0 | 0.0 |
| Mean ± SD | – | 10.8±3.0 | – | 67.8±18.7 | 5.7±4.1a | 128.0±38.9 | 8.0±0a | 108.7±13.7 | 118.2±13.6 | 416.8±90.1 |
253.5±84.0a | 26.0±10.2 | 6.9±1.0a | 49.0±17.1 | 1.3±2.1a |
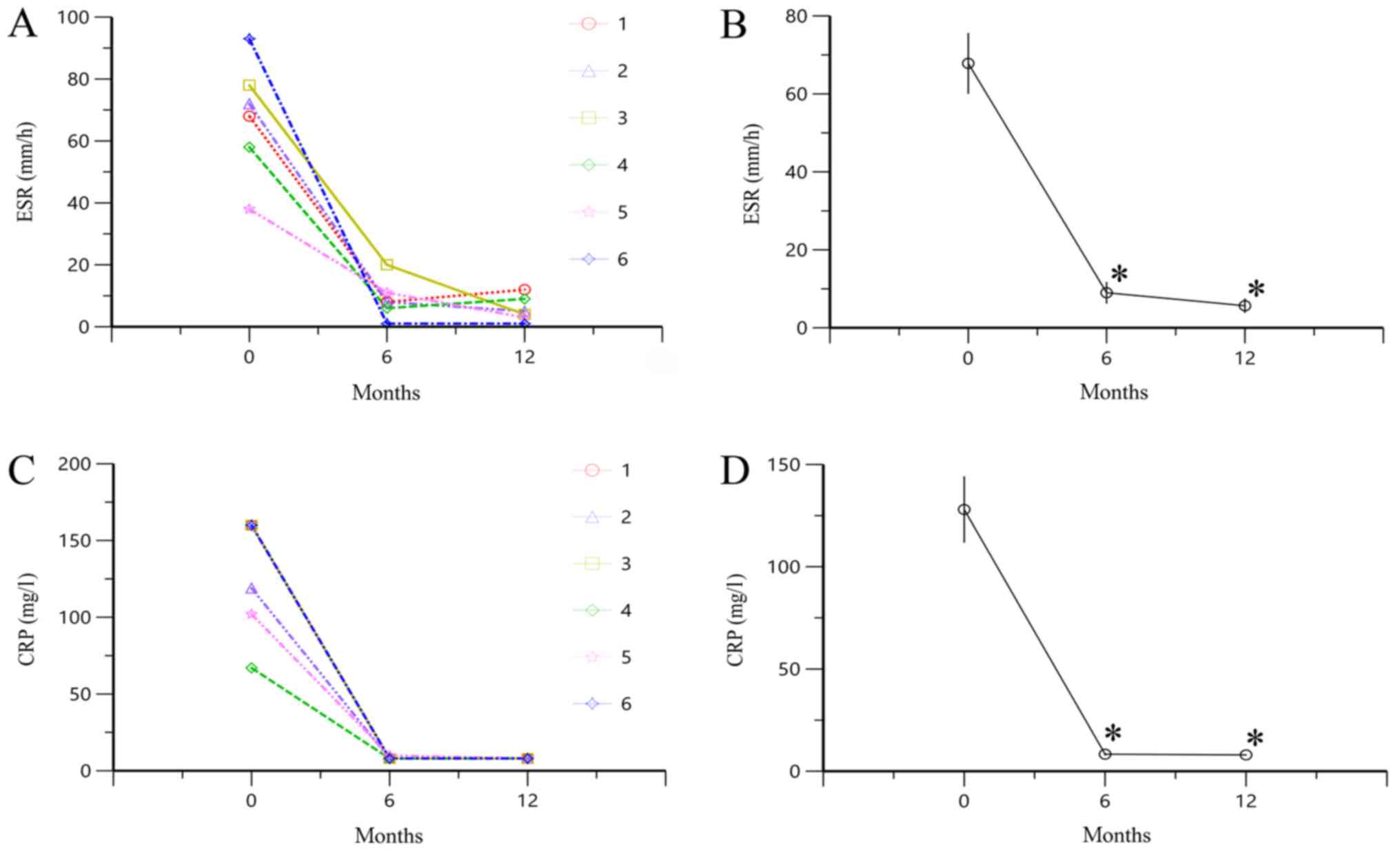
Changes in ESR and CRP level following
treatment with TAC
ESR and CRP level were significantly lower at 6 and
12 months compared with 0 months (all P<0.05; Fig. 1). These results indicated that TAC
could significantly downregulate the high ESR and CRP level in
SOJIA.
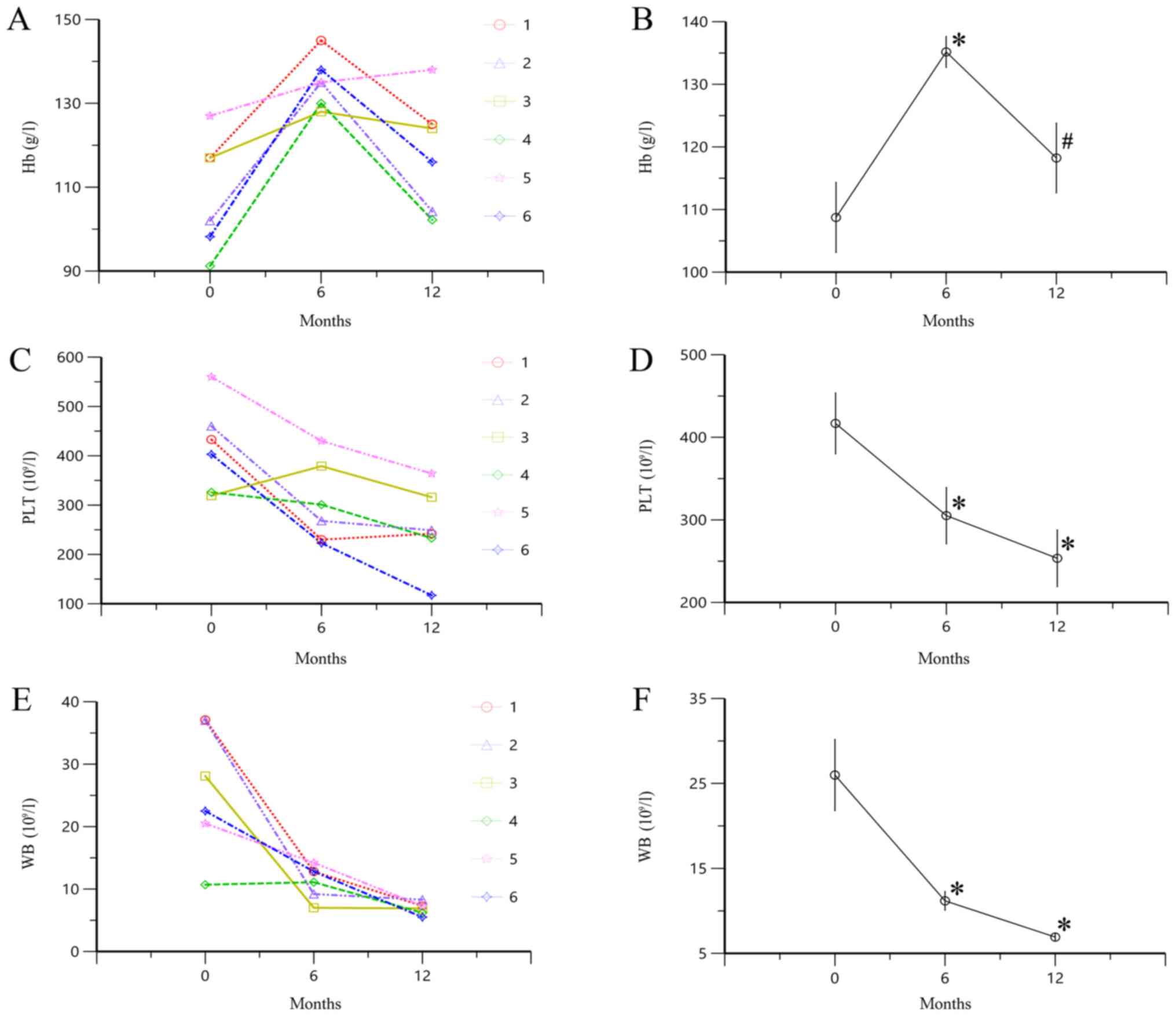
Changes in Hb, PLT and WB levels
following treatment with TAC
Hb level significantly increased at 6 months of
treatment with TAC compared with the baseline level (P<0.05;
Fig. 2A and B). However, following
treatment with TAC for 12 months, the Hb level decreased compared
with the value at 6 months (P<0.05). PLT and WB levels
significantly decreased after 6 and 12 months of treatment with TAC
compared with baseline (all P<0.05; Fig. 2C-F). These results indicated that
administration of TAC significantly decreased the levels of PLT and
WB in patients with SOJIA. However, the level of Hb increased at 6
months, followed by a decrease at 12 months of treatment.
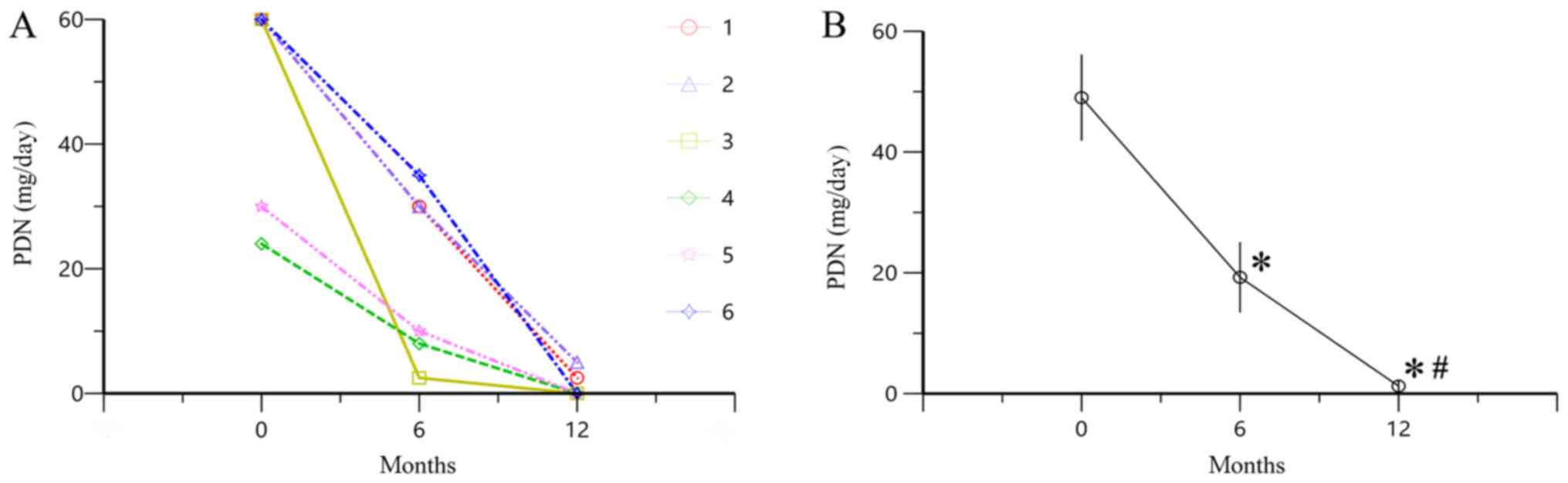
Changes in PDN dose following
treatment with TAC
Following treatment with TAC for 6 and 12 months,
PDN dose was decreased compared with the baseline (P<0.05;
Fig. 3), indicating that treatment
with TAC reduced the dose of PDN required for the treatment of
patients with SOJIA.
Changes in the expression levels of
IL-6 following treatment with TAC
Following treatment with TAC for 12 months, the
expression levels of IL-6 decreased significantly compared with the
baseline level (Table II). These
results indicated that administration of TAC significantly
decreased the expression levels of IL-6 in patients with SOJIA.
 | Table II.Alterations in IL-6 expression levels
among the 6 patients. |
Table II.
Alterations in IL-6 expression levels
among the 6 patients.
|
| IL-6, pg/ml |
|---|
|
|
|
|---|
| Patient no. | Prior to treatment
with TAC | Following treatment
with TAC for 12 months |
|---|
| 1 | 265.90 | 15.04 |
| 2 | 50.00 | 8.70 |
| 3 | 112.90 | 9.05 |
| 4 | >1000.00 | 1.92 |
| 5 | 198.00 | 9.13 |
| 6 | 317.80 | 2.35 |
| Mean ± SD | 324.10±345.23 |
7.70±4.91a |
Discussion
A proportion of patients with SOJIA continue to
require long-term corticosteroid therapy for disease control.
However, repeated and prolonged steroid therapy increases the risk
of obesity, cushingoid appearance, hypertension, growth
retardation, osteoporosis, infections and psychological problems
(13). Therefore, a safe and
effective therapeutic strategy for controlling SOJIA is required
(14).
Previous studies have identified the importance of
suppressing the pathogenic effects of IL-6 and other
proinflammatory cytokines in treating SOJIA (14–16). TAC
effectively suppresses the production of TNF-α, IL-1β and IL-6
through T cell activation (9,12), and
has been successfully used without serious adverse effects to treat
rheumatoid arthritis in adults and the elderly patients (8,9).
Therefore, it was hypothesized that TAC may be safe and effective
to treat SOJIA.
In the current study, it was identified that TAC
could significantly downregulate the high ESR, CRP, PLT and WB
levels in patients with SOJIA. The dose of the concomitantly
administered PDN was gradually reduced during the 12 months of
treatment with TAC. The current study indicated that TAC may reduce
the required PDN dose and may be an effective treatment strategy
for SOJIA.
The mechanism by which TAC effectively ameliorates
SOJIA requires further investigation. Previously, the successful
use of recombinant homolog of IL-6 receptor antagonist (IL-6Ra),
which competitively inhibits binding of IL-6 to the IL-6 receptor,
has been reported in the treatment of SOJIA (14,17). The
studies verified that IL-6 is a therapeutic target in SOJIA
(14,17). In the current study, it was
identified that TAC effectively suppresses IL-6 production;
therefore, this may be the mechanism by which TAC exerts its
therapeutic effects in SOJIA.
Furthermore, previous studies have reported the use
of TAC for the treatment of patients with SOJIA. Shimizu et
al (18) reported a case of a
6-year-old Japanese female who presented with a 6-month history of
polyarthritis and was diagnosed with poly-JIA. This study
identified TAC as a potential alternative when treating patients
with refractory polyarticular JIA (18). In addition, Tanaka et al
(19) reported using TAC to treat
difficult cases of SOJIA, including an 8.5-year-old Japanese male
with a 6-year history of SOJIA, and a 5-year-old Japanese female
with a 1.5-year history of refractory SOJIA. However, these studies
both focused on Japanese children. To the best of our knowledge,
this is the first study to report the treatment of SOJIA with TAC
in Chinese children, including three males and three females, whose
duration of disease ranged from 1.1 to 5.0 years.
In conclusion, TAC may be an effective treatment
strategy for patients with SOJIA. However, the current study was
performed at a single center. Therefore, further multicenter and
prospective studies with a larger number of patients are
required.
Acknowledgements
Not applicable.
Funding
The present study was supported by Clinical Pharmacy
Key Specialty Construction Project of Shanghai (grant no. YZ2017/5)
and Wuxi Science and Technology Development Guidance Plan (Medical
and Health Care; grant no. CSZON1744).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and DW conceived and designed the study. DW and
XC collected and analyzed data. DW and XC wrote the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of Children's Hospital of Fudan University. All
procedures performed in studies involving human participants were
in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Declaration of
Helsinki and its later amendments or comparable ethical standards.
Informed consent was obtained from all parents/legal guardians of
the participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schulert GS, Minoia F, Bohnsack J, Cron
RQ, Hashad S, Kon EPI, Kostik M, Lovell D, Maritsi D, Nigrovic PA,
et al: Effect of biologic therapy on clinical and laboratory
features of macrophage activation syndrome associated with systemic
juvenile idiopathic arthritis. Arthritis Care Res (Hoboken).
70:409–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cimaz R: Systemic-onset juvenile
idiopathic arthritis. Autoimmun Rev. 15:931–934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allantaz F, Chaussabel D, Stichweh D,
Bennett L, Allman W, Mejias A, Ardura M, Chung W, Smith E, Wise C,
et al: Blood leukocyte microarrays to diagnose systemic onset
juvenile idiopathic arthritis and follow the response to IL-1
blockade. J Exp Med. 204:2131–2144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quartier P, Allantaz F, Cimaz R, Pillet P,
Messiaen C, Bardin C, Bossuyt X, Boutten A, Bienvenu J, Duquesne A,
et al: A multicentre, randomised, double-blind, placebo-controlled
trial with the interleukin-1 receptor antagonist anakinra in
patients with systemic-onset juvenile idiopathic arthritis (ANAJIS
trial). Ann Rheum Dis. 70:747–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Benedetti F, Massa M, Robbioni P,
Ravelli A, Burgio GR and Martini A: Correlation of serum
interleukin-6 levels with joint involvement and thrombocytosis in
systemic juvenile rheumatoid arthritis. Arthritis Rheum.
34:1158–1163. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeWitt EM, Kimura Y, Beukelman T, Nigrovic
PA, Onel K, Prahalad S, Schneider R, Stoll ML, Angeles-Han S,
Milojevic D, et al: Consensus treatment plans for new-onset
systemic juvenile idiopathic arthritis. Arthritis Care Res
(Hoboken). 64:1001–1010. 2012.PubMed/NCBI
|
|
7
|
Vastert SJ, de Jager W, Noordman BJ,
Holzinger D, Kuis W, Prakken BJ and Wulffraat NM: Effectiveness of
first-line treatment with recombinant interleukin-1 receptor
antagonist in steroid-naive patients with new-onset systemic
juvenile idiopathic arthritis: Results of a prospective cohort
study. Arthritis Rheumatol. 66:1034–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawai S and Yamamoto K: Safety of
tacrolimus, an immunosuppressive agent, in the treatment of
rheumatoid arthritis in elderly patients. Rheumatology (Oxford).
45:441–444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo H, Abe T, Hashimoto H, Uchida S,
Irimajiri S, Hara M and Sugawara S: Efficacy and safety of
tacrolimus (FK506) in treatment of rheumatoid arthritis: A
randomized, double blind, placebo controlled dose-finding study. J
Rheumatol. 31:243–251. 2004.PubMed/NCBI
|
|
10
|
Segarra A, Vila J, Pou L, Majo J, Arbos A,
Quiles T and Piera LL: Combined therapy of tacrolimus and
corticosteroids in cyclosporin-resistant or -dependent idiopathic
focal glomerulosclerosis: A preliminary uncontrolled study with
prospective follow-up. Nephrol Dial Transplant. 17:655–662. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki K, Tsugawa K and Tanaka H:
Tacrolimus for the treatment of focal segmental glomerulosclerosis
resistant to cyclosporine A. Pediatr Nephrol. 21:1913–1914. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mok CC, Tong KH, To CH, Siu YP and Au TC:
Tacrolimus for induction therapy of diffuse proliferative lupus
nephritis: An open-labeled pilot study. Kidney Int. 68:813–817.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hahn D, Hodson EM, Willis NS and Craig JC:
Corticosteroid therapy for nephrotic syndrome in children. Cochrane
Database Syst Rev. Cd001533. 2015. View Article : Google Scholar
|
|
14
|
Tanaka H, Tsugawa K, Nakahata T, Suzuki K
and Ito E: Leukocytapheresis for the treatment of refractory
systemic-onset juvenile idiopathic arthritis. Clin Rheumatol.
26:1014–1016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura Y, Pinho P, Walco G, Higgins G,
Hummell D, Szer I, Henrickson M, Watcher S and Reiff A: Etanercept
treatment in patients with refractory systemic onset juvenile
rheumatoid arthritis. J Rheumatol. 32:935–942. 2005.PubMed/NCBI
|
|
16
|
Quartier P, Taupin P, Bourdeaut F, Lemelle
I, Pillet P, Bost M, Sibilia J, Kone-Paut I, Gandon-Laloum S,
LeBideau M, et al: Efficacy of etanercept for the treatment of
juvenile idiopathic arthritis according to the onset type.
Arthritis Rheum. 48:1093–1101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokota S: Interleukin 6 as a therapeutic
target in systemic-onset juvenile idiopathic arthritis. Curr Opin
Rheumatol. 15:581–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimizu M, Ueno K, Ishikawa S, Tokuhisa Y,
Inoue N and Yachie A: Treatment of refractory polyarticular
juvenile idiopathic arthritis with tacrolimus. Rheumatology
(Oxford). 53:2120–2122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka H, Tsugawa K, Suzuki K, Oki ES,
Nonaka K, Kimura S and Ito E: Treatment of difficult cases of
systemic-onset juvenile idiopathic arthritis with tacrolimus. Eur J
Pediatr. 166:1053–1055. 2007. View Article : Google Scholar : PubMed/NCBI
|