Introduction
Uterine fibroid is a common gynecological benign
tumor, in which the age of most patients is more than 35 years
(1). According to reports in the
literature, uterine fibroid can cause increased menstrual blood
volume, prolonged menstruation, pelvic distention, soreness of
waist or even infertility, due to differences in the size and
location of fibroid, severely affecting the quality of lives
(2,3). With the development of society, more
attention has been paid by women to the quality of life, physical
integrity, uterine physiological function and abdominal beauty
(4). According to reports in the
literature, the endometrial receptivity can be evaluated by a good
congestive state of endometrium that is the basic condition for
embryo implantation and reflects the capacity of an embryo to be
accepted (5). The indicator for
assessing the congestive state of endometrium is usually the
uterine arterial blood flow parameter, mainly pulsatility index
(PI) and resistance index (RI), and the smaller the value is, the
more favorable the embryo implantation is (6). Testing the function of sex hormone to
assess ovarian can indirectly reflect the health of the uterus. Sex
hormone mainly includes follicle stimulating hormone (FSH),
luteinizing hormone (LH) and estradiol (E2). When
ovarian failure occurs, concentrations of FSH and LH will increase
(7). Therefore, the clinical use of
minimally invasive technique in the treatment of uterine fibroid is
increasing and requirements are also gradually increasing.
High intensity focused ultrasound (HIFU) technology
is new for the treatment of tumors with non-invasiveness (8). The main principle is to use ultrasonic
waves with characteristics such as tissue penetrating and
focusability to concentrate the low-intensity ultrasonic waves on
the lesions in the body, so that the focus area rapidly warms up to
60–100°C, resulting in coagulation necrosis of the target tissues,
but at the same time, it does not damage the tissues outside the
target area. Detection of pathology and related enzymes in the
tissues around the target area is strong evidence, and no
significant changes have been found (9,10). The
advantage of HIFU is that it has the same effect as myomectomy and
can cause necrosis of fibroid tissues, but it can avoid the damage
of ultrasonic waves to normal tissues, so as to achieve the effect
of minimally invasive treatment. This has been confirmed by
clinical manifestations of patients and imaging (11,12). At
the same time, HIFU has played a safe and effective role in
patients with a willingness to have pregnancy, not only to allow
uterine cavity a good recovery, but also to retain pregnancy
ability (13).
This study investigated the effect of HIFU uterine
fibroid ablation on the endometrial receptivity and sex hormone
level in uterine fibroid patients and the analysis of influencing
factors for treatment rate, to provide the decision-making basis
for selection of HIFU ablation in the treatment of uterine fibroid
indications, and optimization and standardization of treatment
schemes.
The study was approved by the Ethics Committee of
Jining Maternity and Child Care Hospital (Jining, China). Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Materials and methods
General information
A retrospective analysis of 266 uterine fibroid
patients, who meet the symptoms of uterine fibroid, confirmed
clinically and by imaging in the Jining Maternity and Child Care
Hospital from October 2013 to October 2016, was performed. The
observation group was treated with HIFU ablation, a total of 143
cases, 163 fibroids, with an average age of 36.25±7.13 years. The
control group was treated with myomectomy, a total of 123 cases,
140 fibroids, with an average age of 35.75±6.42 years. There was no
significant difference in general information between the two
groups (P>0.05). All patients in pregnancy and lactation,
suffering from other gynecological diseases, heart disease,
malignant tumors and coagulation dysfunction were excluded, and
patients who had not taken hormone drugs within 6 months were
included (Table I).
 | Table I.General information (n, %). |
Table I.
General information (n, %).
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Factors | Observation | Control | t/χ2 | P-value |
|---|
| Age (years) | 36.25±7.13
(n=143) | 35.75±6.42
(n=123) | 0.597 | 0.551 |
| Tumor diameter
(cm) | (n=163) | (n=140) | 0.212 | 0.729 |
|
<4 | 80 (49.08) | 65 (46.43) |
|
|
| ≥4 | 83 (50.92) | 75 (53.57) |
|
|
| Tumor volume
(cm3) | (n=163) | (n=140) | 0.196 | 0.730 |
|
<54 | 81 (49.69) | 66 (47.14) |
|
|
| ≥54 | 82 (50.31) | 74 (52.86) |
|
|
| Fibroid location | (n=163) | (n=140) | 0.158 | 0.722 |
| Anterior
wall/Posterior wall | 100 (61.35) | 89 (63.57) |
|
|
| Uterine
fundus | 63 (38.65) | 51 (36.43) |
|
|
| Fibroid target skin
distance (cm) | (n=163) | (n=140) | 0.198 | 0.729 |
|
<7 | 88 (53.99) | 72 (51.43) |
|
|
| ≥7 | 75 (46.01) | 68 (48.57) |
|
|
| Fibroid type | (n=163) | (n=140) | 0.073 | 0.795 |
| Muscle
wall | 121 (74.23) | 102 (72.86) |
|
|
|
Non-muscle wall | 42 (25.77) | 38 (27.14) |
|
|
Treatment methods
JC-200 type HIFU (Chongqing Haifu Medical Technology
Co., Ltd., Chongqing, China) was used for ablation therapy within
one week after menstruation in observation group. The treatment
diameter was 195 mm, and the treatment power was 160–420 W.
Ultrasound scanning located the extent of fibroid lesions, captured
dynamic images and located planar areas. Ultrasound probes were
used to determine the number, size and location of fibroids, and
lesion target areas were differentiated into different treatment
levels. Patients were placed in a supine position and a
dot-line-surface ablation treatment was performed through the focus
area according to the treatment plan. At the same time, routine
ultrasound examination, ultrasound contrast and MRI examination
were performed at 1 month before and within 6 months after
treatment to determine the therapeutic effect.
The control group was treated with myomectomy,
including transabdominal, laparoscope and transvaginal
myomectomy.
Observation indicators
The PI and RI of the uterine arterial blood flow
were measured during the luteal phase of menstruation by
transvaginal ultrasonography at 1 month before and within 6 months
after operation. At the same time, the sex hormone level was
measured, and 4 ml of fasting cubital venous blood were extracted.
Serum LH, FSH and E2 were detected by chemical
immunofluorescence.
Evaluation of curative effect
The ablation rate of fibroid was calculated
according to the following formula and evaluation criteria, and the
curative effect was evaluated (14)
(Table II):
 | Table II.Modified RECIST criteria for
evaluating the curative effect of cancer therapy. |
Table II.
Modified RECIST criteria for
evaluating the curative effect of cancer therapy.
| Ablation rate | Evaluation of
effect |
|---|
| Ablation rate
<50% | Effective |
| >0 ablation rate
≤50% | Marked effective |
| Ablation rate =0 | Invalid |
Preoperative uterine fibroid volume =1/6× π × long
diameter × wide diameter × thick diameter.
Ablation rate = fibroid volume after
ablation/preoperative uterine fibroid volume ×100%.
Statistical analysis
SPSS20.0 statistical software (IBM Corp., Armonk,
NY, USA) was used for data analysis. Chi-square test was used for
count data, t-test for measurement data, paired t-test for
comparison between before and after treatment in the group, and
logistic, univariate and multivariate regression analyses for the
influencing factors for HIFU treatment rate. ANOVA was used for
comparison beween multiple groups with LSD test. P<0.05 was
considered to indicate a statistically significant difference.
Results
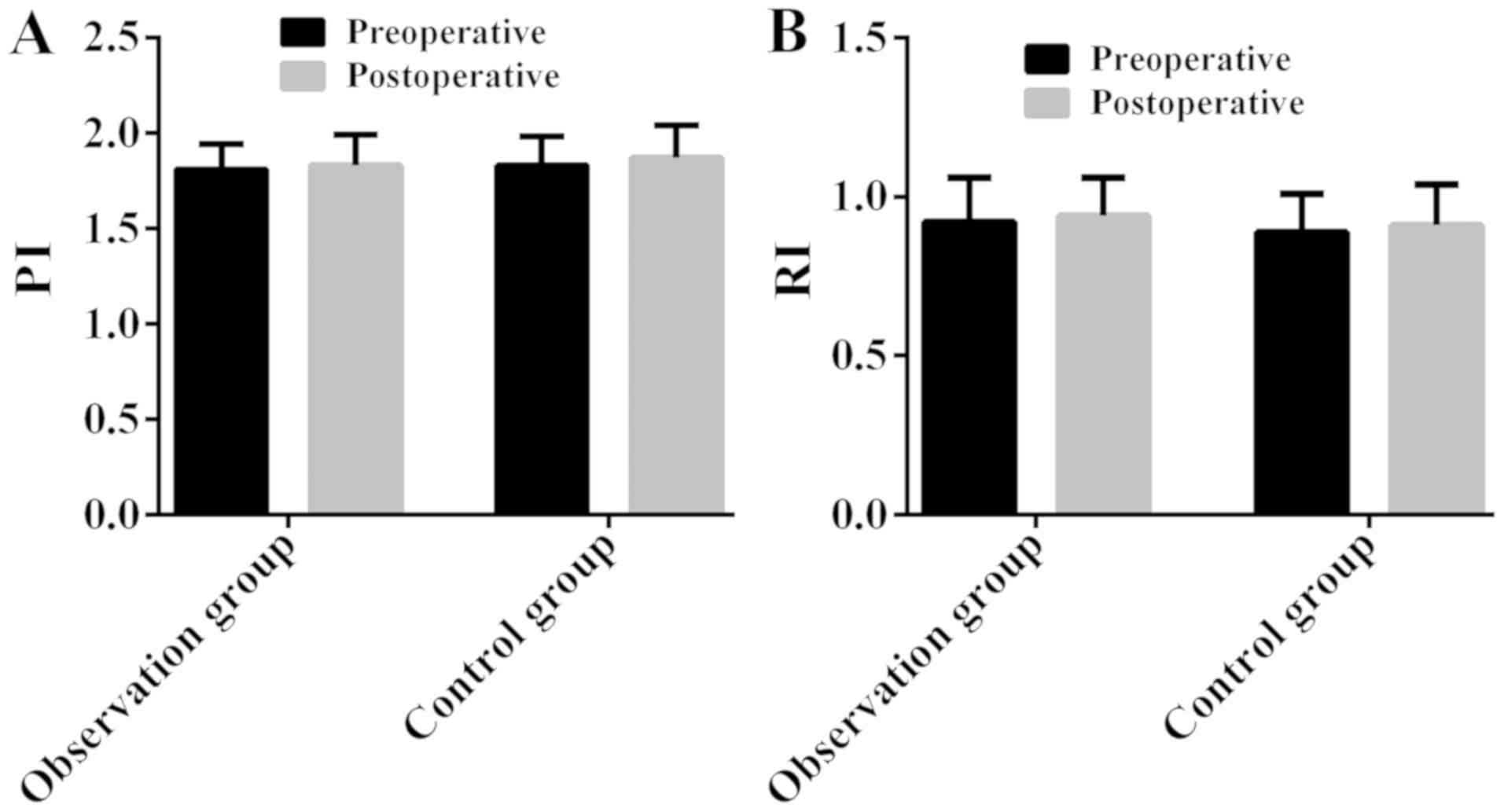
Comparison of preoperative and
postoperative PI and RI between the two groups
There was no significant difference in preoperative
and postoperative PI and RI between the two groups (P>0.05); no
significant difference between preoperative PI and postoperative PI
in the same group (P>0.05), neither RI (P>0.05) (Fig. 1; Table
III).
 | Table III.Comparison of preoperative and
postoperative PI and RI between the two groups. |
Table III.
Comparison of preoperative and
postoperative PI and RI between the two groups.
|
|
| Groups |
|
|
|---|
|
|
|
|
|
|
|---|
| Indicators | Time | Observation
(n=143) | Control (n=123) | t value | P-value |
|---|
| PI | Preoperative | 1.81±0.13 | 1.83±0.15 | 1.165 | 0.245 |
|
| Postoperative | 1.83±0.16 | 1.86±0.17 | 1.919 | 0.056 |
|
| t value | 1.160 | 1.957 |
|
|
|
| P-value | 0.247 | 0.052 |
|
|
| RI | Preoperative | 0.92±0.14 | 0.89±0.12 | 1.860 | 0.064 |
|
| Postoperative | 0.94±0.12 | 0.91±0.13 | 1.956 | 0.051 |
|
| t value | 1.297 | 1.254 |
|
|
|
| P-value | 0.196 | 0.211 |
|
|
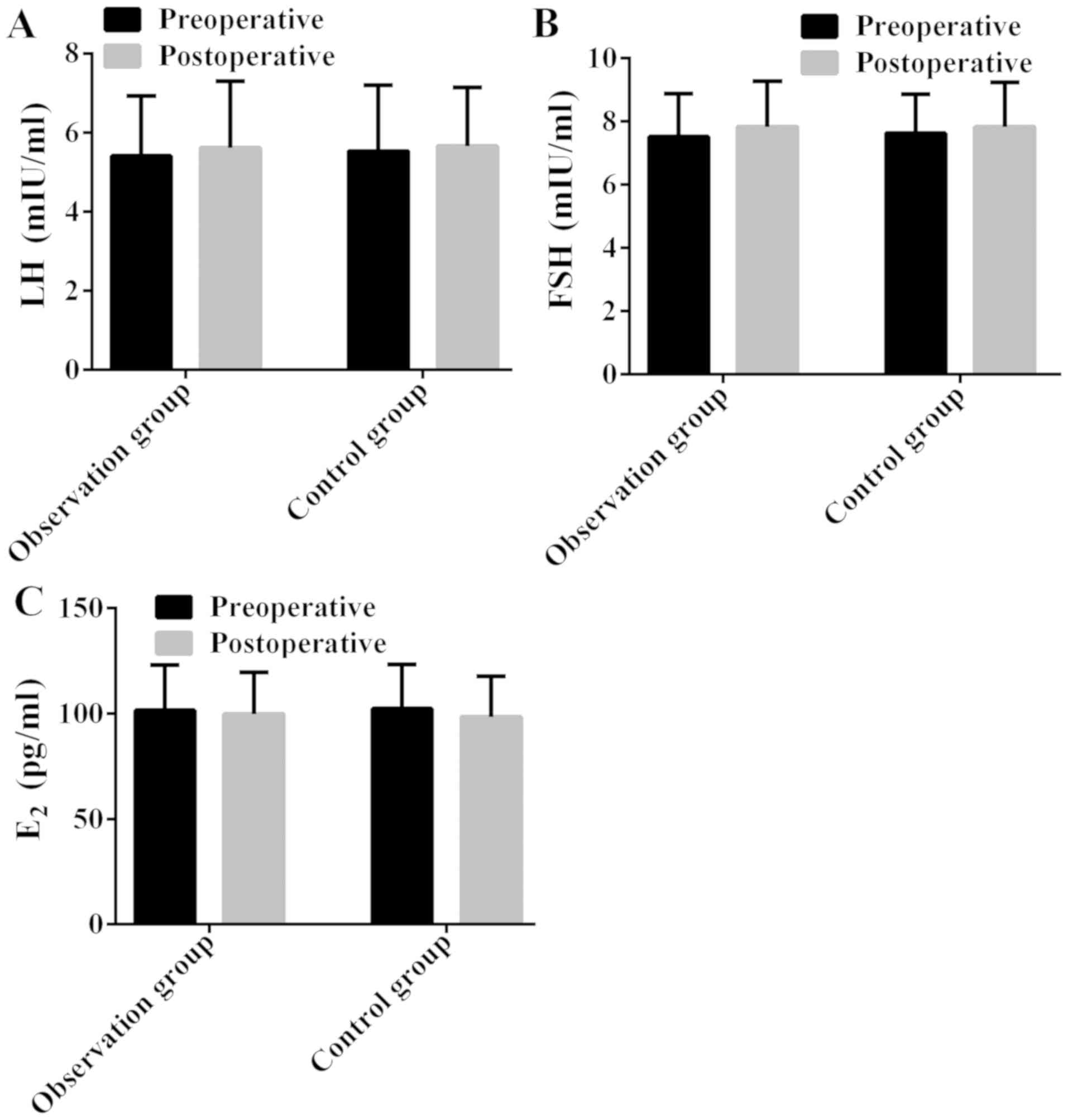
Comparison of preoperative and
postoperative sex hormone level between the two groups
There was no significant difference in preoperative
and postoperative LH, FSH and E2 between the two groups
(P>0.05); no significant difference between preoperative LH and
postoperative LH in the same group (P>0.05), neither FSH or
E2 (P>0.05) (Fig. 2;
Table IV).
 | Table IV.Comparison of preoperative and
postoperative sex hormone level between the two groups. |
Table IV.
Comparison of preoperative and
postoperative sex hormone level between the two groups.
|
|
| Groups |
|
|
|---|
|
|
|
|
|
|
|---|
| Items | Time | Observation
(n=143) | Control
(n=123) | t value | P-value |
|---|
| LH (mIU/ml) | Preoperative | 5.41±1.52 | 5.53±1.67 | 0.613 | 0.540 |
|
| Postoperative | 5.62±1.68 | 5.66±1.48 | 0.205 | 0.838 |
|
| t value | 1.108 | 0.646 |
|
|
|
| P-value | 0.269 | 0.519 |
|
|
| FSH (mIU/ml) | Preoperative | 7.51±1.36 | 7.62±1.32 | 0.667 | 0.506 |
|
| Postoperative | 7.83±1.45 | 7.82±1.42 | 0.057 | 0.955 |
|
| t value | 1.925 | 1.177 |
|
|
|
| P-value | 0.055 | 0.241 |
|
|
| E2
(pg/ml) | Preoperative | 101.56±21.43 | 102.34±20.95 | 0.299 | 0.765 |
|
| Postoperative | 99.82±19.64 | 98.42±19.13 | 0.587 | 0.558 |
|
| t value | 0.716 | 1.532 |
|
|
|
| P-value | 0.475 | 0.127 |
|
|
Relationship between HIFU treatment
rate and clinical pathology of uterine fibroid
There was no significant association between HIFU
treatment rate and age, tumor diameter, tumor volume, fibroid type
and ultrasonic echo intensity (P>0.05); but an association
between HIFU treatment rate and fibroid location, fibroid target
skin distance and ultrasound contrast intensity (P<0.05)
(Table V).
 | Table V.Relationship between HIFU treatment
rate and clinical pathology of uterine fibroid (n, %). |
Table V.
Relationship between HIFU treatment
rate and clinical pathology of uterine fibroid (n, %).
| Factors | Effective
(n=123) | Marked effective
(n=40) | χ2 | P-value |
|---|
| Age (years) |
|
| 0.623 | 0.469 |
|
<35 | 58 (47.15) | 16 (40.00) |
|
|
|
≥35 | 65 (52.85) | 24 (60.00) |
|
|
| Tumor diameter
(cm) |
|
| 0.918 | 0.367 |
|
<4 | 63 (51.22) | 17 (42.50) |
|
|
| ≥4 | 60 (48.78) | 23 (57.50) |
|
|
| Tumor volume
(cm3) |
|
| 1.097 | 0.363 |
|
<54 | 64 (52.03) | 17 (42.50) |
|
|
|
≥54 | 59 (47.97) | 23 (57.50) |
|
|
| Fibroid
location |
|
| 4.478 | 0.036 |
|
Anterior wall/ | 76 (61.79) | 32 (80.00) |
|
|
|
Posterior wall |
| Uterine
fundus | 47 (38.21) | 8 (20.00) |
|
|
| Fibroid target skin
distance (cm) |
|
| 8.223 | 0.006 |
|
<7 | 69 (56.10) | 12 (30.00) |
|
|
| ≥7 | 54 (43.90) | 28 (70.00) |
|
|
| Fibroid type |
|
| 0.505 | 0.553 |
| Muscle
wall | 85 (69.11) | 30 (75.00) |
|
|
|
Non-muscle wall | 38 (30.89) | 10 (25.00) |
|
|
| Ultrasound contrast
intensity |
|
| 14.466 | 0.001 |
| Low
intensity | 24 (19.51) | 19 (47.50) |
|
|
| Equal
intensity | 41 (33.33) | 13 (32.50) |
|
|
| High
intensity | 58 (47.15) | 8 (20.00) |
|
|
| Ultrasonic echo
intensity |
|
| 0.180 | 0.914 |
| Low
echo | 54 (43.90) | 18 (45.00) |
|
|
| Equal
echo | 47 (38.21) | 16 (40.00) |
|
|
| High
echo | 22 (17.89) | 6 (15.00) |
|
|
Analysis of influencing factors for
HIFU treatment rate
Statistically different factors in Table V were used as independent variables
for univariate/multivariate regression analysis. Results of
univariate analysis showed that HIFU treatment rate was associated
with fibroid location, fibroid target skin distance and ultrasound
contrast intensity (P<0.05), but it was not associated with age,
tumor diameter, tumor volume, fibroid type and ultrasonic echo
intensity (P>0.05). Results of multivariate analysis showed that
fibroid location and ultrasound contrast intensity were independent
influencing factors for HIFU treatment rate, and the difference was
statistically significant (P<0.05) (Tables VI and VII).
 | Table VI.Univariate analysis of influencing
factors for HIFU treatment rate. |
Table VI.
Univariate analysis of influencing
factors for HIFU treatment rate.
| Items | OR | 95% CI | P-value |
|---|
| Fibroid
location | 6.453 | 3.562–10.245 | 0.026 |
| Fibroid target skin
distance (cm) | 3.012 | 2.033–11.859 | 0.035 |
| Ultrasound contrast
intensity | 7.965 | 1.754–9.523 | 0.019 |
| Age (years) | 5.168 | 3.468–16.255 | 0.216 |
| Tumor diameter
(cm) | 8.629 | 4.256–12.856 | 0.082 |
| Tumor volume
(cm3) | 8.236 | 5.826–16.544 | 0.071 |
| Fibroid type | 6.102 | 3.719–18.408 | 0.104 |
| Ultrasonic echo
intensity | 7.338 | 2.913–15.742 | 0.069 |
 | Table VII.Multivariate analysis of influencing
factors for HIFU treatment rate. |
Table VII.
Multivariate analysis of influencing
factors for HIFU treatment rate.
| Items | OR | 95% CI | P-value |
|---|
| Fibroid
location | 7.216 | 3.456–9.541 | 0.032 |
| Ultrasound contrast
intensity | 8.015 | 3.568–12.482 | 0.015 |
Discussion
Uterine fibroid is a gynecological disease with a
high incidence, prevalent in menopausal women and lacking typical
clinical symptoms. Only a small proportion of patients of
childbearing age will consult a doctor because of increased
menstrual blood volume (15). For
the treatment of uterine fibroid, myomectomy, interventional
therapy and drug therapy may not be accepted by the majority of
patients, due to risks such as large wound area, long recovery
period, metabolic disorder, decreased immune function, decreased
sexual desire, and easy to reduce the ovarian function (16–18).
Therefore, in clinical practice, the treatment of retaining the
uterus has become the norm.
Results of this study showed that there was no
significant difference in preoperative and postoperative PI and RI
between the two groups (P>0.05); no significant difference
between preoperative PI and postoperative PI in the two groups
(P>0.05), neither RI (P>0.05). It suggests that HIFU
treatment has no effect on endometrial receptivity and it is safe
and effective. There was no significant difference in preoperative
and postoperative LH, FSH and E2 between the two groups
(P>0.05); no significant difference between preoperative LH and
postoperative LH in the same group (P>0.05), or FSH and
E2 (P>0.05). The study results of Fu et al
(19) are different from ours. HIFU
was effective in the treatment of uterine fibroid and can
significantly improve its clinical symptoms. Serum LH level after
treatment was significantly higher than that before treatment
(P<0.05), and serum E2 and FSH levels after treatment
were significantly lower than those before treatment (P<0.05).
It may be due to the large difference in the sample size, and the
control group set up in this study was treated with myomectomy.
However, study of Yang et al only compared HIFU between
before and after treatment (16).
Study of Rueff et al (20)
showed that there was no significant difference in serum FSH level
between before and after treatment with HIFU in uterine fibroid
patients. This is basically consistent with the results of this
study that both LH and FSH slightly improved. The observation group
was grouped through imaging examination according to the ablation
rate after treatment. Results of logistic univariate analysis
showed that HIFU treatment rate was associated with fibroid
location, fibroid target skin distance and ultrasound contrast
intensity (P<0.05), but it was not associated with age, tumor
diameter, tumor volume, fibroid type and ultrasonic echo intensity
(P>0.05). Results of multivariate analysis showed that fibroid
location and ultrasound contrast intensity were independent
influencing factors for HIFU treatment rate, and the difference was
statistically significant (P<0.05). This is basically consistent
with the findings of Donnez et al (21), which showed that the treatment rate
was related to fibroid location. According to reports in the
literature (22), the fibroid is
located on the anterior wall and the anterior uterine fundus can be
better focused on in the lesion, due to the proximity of the
bladder and the short target skin distance; at the same time, it is
far away from the intestinal tract, sacral bone and peripheral
nerves. Therefore, the safety and the tolerance of patients with
anterior wall and anterior uterine fundus are better, and HIFU
treatment rate is also higher. The study results of Chen et
al (23) showed that the higher
the ultrasound contrast intensity was, the richer the perfusion of
uterine fibroid was, resulting in a lower HIFU treatment rate. The
reason may be that after the absorption of ultrasound energy, the
blood flow will leave the focus area of the lesion as the
circulation moves, leading to a decrease in heat accumulation that
is the key of the treatment of uterine fibroid with HIFU (24).
In conclusion, treatment of uterine fibroid with
HIFU has no effect on the patient's endometrial receptivity and sex
hormone level. Fibroid location and ultrasound contrast intensity
are independent risk factors for HIFU treatment. This study
provides guidance for the clinical optimization of treatment
methods and is more conducive to the promotion of HIFU ablation
therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and BG conceived and designed the study. YD, XG
and DS collected and analyzed the data. YC, YD and BG wrote the
manuscript and revised it critically. CX was responsible for
observation indicators analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jining Maternity and Child Care Hospital (Jining, China). Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rizzello A, Franck J, Pellegrino M, De
Nuccio F, Simeone P, Fiore G, Di Tommaso S, Malvasi A, Tinelli A,
Fournier I, et al: A Proteomic Analysis of Human Uterine Myoma.
Curr Protein Pept Sci. 18:167–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nelson AL and Massoudi N: New developments
in intrauterine device use: Focus on the US. Open Access J
Contracept. 7:127–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donnez J and Dolmans MM: Uterine fibroid
management: From the present to the future. Hum Reprod Update.
22:665–686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiaffarino F, Cipriani S, Ricci E, La
Vecchia C, Chiantera V, Bulfoni A and Parazzini F: Alcohol
consumption and risk of uterine myoma: A systematic review and meta
analysis. PLoS One. 12:e01883552017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giudice LC: Potential biochemical markers
of uterine receptivity. Hum Reprod. 14 Suppl 2:3–16. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoozemans DA, Schats R, Lambalk NB,
Homburg R and Hompes PG: Serial uterine artery Doppler velocity
parameters and human uterine receptivity in IVF/ICSI cycles.
Ultrasound Obstet Gynecol. 31:432–438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan AT, Shehmar M and Gupta JK: Uterine
fibroids: Current perspectives. Int J Womens Health. 6:95–114.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo Q, Zhao W, Xu F, Zhang S and Chen P:
Validity of high intensive focused ultrasound in the treatment of
uterus myoma and adenomyoma in Hebei Province: A report of 166
cases. Zhonghua Yi Xue Za Zhi. 95:693–696. 2015.(In Chinese).
PubMed/NCBI
|
|
9
|
Pron G: Magnetic resonance-guided
high-intensity focused ultrasound (MRgHIFU) treatment of
symptomatic uterine fibroids: An evidence-based analysis. Ont
Health Technol Assess Ser. 15:1–86. 2015.
|
|
10
|
Daher S, Massarwa M, Benson AA and Khoury
T: Current and future treatment of hepatocellular carcinoma: An
updated comprehensive review. J Clin Transl Hepatol. 6:69–78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trefoux Bourdet A, Luton D and Koskas M:
Clinical utility of ulipristal acetate for the treatment of uterine
fibroids: Current evidence. Int J Womens Health. 7:321–330.
2015.PubMed/NCBI
|
|
12
|
Yin H, Lo JH, Kim JY, Marsh EE, Kim JJ,
Ghosh AK, Bulun S and Chakravarti D: Expression profiling of
nuclear receptors identifies key roles of NR4A subfamily in uterine
fibroids. Mol Endocrinol. 27:726–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lumsden MA: Modern management of fibroids.
Obstetrics, Gynaecol Reprod Med. 20:82–86. 2010. View Article : Google Scholar
|
|
14
|
Ghobrial FEI, Eldin MS, Razek AAKA, Atwan
NI and Shamaa SSA: Computed tomography assessment of hepatic
metastases of breast cancer with revised response evaluation
criteria in solid tumors (RECIST) criteria (Version 1.1):
Inter-observer agreement. Pol J Radiol. 82:593–597. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen LS and Valle RF: Role of vaginal
sonography and hysterosonography in the endoscopic treatment of
uterine myomas. Fertil Steril. 73:197–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang R, Xu T, Fu Y, Cui S, Yang S and Cui
M: Leiomyomatosis peritonealis disseminata associated with
endometriosis: A case report and review of the literature. Oncol
Lett. 9:717–720. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szkodziak P, Szkodziak F, Trzeciak K and
Czuczwar P: Minimally invasive procedures in the management of
uterine fibroids. Przegl Menopauz. 16:122–125. 2017.
|
|
18
|
Zhou J and Qu F: Treating gynaecological
disorders with traditional Chinese medicine: A review. Afr J Tradit
Complement Altern Med. 6:494–517. 2009.PubMed/NCBI
|
|
19
|
Fu X, Huang F, Chen Y, Deng Y and Wang Z:
Application of dexmedetomidine-remifentanil in high-intensity
ultrasound ablation of uterine fibroids: A randomised study. BJOG.
124 Suppl 3:23–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rueff LE and Raman SS: Clinical and
technical aspects of MR-guided high intensity focused ultrasound
for treatment of symptomatic uterine fibroids. Semin Intervent
Radiol. 30:347–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donnez J, Arriagada P, Donnez O and
Dolmans MM: Emerging treatment options for uterine fibroids. Expert
Opin Emerg Drugs. 23:17–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long L, Chen J, Xiong Y, Zou M, Deng Y,
Chen L and Wang Z: Efficacy of high-intensity focused ultrasound
ablation for adenomyosis therapy and sexual life quality. Int J
Clin Exp Med. 8:11701–11707. 2015.PubMed/NCBI
|
|
23
|
Chen L, ter Haar G, Hill CR, Dworkin M,
Carnochan P, Young H and Bensted JP: Effect of blood perfusion on
the ablation of liver parenchyma with high-intensity focused
ultrasound. Phys Med Biol. 38:1661–1673. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elhelf IAS, Albahar H, Shah U, Oto A,
Cressman E and Almekkawy M: High intensity focused ultrasound: The
fundamentals, clinical applications and research trends. Diagn
Interv Imaging. 99:349–359. 2018. View Article : Google Scholar : PubMed/NCBI
|