Introduction
Total knee arthroplasty (TKA) has become one of the
most effective surgery methods for patients with osteoarticular
diseases (1). Pain, hypoxemia and
vein thrombosis are the most common complications for patients in
post-TKA, which can severely affect patient quality of life
(2–4). The factors involved in the
complications of TKA are diverse, including the intraoperative
state of patients, anesthesia application or nursing (5,6). Nursing
intervention may serve a critical function in the process of TKA,
and can alleviate the pain in post-TKA (7). In addition, the complication of pain in
post-operative TKA still remains a challenge to patients (8). Hence, it will be beneficial to explore
novel nursing methods and to develop effective strategies for
alleviating pain in post-TKA.
Tetramethylpyrazine (TMP) is an alkaloid monomer
that exists in the roots or stems of the Chinese herbal medicine,
Ligusticum wallichii (9).
Previous studies have reported that TMP primarily acts as an
antioxidant, calcium antagonist, inhibitor of platelet aggregation,
promoter of microcirculation and blood clots, and regulator of the
immune response (10,11). TMP has been widely used in the
treatment of cardiovascular disease (12,13).
Furthermore, as an anti-inflammatory factor, TMP serves a critical
function in numerous types of disease. For example, TMP was
identified to reduce the inflammatory mediators induced by amyloid
in rat brain microglia (14), and
the expression of hypoxia-inducible factor 1α and tumor necrosis
factor (TNF)-α were suppressed by TMP in middle cerebral artery
occlusion-induced brain ischemia (15). TMP has also been reported to serve a
function in alleviating pain following surgery, such as chronic
pelvic pain syndrome and neuropathic pain (16,17).
However, the mechanisms by which TMP alleviates pain in
post-surgery patients remain unclear.
In the current study, the clinical data of patients
who received TKA surgery were collected and the effects of nursing
cooperation on the degree of pain post-TKA were evaluated.
Subsequently, a rat TKA model was established and the potential
effects of TMP on the degree of pain were analyzed. A possible
molecular mechanism of TMP was also investigated. The present study
aimed to illustrate the effect of nursing cooperation on pain
degree in patients after TKA and to investigate the potential
benefits of TMP for relieving pain in clinical TKA patients.
Materials and methods
Patient recruitment
A total of 26 patients who received TKA in the
Surgery Center of The First Affiliated Hospital of Harbin Medical
University (Harbin, China) between June 2014 and March 2016 were
enrolled in this study. All the patients provided their informed
consent and the study was approved by the Ethics Committee of The
first Affiliated Hospital of Harbin Medical University, in
accordance with the Declaration of Helsinki.
Nursing cooperation and TKA
surgery
For the procedure of TKA surgery (18), a total of 13 patients received
nursing cooperation (18) and the
surgery was performed as follows: i) Preoperative preparation
included visiting patients, talking with patients to understand
psychological and physical conditions. Furthermore, the nurse
explained the anesthesia, treatment and operation procedure to the
patients, and alleviated anxiety. Also, patients were informed that
the sounds created by the bone hammer, electric saw or electric
drill were normal, and that there was no need to be alarmed. The
nurse gained the patients' trust so that good cooperation could be
obtained during surgery. ii) Circulating nurse: The effective
venous infusion channel and other necessary care were prepared by
the circulating nurse. During anesthesia, under the help of the
circulating nurse, the tracheal intubation or lumbar hemp joint
puncture was performed according to the surgery. Hemostatic start
time was recorded at the beginning of surgery. Patient status was
closely observed to ensure the transfusion was unobstructed, and to
guarantee the strict supervision of aseptic technique. For controls
(n=13), patients received TKA surgery and were cared for using
conventional nursing protocol (18).
Following TKA (n=13), continuous passive motion within 5 sec was
applied for patients at 6, 12, 24 and 48 h. Pain was evaluated
based on the VAS (0, no pain; 10, unbearable sharp pain) (19). ‘Rest’ indicated patients that did not
perform any strenuous physical activity after TAK. ‘Sport’
indicated patients walked after TAK.
Rat model construction and TMP
treatment
This animal experiment was approved by the Animal
Care Committee of Harbin Medical University Medical Center (Harbin,
China). Sprague Dawley rats (n=40) were purchased from Harbin
Medical University Medical Center and bred in the Experimental
Animal Center. In order to construct the TKA model, rats were
anesthetized with 3.5% chloral hydrate (for 5 min) in oxygen and
placed in the supine position. The left knee was then bent by 90°
and firmly supported on either side by the blunt ends of
sterectactic ear bars. Then, a 0.5 cm long skin incision was made
over the patellar tendon under sterile conditions. The fascia over
the muscle and tendon was scraped away with an elevator, then the
lateral side of the tendon was freed from the underlying fascia
with the top of a scalpel blade. Then, the tendon was removed
laterally by ~3 mm and held in place with a retractor.
Subsequently, a hole (1.4 mm diameter, 0.5 mm deep) was generated
using a diamond drill bur, in both the femur and tibia, at 2 mm
above and below the knee joint, respectively. Finally, the
retractor was removed to allow the patella tendon to return to
midline. Then the incision was sutured with nylon and 0.3 ml
penicillin was provided at the incision for preventing bacterial
infection. Rats were randomly divided into TKA+TMP+Interferon γ
group, TKA+TMP group, TKA group, and sham group with 10 rats in
each group. For the TKA + TMP group (17), the holes were immediately filled with
100 mg/kg TMP (batch no. 09110931; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) during surgery. The TKA group was administered
with normal saline at the same dose. Rats in the sham group only
underwent a skin incision. All rats were administered with the same
duration of exposure to chloral hydrate. All rats were allowed to
recover for 36 h before behavioral testing.
Pain threshold assay
Mechanical withdrawal threshold (MWT) was evaluated
using an electronic pain measuring instrument (von Frey filaments,
Stoelting Co., Wood Dale, IL, USA), as previously described
(20). Briefly, the rats in each
group were acclimated in transparent plastic cages with mesh floors
for 30 min. Pressure was applied to the plantar surface of each
hind paw from using calibrated electronic von Frey filaments and
held for 5 sec. The force applied at the time of sharp withdrawal
was recorded. MWT was measured in triplicate and the results were
averaged.
Enzyme-linked immunosorbent assay
(ELISA)
Inflammatory cytokine levels in rat serum, including
IL-6, IL-10 and TNF-α, were assessed using ELISA (21). Briefly, rats were anesthetized
halothane (3% for induction and 1.5% for maintenance; 40 mg/kg) and
0.5 ml trunk blood was collected at 48 h post-surgery among
different groups to avoid any substantial time lag in samples
collection. Blood samples were centrifuged at 600 × g for 15 min at
4°C, and supernatants were stored at −20°C until analysis.
Pre-treated microtiter plates were coated with 50 µg/ml calf thymus
antibodies [IL-6 (cat. no. D6000B), IL-10 (cat. no. R1000), and
TNF-α (cat. no. RTA00); R&D Systems, Inc., Minneapolis, MN,
USA] for 2 h at 37°C, and then stored overnight at 4°C. Then,
plates were washed with PBS buffer containing 0.05% Tween-20 (PBST)
3 times, followed by blocking with 5% goat serum (Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany) in PBST for 1 h. Then, serum
samples [diluted at 1:100 in PBST containing 10% calf serum
(Sigma-Aldrich, Merck KGaA) and 5% goat serum] were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
(Sigma-Aldrich, Merck KGaA) for 2 h at 37°C. The reaction was
blocked with 2N H2SO4 and absorbance was
measured using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 492 nm.
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
Total mRNA from rat serum was isolated using TRIzol
reagent as previously described (22). cDNA was produced using reverse
transcriptase (iScript™ cDNA Synthesis kit; Bio-Rad
Laboratories, Inc.) according to manufacturer's protocol. The
expression levels of mRNAs were measured by SYBR Green-based qPCR
(SYBR Green Master mix, Thermo Fisher Scientific, Inc.) with the
following conditions: 95°C for 10 min, 40 cycles of 95°C for 30 sec
and 58°C for 30 sec. GAPDH was used as the internal control.
Primers used for target amplification were as follows: JAK, sense:
5′-GAAGGAATGGGATTGACGAT-3′, antisense: 5′-GCAGCCTGTGTTGTTGTCTT-3′;
STAT3, sense: 5′-ACCAGCAGTATAGCCGCTTC-3′, antisense:
5′-GCCACAATCCGGGCAATCT-3′; and GAPDH, sense:
5′-TATGATGATATCAAGAGGGTAGT-3′, anti-sense:
5′-TGTATCCAAACTCATTGTCATAC-3′. Relative expression levels of genes
were analyzed using the 2−ΔΔCq method (23).
Western blot analysis
Synovial tissues of knee were isolated from rats and
lysed with radioimmunoprecipitation assay lysis buffer (Sangon
Biotech Co., Ltd., Shanghai, China) containing phenylmethanesufonyl
fluoride (Sigma-Aldrich; Merck KGaA, Germany), then centrifuged at
10,500 r/g for 10 min at 4°C. The supernatant was collected for the
measurement of protein concentration using a BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). For western blotting, 30
µg protein per cell lysate was subjected to 12% sodium
dodecylsulfate-polyacrylamide gel electrophoresis, then transferred
onto polyvinylidene difluoride (PVDF) membranes. The PVDF membranes
were blocked in Tris-buffered saline with Tween-20 (TBST)
containing 5% non-fat milk for 1 h at room temperature. The
membranes were incubated with rabbit anti-JAK (cat. no. 702434;
1:100 dilution) and -STAT3 (cat. no. 710077; 1:100 dilution;
Invitrogen; Thermo Fisher Scientific, Inc.) and mouse anti-GAPDH
antibody (cat. no. MA5-15738-1MG; 1:5,000 dilution; Invitrogen;
Thermo Fisher Scientific, Inc.) overnight at 4°C. Then, the
membranes were incubated with HRP-conjugated goat anti-rabbit (cat.
no. 62272) or -mouse (cat. no. 35518) secondary antibody (1:1,000
dilution; Invitrogen, Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Finally, the PVDF membranes were washed 3
times with 1X TBST buffer (10 min/wash). The signals were detected
after incubation with a chromogenic substrate using the enhanced
chemiluminescence reagent kit (EMD Millipore, Bedford, MA, USA).
GAPDH served as the internal control. Rats treated with 10 ng/ml
interferon (IFN)-γ for 48 h (Sigma-Aldrich; Merck KGaA) were
introduced as an additional control group.
Statistical analysis
All experiments were independently conducted 3
times. All statistical analyses were performed on GraphPad Prism
5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are
expressed as the mean ± standard error of the mean. Independent
sample t-test was used to calculate the difference between two
groups. One-way analysis of variance with Tukey's post hoc test was
used to calculate the difference among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Nursing cooperation assists in
alleviating pain in post-TKA
To analyze whether nursing cooperation could assist
in alleviating pain in post-TKA, the VAS pain scores from patients
who received TKA surgery were assessed (Table I). The results indicated that
following surgery, the VAS pain score was significantly higher at 6
and 12 h in the control group (~3.4 at 6 h;~4.3 at 12 h) compared
with the nursing cooperation group (~2.1 at 6 h;~3.1 at 12 h;
P<0.05). At 24 and 48 h post-TKA surgery, the VAS pain scores
were separated into two subgroups: Rest group and sport group. At
each time point, the pain score at rest status was lower compared
with sport status. In addition, the VAS pain scores for patients in
the nursing cooperation groups were lower compared with the control
group at 24 and 48 h for the rest and sport subgroups (all
P<0.05). These data suggested that nursing cooperation for TKA
may serve a critical function in determining pain in patients
following TKA surgery.
 | Table I.VAS pain scores for patients following
TKA surgery in two groups. |
Table I.
VAS pain scores for patients following
TKA surgery in two groups.
|
| Time after surgery
(h) |
|---|
|
|
|
|---|
|
|
|
| 24 | 48 |
|---|
|
|
|
|
|
|
|---|
| Group | 6 | 12 | Rest | Sport | Rest | Sport |
|---|
| Control | 3.4±0.4 | 4.3±1.2 | 2.3±0.8 | 4.8±1.2 | 1.9±0.7 | 2.7±0.7 |
| Nursing
cooperation | 2.1±0.3a | 3.1±0.9a | 1.4±0.9a | 2.9±0.8a | 0.7±0.6a | 1.5±0.6a |
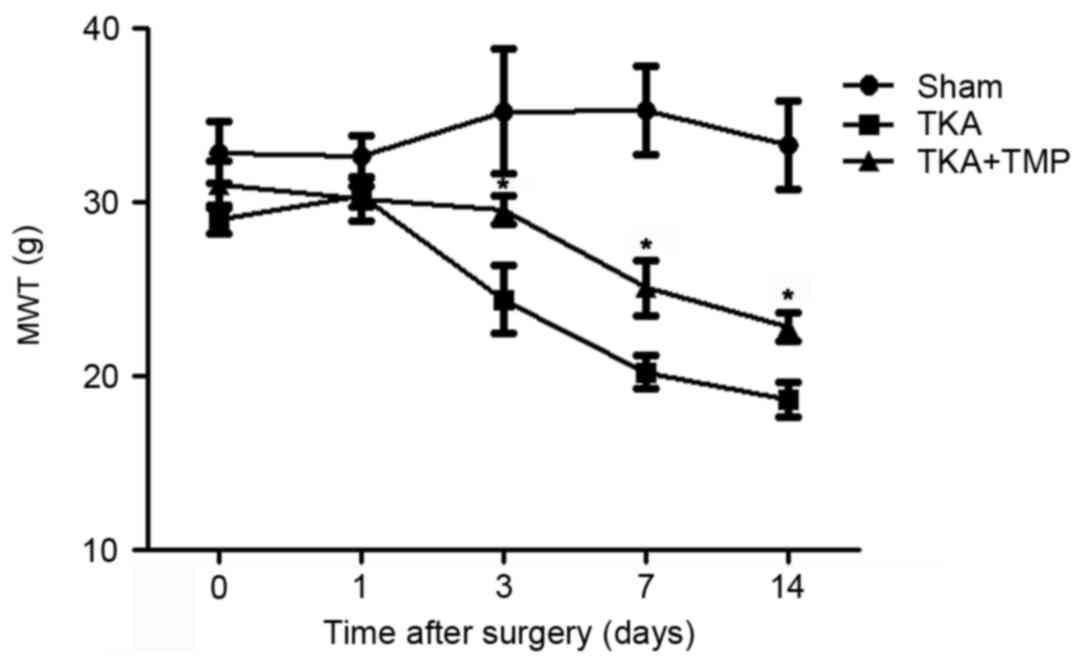
TMP alleviates pain in rats undergoing
TKA
Pain in post-TKA has become a common issue for
patients in the clinic, yet there are few useful strategies for
treating this problem. To investigate the effects of TMP on the
pain of patients undergoing TKA, a TKA rat model was established.
The effect of TMP on rat pain following TKA surgery at 1, 3, 7 and
14 days was evaluated by the mechanical stimulation method
(Fig. 1). MWT decreased over time
following surgery in TKA rats compared with the sham group.
However, MWT was significantly increased by the application of TMP
at 3, 7 and 14 days after surgery (P<0.05).
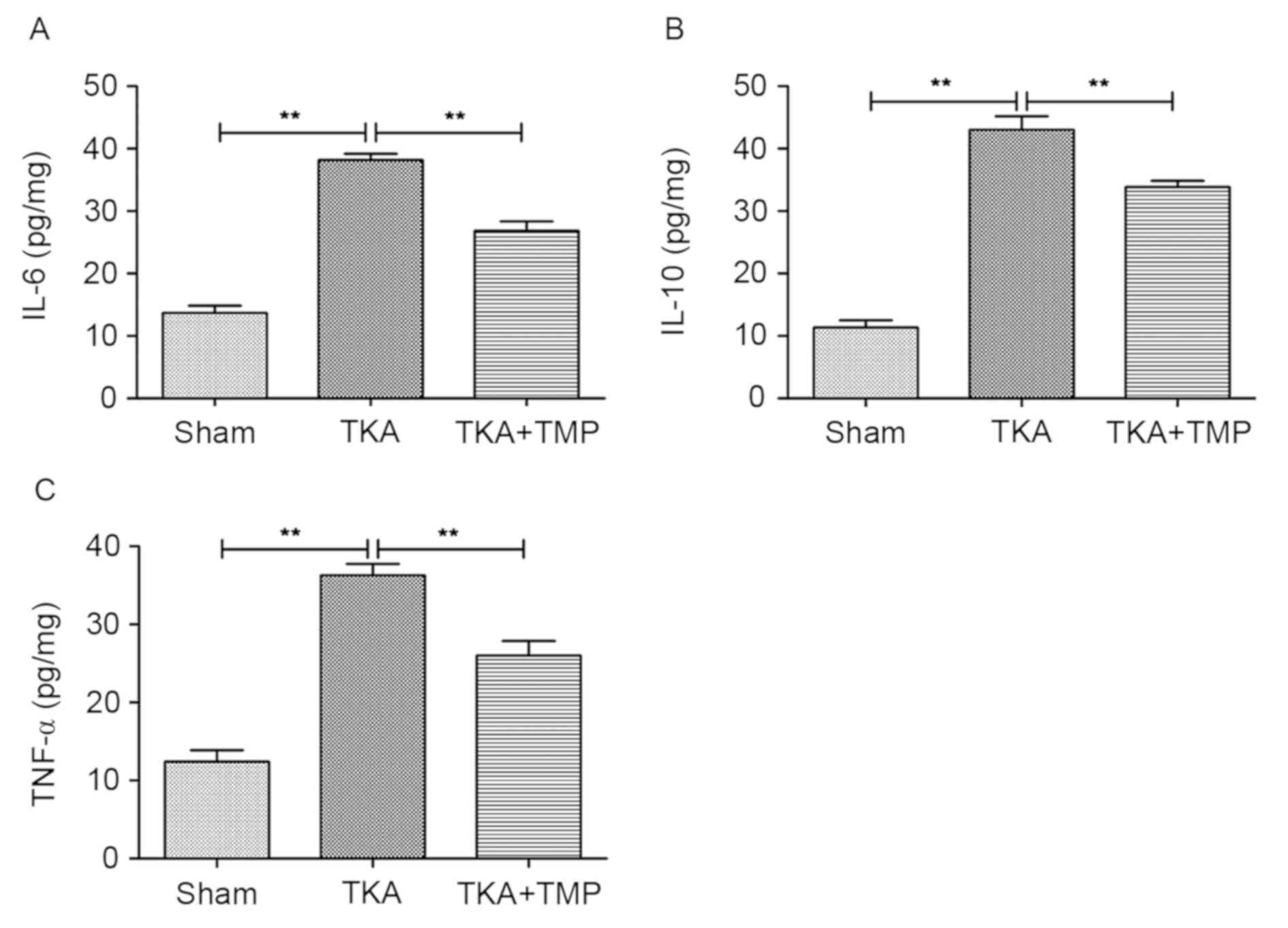
TMP reduces inflammatory cytokine
secretion
To analyze the effects of TMP on inflammation in
rats following TKA, the levels of cytokines IL-6, IL-10 and TNF-α
were detected using ELISA (Fig. 2).
The results indicated that the levels of the three cytokines were
significantly increased in TKA rats compared with the sham group
(P<0.01). By contrast, their levels were significantly decreased
in the TMP-treated group compared with the TKA group (P<0.01).
However, the levels were still higher compared with the sham group.
These results suggested that TMP application may reduce
inflammatory cytokine secretion to a certain extent following
TKA.
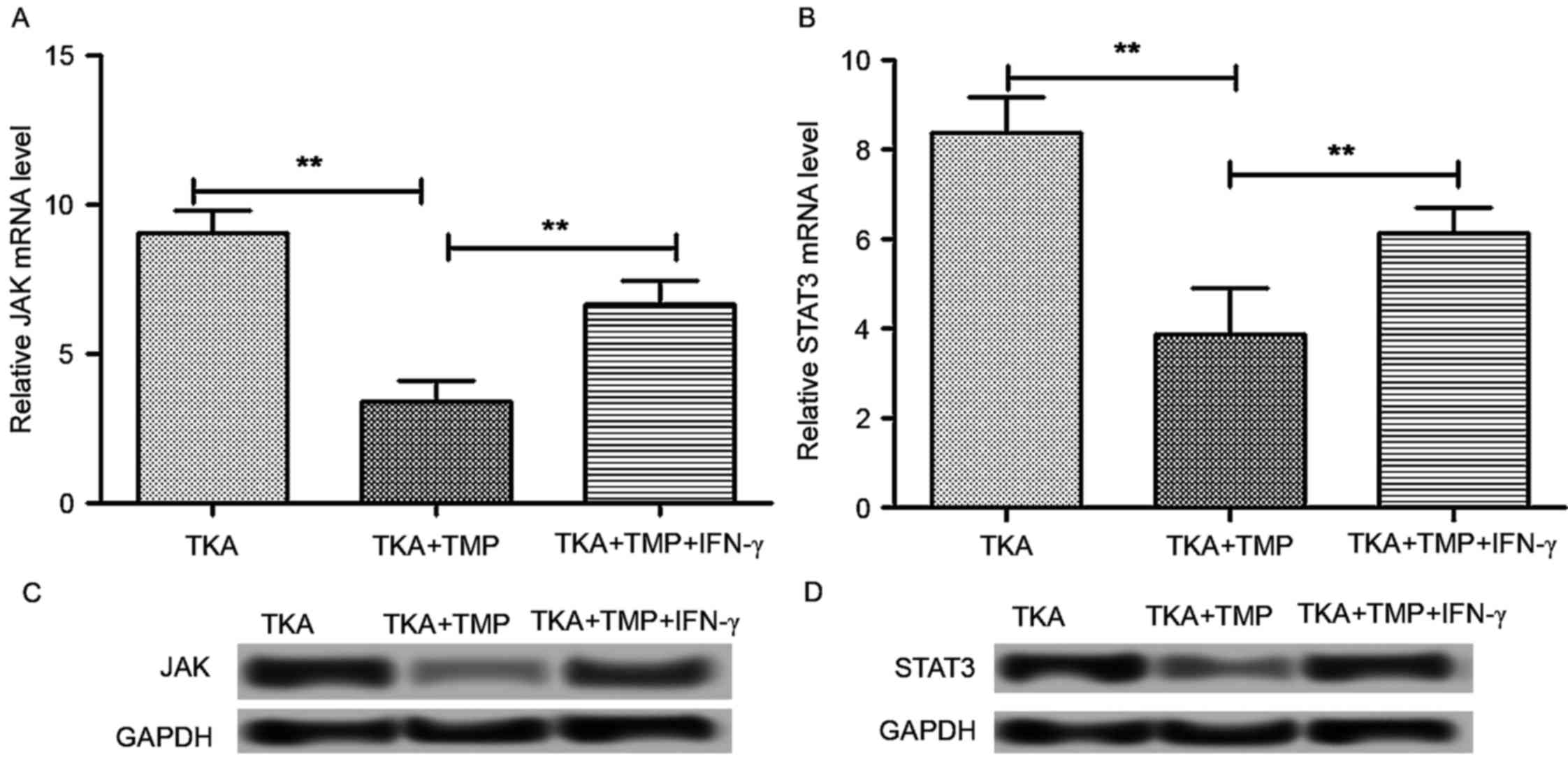
TMP suppresses the activation of
JAK/STAT3 signaling
It has previously been reported that the JAK/STAT3
signaling pathway is involved in mechanisms of pain, including
neuropathic pain (23). Therefore,
it was hypothesized that JAK/STAT3 signaling may be involved in the
effects of TMP on pain following TKA surgery. Tissues from rats at
day 14 post-surgery were used to detect the protein and mRNA
expression of JAK and STAT3 (Fig.
3). The results indicated that the mRNA expression level of JAK
and STAT3 was significantly decreased in TKA rats at 14 days
following application of TMP (P<0.01). The protein expression of
JAK and STAT3 exhibited a similar trend. These results suggested
that TMP inhibits JAK/STAT3 signaling.
In addition, IFN-γ has been demonstrated to be an
activator for JAK/STAT3 signaling (24,25).
Therefore, IFN-γ treatment (10 ng/ml for 48 h) was introduced as an
additional control group to verify the influence of TMP on
JAK/STAT3 signaling. The results indicated that the mRNA expression
of JAK and STAT3 was significantly increased in the TKA + TMP +
IFN-γ group compared with the TKA + TMP group (P<0.01). The
protein expression of JAK and STAT3 exhibited a similar trend.
These results supported the proposal that TMP alleviates pain
through regulating the JAK/STAT3 signaling pathway.
TMP alleviates TKA pain by regulating
the JAK/STAT3 signaling pathway
To further verify the effects of TMP on the
JAK/STAT3 signaling pathway, MWT was measured in rats using
mechanical stimulation (Fig. 4). As
predicted, MWT was markedly decreased in TKA rats compared with the
sham group, while this effect was reversed by the application of
TMP at days 3, 7 and 14 (P<0.05), indicating that TMP suppresses
pain caused by TKA. Additionally, the results revealed that the
effect of TMP on pain caused by TKA was enhanced by the application
of IFN-γ at days 3, 7 and 14 (P<0.05).
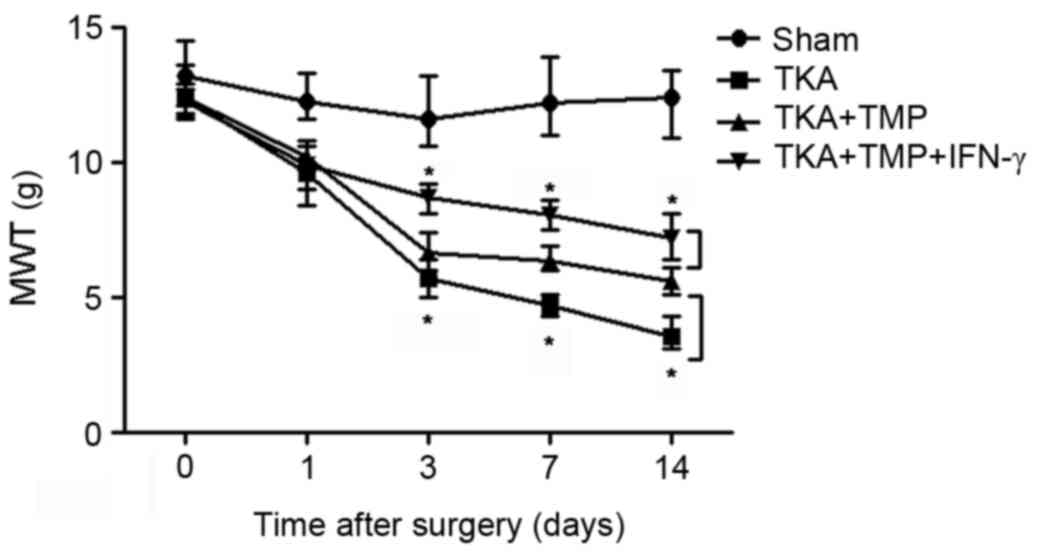 | Figure 4.TMP alleviates pain caused by TKA via
regulating the JAK/STAT3 signaling pathway. MWT was markedly
decreased in rats with TKA compared with the sham group, but TMP
treatment increased MWT significantly compared with the TKA group.
However, the activator of JAK/STAT3 signaling, IFN-γ, enhanced the
effect of TMP on MWT. *P<0.05. TMP, tetramethylpyrazine; TKA,
total knee arthroplasty; MWT, mechanical weight threshold; JAK,
Janus kinase; STAT3, signal transducer and activator of
transcription 3; IFN, interferon. |
Discussion
Accumulating evidence supports TKA as a treatment
strategy for patients who are suffering from osteoarticular
diseases (1,26). However, patients who receive TKA
often experience complications in post-TKA, including pain
(3,4,27). In
the present study, the effect of nursing cooperation on the degree
of pain experienced by patients in post-TKA was evaluated based on
clinical data. Subsequently, a drug treatment was evaluated for the
alleviation of pain caused by TKA and its possible molecular
mechanism was investigated. Consistent with previous evidence
(28), the present results revealed
that the VAS pain score for patients in post-TKA who received
nursing cooperation during TKA was lower compared with the control
group. This indicates a beneficial role of nursing cooperation in
alleviating pain in patients following TKA.
Postoperative analgesia is crucial for patients with
TKA. Accordingly, in the present study, an anti-inflammatory agent,
TMP, was evaluated for treating pain in post-TKA. The major causes
for pain in post-TKA are inflammation, nerve pain or mechanical
stimulation (29). In accordance
with previous studies (30,31), the present data revealed that MWT for
rats in post-TKA was significantly decreased compared with the
control, and levels of inflammatory cytokines were increased
(Figs. 1 and 2). Thompson et al (32) demonstrated that TMP could reduce the
level of IL-10 in post-spinal cord injury. Cytokines, including
IL-6 and TNF-α, were also suppressed by TMP in other inflammatory
conditions, including brain ischemia (15). In the present study, MWT was
increased and levels of IL-10, IL-6 and TNF-α were reduced by TMP
treatment. Therefore, it was speculated that TMP could alleviate
pain and inhibit inflammation in post-TKA.
Furthermore, the possible molecular mechanism of TMP
in alleviating pain was explored. The JAK/STAT3 signaling pathway
is not only involved in various biological processes, including
cell stress, growth, differentiation and anti-inflammatory effects,
but is also involved in the signal transduction of numerous
anti-inflammatory factors (33).
Molet et al (34) reported
that activation of the microglial JAK/STAT3 signaling pathway
promoted nerve pain in spinal neurons and astrocytes, suggesting
that activation of JAK/STAT3 is an indicator of nerve pain.
Similarly, in the present study, the expression of JAK or STAT3 was
higher in rats with TKA compared with the control, indicating that
JAK/STAT3 signaling is involved in the pain following TKA. The
effects of TMP on pain via the JAK/STAT3 signaling pathway have not
been fully discussed previously. However, Gao et al
(35) demonstrated that TMP
inhibited JAK/STAT3 signal transduction in cardiomyocyte
hypertrophy. In the present study, the expression of JAK/STAT3
protein was suppressed by the application of TMP, implying that TMP
alleviates pain in post-TKA by inhibiting the JAK/STAT3 signaling
pathway. In addition, IFN-γ is an inducer of JAK/STAT3 signaling,
and its application can result in inflammation or pain occurrence
(36). In the present study,
application of IFN-γ increased MWT in post-TKA rats, which
indicated an enhanced effect of TMP on JAK/STAT3 signaling for
alleviating pain.
In summary, the data presented in the present study
suggests that nursing cooperation may serve a critical function
during the process of TKA and may reduce the degree of pain
experienced by patients following TKA. In addition, the present
study suggests that TMP may alleviate pain following TKA via
suppressing the JAK/STAT3 signaling pathway. The current study
highlights the importance of nursing cooperation during TKA, and a
possible application of TMP for the clinical treatment of pain in
post-TKA. However, further studies are required to explore the
detailed mechanism of TMP in alleviating pain for patients
following TKA.
References
|
1
|
Wakabayashi H, Takigawa S, Hasegawa M,
Kakimoto T, Yoshida K and Sudo A: Polyarticular late infection of
total joint arthroplasties in a patient with rheumatoid arthritis
treated with anti-interleukin-6 therapy. Rheumatology (Oxford).
53:1150–1151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eskander MS, Mcphee E, Eskander JP,
Nascimento R, Mccormick JJ, Hao S, Shepro D and Johnson K: A left
knee wound complication by non-Hodgkins lymphoma in bilateral total
knee arthroplasties. Arch Orthop Trauma Surg. 128:1387–1390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belmont PJ Jr, Goodman GP, Waterman BR,
Schoenfeld AJ and Bader JO: Thirty-day postoperative complications
and mortality following total knee arthroplasty: Incidence and risk
factors among a national sample of 15,321 patients. J Bone Joint
Surg Am. 96:20–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pugely AJ, Martin CT, Gao Y,
Mendoza-Lattes S and Callaghan JJ: Differences in short-term
complications between spinal and general anesthesia for primary
total knee arthroplasty. J Bone Joint Surg Am. 95:193–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nuelle DG and Mann K: Minimal incision
protocols for anesthesia, pain management, and physical therapy
with standard incisions in hip and knee arthroplasties: The effect
on early outcomes. J Arthroplasty. 22:20–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allepuz A, Espallargues M, Moharra M,
Comas M and Pons JM: Research Group on Support Instruments-IRYSS
Network: Prioritisation of patients on waiting lists for hip and
knee arthroplasties and cataract surgery: Instruments validation.
BMC Health Serv Res. 8:752008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hejblum G, Atsou K, Dautzenberg B and
Chouaid C: Cost-benefit analysis of a simulated institution-based
preoperative smoking cessation intervention in patients undergoing
total hip and knee arthroplasties in France. Chest. 135:477–483.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharkey PF, Lichstein PM, Chao S, Tokarski
AT and Parvizi J: Why are total knee arthroplasties failing
today-has anything changed after 10 years? J Arthroplasty.
29:1774–1778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni X, Wong SL, Wong CM, Lau CW, Shi X, Cai
Y and Huang Y: Tetramethylpyrazine protects against hydrogen
peroxide-provoked endothelial dysfunction in isolated rat aortic
rings: Implications for antioxidant therapy of vascular diseases.
Evid Based Complement Alternat Med 2014. 6271812014.
|
|
10
|
Gaetano GD, Bottecchia D and Vermylen J:
Retraction of reptilase-clots in the presence of agents inducing or
inhibiting the platelet adhesion-aggregation reaction. Thromb Res.
2:71–84. 1973. View Article : Google Scholar
|
|
11
|
Sarkar S, Freedman J and Varghese S:
Methods and kits for sustaining eNOS activity to inhibit platelet
aggregation, and clot retraction, and promote fibrinolysis.
Journal. 2011.
|
|
12
|
Wang GF, Shi CG, Sun MZ, Wang L, Wu SX,
Wang HF, Xu ZQ and Chen DM: Tetramethylpyrazine attenuates
atherosclerosis development and protects endothelial cells from
ox-LDL. Cardiovasc Drugs Ther. 27:199–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li WS, Yang C, Shan L, Zhang Z, Wang Y,
Kwan YW, Lee SM, Hoi MP, Chan SW, Cheung AC, et al: Relaxation
effect of a novel Danshensu/tetramethylpyrazine derivative on rat
mesenteric arteries. Eur J Pharmacol. 761:153–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim M, Kim SO, Lee M, Lee JH, Jung WS,
Moon SK, Kim YS, Cho KH, Ko CN and Lee EH: Tetramethylpyrazine, a
natural alkaloid, attenuates pro-inflammatory mediators induced by
amyloid β and interferon-γ in rat brain microglia. Eur J Pharmacol.
740:504–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang Y, Hsiao G, Chen SH, Chen YC, Lin
JH, Lin KH, Chou DS and Sheu JR: Tetramethylpyrazine suppresses
HIF-1alpha, TNF-alpha, and activated caspase-3 expression in middle
cerebral artery occlusion-induced brain ischemia in rats. Acta
Pharmacol Sin. 28:327–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanders HR and Albitar M: TMPRSS2 for the
diagnosis of prostate disease. Journal. 2013.
|
|
17
|
Leng YF, Gao XM, Wang SX and Xing YH:
Effects of tetramethylpyrazine on neuronal apoptosis in the
superficial dorsal horn in a rat model of neuropathic pain. Am J
Chin Med. 40:1229–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macario A, Schilling P, Rubio R and
Goodman S: Economics of one-stage versus two-stage bilateral total
knee arthroplasties. Clin Orthop Relat Res. 414:149–156. 2003.
View Article : Google Scholar
|
|
19
|
Kelly AM: The minimum clinically
significant difference in visual analogue scale pain score does not
differ with severity of pain. Emerg Med J. 18:205–207. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vivancos GG, Verri WA Jr, Cunha TM, Schivo
IR, Parada CA, Cunha FQ and Ferreira SH: An electronic
pressure-meter nociception paw test for rats. Braz J Med Biol Res.
37:391–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li P, Xie TB, Yi ZR, Wei LL, Wang DM,
Zhang JY, Ou X, Chen F and Luo X: Research on detection results of
aflatoxin M1 enzyme-linked immune sorbent assay detection kit. J
Food Saf Qual. 6:2297–2302. 2015.
|
|
22
|
Rancourt MF, Kemp KA, Plamondon SM, Kim PR
and Dervin GF: Unicompartmental knee arthroplasties revised to
total knee arthroplasties compared with primary total knee
arthroplasties. J Arthroplasty. 27 (8 Suppl):S106–S110. 2012.
View Article : Google Scholar
|
|
23
|
Dominguez E, Rivat C, Pommier B, Mauborgne
A and Pohl M: JAK/STAT3 pathway is activated in spinal cord
microglia after peripheral nerve injury and contributes to
neuropathic pain development in rat. J Neurochem. 107:50–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takaoka A, Tanaka N, Mitani Y, Miyazaki T,
Fujii H, Sato M, Kovarik P, Decker T, Schlessinger J and Taniguchi
T: Protein tyrosine kinase Pyk2 mediates the Jak-dependent
activation of MAPK and Stat1 in IFN-gamma, but not IFN-alpha,
signaling. EMBO J. 18:2480–2488. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim J, Yoon Y, Jeoung D, Kim YM and Choe
J: Interferon-γ stimulates human follicular dendritic cell-like
cells to produce prostaglandins via the JAK-STAT pathway. Mol
Immunol. 66:189–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caplan N and Kader DF: A comparison of
four models of total knee-replacement prostheses. Banaszkiewicz P
and Kader D; Classic Papers in Orthopaedics, : Springer. (London).
169–171. 2014.
|
|
27
|
Cobb JP: Patient safety after partial and
total knee replacement. Lancet. 384:1405–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akyol O, Karayurt O and Salmond S:
Experiences of pain and satisfaction with pain management in
patients undergoing total knee replacement. Orthop Nurs. 28:79–85.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanavalee A, Honsawek S, Rojpornpradit T,
Sakdinakiattikoon M and Ngarmukos S: Inflammation related to
synovectomy during total knee replacement in patients with primary
osteoarthritis: A prospective, randomised study. J Bone Joint Surg
Br. 93:1065–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balandraud N, Meynard JB, Auger I, Sovran
H, Mugnier B, Reviron D, Roudier J and Roudier C: Epstein-Barr
virus load follow up in rheumatoid arthritis patients treated with
TNF-α inhibitors. Arth Res Ther. 6:912004. View Article : Google Scholar
|
|
31
|
Berbari E, Mabry T, Tsaras G, Spangehl M,
Erwin PJ, Murad MH, Steckelberg J and Osmon D: Inflammatory blood
laboratory levels as markers of prosthetic joint infection: A
systematic review and meta-analysis. J Bone Joint Surg Am.
92:2102–2109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thompson CD, Zurko JC, Hanna BF,
Hellenbrand DJ and Hanna A: The therapeutic role of interleukin-10
after spinal cord injury. J Neurotrauma. 30:1311–1324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee C, Lim HK, Sakong J, Lee YS, Kim JR
and Baek SH: Janus kinase-signal transducer and activator of
transcription mediates phosphatidic acid-induced interleukin
(IL)-1beta and IL-6 production. Mol Pharmacol. 69:1041–1047.
2006.PubMed/NCBI
|
|
34
|
Molet J, Mauborgne A, Diallo M, Armand V,
Geny D, Villanueva L, Boucher Y and Pohl M: Microglial Janus
kinase/signal transduction and activator of transcription 3 pathway
activity directly impacts astrocyte and spinal neuron
characteristics. J Neurochem. 136:133–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao MH, Zhang L, Li B, Ren SR and Zhang B:
Effect of tetramethylpyrazine on JAK-STAT signal transduction in
cardiomyocyte hypertrophy. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
27:519–521, 524. 2011.(In Chinese). PubMed/NCBI
|
|
36
|
Delgado M: Inhibition of interferon (IFN)
gamma-induced Jak-STAT1 activation in microglia by vasoactive
intestinal peptide: Inhibitory effect on CD40, IFN-induced
protein-10, and inducible nitric-oxide synthase expression. J Biol
Chem. 278:27620–27629. 2003. View Article : Google Scholar : PubMed/NCBI
|