Introduction
Osteoporosis is a common metabolic bone disease
characterized by decreased bone mass, reduced bone density and
destruction of the microstructure of the bone, which leads to
consequent increase in bone fragility (1). Its occurrence and development is an
extremely complicated biological process involves in multiple
factors, multi-genes and multi-stage experiences (2–4).
Although some osteoporosis-related genes and pathways have been
revealed by many scholars (5,6), the
underlying pathogenesis of osteoporosis still remains obscure.
Bisphosphonates (BO), which can inhibit bone
resorption, are currently the most commonly used and effective
drugs in the treatment of osteoporosis. However, it has been
reported to have severe side effects, such as
bisphosphonate-related osteonecrosis of the jaw (7). Therefore, understanding the molecular
mechanism of osteoporosis, identifying important pathways and genes
and taking these signaling pathways as targeting points are
important strategies to develop new anti-osteoporosis drugs or
prevent osteoporosis. Current advances in high-throughput
experimental techniques are of great help in accelerating the
identification of the key genes and pathways involved in
osteoporosis (8,9).
The Markov chain is a kind of stochastic process
with the so-called ‘Markov property’. The process has the following
characteristics: The next state only depends on the current state,
not related to the previous state. Markov chain integral cleverly
disassembles analytic integrals into a probabilistic expectation
problem and obtains approximate expectations through a large number
of samples. Gibbs sampling is a very popular Bayesian method
(10). It is assumed that the
subjects studied have a certain understanding before sampling, and
the prior distribution is often used to describe this
understanding. Then, based on the extracted samples, the prior
knowledge is corrected to obtain the posterior distribution. The
inference of various statistics are based on the posterior
distribution (11). According to the
probability distributions, researchers can find disturbed pathways
and genes in the pathology of complex diseases. In this study, we
attempted to predict the criticality pathways and genes related to
osteoporosis by Markov methods and Gibbs sampling.
Materials and methods
Data collection
The transcriptomic profiles of osteoclasts treated
with bisphosphonates (E-GEOD-63009) were obtained from the
ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/). A total of 9
samples were selected for analysis. These samples were divided into
two groups: vehicle group (3 samples) and bisphosphonates group (6
simples). After standard preprocessing (12,13), we
converted the expression profile from probe level to gene symbol
level, and removed the duplicated symbols. Finally, a total of
20,514 genes were obtained in each group.
Metabolic pathway enrichment
For the discovery of disturbed pathways associated
with osteoclasts, all human pathways were obtained from the Kyoto
Encyclopedia of Genes and Genomes (KEGG) database (14), including 287 pathways and 6,894
genes. The expression profiling genes were enriched to the KEGG
pathways. The KEGG pathways, with gene intersections ≥5, were
screened out and 280 pathways were obtained.
Pathway initial state build
According to the enrichment of gene in each pathway,
we calculated the average value of gene expression of each pathway
under vehicle condition (state 1) and bisphosphonate condition
(state 2), which is regarded as the expression of this pathway.
State 1 was considered as the initial state of the pathway and
state 2 was considered as a priori value of the path.
According to Markov chain theory, the initial
transition probability is obtained from the expression of state 1
and state 2 in the access system, and state three is derived from
state 2. State n is sequentially deduced, and state n is only
related to the state n-1. The original state of the system has no
relation with other Markov processes.
Gibbs sampling
To obtain a Markov chain, Gibbs sampling was
performed. An empty set of Gibbs sampling was defined first, and
the initial state and priori value data sets were put into the
empty set for Gibbs sampling. A k-dimensional vector (k = 280, 280
KEGG pathways), was initialized one of the elements was extracted
in turn and the posterior value of the element was calculated. This
process was looped k times to generate a new k-dimensional vector,
and defined as state 3. After 10,000 times (n=10,000) Gibbs
sampling, a k-dimensional vector with 10,000 samples is constructed
to obtain a Markov chain, and it is considered that the samples
generated from 2,000 begin to follow the distribution of the real
samples.
Analysis of the disturbed
pathways
Through Gibbs sampling, the posterior probability of
each pathway was obtained. The probability (α) of occurrence
of each pathway was defined as formula 1.
α = ∑i = 200010000Pi10000 − 2000 +1
where Pi represents the posterior
value of this pathway in the ith sample. Based on the
gene expression values under vehicle condition and bisphosphonate
condition, the pathway expression differences between these two
conditions were calculated by t-test. Next, the pathways were
ranked according to the P-values. Combining the rank and values and
α, correction coefficient (c) was calculated as
formula 2.
c = 1 − rankin
In formula 2, n, represents the number of
pathways, ranki represents the rank of pathway
i.
Subsequently, the adjusted probability
(αadj) was calculated. Pathways with
αadj ≥0.75 were screened out and considered as
the differential pathways.
Identifying key genes
After finding the key pathways, we identified the
hub genes from these pathways using Gibbs sampling. The genes in
the key pathways were transformed into Markov chains in order to
identify key genes by Gibbs sampling. Similar with the
identification of key pathways, the posterior probability of each
gene in the key pathways was obtained and the probability of each
gene was calculated using formula one, where Pi
represents the posterior value of this gene in the ith
sample. Similarly, gene expression variation was taken into account
to adjust the probability. According to the expression level of the
genes in the key pathways in different states, the gene expression
differences between vehicle group and bisphosphonates group were
calculated by Student's t-test. Subsequently, the adjusted
probability of the genes in the key pathways was calculated and the
genes were ranked according to the adjusted probability. Genes with
αadj ≥0.75 were screened out and considered as
hub genes.
Results
Data preprocessing and pathway
enrichment
The expression data sets of osteoclasts were
acquired from the ArrayExpress database, containing 6 samples
treated with bisphosphonates and 3 samples not treated by
bisphosphonates. After preprocessing, a total of 20,514 genes were
obtained for the next analysis. Among 287 human pathways obtained
from KEGG database, 280 pathways with at least 5 genes were
selected for the next analysis.
Perturbed pathways in
bisphosphonate-treated osteoclasts
Based on the preprocessed expression data and the
screened pathways, the posterior probability of each pathway was
obtained by Gibbs sampling. The probability α of occurrence
of each pathway was calculated and adjusted. The probability
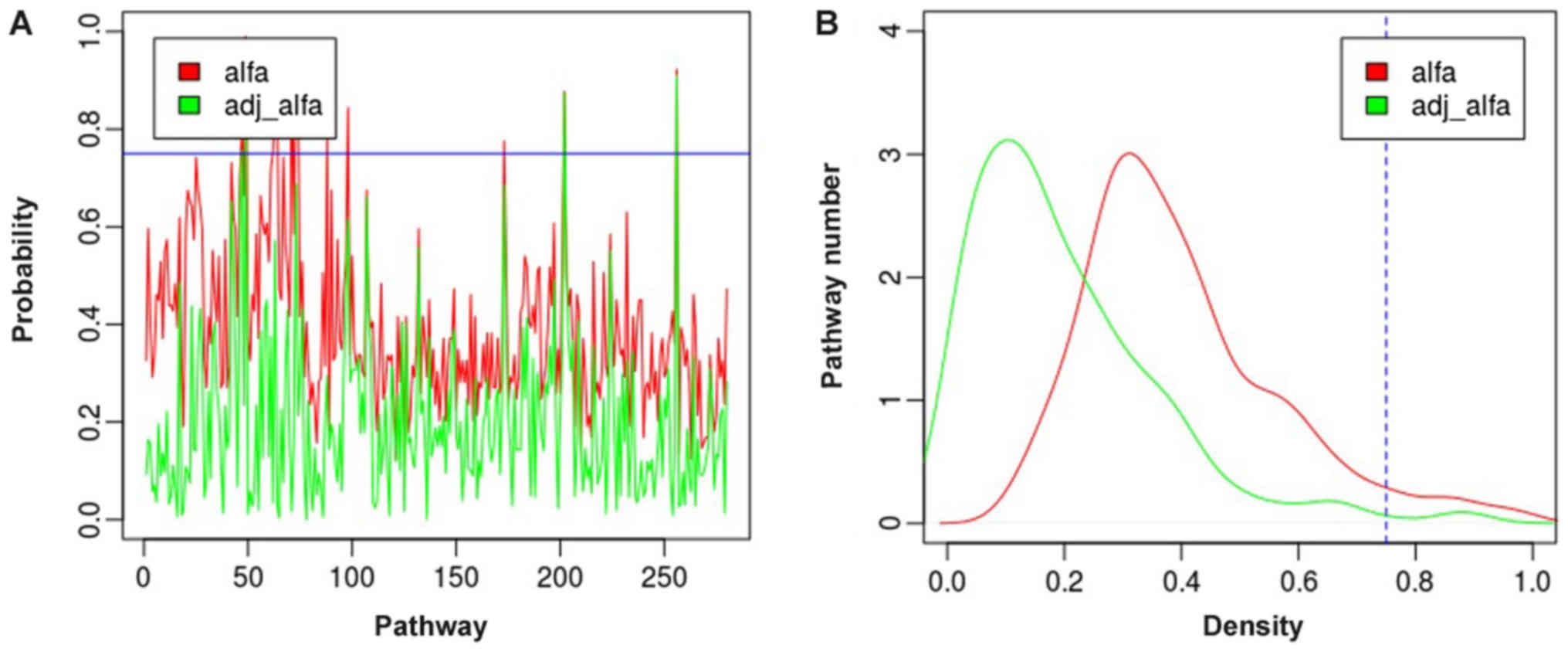
distribution of the 280 pathways is shown in Fig. 1. Under the criterion of
αadj ≥0.75, we obtained 4 pathways in total. The
4 pathways are Maturity onset diabetes of the young (hsa04950,
αadj=0.909), Olfactory transduction (hsa04740,
αadj=0.874), Cyanoamino acid metabolism
(hsa00460, αadj=0.859), Taurine and hypotaurine
metabolism (hsa00430, αadj=0.756), respectively.
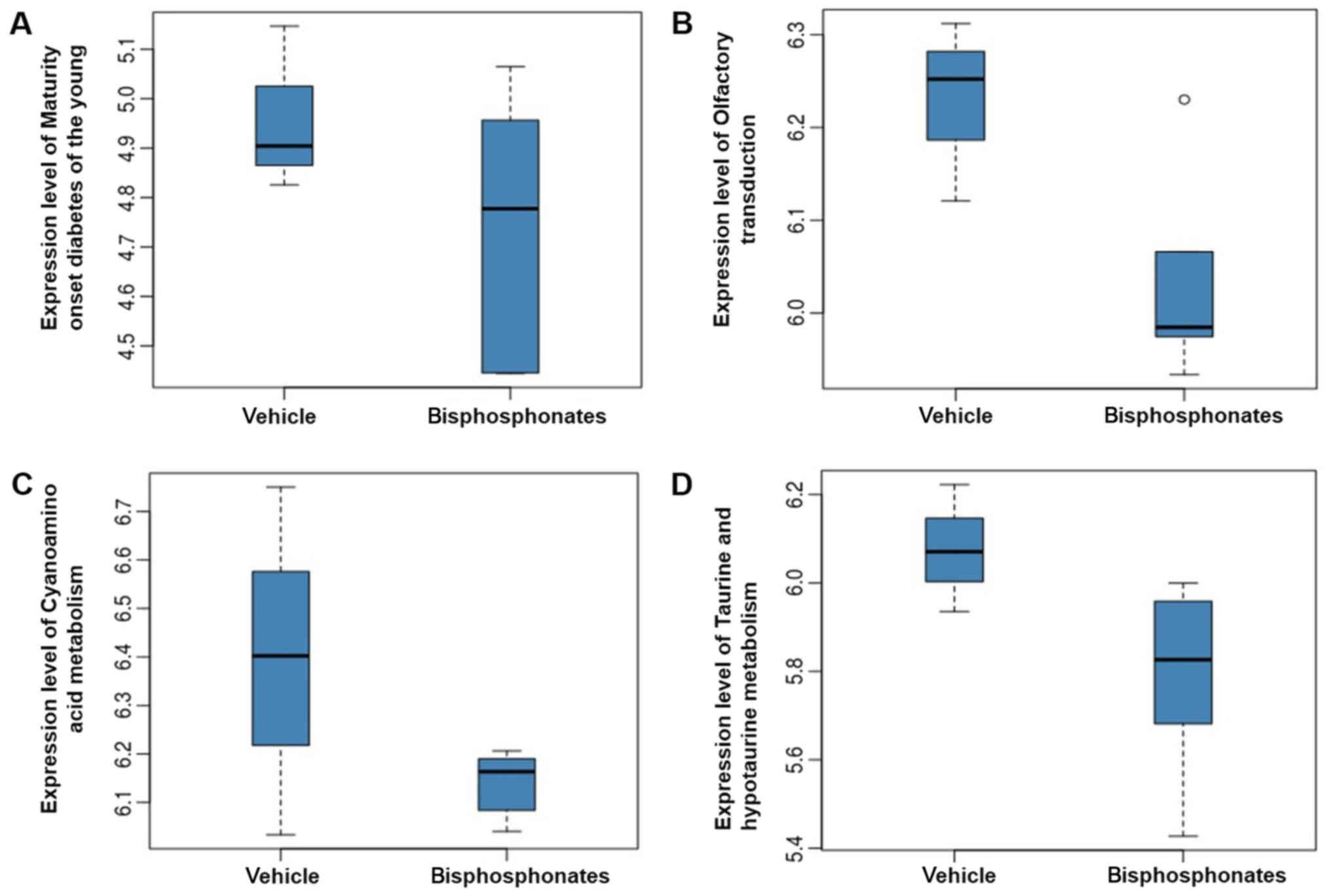
The expression levels of hsa04950, hsa04740, hsa00460 and hsa00430
in vehicle group and bisphosphonates group are shown in Fig. 2. It was obviously observed that the
expression levels of these four pathways were decreased in
bisphosphonate groups relative to the vehicle groups.
Perturbed genes in
bisphosphonate-treated osteoclasts
In order to identify the hub genes in the key
pathways we obtained previously, Gibbs sampling was performed
again. The genes in the key pathways were analyzed and a total of
263 genes were obtained for the next analysis. After Gibbs
sampling, the probability (α) of occurrence of each gene in
these 4 pathways was calculated and adjusted as described in the
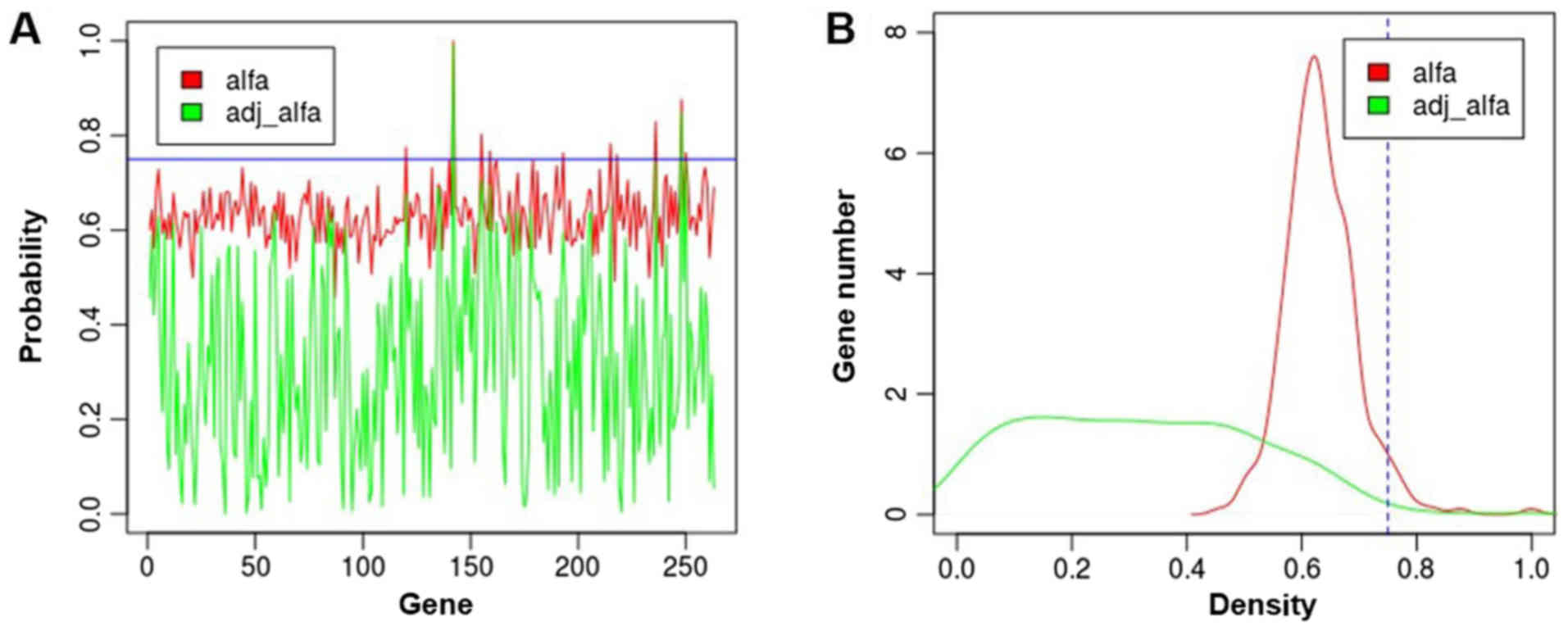
methods. The probability distribution of the 263 genes is shown in
Fig. 3. The top 5 genes ranked
according to αadj are listed in Table I.
 | Table I.The top 5 genes ranked according to
αadj. |
Table I.
The top 5 genes ranked according to
αadj.
| Gene | P-value | Rank p | R-value | α |
αadj |
|---|
| OR2A4 | 0.012 | 2 | 0.992 | 0.999 | 0.992 |
| NKX2-2 | 0.041 | 8 | 0.970 | 0.876 | 0.849 |
| CLCA1 | 0.119 | 27 | 0.897 | 0.829 | 0.744 |
| PAX6 | 0.038 | 7 | 0.973 | 0.763 | 0.743 |
| OR10H2 | 0.131 | 31 | 0.882 | 0.802 | 0.707 |
The genes with αadj ≥0.75 were
selected as hub genes. Finally, two hub genes related to
osteoporosis were screened out, including OR2A4
(αadj=0.992) and NKX2-2
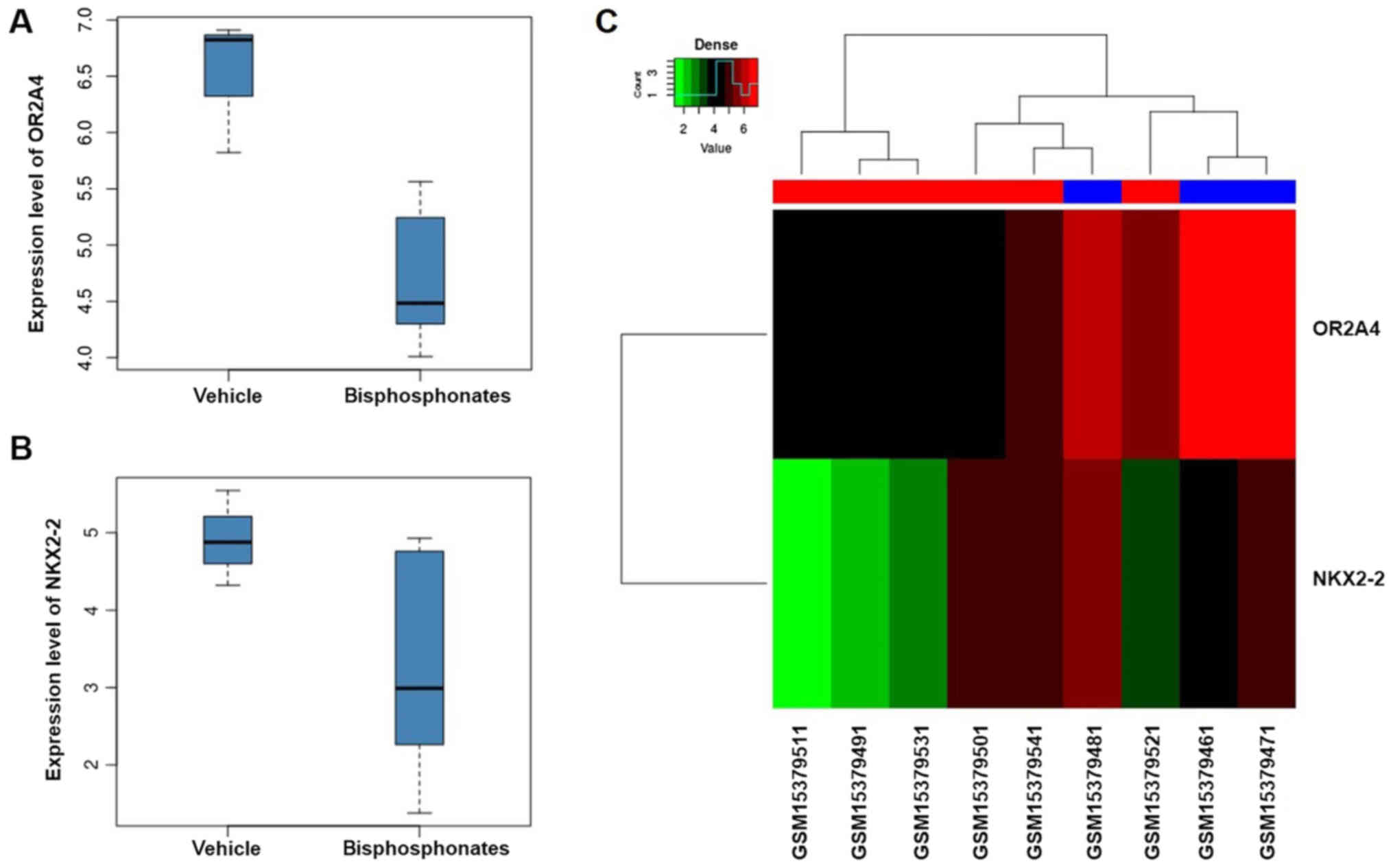
(αadj=0.849). The expression levels of these two
genes in vehicle group and bisphosphonates group are shown in
Fig. 4A and B. We found that both
the expression of OR2A4 and NKX2-2 decreased in
bisphosphonate group compared to vehicle group. The heatmap of two
hub genes is shown in Fig. 4C. We
found that the distribution of these 2 genes in 9 samples was
consistent with the results observed above.
Discussion
In this study, four perturbed biological pathways
(Maturity onset diabetes of the young, Olfactory transduction,
Cyanoamino acid metabolism, Taurine and hypotaurine metabolism),
that may be associated with osteoporosis were identified by Gibbs
sampling. Among these 4 pathways, Maturity onset diabetes of the
young (MODY) pathway showed the highest αadj
value (0.909). MODY is a monogenic form of type 2 diabetes, with
the characteristic of early onset and autosomal dominant
inheritance (15). Six genes are
known to cause MODY, including HNF4α (MODY1),
HNF1α (MODY3), PDX1 (MODY4),
HNF1β (MODY5), NEUROD1 (MODY6) and
glucokinase catalyzes (16). In
bisphosphonates treated osteoclasts, we found that the levels of
HNF4α, PDX1 (MODY4) and HNF1β had almost no
change, while the levels of HNF1α and NEUROD1 were
upregulated. It has been reported that diabetes mellitus can be
complicated by osteoporosis which called diabetic osteoporosis, but
its correlation has not been widely recognized (17). Many researchers have demonstrated
that in type 1 diabetes patients, the bone mass was reduced and
fracture risk was increased. However, in type 2 diabetes, this risk
is controversial (17). Some
scholars have suggested that type 2 diabetes patients may possess a
reduced risk of osteoporosis with an increased bone mineral density
and body weight (18). While, recent
studies have demonstrated that fracture risk increased in type 2
diabetes patients, even though the bone mineral density increased
or was independent (19–21). Here, we found that MODY pathway was
disturbed in osteoclasts treated with bisphosphonates, indicating
that this pathway was associated with the activity of osteoclasts
and played critical roles in the development of osteoporosis.
Furthermore, we identified two hub genes
(OR2A4 and NKX2-2) that may be associated with
osteoporosis using Gibbs sampling. Among these two genes,
NKX2-2 was involved in MODY pathway and gained the second
highest αadj value 0.849. NKX2-2 (named as NKX2.2
or NKX2B), contains a homeobox domain and is a member of NK2 family
(22). This protein has been
suggested to be involved in the neuronal development (23) and the differentiation of pancreatic β
cells. Sussel et al have reported that lacking NKX2.2 caused
an incompletely differentiated state of pancreatic β cells and
NKX2.2 null mice suffered from diabetes immediately after birth due
to lack of insulin (24). Hence
NKX2.2 played a critical role in the development of diabetes which
have a certain relationship with osteoporosis. Furthermore, Smith
et al reported that inhibiting the expression of NKX2.2 by
RNAi could suppress oncogenesis of Ewing's sarcoma (25). This observation implied that NKX2.2
has the potential to serve as a therapeutic target for Ewing's
sarcoma which is a malignant bone tumor composed of small round
cells (25). Although the exact
relationship between MODY pathway and NKX2-2 gene and
osteoporosis has not been reported yet, the decreased expression of
MODY pathway and NKX2-2 in bisphosphonate treated
osteoclasts indicating they may play critical roles in osteoporosis
development.
OR2A4 (olfactory receptor, family 2, subfamily A,
member 4) which gained the highest αadj value
0.992, is a member of olfactory receptors that involves in
olfactory transduction pathway. Olfactory receptors are expressed
in a series of tissues. Tsai et al have demonstrated that
OR2A4 was expressed in human skin cells and affected
cytokinesis and cell proliferation (26). To this date, few studies have
demonstrated the direct association between olfactory transduction
pathway/olfactory receptors and osteoporosis. Besides, the
relationships between osteoporosis and the other two pathways we
identified in this study (Cyanoamino acid metabolism pathway and
Taurine and hypotaurine metabolism pathway) also have limited
studies. Further investigation may reveal new mechanisms of
osteoporosis and provide new targets for osteoporosis
treatment.
In conclusion, we found 4 disturbed pathways
(Maturity onset diabetes of the young, Olfactory transduction,
Cyanoamino acid metabolism, Taurine and hypotaurine metabolism) and
two hub genes (OR2A4 and NKX2-2) in osteoclasts
treated with bisphosphonates using Gibbs sampling. These results
give us a better understanding of the potential mechanism of
bisphosphonate treatment of osteoporosis and the pathogenesis of
osteoporosis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Fujian Province (2016J01604).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ conceived the study and drafted the manuscript.
BZ and FW acquired the data; LZ, BZ and FW analyzed the data. XHC
contributed to the analysis of the data and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maraka S and Kennel KA: Bisphosphonates
for the prevention and treatment of osteoporosis. BMJ.
351:h37832015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ralston SH and Uitterlinden AG: Genetics
of osteoporosis. Endocr Rev. 31:629–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pietschmann P, Rauner M, Sipos W and
Kerschan-Schindl K: Osteoporosis: An age-related and
gender-specific disease - a mini-review. Gerontology. 55:3–12.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seeman E: Bone quality: The material and
structural basis of bone strength. J Bone Miner Metab. 26:1–8.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thouverey C and Caverzasio J: Focus on the
p38 MAPK signaling pathway in bone development and maintenance.
Bonekey Rep. 4:7112015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abu-Amer Y: NF-kappaB signaling and bone
resorption. Osteoporos Int. 24:2377–2386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mukudai Y, Kondo S, Koyama T, Li C, Banka
S, Kogure A, Yazawa K and Shintani S: Potential anti-osteoporotic
effects of herbal extracts on osteoclasts, osteoblasts and
chondrocytes in vitro. BMC Complement Altern Med. 14:292014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Z, Gao M, Liu Q and Tao MDJ:
Comprehensive transcriptome analysis of mesenchymal stem cells in
elderly patients with osteoporosis. Aging Clin Exp Res. 27:595–601.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Zoelen EJ, Duarte I, Hendriks JM and
van der Woning SP: TGFβ-induced switch from adipogenic to
osteogenic differentiation of human mesenchymal stem cells:
Identification of drug targets for prevention of fat cell
differentiation. Stem Cell Res Ther. 7:1232016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lavine M, West M and Praeger A: A Bayesian
method for classification and discrimination. Can J Stat.
20:451–461. 1992. View
Article : Google Scholar
|
|
11
|
Mclachlan GJ and Krishnan T: The EM
algorithm and extensions. 2nd. John Wiley & Sons; Hoboken, NJ:
2007
|
|
12
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Irizarry RA, Bolstad BM, Collin F, Cope
LM, Hobbs B and Speed TP: Summaries of affymetrix GeneChip probe
level data. Nucleic Acids Res. 31:e152003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aoki-Kinoshita KF and Kanehisa M: Gene
annotation and pathway mapping in KEGG. Methods Mol Biol.
396:71–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamagata K, Furuta H, Oda N, Kaisaki PJ,
Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M and Bell GI:
Mutations in the hepatocyte nuclear factor-4alpha gene in
maturity-onset diabetes of the young (MODY1). Nature. 384:458–460.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winckler W, Weedon MN, Graham RR,
McCarroll SA, Purcell S, Almgren P, Tuomi T, Gaudet D, Boström KB,
Walker M, et al: Evaluation of common variants in the six known
maturity-onset diabetes of the young (MODY) genes for association
with type 2 diabetes. Diabetes. 56:685–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leidig-Bruckner G, Grobholz S, Bruckner T,
Scheidt-Nave C, Nawroth P and Schneider JG: Prevalence and
determinants of osteoporosis in patients with type 1 and type 2
diabetes mellitus. BMC Endocr Disord. 14:332014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heath H III, Melton LJ III and Chu CP:
Diabetes mellitus and risk of skeletal fracture. N Engl J Med.
303:567–570. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janghorbani M, Van Dam RM, Willett WC and
Hu FB: Systematic review of type 1 and type 2 diabetes mellitus and
risk of fracture. Am J Epidemiol. 166:495–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwartz AV, Sellmeyer DE, Ensrud KE,
Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM and Cummings
SR; Study of Osteoporotic Features Research Group: Older women with
diabetes have an increased risk of fracture: A prospective study. J
Clin Endocrinol Metab. 86:32–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strotmeyer ES, Cauley JA, Schwartz AV,
Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de
Rekeneire N, Harris TB, et al: Diabetes is associated independently
of body composition with BMD and bone volume in older white and
black men and women: The Health, Aging, and Body Composition Study.
J Bone Miner Res. 19:1084–1091. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lessnick SL and Owen LA: NKX2-2 (NK2
homeobox 2). Atlas Genet Cytogenet Oncol Haematol. 12:375–376.
2008.
|
|
23
|
Briscoe J, Sussel L, Serup P,
Hartigan-O'Connor D, Jessell TM, Rubenstein JL and Ericson J:
Homeobox gene Nkx2.2 and specification of neuronal identity by
graded Sonic hedgehog signalling. Nature. 398:622–627. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sussel L, Kalamaras J, Hartigan-O'Connor
DJ, Meneses JJ, Pedersen RA, Rubenstein JL and German MS: Mice
lacking the homeodomain transcription factor Nkx2.2 have diabetes
due to arrested differentiation of pancreatic beta cells.
Development. 125:2213–2221. 1998.PubMed/NCBI
|
|
25
|
Smith R, Owen LA, Trem DJ, Wong JS,
Whangbo JS, Golub TR and Lessnick SL: Expression profiling of
EWS/FLI identifies NKX2.2 as a critical target gene in Ewing's
sarcoma. Cancer Cell. 9:405–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai T, Veitinger S, Peek I, Busse D,
Eckardt J, Vladimirova D, Jovancevic N, Wojcik S, Gisselmann G,
Altmüller J, et al: Two olfactory receptors-OR2A4/7 and
OR51B5-differentially affect epidermal proliferation and
differentiation. Exp Dermatol. 26:58–65. 2017. View Article : Google Scholar : PubMed/NCBI
|