Introduction
Septic shock is caused by the immerse release of
inflammatory mediators or cytokines triggered by pathogens and
their released toxin products, which further leads to increased
vascular dilatation and capillary permeability as well as
hypovolemia induced insufficiency of tissue perfusion, thereby
leading to sepsis syndrome associated with shock (1). It has been reported that septic shock
is caused by excessive stimulation of the immune system by
bacterial toxins, resulting in excessive proinflammatory cytokine
production, including tumor necrosis factor-α (TNF-α),
interleukin-l (IL-1) and IL-6 (2).
In the United States, there are approximately 750,000 patients with
septic shock each year and nearly 250,000 of them will die
(3). Although kinds of advanced
methods have been applied to the treatment of septic shock, few
have been able to bring down the high mortality rate of septic
shock. In the intensive care unit (ICU), the in-hospital mortality
rate of the patients with systemic septic shock is 29% (4), and the 30 day mortality rate is up to
54% (5).
When serious microbial infection or extensive tissue
damage occurs, alarmin proteins released from necrotic cells,
together with pathogen associated molecular patterns (PAMPs)
expressed by nonpathogenic bacteria, are collectively referred to
as damage associated molecular patterns (DAMPs). High concentration
of DAMPs will lead to a comprehensive, systematic immune response
(both innate and adaptive immunity are involved), resulting in
inflammatory cascade (6).
Specifically, neutrophils, macrophages and other immune cells
produce and release a large number of cytokines (such as IL-1, IL-6
and TNF-α), chemotactic factors, complement activated products and
other mediators. This proinflammatory environment can lead to more
powerful release of secondary medium (such as lipid factors and
active oxygen). Due to the excessive stimulation of immune cells,
the body produces many abnormal cytokines (called cytokine storm),
which results in the excessive expression of the beneficial
anti-infection response as well as occurrence of destructive
inflammation (7).
Studies have shown that during the progression from
sepsis to septic shock, immunosuppression (including the complete
shutdown of important intracellular signaling pathways and acquired
immune dysfunction) is an important cause of sepsis exacerbation
and even death (8). It is worth
noting that in sepsis, neutrophils may have ‘paralysis of the
immune function’ (9). As important
effector cells of innate immunity, neutrophils was the first
responder that migrate to infection site at early stages of
infection. Neutrophils roll along and adhere to the endothelial
cells, which was mediated by selectin, immunoglobulin superfamily
and integrin molecules (10). Once
transpassing endothelial membrane, neutrophils was guided by
chemokines to move along the gradient towards the inflammatory site
(11), and finally kill pathogenic
bacteria by phagocytosis and release of hyperoxide and protease. It
can be seen that the normal migration of inflammatory cells to
inflammatory sites is an important prerequisite for anti-infection
response.
Septic shock is the result of the host's immune
response to the pathogen. Different pathogen patterns were
identified by pattern recognition receptor family the toll-like
receptors (TLR). Different types of TLR are responsible for
different pathogen groups. For instance, TLR-4 is the main
transmembrane receptor for the transduction of G- bacterial
endotoxin (LPS) signal. Once TLR-4 recognizes LPS, it initiates the
immune process, and increases the TREM-1 expression (12) that can enhance the cytokine cascade
after infection, hence it plays a core role in the occurrence and
development of sepsis (13,14).
Pseudomonas aeruginosa-mannose sensitive
hemagglutinin (PA-MSHA) is a biologic drug that has the function of
bidirectional immunomodulation and killing tumor cells, which has
been widely used in tumor adjuvant therapy (15,16). The
mechanism of PA-MSHA participating in the anti-infection process
has also become a research hot spot in recent years. Recent studies
have found that PA-MSHA is the natural ligand of TLR-4 (17,18),
which can increase the proportion of CD4+
CD25+ Foxp3+ regulatory T cells (Treg) in
peripheral blood cells, thereby regulating immune response
(19,20). Therefore, we speculate that PA-MSHA
may play a role in regulating tissue immunity and inflammation in
septic shock.
In this experiment, PA-MSHA pretreatment was
administered to the rat model of septic shock. The expression of
ascite cytokines, intestinal mucosal cytokines, adhesion molecule
and peripheral blood neutrophils TLR4 was observed. The preventive
effect of PA-MSHA on infective intestinal injury is discussed. Our
study will offer novel therapeutic strategies in septic shock
treatments.
Materials and methods
Animals and grouping
SPF male SD rats (aged 8–10 weeks), weighing 240–260
g, were randomly divided into 3 groups (n=10), the blank control,
the septic shock and the PA-septic shock group. Rats were housed in
a temperature controlled room (21±2°C) with a relative humidity
range from 30 to 40% on a 12:12 h light/dark cycle (lights on at
06:00). All rats had free access to water and food. Each rat of
blank control group was injected subcutaneously with normal saline,
0.3 ml/day for 7 days. The septic shock group was given 0.3 ml/day
by subcutaneous injection of saline injection for 7 days. In
PA-septic shock group, PA-MSHA was injected subcutaneously at 0.3
ml/day. After continuous administration for 7 days, the rat model
of septic shock was established by replicating the CLP model at day
8. After 14.5 h, mean arterial pressure (MAP) of rats were
monitored every 30 min. In this study, when the MAP of rats was
reduced to 70% or below (excluding low blood pressure caused by
other causes such as massive bleeding), it was considered that the
septic shock model preparation succeeded.
This study was approved by the Animal Ethics
Committee of Nanjing First Hospital, Nanjing Medical University
Animal Center (Nanjing, China).
Specimen collecting and testing
Peritoneal lavage and bacterial
culture
After being anesthetized, the rats were placed in
supine position on the sterile operation table. Conventional
surgery area disinfection was conducted. Then incision of the neck
skin along the midline was performed, and the right common carotid
artery was separated layer by layer. After the puncture needle was
fixed, the monitor was connected to monitor the vital signs. The
abdominal cavity was opened, and RPMI-1640 culture fluid was used
to lavage the abdominal cavity with 5 ml/times 4 times. The
irrigation solution was then pipetted into two 10-ml centrifuge
tubes (the recovery rate is >80%, which is defined as the
effective recovery of more than 16 ml). The two 10-ml centrifuge
tubes were separated into tube 1 and 2. Tube 1 was centrifuged at
1,500 × g for 5 min at 20°C and the supernatant was sub-packed into
2 ml EP tubes, preserved at −60°C in a refrigerator. The irrigation
solution in tube 2 was used for bacterial culture.
Abdominal aorta blood sampling
Obtained blood (3 ml) was injected into the blood
culture bottle for blood culture. The residual arterial blood was
added to 4.5% EDTA anticoagulant for neutrophils isolation.
Peripheral blood neutrophils
isolation
Anticoagulant and 2% Dextran solution was mixed at
1:1 volume, gently whirl mixed and left static for 1 h. The white
blood cell suspension in the transparent supernatant was then
collected and pipetted into another tube. After 10 min of
centrifugation at 1,500 × g at 20°C, the supernatant was discarded,
5 ml 0.1% EDTA liquid was added to the white cells of test tube
wall. A total of 1.5 ml 0.1% EDTA liquid was added to washing and
discarded supernatant to make the suspended white cell suspension
in another tube. A total of 2 ml 75% Percoll separation liquid was
added, followed by addition of 2 ml 60% Percoll separation liquid.
Percoll stratification with different densities was observed by the
naked eye. The suspended white cell suspension was gently laid on
the 60% Percoll separation liquid and placed in a horizontal
hanging basket centrifuge. The cloud layer cells were fully
absorbed by the PAP suction tube, and the centrifuge tube was
placed in the tube, and the 0.1% EDTA liquid was centrifuged at
1,500 × g for 3 min at 20°C with 6 ml 2 times. After
centrifugation, the neutrophils were washed and resuspended with 1
ml buffer.
Flow cytometry
Human TruStain FcX™ (cat. no. 422301; BioLegend, San
Diego, CA, USA), used as the blocking solution, was added for
incubation at room temperature for 5 min. The neutrophil suspension
was divided into 2 test tubes, TLR-4 and IgG tube. A total of 2 µl
anti-TLR4 antibody (ab45104; Abcam, Cambridge, MA, USA) was added
into the TLR-4 tube, and 2 µl mouse IgG2b-isotype control (ab91532;
Abcam) added into IgG tube. Goat anti-mouse IgG (H+L)
Cross-Adsorbed secondary antibody (FITC) (cat. no. 31541) was
purchased from Thermo Fisher Scientific, Inc., (Waltham, MA, USA)
and incubated at 20°C for 30 min. Both tubes were incubated in the
dark for 30 min at room temperature. The samples were washed in
phosphate-buffered saline (PBS) 3 times and measurements were
performed by a flow cytometer (FACSCalibur; BD Biosciences, San
Jose, CA, USA). Data were obtained and analyzed using the CellQuest
professional software (BD Biosciences)
Transwell chamber method
The neutrophils were diluted and isolated with the
buffer solution, and the cell density was adjusted to
1×106/ml. The chemotactic solution was prepared by fMLP
and buffer solution at 10−4 M. A total of 600 µl fMLP
chemotaxis solution or buffer solution was added into the lower
chamber of culture plate. The upper chamber of Transwell chamber
was placed into the culture plate with 100 µl diluted neutrophils
injected into the Transwell chamber. The Transwell chamber was
placed at 37°C 5% CO2 incubator and incubated for 60
min. Finally, the Transwell chamber was taken out of the incubator
to terminate the chemotaxis, then fixed for 3 min in methanol,
stained with Wright-Giemsa staining. Stained cells were counted and
photographed by microscopy.
Exploratory laparotomy and specimen
collection
Exploratory laparotomy was conducted to observe the
color and shape of the intestine. Jejunum tissue (4 cm) was removed
at 10 cm away from the distal end to 10 cm of pileus ventriculi.
The tissue was repeatedly washed with normal saline. Blood, feces
and intestinal juice from internal and external side of intestinal
canal were washed away. The tissue was put into the aseptic
cryopreservation tube, then immediately stored in the liquid
nitrogen for preservation.
Detection of IL-1β, IL-6 and TNF-α in
peritoneal lavage fluid and intestinal tissue
The tissue was thawed, rinsed, wiped dry by clean
filter paper, and ground into homogenates. Homoegenates were
centrifuged at 1,300 × g for 6 min at 4°C to harvest the
supernatant. ELISA kit was used for detection of IL-1β, IL-6 and
TNF-α concentration, strictly according to the instructions. Rat
IL-1β ELISA kit (cat. no. 69-30375); rat IL-6 ELISA kit (cat. no.
69-30490) and rat TNF-α ELISA kit (cat. no. 69-25328) were
purchased from Moshake Biotechnology Company (Wuhan, China;
http://www.mskbio.com/index.aspx).
Standard curve was made to calculate the concentration of IL-1β,
IL-6 and TNF-α.
Western blotting detection of TLR-4,
ICAM-1 and VCAM-1 expression in intestinal tissue
Intestinal tissue protein samples were centrifuged
at 10,000 × g for 5 min at 4°C and the supernatant was taken for
electrophoresis. The sample volume was adjusted to equal amount
according to the protein concentration by using the E-PAGE™ loading
buffer (cat. no. EPBUF01; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Proteins (30 μg) were added into per lane for the
electrophoresis. The extracted proteins were separated using a 10%
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE). After electrophoresis, the separated protein was
transferred to the polyvinylidene fluo-ride (PVDF) membrane by 250
mA constant flow membrane. Then the membrane was incubated for 2 h
in Tris-buffered saline-tween (TBST) containing 5% skimmed milk for
blocking. The primary antibody was diluted with TBST and added to
protein samples for overnight incubation at 4°C in a refrigerator.
The samples were then incubated with peroxidase labelled secondary
antibody at room temperature for 2 h. The UVP chemiluminescence
imaging system was used for continuous collection of images. Rabbit
polyclonal TLR4 antibody (cat. no. ab13556; dilution, 1:500);
rabbit monoclonal ICAM1 antibody (cat. no. ab53013; dilution,
1:500); rabbit monoclonal VCAM1 antibody (cat. no. ab134047;
dilution, 1:500); rabbit polyclonal NF-kB p65 antibody (cat. no.
ab16502; dilution, 1:500); rabbit monoclonal IKB alpha antibody
(cat. no. ab32518; dilution, 1:500); rabbit polyclonal GAPDH
antibody (cat. no. ab37168; dilution: 1:500) and secondary goat
anti-rabbit (HRP) IgG antibody (cat. no. ab6721; dilution, 1:2,000)
were all purchased from Abcam (Cambridge, MA, USA). The images were
semi-quantitative analyzed with the light density value of target
protein/GAPDH. The gray value was analyzed using ImageJ software
(Version 1.38; National Institutes of Health, Bethesda, MA,
USA).
Statistical analysis
SPSS19.0 (IBM Corp., Armonk, NY, USA) software was
used for statistical analysis. The measurement data are presented
as mean ± standard deviation (SD). Comparison between groups was
done using οne-way ANOVA test followed by post-hoc test (Least
Significant Difference). P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of septic shock rat
model
Generally, septic shock CLP model in rats was
duplicated successfully in 16–23 h. After 14.5 h replication of
model, the MAP of rats was monitored. At 14.5–15 h, there was no
significant difference in MAP between groups. At 15.5 h, the MAP of
the rats in the septic shock group was lower than that of the blank
control group (P<0.05), but it did not reach 70% of the control
group MAP. At 16 h, when compared with the control group, MAP of
the other 2 groups was significantly decreased (Fig. 1), down to 70% of the blank control
group, indicating that the model of septic shock rat model was
successfully established. In the blank group, the cultured blood
was bacteria negative, while the cultured blood of the other 2
groups were found to be Escherichia coli (+). Moreover, in
the blank group, the bacterial culture of the peritoneal lavage
fluid was negative, whereas the other 4 groups were found to be
Escherichia coli (+), with other heterozygous bacteria.
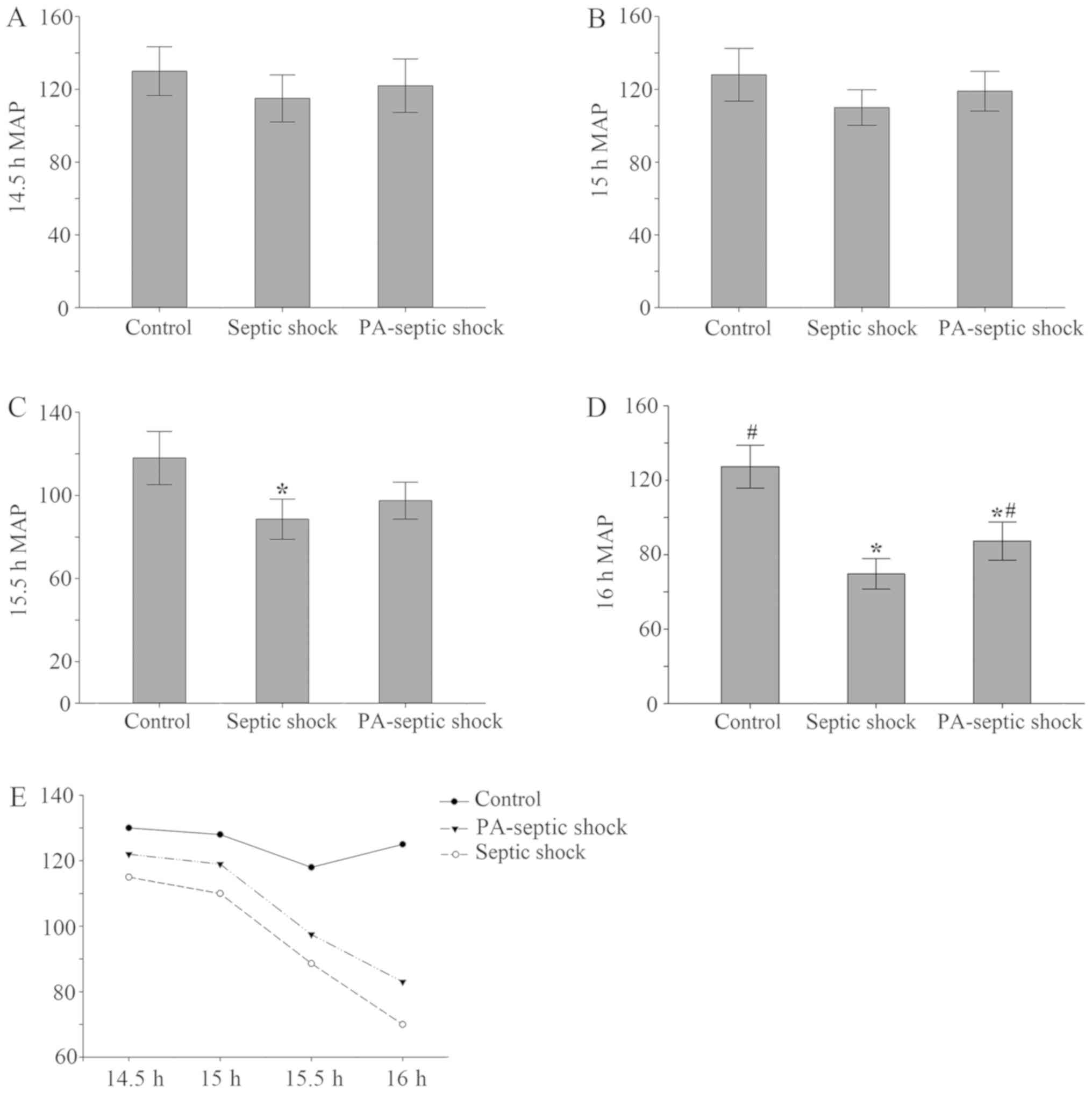 | Figure 1.Changes in average arterial pressure
in each treatment group. (A and B) There was no significant
difference in MAP between 14.5–15 h rats after the establishment of
the model. (C) After 15.5 h the model was established, compared
with the blank control group, the MAP of the rats in the septic
shock group decreased (P<0.05), PA-infection shock group had no
significant changes. (D) Compared with the blank group, the MAP in
the other 2 groups decreased significantly, and compared with the
septic shock group, the mean arterial pressure in the PA-septic
shock group increased. (E) The MAP of the rats in the septic shock
group and the PA-septic shock group showed a progressive decline,
and the MAP in the blank control group did not change obviously.
MAP, mean arterial pressure; PA, Pseudomonas aeruginosa.
*P<0.05, the difference between the control group and the blank
control group was statistically significant. #P<0.05,
the difference was statistically significant compared with that of
the septic shock group. |
Increased expression of IL-1β, IL-6 and TNF-α in
abdominal lavage fluid and intestinal tissue in rats with septic
shock. The RPMI-1640 culture medium was used to wash the abdominal
cavity of rats in each group, and the peritoneal lavage fluid was
taken respectively with the supernatant removed by centrifugation
at 2,000 × g for 3 min at 4°C. The concentration of IL-1β, IL-6 and
TNF-α in each group of lavage liquid was detected by ELISA kit. The
results showed that compared with the blank control group, the
concentrations of IL-1β, IL-6 and TNF-α in peritoneal lavage fluid
of septic shock group and PA-septic shock group were both increased
(P<0.05). Among them, the concentrations of IL-1β, IL-6 and
TNF-α in septic shock group was higher than the PA-septic shock
group (P<0.05) (Fig. 2A-C).
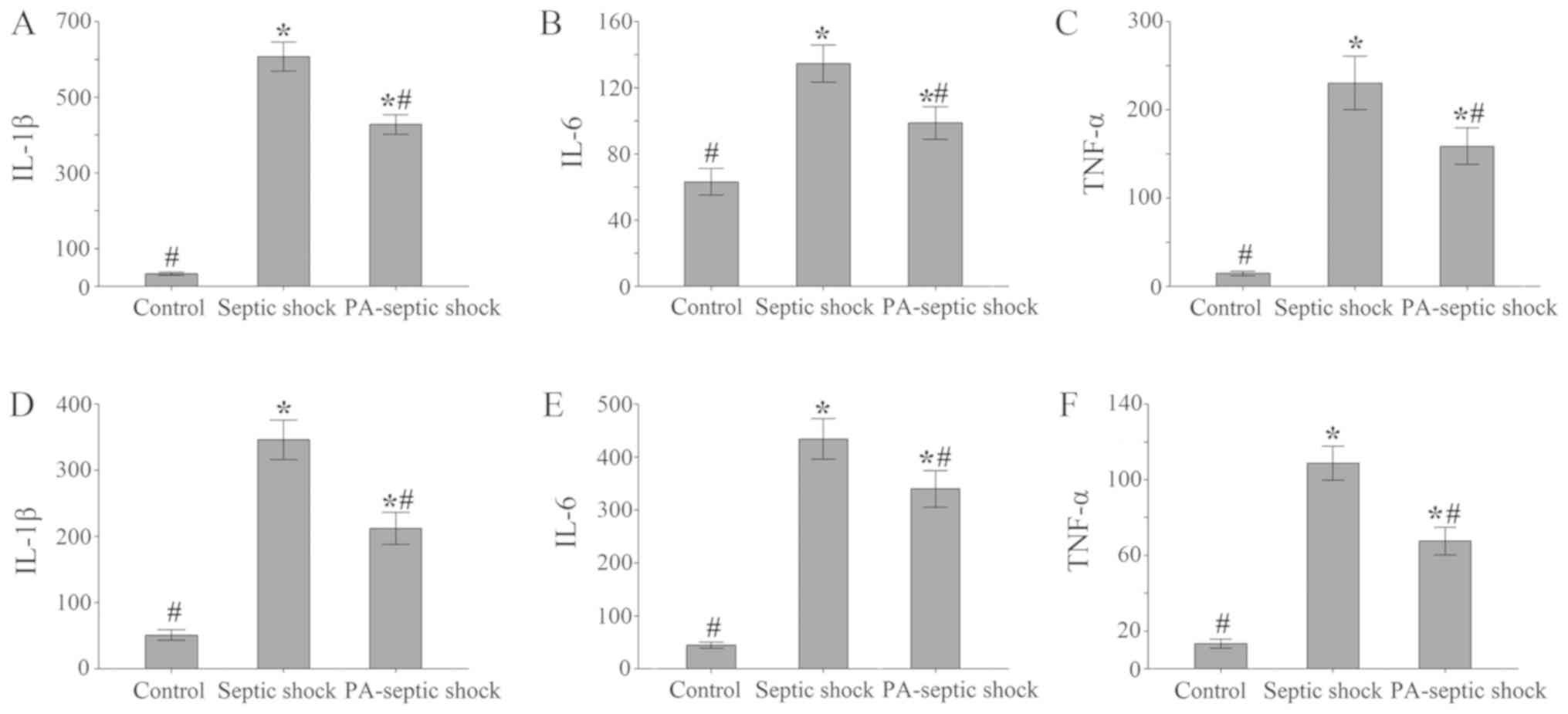 | Figure 2.The concentration of inflammatory
factors in the peritoneal lavage and intestinal tissue of each
treatment group. (A-C) Compared with the blank control group, the
concentration of IL-1, IL-6 and TNF-α in the other 2 groups of
peritoneal lavage fluid increased (P<0.05). Among them, the
infection shock group was higher than the PA-septic shock group
(P<0.05). (D-F) Compared with the blank control group, the
concentration of IL-1, IL-6 and TNF-α in the intestinal homogenate
of the septic shock group and the PA-septic shock group increased.
IL-1, interleukin-l; TNF-α, tumor necrosis factor-α; PA,
Pseudomonas aeruginosa. *P<0.05, the difference between
the control group and the blank control group was statistically
significant. #P<0.05, the difference was
statistically significant compared with that of the septic shock
group. |
Furthermore, compared with the blank control group,
the concentrations of IL-1β, IL-6 and TNF-α in the intestinal
homogenate of both septic shock group and PA-septic shock group
increased (P<0.05) (Fig.
2D-F).
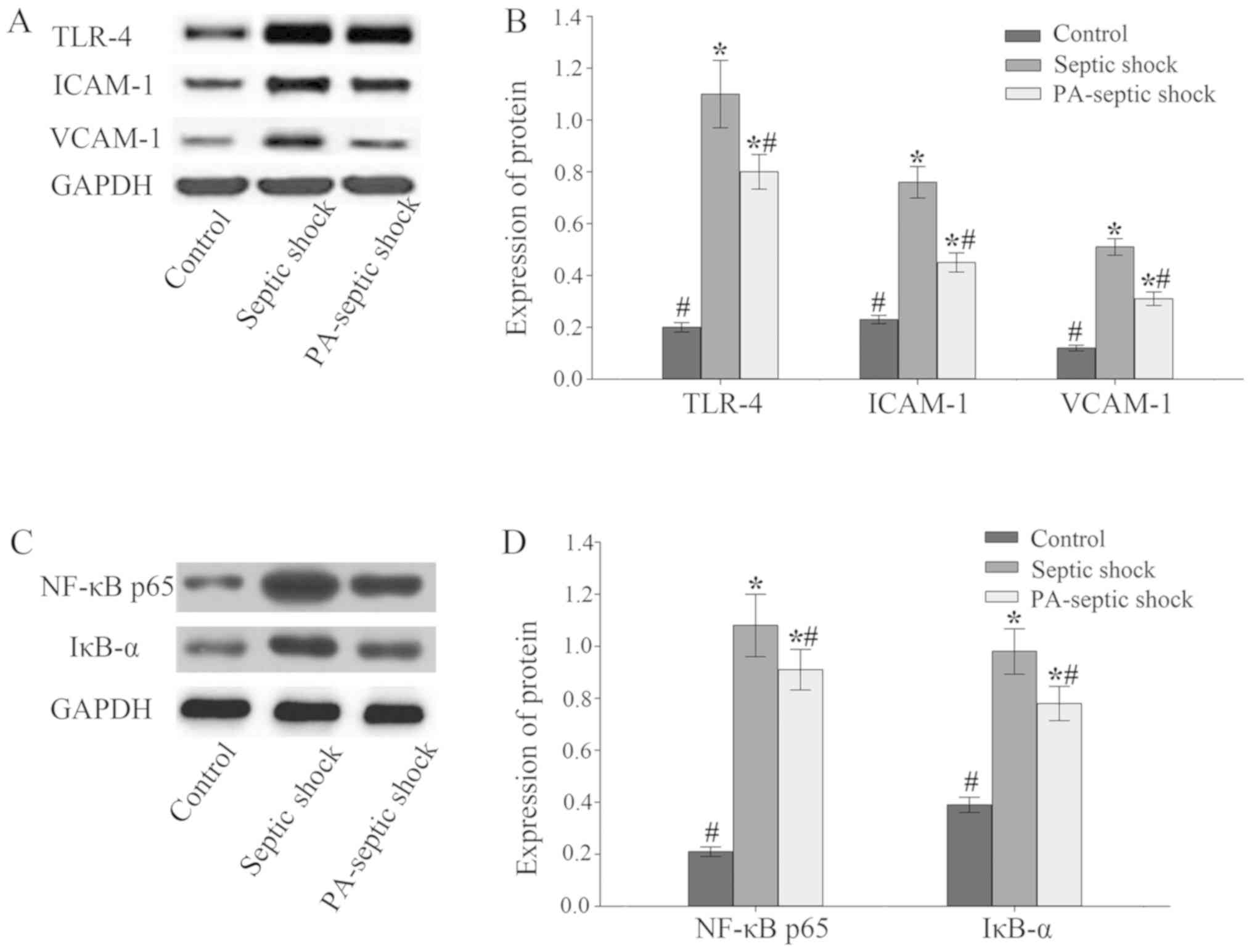
Expression of TLR-4-NF-κB, ICAM-1 and
VCAM-1 in intestinal tissue of septic shock rats increased
significantly
The expression of related proteins in the intestinal
tissue of rats was detected by western blotting. The results showed
that compared with the blank control group, the expression of
TLR-4-NF-κB and ICAM-1 and VCAM-1 in the intestinal tissue of
septic shock group and PA-infected shock group increased
(P<0.05), and the expression level of the septic shock group was
higher than that of the PA-septic shock group (P<0.05) (Fig. 3).
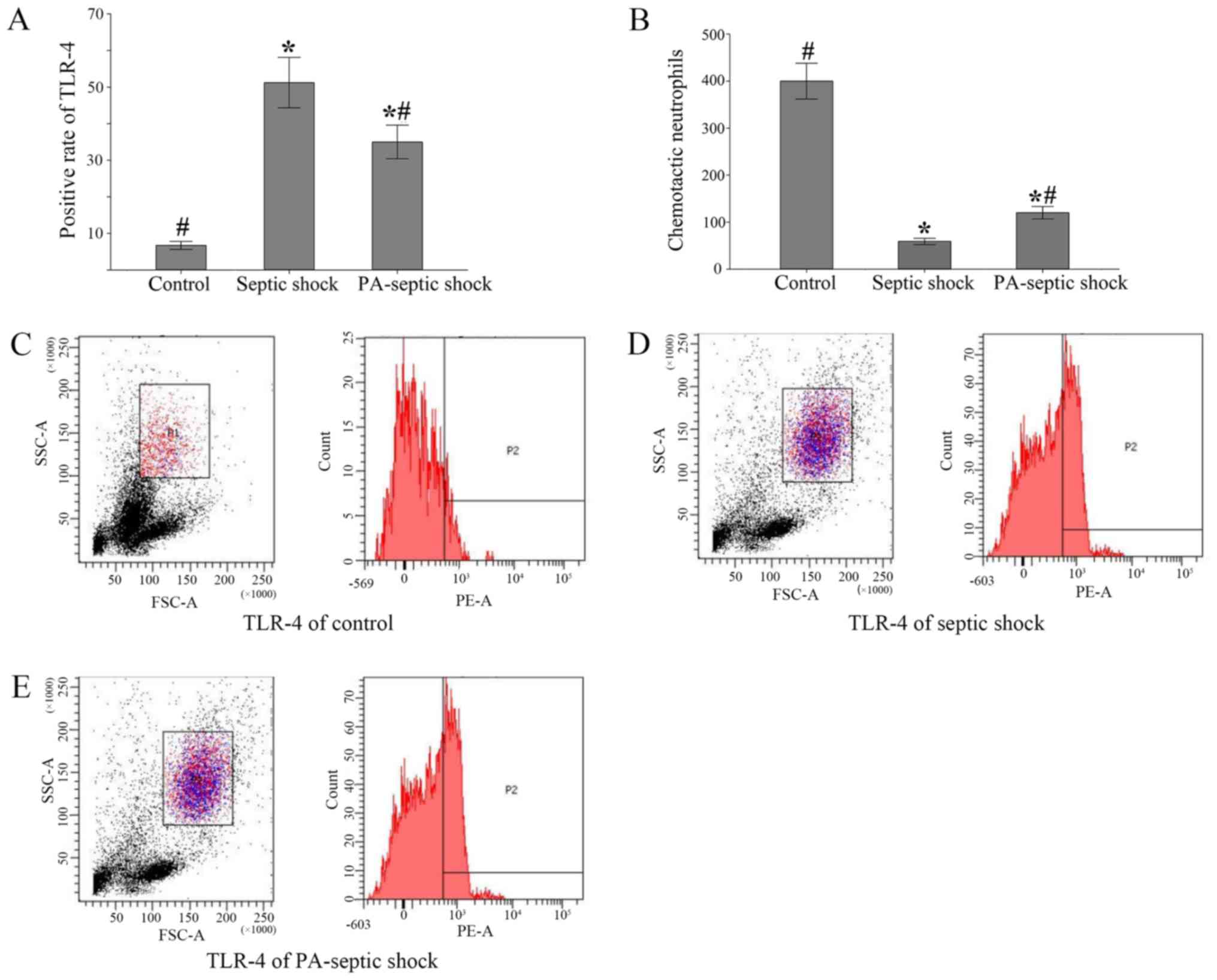
The positive rate of TLR-4 expression
on the surface of peripheral blood neutrophils and the number of
chemotaxis neutrophils in the peripheral blood of rats with septic
shock were significantly increased
Neutrophils were isolated from peripheral blood and
the positive rate of TLR4 expression in neutrophils was detected by
flow cytometry. The positive rate of TLR-4 expression in peripheral
blood of septic shock group and PA-septic shock group was
significantly higher than that in blank control group (P<0.05)
(Fig. 4A). Additionally, the number
of chemotaxis neutrophils in peripheral blood of each group was
detected by Transwell chamber method. It was found that the number
of chemotaxised neutrophils in the peripheral blood of the septic
shock group was fewer than that of the PA-septic shock group
(P<0.05) (Fig. 4B-E).
Discussion
In recent years, immunomodulatory therapy has become
a new direction for the treatment of sepsis. From the perspective
of pathogenic biology, sepsis is potentially a life-threatening
complication of infection, which is initiated by pathogen endotoxin
release to blood circulation (21).
It is a complex, dynamic syndrome with great heterogeneity related
to ethnic, sex, age, genetic and environmental factors (22). Innate immunity is the first line of
defense against pathogenic microorganism infection. The innate
immune system specifically recognizes PAMPs by pathogen recognition
receptors (PPRs), thus effectively initiating the immune response.
As a member of the PPRs family, the TLR is one of the most
important receptors in the innate immune system. TLR-4 is the first
TLR recognized by researches, they are mainly expressed in the
cells involved in host defense functions, such as macrophages,
neutrophils, dendritic cells, lymphocytes, endothelial cells and
epithelial cells. Renal tubular epithelial cells, heart,
respiratory epithelial cells and intestinal epithelial cells also
express TLR-4 (23–25).
Because of its important role in the initial stage
of inflammation, TLR-4 has become a potential target for the
treatment of septic shock. It is known that TLR4 can mediate the
recognition of lipopolysaccharide (LPS) by identifying the
characteristic PAMPs in the outer membrane of gram negative
bacteria, which is an important trigger of inflammatory response in
septic shock (26). In addition to
LPS, various endogenous ligands can be identified by TLR-4, such as
HMGB1 (27). TLR-4 can activate the
transcription factors, such as NF-κB, MCP-1, ICAM-1, and other
inflammatory factors through the MyD88-dependent signaling pathway
(28). In recent studies, blocking
the expression of the TLR4-MD2 complex can prevent septic shock
(29).
PA-MSHA can enhance the immune defense, while on the
one hand, the cellular component of PA-MSHA can activate PPRs such
as TLR-4. As a result, PA-MSHA can activate a variety of immune
cells, such as dendritic cells, macrophages, Treg and NK cells, as
well as help reconstruct the immune surveillance and immune defense
(30). Furthermore, PA-MSHA has
immunogenicity, which can regulate the activation of polyclonal B
cells and produce broad-spectrum, highly effective bactericidal
antibodies. It can effectively defend against the infection caused
by gram-negative bacilli (31).
The aim of this research is to observe the
expression of cytokines, TLR-4, adhesion molecules and the
expression of TLR4 on peripheral blood neutrophils in septic shock
rats after PA-MSHA pretreatment.
The results showed in our rat sepsis model, that the
expression of TLR4 in the intestinal tissue, as well as the rate of
TLR-4 positive cells in the peripheral blood neutrophils were
significantly higher than that in the blank group. PA-MASH
pretreatment could reduce the expression level of TLR-4 in
intestinal tissue and the TLR-4 positive neutrophils in the
intestinal tissue of rats with septic shock. At the same time, the
concentration of inflammatory factors IL-1β, IL-6 and TNF-α in the
abdominal and intestinal tissues of each group also showed the same
trend. This suggested that PA-MSHA can reduce the expression of
TLR-4 receptor, inhibit TLR4-NF-κB proinflammatory factor pathway
and suppress the release of proinflammatory factors, thereby
effectively inhibiting inflammatory reaction and reducing
intestinal mucosal injury in rats.
ICAM-1 and VCAM-1 are important adhesion molecules
that mediate cell adhesion. ICAM-1 and VCAM-1 belong to the
immunoglobulin superfamily, which act as the ligands of leukocyte
adhesion molecule integrin, and play a key role in the process of
the late stage of adherence of leukocytes to endothelial cells and
migration of endothelial cells (32–34).
Their main role is to stabilize intercellular interactions and to
promote adherence of inflammatory cells and endothelial cells
(35). When inflammatory reaction
occurs, with the action of LPS and cytokines such as IL-1, TNF-α,
INF-γ, ICAM-1 was activated on the cell surface especially in
endothelial cells. VCAM-1 activates endothelial cell NADPH oxidase
and shrinks endothelial cells. The opened ‘endothelial cell gate’
is an important channel for inflammatory cells to migrate from
blood to tissue (36).
In this study, the expression levels of ICAM-1 and
VCAM-1 in other two groups were both higher than that in the blank
group. When the septic shock occurs, a large number of inflammatory
mediators are released into the blood, which can promote the
migration of inflammatory cells to the infected site by
upregulating the expression level of ICAM-1 and VCAM-1. On the
other hand, ICAM-1 can further increase the expression of VCAM-1,
which promote inflammatory cells to release more inflammatory
mediators. In addition, inflammatory mediators further exacerbate
excessive ischemia-reperfusion injury, aggravate the damage of
intestinal mucosal barrier function, thereby leading to more
pathogenic bacteria and endotoxin entering into the blood.
Eventually, vicious spiral was formed to accelerate the progression
of septic shock. Furthermore, our study also demonstrated that the
expression levels of ICAM-1 and VCAM-1 in septic shock group were
both higher than those in PA- infected shock group, indicating that
PA-MSHA premedication could inhibit the expression of ICAM-1 and
VCAM-1 in intestinal tract of rats with septic shock. It is
hypothesized that PA-MSHA can reduce the expression of TLR-4 and
further inhibit the TLR4-NF-κB-proinflammatory pathway; As a
result, the release of proinflammatory factors was suppressed,
thereby effectively inhibiting the inflammatory response and
reducing the intestinal mucosal injury in rats. Some studies have
shown that LPS induces the production of ROS by activating the
‘TLR4/MyD88/c-Src/NADPH oxidase’ pathway and triggers the
activation of p38MAPK and ATF2. Activated ATF2 can lead to an
increase in VCAM-1 promoter activity and increase the expression of
VCAM-1 (37). Other studies have
shown that LPS can increase the production of ROS by activating
TLR4/MyD88/TRAF6/c-Src/NADPH oxidase pathway, and then activate
NF-κB, which leads to the increase of ICAM-1 production (38).
In conclusion, PA-MSHA pretreatment can reduce the
systemic and local (intraperitoneal, intestinal) inflammation, thus
preventing the intestinal injury caused by septic shock. Our
results indicated that the underlying mechanisms might include that
PA-MSHA suppressed TLR4-NF-κB-proinflammatory pathway and inhibited
the role of adhesion molecules such as ICAM-1 and VCAM-1. Our study
offers new insight into the future therapy of septic shock.
Acknowledgements
Not applicable.
Funding
This work was supported in part by grants from the
National Natural Science Foundation of China (no. 81701881), and
the Nanjing Medical Science and Technology Development Foundation
(no. YKK17102).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WZ and XW designed the study and performed the
experiments, JS, XS and YX established the animal models, JS and SY
collected the data, WZ and JS analyzed the data, WZ and XW prepared
the manuscript. All authors read and approved the final study.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Nanjing First Hospital, Nanjing Medical University
Animal Center (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferrer R, Martin-Loeches I, Phillips G,
Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C and Levy
MM: Empiric antibiotic treatment reduces mortality in severe sepsis
and septic shock from the first hour: Results from a
guideline-based performance improvement program. Crit Care Med.
42:1749–1755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bassetti M, Righi E, Ansaldi F, Merelli M,
Trucchi C, De Pascale G, Diaz-Martin A, Luzzati R, Rosin C, Lagunes
L, et al: A multicenter study of septic shock due to candidemia:
Outcomes and predictors of mortality. Intensive Care Med.
40:839–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bihari D, Prakash S and Bersten A:
Low-dose vasopressin in addition to noradrenaline may lead to
faster resolution of organ failure in patients with severe
sepsis/septic shock. Anaesth Intensive Care. 42:671–674.
2014.PubMed/NCBI
|
|
4
|
Greeneltch KM, Haudenschild CC, Keegan AD
and Shi Y: The opioid antagonist naltrexone blocks acute endotoxic
shock by inhibiting tumor necrosis factor-alpha production. Brain
Behav Immun. 18:476–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tidswell M and LaRosa SP: Toll-like
receptor-4 antagonist eritoran tetrasodium for severe sepsis.
Expert Rev Anti Infect Ther. 9:507–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ;: The ACCP/SCCM
Consensus Conference Committee. American College of Chest
Physicians/Society of Critical Care Medicine: Definitions for
sepsis and organ failure and guidelines for the use of innovative
therapies in sepsis. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rittirsch D, Flierl MA and Ward PA:
Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 8:776–787.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotchkiss RS and Nicholson DW: Apoptosis
and caspases regulate death and inflammation in sepsis. Nat Rev
Immunol. 6:813–822. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Solomkin JS, Jenkins MK, Nelson RD,
Chenoweth D and Simmons RL: Neutrophil dysfunction in sepsis. II.
Evidence for the role of complement activation products in cellular
deactivation. Surgery. 90:319–327. 1981.PubMed/NCBI
|
|
10
|
Witko-Sarsat V, Rieu P, Descamps-Latscha
B, Lesavre P and Halbwachs-Mecarelli L: Neutrophils: Molecules,
functions and pathophysiological aspects. Lab Invest. 80:617–653.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luster AD: Chemokines - chemotactic
cytokines that mediate inflammation. N Engl J Med. 338:436–445.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Lei X, Li X, Cai JM, Gao F and Yang
YY: Toll-like receptors and radiation protection. Eur Rev Med
Pharmacol Sci. 22:31–39. 2018.PubMed/NCBI
|
|
13
|
Zhang Y, Lu Y, Ma L, Cao X, Xiao J, Chen
J, Jiao S, Gao Y, Liu C, Duan Z, et al: Activation of vascular
endothelial growth factor receptor-3 in macrophages restrains
TLR4-NF-κB signaling and protects against endotoxin shock.
Immunity. 40:501–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castle NA: Aquaporins as targets for drug
discovery. Drug Discov Today. 10:485–493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu WH, Liu ZB, Hou YF, Hong Q, Hu DL and
Shao ZM: Inhibition of autophagy enhances the cytotoxic effect of
PA-MSHA in breast cancer. BMC Cancer. 14:2732014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li T, Dong ZR, Guo ZY, Wang CH, Zhi XT,
Zhou JW, Li DK, Chen ZT, Chen ZQ and Hu SY: Mannose-mediated
inhibitory effects of PA-MSHA on invasion and metastasis of
hepatocellular carcinoma via EGFR/Akt/IκBβ/NF-κB pathway. Liver
Int. 35:1416–1429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou J, Liu Y, Liu Y and Shao Y: The MSHA
strain of Pseudomonas aeruginosa activated TLR pathway and
enhanced HIV-1 DNA vaccine immunoreactivity. PLoS One.
7:e477242012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Luo F, Zhang Y, Wang L, Lin W,
Yang M, Hu D, Wu X and Chu Y: Pseudomonas aeruginosa
mannose-sensitive hemagglutinin promotes T-cell response via
toll-like receptor 4-mediated dendritic cells to slow tumor
progression in mice. J Pharmacol Exp Ther. 349:279–287. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu XF, Wang L, Qu Y, Zhong DW, Miao XY
and Yao HL: Effect of the PA-MSHA vaccine on septic serum-induced
inflammatory response. Mol Med Rep. 7:1350–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wen H: Sepsis induced by cecal ligation
and puncture. Methods Mol Biol. 1031:117–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buras JA, Holzmann B and Sitkovsky M:
Animal models of sepsis: Setting the stage. Nat Rev Drug Discov.
4:854–865. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wiersinga WJ: Current insights in sepsis:
From pathogenesis to new treatment targets. Curr Opin Crit Care.
17:480–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar H and Bot A: Innate immune
recognition mechanisms and translational opportunities. Int Rev
Immunol. 32:113–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar S, Ingle H, Prasad DV and Kumar H:
Recognition of bacterial infection by innate immune sensors. Crit
Rev Microbiol. 39:229–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bachar O, Adner M, Uddman R and Cardell
LO: Toll-like receptor stimulation induces airway
hyper-responsiveness to bradykinin, an effect mediated by JNK and
NF-kappa B signaling pathways. Eur J Immunol. 34:1196–1207. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poltorak A, He X, Smirnova I, Liu MY, Van
Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al:
Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations
in Tlr4 gene. Science. 282:2085–2088. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JS, Svetkauskaite D, He Q, Kim JY,
Strassheim D, Ishizaka A and Abraham E: Involvement of toll-like
receptors 2 and 4 in cellular activation by high mobility group box
1 protein. J Biol Chem. 279:7370–7377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kerger BD, Thuett KA and Finley BL:
Evaluation of four α-diketones for toll-like receptor-4 (TLR-4)
activation in a human transfected cell line. Food Chem Toxicol.
74:117–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daubeuf B, Mathison J, Spiller S, Hugues
S, Herren S, Ferlin W, Kosco-Vilbois M, Wagner H, Kirschning CJ,
Ulevitch R, et al: TLR4/MD-2 monoclonal antibody therapy affords
protection in experimental models of septic shock. J Immunol.
179:6107–6114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G;:
International Sepsis Definitions Conference: 2001
SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions
conference. Intensive Care Med. 29:530–538. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mossman KL, Mian MF, Lauzon NM, Gyles CL,
Lichty B, Mackenzie R, Gill N and Ashkar AA: Cutting edge: FimH
adhesin of type 1 fimbriae is a novel TLR4 ligand. J Immunol.
181:6702–6706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakae H, Endo S, Yamada Y and Inada K:
Bound and soluble adhesion molecule and cytokine levels in patients
with severe burns. Burns. 26:139–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Q, Shao L, He M, Chen H, Liu D, Luo
Y and Dai Y: Inhibitory effects of sasanquasaponin on
over-expression of ICAM-1 and on enhancement of capillary
permeability induced by burns in rats. Burns. 31:637–642. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jersmann HP, Hii CS, Ferrante JV and
Ferrante A: Bacterial lipopolysaccharide and tumor necrosis factor
alpha synergistically increase expression of human endothelial
adhesion molecules through activation of NF-kappaB and p38
mitogen-activated protein kinase signaling pathways. Infect Immun.
69:1273–1279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thomson AJ, Greer MR, Young A, Boswell F,
Telfer JF, Cameron IT, Norman JE and Campbell S: Expression of
intercellular adhesion molecules ICAM-1 and ICAM-2 in human
endometrium: Regulation by interferon-gamma. Mol Hum Reprod.
5:64–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cook-Mills JM: VCAM-1 signals during
lymphocyte migration: Role of reactive oxygen species. Mol Immunol.
39:499–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee IT, Shih RH, Lin CC, Chen JT and Yang
CM: Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1
expression induced by lipopolysaccharide in human renal mesangial
cells. Cell Commun Signal. 10:332012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cho RL, Yang CC, Lee IT, Lin CC, Chi PL,
Hsiao LD and Yang CM: Lipopolysaccharide induces ICAM-1 expression
via a c-Src/NADPH oxidase/ROS-dependent NF-κB pathway in human
pulmonary alveolar epithelial cells. Am J Physiol Lung Cell Mol
Physiol. 310:L639–L657. 2016. View Article : Google Scholar : PubMed/NCBI
|