Introduction
Rosacea is a common chronic inflammatory skin
disease, which affects facial skin blood vessels and sebaceous
glands. The primary symptoms include transient or non-transient
erythema, papules, pustules and telangiectasia, all of which
largely affect the central face. Patients with a prolonged disease
course may develop dark red plaques or rhinophyma (typical
irregular thickening of the nasal skin) due to hyperplasia
(1). According to the National
Rosacea Society Expert Committee, rosacea is classified as mild,
moderate or severe based on severity of the lesions and the
symptoms (2). The protracted disease
course, tendency for recurrence, associated changes in facial
complexion, skin ulceration and scar formation tend to have a
detrimental effect on the physical, mental and social health of the
patients (3).
Primary treatment modalities for rosacea at present
include anti-inflammatory, antipruritic and anti-parasitic agents,
and vasomotor regulation (2).
Current treatments for rosacea mainly focus on alleviating the
syndrome to improve the quality of life in patients, and preventing
disease progression. However, they are not completely effective
possibly due to a lack of clear understanding of the pathogenetic
mechanisms of rosacea. More improved and novel alternative
treatment options are in urgent need (4).
A close association of Demodex mite with
facial dermatitis was previously observed (5). Demodex mite is a microscopic
ectoparasite with a slender body (length, 0.3–0.4 mm), which mainly
feeds off epidermal cells and sebum components. It inhabits human
and mammalian hair follicles and sebaceous glands, especially the
skin over the nose, cheeks, forehead and chin. The human body has
characteristics of high infectivity, and low and conditional
pathogenicity to Demodex mites (6,7). The
infestation rate of Demodex mite is also high in rosacea
patients (5). If ornidazole
treatment is used alone, untreated lesions tend to aggravate and
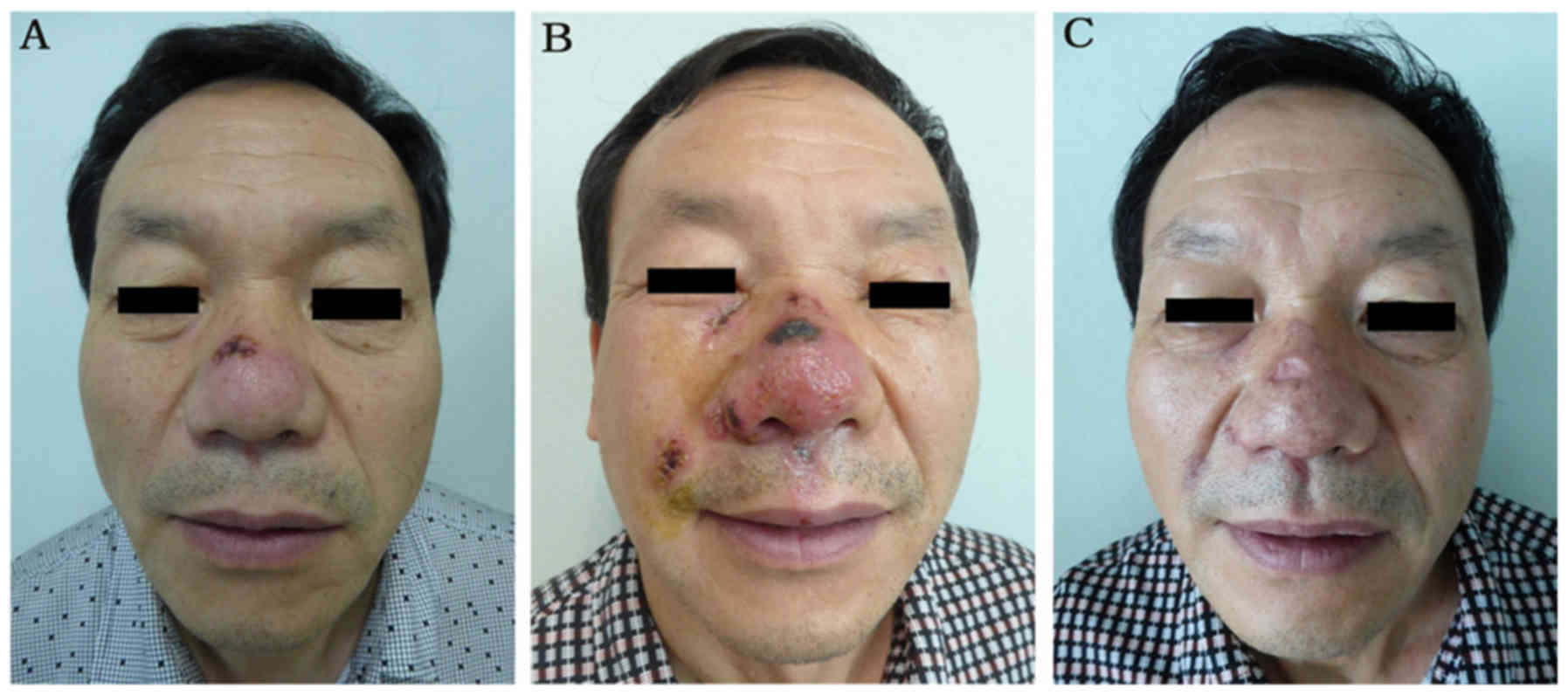
result in ulceration and scar formation (Fig. 1) (8).
Fibroblast growth factor refers to a class of
secreted polypeptide ligands that are widely used for treatment of
burns (including superficial II, deep II, granulation wounds),
chronic wounds (including chronic ulcer), fresh wounds (traumatic,
donor site wound and surgical wounds), and for treatment of
neurological diseases such as Parkinson's disease, and repair of
corneal lesions (9–11). In our previous study, a statistically
significant beneficial effect of topical use of recombinant bovine
basic fibroblast growth factor (rbFGF) gel was observed in patients
with facial dermatitis (8). However,
whether rbFGF improves treatment outcomes in rosacea patients
following anti-Demodex treatment remains unknown. In the
present study, the treatment outcomes of rbFGF in 1,287 rosacea
patients following anti-Demodex treatment were investigated
and significant improvement was observed in the total effective
rates for erythema, papules, desquamation and dryness in the rbFGF
treatment group.
Materials and methods
Subjects
The present study was approved by the Ethics
Committee of Lanzhou General Hospital (Lanzhou, China). In the
present single-blind randomized study, a total of 1,287 patients
with Demodex-mite induced rosacea were treated at the
dermatology outpatient department of Lanzhou General Hospital
between June 2014 and February 2016. The study was focused on
Demodex-mite induced rosacea manifested with papules and
pustule. The inclusion criteria were clinical manifestations of
rosacea, which is defined as persistent erythema of the central
portion of the face, lasting for at least 3 months (detailed
diagnostic criteria for rosacea are listed in Table I) (2)
and positive test results for Demodex mite from a
standardized skin surface biopsy. All patients recruited in the
present study were classified as moderate to severe rosacea
(12). As all patients exhibited
moderate to severe rosacea with primary features including
transient and non-transient erythema, papules and pustules and
telangiectasia, the patients were not divided into subtypes. The
exclusion criteria were pregnant or lactating women; patients who
had cardiovascular, liver, kidney or hematopoietic system disorders
and/or other serious primary diseases, such as heart disease and
diabetes; patients with mental illness or cancer; patients who had
been diagnosed and previously treated for rosacea; and patients who
were allergic to ornidazole or rbFGF gel. Informed consent was
obtained from all enrolled patients. The clinical trial
registration number was ChiCTR-IPR-15006451.
 | Table I.Diagnostic criteria for rosacea,
including primary and secondary features. |
Table I.
Diagnostic criteria for rosacea,
including primary and secondary features.
| Primary features
(presence of one or more of the following) | Secondary features
(may include one or more of the following) |
|---|
| Flushing (transient
erythema) | Burning or stinging
discomfort |
| Nontransient
erythema | Dry appearance and
texture |
| Papules and
pustules | Edema |
| Telangiectasia | Ocular
manifestations |
|
| Peripheral
location |
|
| Phymatous
changes |
|
| Plaque |
Standardized skin surface biopsy
Patients cleaned their faces with gentle cleanser
and water prior to the biopsy. When the skin dried, a sterile sharp
blade was scrapped on adjacent areas on the patients' nose. The
accumulated debris or sebaceous secretions were collected on a
glass slide, a drop of 5% potassium hydroxide was added and the
coverslip was applied. The samples were then o observed under a
light microscope (magnification, ×40).
Bacterial culture
The accumulated debris was plated on an agar plate
(Autobio Diagnostics Co., Ltd., Zhengzhou, China) and incubated at
35°C for 18–24 h. Staphylococci colonies are usually middle-sized,
spherical, flat and smooth, moist with smooth edges, they are
golden-yellow, white, milky white or lemon-yellow, and some
colonies possess β-hemolytic rings. Based on the aforementioned
characteristics, the authors further separated the colonies that
were considered to be staphylococci and performed catalase tests
after 48 h of culturing to confirm if they were. No staphylococci
infections were identified.
Due to potential bacterial infection, 6 patients
were treated with 2% mupirocin (0.2 g/cm2, t.i.d;
Sino-American Tianjin SmithKline & French Laboratories, Ltd.,
Tianjin, China) for 5–10 days and 9 patients were treated with 2%
fusidic acid ointment (0.2 g/cm2, b.i.d; Bright Future
Pharmaceutical Laboratories, Ltd., Hong Kong, China) for 1 to 2
weeks. If the experiment failed, patients were treatment with
compound betamethasone injections (5 mg/ml betamethasone
dipropionate and 2 mg/ml betamethasone sodium phosphate; both Merck
KGaA, Darmstradt, Germany) and rbFGF gel.
Treatment
The enrolled patients were prescribed oral
ornidazole tablets (0.25 g/tablet; 500 mg twice/day; Zhejiang
Aisheng Pharmaceutical Co., Ltd., Zhejiang, China) for 2–4 courses
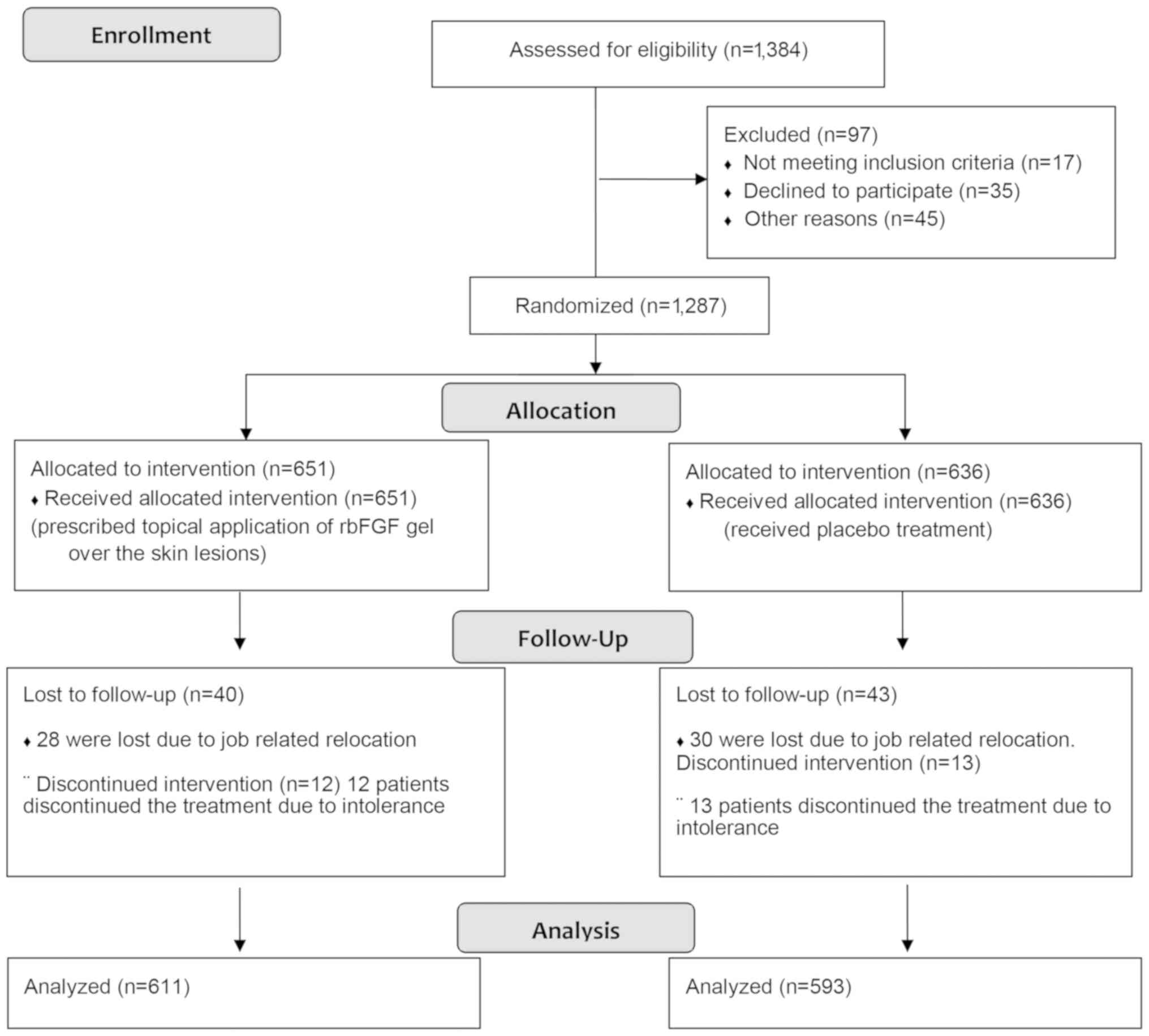
(14 days/course). All patients were then randomly assigned to the
treatment (n=651) or control (n=636) group (Fig. 2). Following treatment with ornidazole
for 7 days, patients in the treatment group were prescribed topical
application of rbFGF gel (21,000 IU/5 g; Zhuhai Yisheng Biological
Pharmaceutical Co., Ltd., Zhuhai, China) over the skin lesions (0.2
g/cm2) for 2–8 weeks, whereas patients in the control
group received rbFGF gel vehicle treatment (containing heparin
sodium, Carbopol®934P, and Triethanolamine which are the
base for the rbFGF gel), unless ulceration occurred.
Total efficacy rate
All patients were scored by the same doctor
following the ornidazole treatment for their primary features,
including erythema, papules, desquamation, dryness and
telangiectasia. Each of the features were counted separately. The
results were graded as absent (0 score), mild (1 score), moderate
(2 score) or severe (3 score) (12–14).
Total efficacy rate and effective sample size were calculated using
the following equation: Effective index = [total score (before
rbFGF treatment-after rbFGF treatment)/total score before
treatment] ×100. The total efficacy index was graded according to
the classification system shown in Table II.
 | Table II.Evaluation of clinical efficacy. |
Table II.
Evaluation of clinical efficacy.
| Grade | Effective
index | Clinical
manifestations |
|---|
| Effective | ≥90% | A decrease in the
majority of skin lesions, e.g., papules and pus blister are not
observed, nasal skin becomes smooth and flat without protrusion,
and pores become small |
| Markedly
improved | ≥60%, <90% | Majority of skin
lesions subsided, e.g., papules, pus basically disappeared, nasal
skin has significantly improved |
| Improved | ≥20%, <60% | Skin lesions have
been reduced, e.g., more than half of the papules, pus blisters
subsided and nasal skin lesions improved |
| Ineffective | <20% | No marked
improvement in skin lesions, e.g., more than half of the papules,
pus blister remained and nasal skin lesions remain unchanged |
Skin biopsy and histological
analysis
As described previously (15), skin biopsy was taken in the clinic
following rbFGF gel treatment. Biopsy tissues were fixed in 10%
formalin (Shanghai Baoman Biotechnology Co., Ltd., Shanghai, China)
for 12 h at room temperature, then dehydrated and embedded in
paraffin (Shanghai Hualing Equipment Factory, Shanghai, China) and
sectioned (4 µm) using a Leica section station (RM2016; Leica
Microsystems GmbH, Wetzlar, Germany). The sections were then
bleached using a pathological bleaching and drying machine
(Changzhou Electronic Instrument Co., Ltd., Changzhou, China),
dewaxed and stained with hematoxylin (Shanghai Chemical Reagent
Co., Ltd., Shanghai, China) and eosin (HE; Third Factory of
Shanghai Reagent Chemicals, Shanghai, China) for 15 min at room
temperature. The HE-stained sections were observed with an optical
microscope (BX53; Olympus Corporation, Tokyo, Japan) under a
magnfication of ×40 or ×100 and diagnosed by a pathologist.
Follow up
Patients in both groups were followed up for 6
months. A total of 83 patients (6.45%) were lost to follow-up and
were excluded from the analysis. Among these, 58 were lost due to
job-related relocation (n=28 from the rbFGF treatment group and
n=30 from control group) and 25 patients discontinued the treatment
due to intolerance (n=12 from rbFGF treatment group and n=13 from
control group).
A total of 71 patients in the control group were
treated with rbFGF due to the occurrence of ulceration during
follow-up. The lesions were scored at the end of the 2nd, 4th and
6th month, and the total effective rate of each lesion index was
statistically analyzed. At the 2nd and 6th week of the rbFGF gel
treatment, routine pathological examination of the facial lesions
was performed to evaluate the rosacea skin lesions (15).
Statistical analysis
All data were analyzed using IBM SPSS 19.0
statistics software (IBM Corp., Armonk, NY, USA). Wilcoxon sign
rank test was used to evaluate the lesion score prior to and
following the rbFGF gel treatment. χ2 test was used to
assess differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
A total of 1,204 [966 female (80.23%) and 238 male
(19.77%)] with Demodex mite-induced rosacea were enrolled in
the present study. The mean age of patients was 32.1 years (range,
18–60 years); the mean duration of disease course was 3.5 years
(range, 0.1–20.0 years). Following treatment with ornidazole for 7
days, all patients were randomized to the rbFGF (n=611; 496 female
and 115 male; mean age, 31.8 years) or control (n=593; 470 female
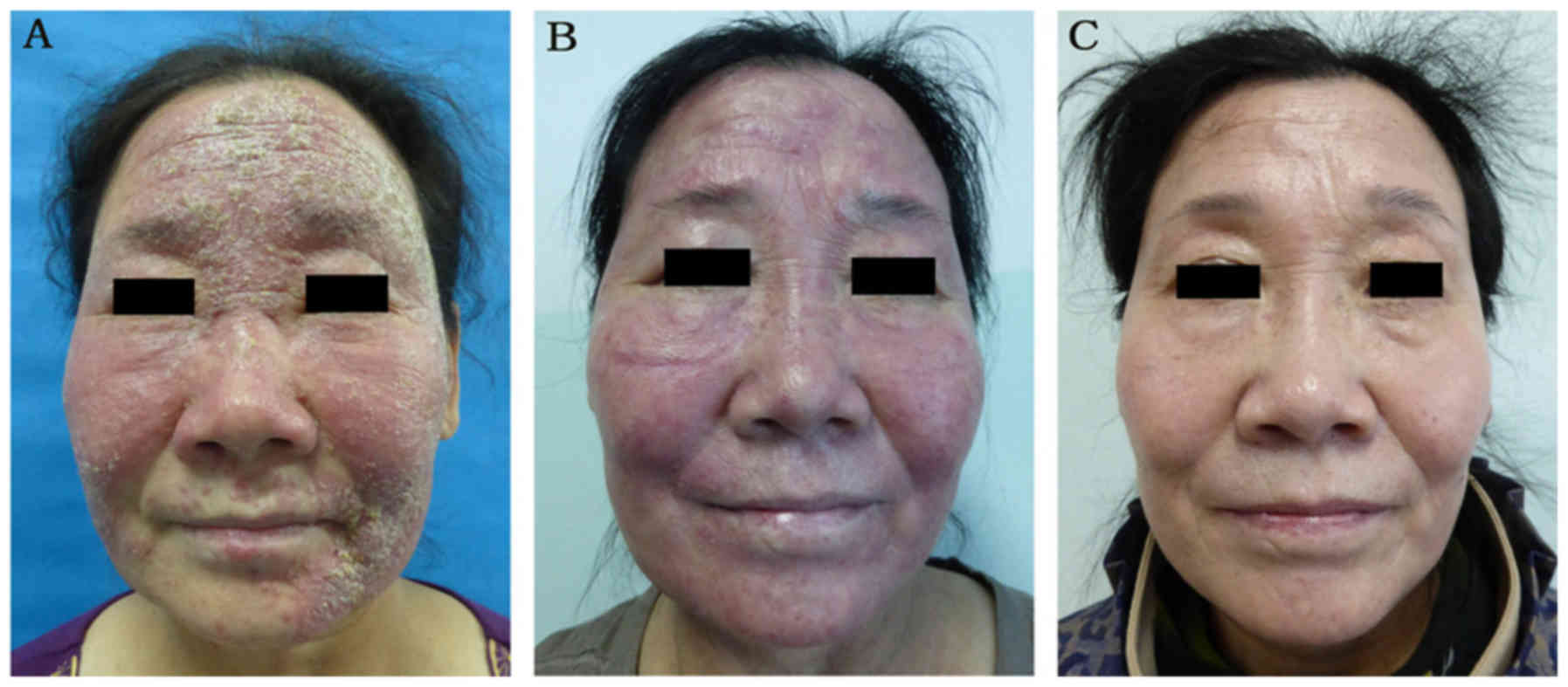
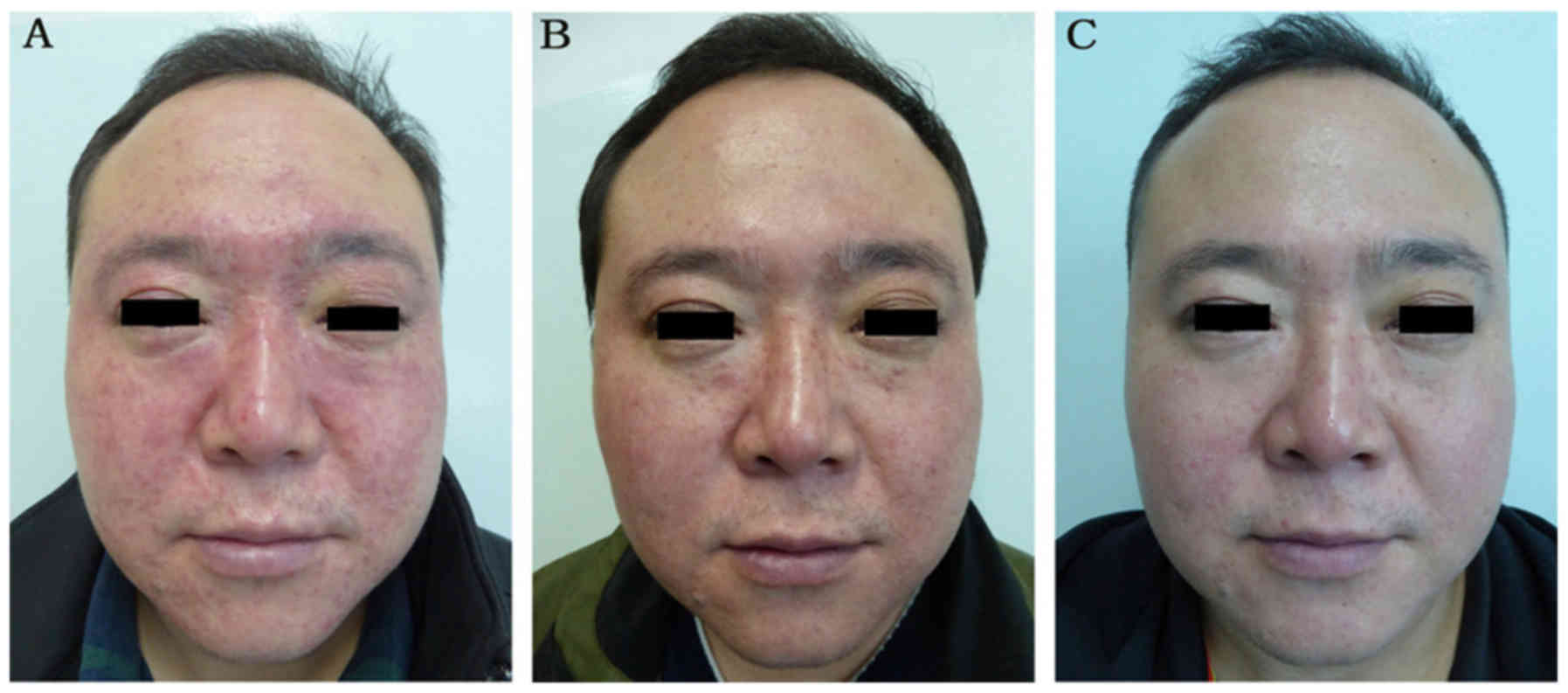
and 123 male; mean age, 32.0 years) groups. Retrospective images of
the treatment and control groups are presented in Figs. 3 and 4, respectively. There were no marked
differences with respect to baseline patient characteristics
between the two groups (Figs. 3A and
4A). The pathological symptoms of
rosacea included superficial dermal tissue necrosis, caseous
necrosis and formation of granuloma structures. The current study
demonstrated that the application of rbFGF gel can effectively
repair the pathological structure of rosacea tissue.
In the pathological analysis of the tissue samples,
neutrophil activity was not observed. Furthermore, there was no
purulent discharge from the facial lesions. Culture of necrotic
tissue secretions did not grow Staphylococcus aureus and
Staphylococcus epidermidis (16). Finally, 6 patients were treated with
2% mupirocin for 5–10 days and 9 patients were treated with 2%
fusidic acid ointment for 1 to 2 weeks; these treatments failed.
Only treatments with compound betamethasone injections and rbFGF
gel were effective.
All patients were followed up for at least 6 months.
At 2-month follow up, 43.37% patients in the treatment group
exhibited a marked improvement (Fig.
3B), compared with 23.95% patients in the control group
(Fig. 4B). Treatment outcome was
assessed as ineffective in 71.16% patients in the control group.
The total effective rates in the treatment and control groups were
81.67 and 28.84%, respectively (P<0.05; Table III). At 4-month follow up, 60.06%
patients in the treatment group exhibited marked improvement
compared with 8.09% in the control group. Treatment outcome in
59.19% of patients in the control group was rated as ineffective.
The total effective rates in the treatment and control groups were
85.11 and 40.81%, respectively (P<0.05; Table III). Similarly, at 6-month follow
up, lesions in the majority of patients (68.74%) in the treatment
group exhibited marked improvement (Fig.
3C), whereas the outcome of ornidazole treatment alone was
rated as effective in 2.87% and ineffective in 44.18% patients
(Fig. 4C). At 6 months, although 83
patients were lost to follow-up, the total effective rates in the
treatment and control groups were 96.56 and 55.82%, respectively
(P<0.05; Table III). The total
efficiency rates for the majority of lesion indicators between the
two groups were statistically significant (P<0.05), with the
exception of telangiectasia (Table
IV), which indicated that application of rbFGF gel
significantly improved the skin lesions.
 | Table III.Treatment outcomes at 2-, 4- and
6-month follow-up: Results of skin lesion assessment. |
Table III.
Treatment outcomes at 2-, 4- and
6-month follow-up: Results of skin lesion assessment.
|
|
| Treatment
outcome |
|
|---|
|
|
|
|
|
|---|
|
|
| Effective | Markedly
improved | Improved | Ineffective |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Group | Follow-up
time-point (months) | n | % | n | % | n | % | n | % | Total effective
rate (%) |
|---|
| rbFGF treatment
(n=611) | 2 | 13 | 2.13 | 221 | 36.17 | 265 | 43.37 | 112 | 18.33 | 81.67 |
|
| 4 | 26 | 4.26 | 367 | 60.06 | 127 | 20.79 | 91 | 14.89 | 85.11 |
|
| 6 | 99 | 16.20 | 420 | 68.74 | 71 | 11.62 | 21 | 3.44 | 96.56 |
| Control
(n=593) | 2 | 0 | 0 | 29 | 4.89 | 142 | 23.95 | 422 | 71.16 | 28.84 |
|
| 4 | 9 | 1.52 | 48 | 8.09 | 185 | 31.20 | 351 | 59.19 | 40.81 |
|
| 6 | 17 | 2.87 | 70 | 11.80 | 244 | 41.15 | 262 | 44.18 | 55.82 |
 | Table IV.Comparison of total effective rate
using lesion indicators between the treatment and control groups at
6-month follow up. |
Table IV.
Comparison of total effective rate
using lesion indicators between the treatment and control groups at
6-month follow up.
|
| Erythema | Papules | Desquamation | Dryness | Telangiectasia |
|---|
|
|
|
|
|
|
|
|---|
| Group | n/total | % | n/total | % | n/total | % | n/total | % | n/total | % |
|---|
| rbFGF
treatment | 243/320 | 76 | 93/291 | 32 | 527/586 | 90 | 551/593 | 93 | 47/223 | 21 |
| Control | 70/317 | 22 | 68/297 | 23 | 267/524 | 51 | 323/548 | 59 | 57/251 | 23 |
| P-value | <0.05 |
| <0.05 |
| <0.05 |
| <0.05 |
| >0.05 |
|
At the end of the follow-up period, primary features
including erythema, papules, desquamation and dry appearance prior
to and following the treatment were analyzed using the Wilcoxon
sign rank test (P<0.05). During the 6-month follow up period,
none of the patients in the treatment group developed ulceration or
scar formation; in contrast, 61% of patients in the control group
exhibited exacerbation of skin lesions, of which 12% developed
ulceration and received rbFGF treatment for a mean duration of 4
weeks. Other patients in the control group also received rbFGF
treatment for 2–8 weeks following the completion of the observation
period. None of these patients experienced scar formation. This
suggested that the topical use of rbFGF gel prevented scar
formation.
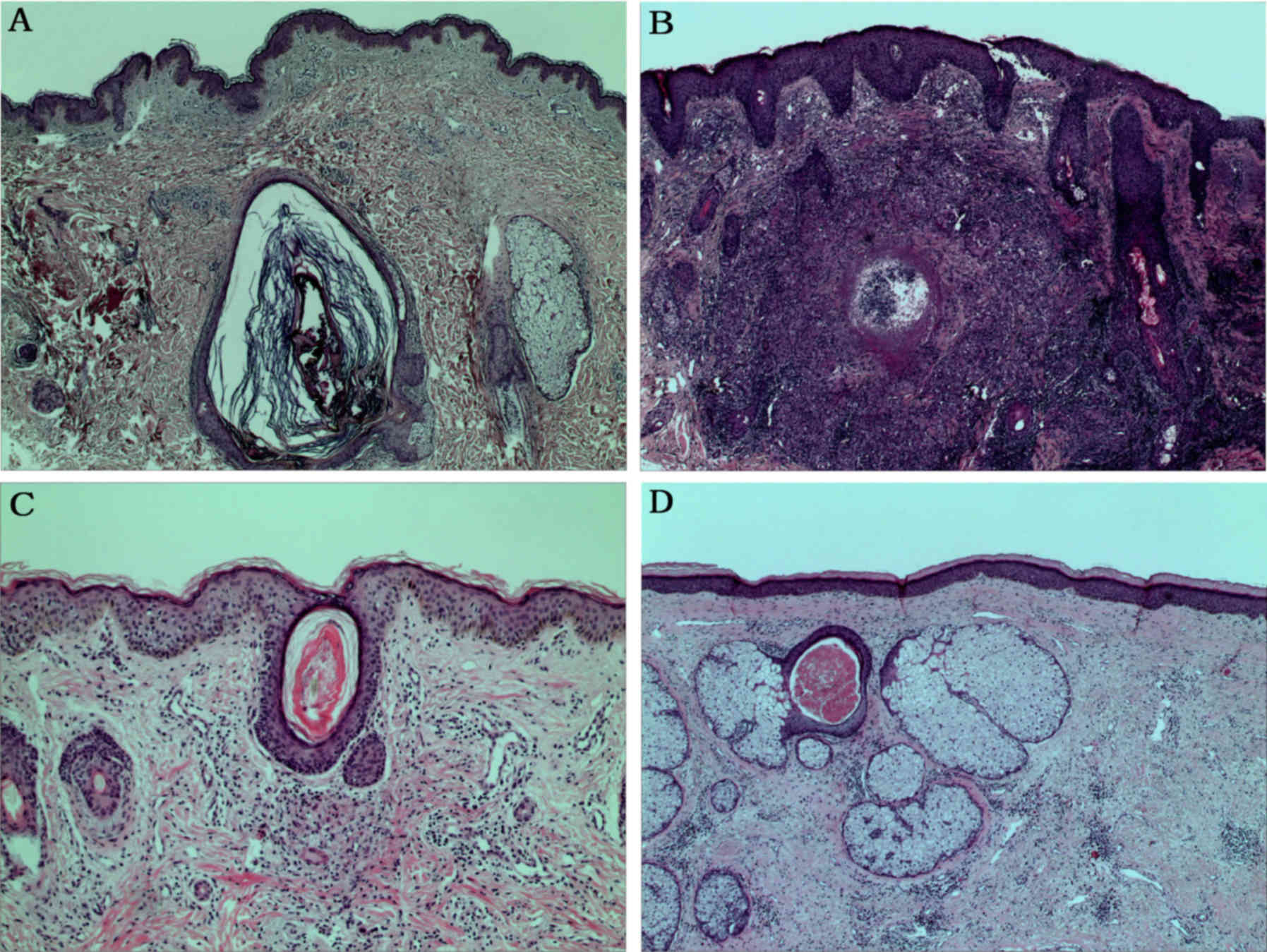
Histological examination of facial lesions was
suggestive of the presence of Demodex mite in the dermis
(Fig. 5A). Key findings were
epidermal hyperplasia, lymphocyte infiltration in the dermis,
epithelioid cells and multinucleated giant cell granulomas
(Fig. 5B). Treatment with rbFGF
treatment markedly alleviated epidermal hyperplasia and reduced the
size of granuloma at 2 weeks (Fig.
5C). The epidermis returned to normal at week 6, the dermal
granulomas were resolved and a large number of telangiectatic
lesions became dilated (Fig.
5D).
Discussion
High infection rates of Demodex have recently
been observed in patients with rosacea; the pathogenetic mechanisms
of the disease mainly comprise mechanical stimulation, secondary
bacterial infection and immune pathology (5). It has also been suggested that
ornidazole-based therapy is highly effective in the treatment of
mite folliculitis (6). Therefore,
ornidazole is preferred over metronidazole due to its longer
biological half-life and fewer side-effects (8). However, the current treatment is not
satisfactory with respect to repair of rosacea-associated skin
lesions (17). In the present study,
the use of rbFGF gel was evaluated for the promotion of healing and
prevention of facial scarring associated with rosacea lesions
following anti-Demodex treatment.
The U.S. Food and Drug Administration (FDA) has
approved several drugs for topical use in treating rosacea, such as
metronidazole, azelaic acid, ivermectin, and brimonidine tartrate
(18). The only approved oral drug
for rosacea is low-dose doxycycline (18–21).
Local treatment is typically prescribed initially, as topical use
of metronidazole, sebacic acid and ivermectin can improve pimples
and pustular rosacea (21).
Metronidazole is a synthetic anti-microbial substance used for the
treatment of infections caused by anaerobic bacteria and protozoa;
azelaic acid is an endogenous dicarboxylic acid with
anti-inflammatory properties, the effect of which is comparable to
or superior than metronidazole (22). The effect of topical ivermectin was
slightly better than that of topical metronidazole in the treatment
of papular pustular rose acne, but both led to relapse in ~66% of
patients within 36 weeks following discontinuation of therapy
(23). The brimonidine gel is a
selective α2-adrenergic receptor agonist with vasoconstrictive
activity for topical treatment of persistent facial erythema
associated with rosacea. However, caution is advised in patients
with depression, cardiovascular disease, Raynaud's phenomenon and
orthostatic hypotension (24).
In our recent study intramuscular injection of
compound betamethasone injection was used for patients with
aggravated rosacea skin lesions, and it was able to obtain better
therapeutic effects (8). However,
attention should be paid to the side effects of systematically
using glucocorticoids, such as local skin pigmentation, delayed
healing of skin ulcers, sodium and water retention, hypokalemia and
osteoporosis; whereas glucocorticoids should not be used when
contraindications exists, such as infection, hypertension,
diabetes, glaucoma and other diseases. RbFGF gel is a better choice
for patients with rosacea and complications with these diseases, as
it avoids the side-effects of glucocorticoids.
FGFs have been demonstrated to serve an important
role in the repair of damaged skin. Wound repair in fibroblast
growth factor receptor 1 and 2 knockout mice was delayed (25). bFGF (also referred to as FGF2) is a
mitogenic cationic polypeptide protein containing 155 amino acids.
Its functional domains consist of a receptor binding domain and a
heparin-binding domain. bFGF binds to the receptor on the target
cell, contributes to the formation, development and maturation of
the skin and its appendages, and serves an important role in skin
regeneration and wound repair (26,27).
Consistent with these findings, the present data also demonstrated
that application of rbFGF alleviated erythema, papules,
desquamation and dryness; it is especially useful in the treatment
of rosacea granuloma lesions and prevents late scar formation. This
may be explained by the following mechanisms: ii) Chemotaxis of
inflammatory cells and fibroblasts into the wound; ii) promotion of
neovascularization at the wound site; iii) regulation of
extracellular matrix formation and degradation; and iv) stimulation
of adjacent cells to produce cytokines. In addition, rbFGF has been
demonstrated to promote proliferation, repair and regeneration of
cells of ectodermal, neuroectodermal and mesodermal origin (such as
epithelial cells, vascular endothelial cells, dermal cells and
fibroblasts), promote capillary regeneration, improve local
circulation and promote wound healing (8,9,28). It also helps repair skin barrier
structure in patients with hormone-dependent dermatitis, reduces
skin sensitivity, improves skin tolerance, increases capillary
elasticity and reduces capillary permeability.
In the pathological analysis of the patient samples,
neutrophil activity was not observed. In addition, there was no
purulent discharge in the facial lesions. Culture of necrotic
tissue secretions did not produce Staphylococcus aureus and
Staphylococcus epidermidis. Finally, the treatment using 2%
mupirocin and 2% fusidic acid ointment all failed. Only the
treatment with compound betamethasone injection (betamethasone
dipropionate 5 mg/ml and betamethasone sodium phosphate 2 mg/ml)
and rbFGF gel was effective. The pathological changes following
rosacea include superficial dermal tissue necrosis, caseous
necrosis and formation of granuloma structure. The application of
rbFGF gel was demonstrated to effectively repair the pathological
structure of rosacea tissue.
The present results suggested that the effect of
rbFGF gel treatment on the rosacea skin lesions following
anti-Demodex were more pronounced as the treatment was
extended. This could be explained by the fact that exogenous bFGF
not only promotes the secretion of endogenous bFGF, but also
stimulates the extracellular matrix and fibroblast protein
synthesis and formation of collagen. However, there was no
significant improvement in telangiectasia, which may be associated
with the expansion of the blood vessels by vascular endothelial
growth factor, angiogenic factor, FGF, transforming growth factor
and other disorders (29).
In the present study, the effective rate was lower
than that in our previous study, which may be attributable to
several causes: The higher efficacy rate in the previous study may
have been due to the relatively small sample size (n=14), compared
with the larger sample size in the present study (n=1,204).
Difference in the sample size may result in variable effective
rate. Furthermore, the patients enrolled in the present study had
symptoms of erythema, papules, desquamation, dryness and
telangiectasia, which suggests a more severe state of disease; this
may also explain the relatively lower effective rate.
Unfortunately, there was no internal control due to
the disapproval of the Ethics Committee of Lanzhou General
Hospital. As an alternative, an rbFGF gel vehicle control was used
as and external control, in accordance with previous studies
(30–32). Most importantly, the effectiveness of
rbFGF treatment was evaluated by the pathological changes in the
patients prior to and following the treatment.
The present single-blind study was focused on
Demodex mite-induced rosacea manifested with papules and
pustule. All patients included in the study were classified as
moderate to severe rosacea with primary features including
transient and non-transient erythema, papules and pustules and
telangiectasia (12). As such,
patients were not further divided into subtypes. In the patients'
interest, doctors were allowed to decide whether the patients from
the control group should be moved to the treatment group. The
disadvantages of the single-blind study are similar to those of
double-blind studies and include potential influence on data
collection, analysis by investigators and the cross-over effect
(33).
The cause of rosacea is unknown, but it may be due
to a variety of hereditary and environmental factors (1,18). Our
future studies will explore the effect of rbFGF on the
Demodex-mite negative rosacea induced skin lesions and its
underlying mechanisms. The sample size may also be increased for
pathological analysis. A further double-blind study with longer
duration of follow-up and independent evaluators will be conducted
in the near future to validate the present findings.
In conclusion, rbFGF may repair skin ulceration and
scar formation in patients with rosacea skin lesions. Early
introduction and long duration of recombinant bovine basic
fibroblast growth factor gel may accelerate the effective treatment
of papules, desquamation and dryness.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the experiments and performed
pathological experiments. XLL collected and interpreted the data.
YJS collected the follow-up data. LZ analyzed the data. JHZ
collected and analyzed the data, and wrote the manuscript. These
authors contributed equally to this work.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Lanzhou General Hospital (Lanzhou, China) and all
patients provided written informed consent.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weinkle AP, Doktor V and Emer J: Update on
the management of rosacea. Clin Cosmet Investig Dermatol.
8:159–177. 2015.PubMed/NCBI
|
|
2
|
Gallo RL, Granstein RD, Kang S, Mannis M,
Steinhoff M, Tan J and Thiboutot D: Standard classification and
pathophysiology of rosacea: The 2017 update by the National Rosacea
Society Expert Committee. J Am Acad Dermatol. 78:148–155. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Egeberg A, Hansen PR, Gislason GH and
Thyssen JP: Patients with Rosacea have increased risk of depression
and anxiety disorders: A Danish Nationwide Cohort Study.
Dermatology. 232:208–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Libon F, El Hayderi L, Nikkels-Tassoudji
N, Dezfoulian B and Nikkels AF: Rosacea. Rev Med Liege. 70:179–185.
2015.(In French). PubMed/NCBI
|
|
5
|
Gonzalez-Hinojosa D, Jaime-Villalonga A,
Aguilar-Montes G and Lammoglia-Ordiales L: Demodex and rosacea: Is
there a relationship? Indian J Ophthalmol. 66:36–38. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen PR: Follicular contact dermatitis
revisited: A review emphasizing neomycin-associated follicular
contact dermatitis. World J Clin Cases. 2:815–821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang YS and Huang YC: Role of Demodex
mite infestation in rosacea: A systematic review and meta-analysis.
J Am Acad Dermatol. 77:441–447.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo Y, Sun YJ, Zhang L and Luan XL:
Treatment of mites folliculitis with an ornidazole-based sequential
therapy: A randomized trial. Medicine (Baltimore). 95:e41732016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zarei F and Soleimaninejad M: Role of
growth factors and biomaterials in wound healing. Artif Cells
Nanomed Biotechnol. 1–6. 2018.PubMed/NCBI
|
|
10
|
Jinfeng L, Yunliang W, Xinshan L, Shanshan
W, Chunyang X, Peng X, Xiaopeng Y, Zhixiu X, Honglei Y, Xia C, et
al: The effect of MSCs derived from the human umbilical cord
transduced by fibroblast growth factor-20 on Parkinson's disease.
Stem Cells Int. 2016:50167682016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meduri A, Scorolli L, Scalinci SZ, Grenga
PL, Lupo S, Rechichi M and Meduri E: Effect of the combination of
basic fibroblast growth factor and cysteine on corneal epithelial
healing after photorefractive keratectomy in patients affected by
myopia. Indian J Ophthalmol. 62:424–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilkin J, Dahl M, Detmar M, Drake L,
Feinstein A, Odom R and Powell F: Standard classification of
rosacea: Report of the National Rosacea Society Expert Committee on
the classification and staging of rosacea. J Am Acad Dermatol.
46:584–587. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rong L, Ding T and Zhou Y: Different
methods in the treatment of rosacea and effect observation of
staging. Sichuan Medical J. 35:444–445. 2014.
|
|
14
|
Tan JK: Evaluation of clinical severity by
acne grading and lesion counting. Springer Berlin. (Heidelberg).
325–330. 2014.
|
|
15
|
Luo Y, Zhang LI, Sun YJ, Du H and Yang GL:
Syringotropic mycosis fungoides responding well to VELP
chemotherapy: A case report. Exp Ther Med. 11:2254–2258. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alebachew T, Yismaw G, Derabe A and Sisay
Z: Staphylococcus aureusburn wound infection among patients
attending yekatit 12 hospital burn unit, addis ababa, ethiopia.
Ethiop J Health Sci. 22:2092012.PubMed/NCBI
|
|
17
|
Del Rosso JQ, Tanghetti EA, Baldwin HE,
Rodriguez DA and Ferrusi IL: The burden of illness of
erythematotelangiectatic rosacea and papulopustular rosacea:
Findings from a web-based survey. J Clin Aesthet Dermatol.
10:17–31. 2017.PubMed/NCBI
|
|
18
|
Rainer BM, Kang S and Chien AL: Rosacea:
Epidemiology, pathogenesis, and treatment. Dermatoendocrinol.
9:e13615742017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mikkelsen CS, Holmgren HR, Kjellman P,
Heidenheim M, Kappinnen A, Bjerring P and Huldt-Nystrøm T: Rosacea:
A clinical review. Dermatol Reports. 8:63872016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tüzün Y, Wolf R, Kutlubay Z, Karakuş O and
Engin B: Rosacea and rhinophyma. Clin Dermatol. 32:35–46. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oge LK, Muncie HL and Phillips-Savoy AR:
Rosacea: Diagnosis and treatment. Am Fam Physician. 92:187–196.
2015.PubMed/NCBI
|
|
22
|
van Zuuren EJ, Fedorowicz Z, Carter B, van
der Linden MM and Charland L: Interventions for rosacea. Cochrane
Database Syst Rev. CD003262. 2015. View Article : Google Scholar
|
|
23
|
Ebbelaar CCF, Venema AW and Van Dijk MR:
Topical ivermectin in the treatment of papulopustular rosacea: A
systematic review of evidence and clinical guideline
recommendations. Dermatol Ther (Heidelb). 8:379–387. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piwnica D, Pathak A, Schäfer G and
Docherty JR: In vitro safety pharmacology profiling of topical
α-adrenergic agonist treatments for erythema of rosacea. Drugs R D.
18:87–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Piwnica D, M Pathak A, Schafer G and
Docherty JR: In vitro safety pharmacology profiling of
topical alpha-adrenergic agonist treatments for erythema in wounded
skin. J Cell Sci. 125:5690–5701. 2012.PubMed/NCBI
|
|
26
|
Zbinden A, Browne S, Altiok EI, Svedlund
FL, Jackson WM and Healy KE: Multivalent conjugates of basic
fibroblast growth factor enhance in vitro proliferation and
migration of endothelial cells. Biomater Sci. 6:1076–1083. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen G, Ren J, Deng Y, Wu X, Huang J, Wang
G, Zhao Y and Li J: An injectable, wound-adapting, self-healing
hydrogel for fibroblast growth factor 2 delivery system in tissue
repair applications. J Biomed Nanotechnol. 13:1660–1672. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu Y, Cao C, Wu Q, Huang A, Song Y, Li H,
Zuo Y, Chu C, Li J and Man Y: The dual delivery of KGF and bFGF by
collagen membrane to promote skin wound healing. J Tissue Eng Regen
Med. 12:1508–1518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marcelo KL, Goldie LC and Hirschi KK:
Regulation of endothelial cell differentiation and specification.
Circ Res. 112:1272–1287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taieb A, Ortonne JP, Ruzicka T,
Roszkiewicz J, Berth-Jones J, Peirone MH and Jacovella J;:
Ivermectin Phase III study group: Superiority of ivermectin 1%
cream over metronidazole 0.75% cream in treating inflammatory
lesions of rosacea: A randomized, investigator-blinded trial. Br J
Dermatol. 172:1103–1110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DuBois J, Dover JS, Jones TM, Weiss RA,
Berk DR and Ahluwalia G: Phase 2 randomized, dose-ranging study of
oxymetazoline cream for treatment of persistent facial erythema
associated with rosacea. J Drugs Dermatol. 17:308–316.
2018.PubMed/NCBI
|
|
32
|
Gold LS, Papp K, Lynde C, Lain E,
Gooderham M, Johnson S and Kerrouche N: Treatment of rosacea with
concomitant use of topical ivermectin 1% cream and brimonidine
0.33% gel: A randomized, vehicle-controlled study. J Drugs
Dermatol. 16:909–916. 2017.PubMed/NCBI
|
|
33
|
Friedman LM, Furberg CD, DeMets D,
Reboussin DM and Granger CB: Fundamentals of clinical trials.
Springer. 2015.
|