Introduction
As a common clinical complication during pregnancy
that threatens maternal and fetal health, pregnancy-induced
hypertension syndrome, referred to as pregnancy-induced
hypertension (PIH), is one of high risk factors for death in
pregnant women (1). The patients
with PIH are mainly characterized by systemic small vasospasm that
results in multiple organ damage throughout the body (2), and causes abnormal maternal uterine
placenta function in pregnant women and thereby fetal hypoxia. As a
result, the possibility of neonatal death is increased (3). PIH complicated with heart failure
refers to the symptoms of myocardial damage in pregnant women with
PIH. Heart failure, a severe complication of PIH and an important
factor causing death in pregnant women, seriously threatens the
safety of the pregnant woman and the fetus (4). At present, there are few biomarkers for
the diagnosis and confirmation of PIH complicated with heart
failure in clinical practice. This leads to the delayed and unclear
diagnosis of the disease, delays the optimal treatment opportunity
and endangers both the maternal and fetal life.
microRNA (miR) is a non-coding, single-stranded
small RNA molecule, and has been considered to be involved in the
occurrence of cardiovascular diseases (5). Currently, miR is widely used in the
marker examination of malignant tumors (6), which is also manifested in blood
according to a study as science and technology and scientific
research levels continue to improve (7). miR-21, which plays an important role in
the development of the cardiovascular system and the occurrence of
diseases, is abnormally expressed in PIH complicated with heart
failure, so it is expected to be a biomarker for the disease
(8). A study has confirmed (9) that miR-21 is significantly
differentially expressed in the serum of patients with heart
failure and healthy individuals.
B-type natriuretic peptide (BNP) is a neurohormone
secreted when the ventricular volume is enlarged and the pressure
is overloaded (10). It is widely
distributed in the tissues of heart, brain, spinal cord and lung,
of which the content is highest in the heart. BNP has high
sensitivity and specificity to the heart, and is not affected by
subjective factors. In recent years, with continuous studies of its
expression and role in heart diseases, BNP has been found to play
an important role in evaluating heart failure (11). The study by McMurray et al
(12) shows that BNP accurately
reflects the degree of heart failure.
At present, there are few studies on the correlation
of miR-21 and BNP with PIH complicated with heart failure and their
diagnostic values. Therefore, in the present study, miR-21 and BNP
expression levels in the plasma of patients were compared between
the PIH group and the PIH complicated with the heart failure group,
to evaluate the diagnostic values of miR-21 and BNP for PIH
complicated with heart failure, in order to provide a basis for the
early diagnosis and treatment of the disease.
Patients and methods
General information
Sixty patients with PIH complicated with heart
failure admitted to Affiliated Hospital of Chengde Medical
University (Chengde, China) from July 2016 to July 2017 were
enrolled as the experimental group and divided into three grades
based on the NYHA cardiac function classification (13). Cardiac function is generally divided
into four grades, but heart failure into three grades by NYHA.
Heart failure classification is slightly supplemented according to
the NYHA classification. Fifty-two patients had a gestational week
of ≤37 weeks and 8 patients had a gestational week of >37 weeks,
with an average gestational week of 36.7±2.2 weeks. There were 18
patients in grade II, the daily physical activity of patients with
heart disease is slightly restricted. Patients have no subjective
symptoms when having a rest, but they have fatigue, palpitation and
dyspnea or angina pectoris when carrying out normal activities,
aged 23–35 years, with an average age of 27.15±2.2 years. There
were 22 patients in grade III, the physical activity of patients
with heart disease is obviously restricted, and patients have
fatigue, palpitation, dyspnea or angina pectoris when carrying out
slight activities, aged 23–36 years, with an average age of
28.7±3.5 years. There were 20 patients in grade IV, patients with
heart disease have severe heart failure symptoms and are unable to
engage in any physical activity. When having a rest, they have
heart failure symptoms that are significantly aggravated after
physical activities, aged 24–38 years, with an average age of
30.4±7.1 years. Thirty-five normal pregnant women were enrolled as
the control group, aged 21–34 years, with an average age of
25.8±4.3 years. Patients with PIH complicated heart failure were in
line with the diagnostic criteria for hypertensive heart failure
during pregnancy (14).
Inclusion and exclusion criteria
The inclusion criteria were the following: i)
Patients without complete left bundle branch block, acute
myocardial infarction, pericardial effusion and hypertrophic
cardiomyopathy were included; ii) patients without acute and
chronic hepatitis, acute and chronic renal failure; iii) patients
without pulmonary heart disease, pneumothorax and pulmonary
infection; iv) patients who had not taken drugs affecting
aldosterone metabolism in the body within 2 weeks; and v) patients
without multiple organ failure. The exclusion criteria were the
following: i) Patients who recently had acute myocardial infarction
or recent acute myocarditis were excluded; ii) patients with acute
and chronic infections; iii) patients with hyperthyroidism, sepsis,
diabetes insipidus and tumors; iv) patients with primary
aldosteronism; v) patients with bronchial asthma; and vi) patients
with simple gestational hypertension or simple heart failure. The
study was approved by the Ethics Committee of Affiliated Hospital
of Chengde Medical University. Patients or their families signed a
full informed consent form.
Equipment
Reagents and instruments
RNA extraction kit (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China), spectrophotometer (Shanghai
INESA Analytical Instrument Co., Ltd., Shanghai, China), RNA
Reverse Transcription kit (Shanghai ShineGene Molecular
Biotechnology Co., Ltd., Shanghai, China), Real-Time fluorescence
quantification PCR kit (Aidlab Biotechnologies Co., Ltd., Beijing,
China), U6 and miR-21 primer sequences (Sangon Biotech Co., Ltd.,
Shanghai, China), the automatic biochemical analyzer SQChemray240
of the laboratory medicine for the detection of BNP (Shanghai
Huanxi Medical Devices Co., Ltd., Shanghai, China).
Methods
Total RNA extraction and cDNA
synthesis
Total RNA was extracted in strict accordance with
the instructions of the serum RNA extraction kit. A
spectrophotometer was used to detect the optical density (OD), with
the reference value of the qualified samples between 1.8 and 2.2.
Reverse transcription was performed in accordance with the
instructions of the Reverse Transcription kit. The RT reaction
solution (20 µl of the reaction system) was prepared as follows: 2
µl of miScript Reverse Transcriptase Mix; 4 µl of 5X miScript
HiSpec buffer; 5 µl of RNase-free water; 2 µl of 10X miScript
Nucleics Mix; 7 µl of Template RNA (2 µg of RNA content per
reaction system, the RNA required was calculated based on the RNA
concentration extracted, and RNase-free water was used to make up
to 7 µl). Reaction conditions were as follows: at 16°C for 30 min,
at 42°C for 30 min and at 75°C for 15 min. After the reverse
transcription, cDNA was placed in a refrigerator at −20°C for later
use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
With U6 as an internal reference gene, the reaction
system was 20.00 µl: each 0.5 µl of positive and negative primers
(Table I), 1.0 µl of cDNA product,
10 µl of 2X miR qPCR Mix, and DEPC were used to make up to 20 µl.
Amplification conditions were: at 94°C for 3 min, at 94°C for 35
sec, at 60°C for 35 sec, and at 72°C for 60 sec, for a total of 40
cycles. The cycle number Cq value was obtained from the qPCR curve,
and 2−ΔCq was used to calculate the miR-21 expression
(15).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Upstream primers | Downstream
primers |
|---|
| U6 internal
reference |
5-ATTGGAACGATACAGAGAAGATT-3 |
5-GGAACGCTTCACGAATTTG-3 |
| miR-21 |
5-ACGTTGTGTAGCTTATCAGACTG-3 |
5-AATGGTTGTTCTCCACACTCTC-3 |
The automatic biochemical analyzer SQChemray240 of
the laboratory medicine and supporting reagents were used to
determine BNP with spectrophotometry in strict accordance with the
instructions.
Statistical analysis
SPSS 17.0 (Keruanwang Technology Co., Ltd., Tianjin,
China) statistical software was used for the statistical analysis
of the research data. The t-test was used for the comparison
between the two groups, and analysis of variance (ANOVA) and Least
Significant Difference test was used for comparison among multiple
groups. Measurement data were expressed as mean ± standard
deviation (mean ± SD), and count data were expressed as rate (%),
tested by χ2 test. Logistic univariate and multivariate
regression analysis were used for disease risk factors, Spearmans
correlation analysis for correlation and ROC curve for judging the
predictive value and cut-point of indicators. P<0.05 indicates a
statistically significant difference.
Results
General information
There were no significant differences in age,
ethnicity, height, body weight, history of diabetes mellitus,
smoking and drinking between the experimental group and the control
group (P<0.05), but there were differences in hypoproteinemia,
blood pressure, six-minute walk test (6MWT), left ventricular
ejection fraction (LVEF) and left ventricular end diastolic
diameter (LVEDD) (P>0.05) (Table
II).
 | Table II.Basic information of the experimental
and control groups [n, (%)]. |
Table II.
Basic information of the experimental
and control groups [n, (%)].
| Basic
information | Experimental group
(n=60) | Control group
(n=35) | t/χ2 | P-value |
|---|
| Age (years) |
|
| 0.513 | 0.473 |
|
<30 | 28 (46.67) | 19 (54.29) |
|
|
| ≥30 | 32 (53.33) | 16 (45.71) |
|
|
| Ethnicity |
|
| 0.118 | 0.732 |
| Han | 56 (93.33) | 32 (91.43) |
|
|
|
Others | 4 (6.67) | 3 (8.57) |
|
|
| Height (cm) |
|
| 0.085 | 0.771 |
|
<163 | 31 (51.67) | 17 (48.57) |
|
|
| ≥163 | 29 (48.33) | 18 (51.43) |
|
|
| Body weight (kg) |
|
| 1.688 | 0.194 |
|
<50 | 34 (56.67) | 15 (42.86) |
|
|
| ≥50 | 26 (43.33) | 20 (57.14) |
|
|
| Hypoproteinemia |
|
| 4.231 | 0.040 |
| Yes | 13 (21.67) | 2 (5.71) |
|
|
| No | 47 (78.33) | 33 (94.29) |
|
|
| Anemia |
|
| 4.117 | 0.042 |
|
Yes | 10 (16.67) | 1 (2.86) |
|
|
| No | 50 (83.33) | 34 (97.14) |
|
|
| History of diabetes
mellitus |
|
| 0.073 | 0.788 |
|
Yes | 8 (13.33) | 4 (11.43) |
|
|
| No | 52 (86.67) | 31 (88.57) |
|
|
| Smoking |
|
| 0.002 | 0.968 |
|
Yes | 5 (8.33) | 3 (8.57) |
|
|
| No | 55 (91.67) | 32 (91.43) |
|
|
| Drinking |
|
| 0.137 | 0.711 |
|
Yes | 7 (11.67) | 5 (14.29) |
|
|
| No | 53 (88.33) | 30 (85.71) |
|
|
| Blood pressure
(mmHg) |
|
Systolic pressure | 159.78±18.32 | 123.71±11.34 | 10.520 | <0.001 |
|
Diastolic pressure | 107.72±2.67 | 86.45±15.28 | 10.550 | <0.001 |
| 6MWT (m) | 298.56±70.93 | 526.79±102.61 | 12.79 | <0.001 |
| LVEF (%) | 40.26±17.54 | 59.67±12.43 | 5.735 | <0.001 |
| LVEDD (mm) | 62±6.39 | 53.01±1.21 | 8.220 | <0.001 |
miR-21 expression level in the two
groups of patients
The miR-21 expression level in the experimental
group was 1.553±0.513 in grade II, 1.654±0.493 in grade III and
1.948±0.651 in grade IV, and that in the control group was
0.927±0.231. The miR-21 expression level in the control group was
compared with that in grades II, III and IV in the experimental
group, all P<0.05; that in grade II was compared with that in
grade III, P>0.05; that in grade III was compared with that in
grade IV, P<0.05; that in grade II was compared with that in
grade IV, P<0.05 (Table
III).
 | Table III.miR-21 expression level in two groups
of patients. |
Table III.
miR-21 expression level in two groups
of patients.
| Groups | n | miR-21 |
|---|
| Experimental
group |
| Grade
II | 18 |
1.553±0.513a |
| Grade
III | 22 |
1.654±0.493a,c |
| Grade
IV | 20 |
1.948±0.651a,b,d |
| Control group | 35 | 0.927±0.231 |
| F | – | 79.546 |
| P-value | – | <0.001 |
BNP expression level in two groups of
patients
BNP expression level in the experimental group was
165.59±82.52 pg/ml in grade II, 786.75±121.99 pg/ml in grade III
and 1018.48±109.54 pg/ml in grade IV, and that in the control group
was 95.36±34.65 pg/ml. The BNP expression level in the control
group was compared with that in grades II, III and IV in the
experimental group, all P<0.05; that in grade II was compared
with that in grade III, P<0.05; that in grade III was compared
with that in grade IV, P<0.05; that in grade II was compared
with that in grade IV, P<0.05 (Table
IV).
 | Table IV.BNP expression level in two groups of
patients (pg/ml). |
Table IV.
BNP expression level in two groups of
patients (pg/ml).
| Groups | n | BNP |
|---|
| Experimental
group |
| Grade
II | 18 |
165.59±82.52a |
| Grade
III | 22 |
786.75±121.99a,b |
| Grade
IV | 10 |
1018.48±109.54a–c |
| Control group | 35 | 95.36±34.65 |
| F | – | 46.670 |
| P-value | – | <0.001 |
ROC curve for evaluation of diagnostic
value
By ROC curve fitting, in the diagnosis of PIH
complicated with heart failure, the odds ratio (OR) of miR-21 was
1.113, the sensitivity was 88.53% and the specificity was 88.33%,
with an area under curve (AUC) of 0.889 and a 95% CI of
82.05–95.76%; the OR of BNP was 123, the sensitivity was 88.57% and
the specificity was 63.33%, with an AUC of 0.747 and a 95% CI of
64.95–84.38% (Fig. 1 and Table V).
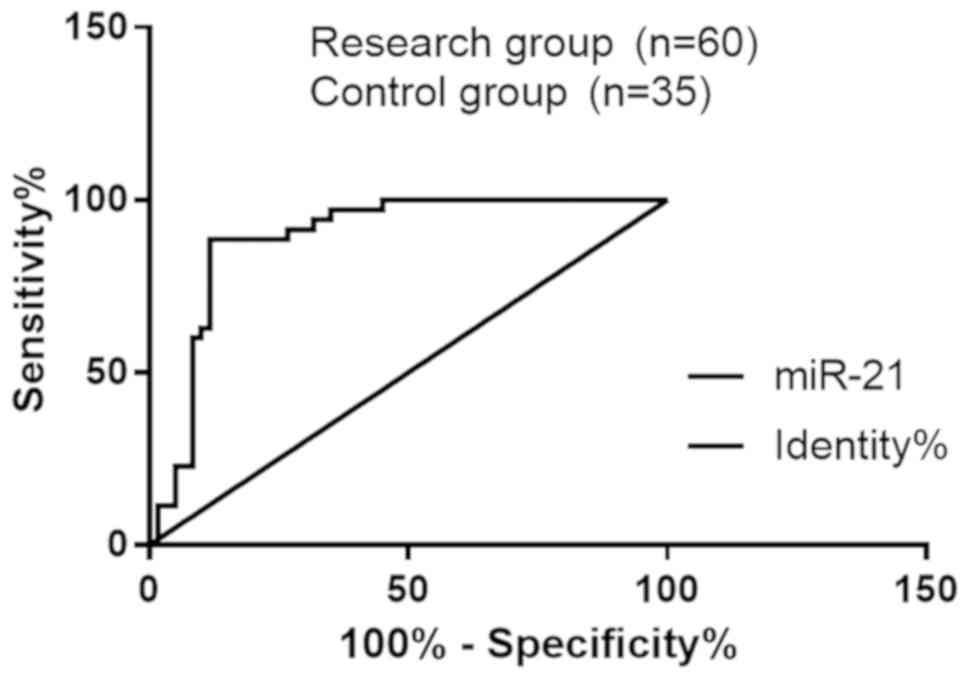 | Figure 1.Diagnostic value of miR-21 and BNP for
PIH complicated with heart failure. The analysis of ROC curve
showed that in the diagnosis of PIH complicated with heart failure,
the OR of miR-21 was 1.113, the sensitivity was 88.53% and the
specificity was 88.33%, with an AUC of 0.889 and a 95% CI of
82.05–95.76%; the OR of BNP cut-off value was 123, the sensitivity
was 88.57% and the specificity was 63.33%, with an AUC of 0.747 and
a 95% CI of 64.95–84.38%. BNP, B-type natriuretic peptide; PIH,
pregnancy-induced hypertension; ROC, receiver operating
characteristic; AUC, area under curve; CI, confidence interval; OR,
odds ratio. |
 | Table V.Diagnostic value of miR-21 and BNP
for PIH complicated with heart failure. |
Table V.
Diagnostic value of miR-21 and BNP
for PIH complicated with heart failure.
| Items | AUC | OR | 95% CI (%) | P-value | Specificity
(%) | Sensitivity
(%) |
|---|
| miR-21 | 0.889 | 1.113 | 82.05–95.76 | <0.001 | 85.71 | 88.33 |
| BNP | 0.747 | 123 | 64.95–84.38 | <0.001 | 62.85 | 88.33 |
Correlation analysis of miR-21
expression with BNP in PIH complicated with heart failure
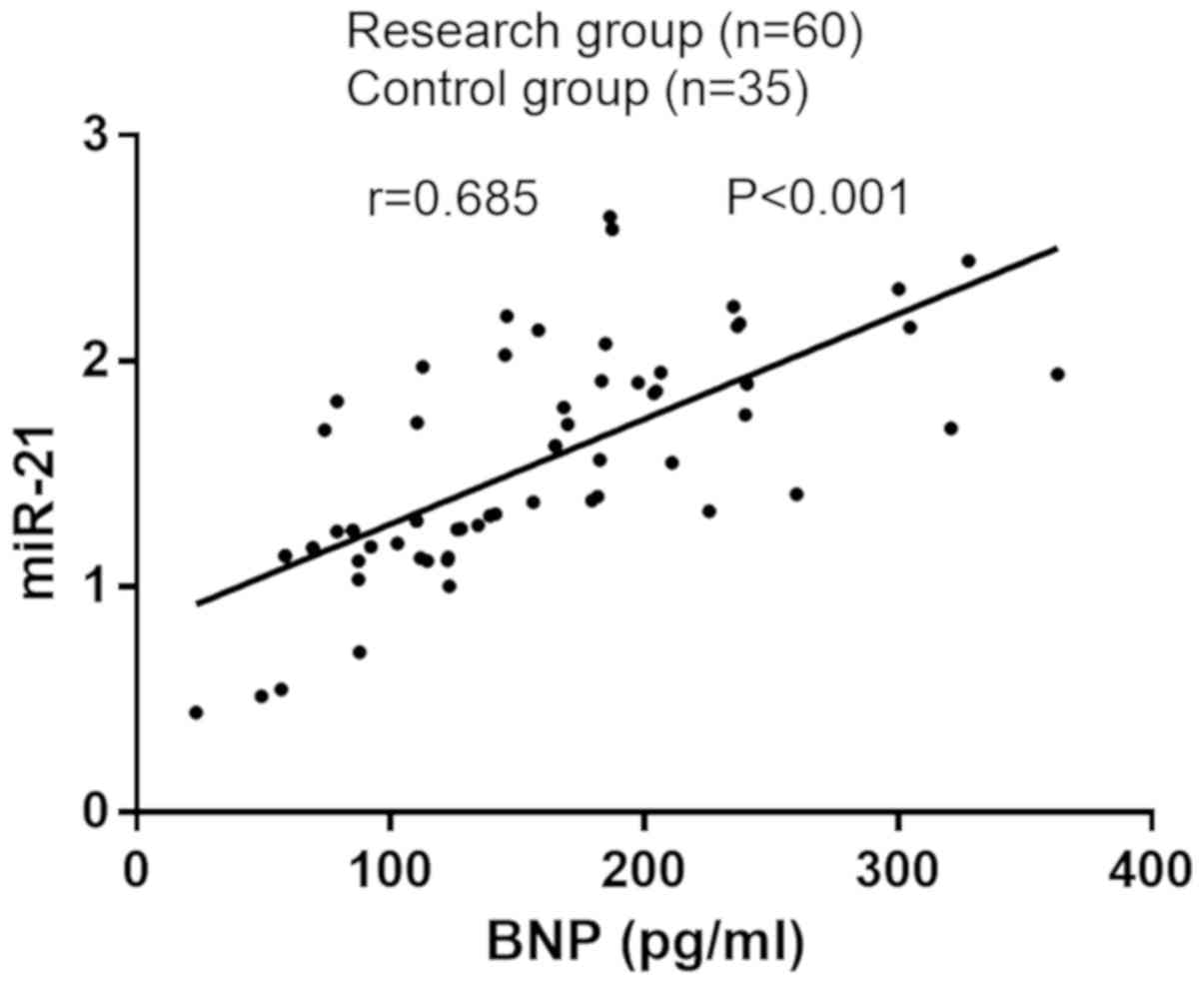
The plasma miR-21 expression was positively
correlated with BNP in patients with PIH complicated with heart
failure (r=0.685, P<0.001) (Fig.
2).
Analysis of risk factors for PIH
complicated with heart failure
Logistic univariate analysis on risk factors for PIH
complicated with heart failure showed that the blood pressure
(P=0.018), 6MWT (P=0.006), LVEF (0.027) and LVEDD (0.036) of
patients were correlated with PIH complicated with heart failure
(Table VI). To control the
influence of confounding factors on this outcome, logistic
multivariate analysis was used on risk factors for PIH complicated
with heart failure. The results showed that blood pressure (OR,
3.045; 95% CI, 0.935–11.442), 6MWT (OR, 9.547; 95% CI,
4.671–13.072), LVEF (OR, 8.665; 95% CI, 5.728–15.569) and LVEDD
(OR, 8.665; 95% CI, 5.728–15.569) were independent risk factors for
the disease (Table VII).
 | Table VI.Logistic univariate analysis of risk
factors for PIH complicated with heart failure. |
Table VI.
Logistic univariate analysis of risk
factors for PIH complicated with heart failure.
| Clinical
parameters | Coefficient | Standard error | Wald value | P-value | OR value | 95% CI |
|---|
| Blood pressure | 0.302 | 0.117 | 10.157 | 0.001 | 4.528 | 1.478–8.537 |
| 6MWT | 1.132 | 0.500 | 3.467 | 0.046 | 3.101 | 0.897–10.077 |
|
Hypoproteinemia | 1.132 | 0.500 | 3.467 | 0.056 | 3.101 | 0.897–10.077 |
| LVEF | 1.431 | 0.495 | 6.491 | 0.043 | 4.485 | 1.471–11.436 |
| Anemia | 0.302 | 0.117 | 5.157 | 0.061 | 3.528 | 1.478–8.537 |
| LVEDD | 1.041 | 0.576 | 3.465 | 0.004 | 3.045 | 0.935–11.442 |
 | Table VII.Logistic multivariate analysis of
risk factors for PIH complicated with heart failure. |
Table VII.
Logistic multivariate analysis of
risk factors for PIH complicated with heart failure.
| Clinical
parameters | Coefficient | Standard error | Wald value | P-value | OR value | 95% CI |
|---|
| Blood pressure | 0.302 | 0.117 | 10.157 | 0.001 | 4.528 | 1.478–8.537 |
| 6MWT | 1.431 | 0.495 | 6.491 | 0.001 | 4.485 | 1.471–11.436 |
| LVEF | 1.041 | 0.576 | 3.465 | 0.001 | 3.045 | 0.935–11.442 |
| LVEDD | 1.954 | 0.392 | 20.329 | 0.001 | 9.547 | 4.671–13.072 |
Discussion
Pregnancy-induced hypertension (PIH) complicated
with heart failure is a common disease during pregnancy and
characterized by no history of hypertension and heart-related
diseases before pregnancy. Heart failure that is complicated with
PIH, is mainly characterized by myocardial injury, which is a heart
failure syndrome and the second cause of pregnant and maternal
death (16). The main manifestation
of such heart failure is arteriolar spasm, mainly systemic
symptoms, resulting in the ischemia and hypoxia of the tissues and
organs of the human body, and thereby myocardial ischemia and
injury and an increase in cardiac load. Severe PIH easily cause
this disease that leads to fetal distress, intrauterine growth
retardation, fetal death and neonatal or maternal death (17).
At present, there is no precise factor or technical
means for the early diagnosis of PIH complicated with heart
failure. Kumarswamy et al (18) have pointed out that the miR-2
expression level in the plasma of patients with heart failure is
significantly higher than that in the normal individuals. Berger
et al (19) suggested that
the BNP expression level in the plasma of patients with heart
failure is significantly higher than that in the normal
individuals, which is important for diagnosing and classifying
heart failure. Du et al (20)
also proposed that the BNP expression level in patients with PIH is
significantly increased, with a diagnostic value. In the historical
literature search, there are few studies on the correlation of
miR-21 and BNP with PIH complicated with heart failure and their
diagnostic values, which were detected and analyzed in the present
study for clarification.
The present study found that the miR-21 expression
level in patients with PIH complicated with heart failure was
higher than that in the normal individuals, and increased in line
with with the increase in the heart failure grade. When the human
heart load is increased, the miR-21 expression level is also
increased, which stimulates and activates the genetic program for
cardiac fibrosis, and then fibrotic changes take place in
myocardial cells that lose normal physiological functions (21). The study by Wu et al (22) also confirmed that miR-21 regulates
and activates multiple factor signaling pathways, the expression
level of which is significantly upregulated in cardiac fibroblasts
of heart failure, and the signal transduction pathway of ERK-MAP
kinase is thus enhanced, which causes the proliferation and
fibrosis of cardiac fibroblasts. Adams et al (11) and Stokes et al (23) proposed in their studies that BNP
expression in patients with heart failure is higher than that in
normal individuals. This study showed that the BNP expression level
in patients with PIH complicated with heart failure was
significantly higher than that in normal individuals, which was
significantly higher with the increase in heart failure grade. BNP
is a peptide neurohormone mainly secreted by ventricular muscle
cells. It is currently believed that the cardiac volume load is
increased during heart failure, and acute decompensated high wall
pressure in patients leads to a significant increase in the BNP
expression level. This is consistent with the views of Kovács et
al (24). The ROC curve was used
for judging the predictive value and cut-point of indicators. The
results showed that the OR of miR-21 was 1.113, the sensitivity was
88.53%, and the specificity was 88.33%, with an AUC of 0.889 and a
95% CI of 82.05–95.76%; the OR of BNP was 123, the sensitivity was
88.57%, and the specificity was 63.33%, with an AUC of 0.747 and a
95% CI of 64.95–84.38%. It is indicated that miR-21 and BNP have
higher diagnostic values for PIH complicated with heart failure.
Cavagna et al (25) and
Cengiz et al (26),
respectively pointed out that miR-21 and BNP have higher diagnostic
values for PIH complicated with heart failure. This study also
found that expression of miR-21 and BNP in patients with PIH
complicated with heart failure was increased in line with the heart
failure grade. The correlation analysis of miR-21 and BNP with PIH
complicated with heart failure showed that miR-21 and BNP were
positively correlated with PIH complicated with heart failure
(r=0.685, P<0.001). In the present study, risk factors for the
prognosis of PIH complicated with heart failure were also analyzed.
The results showed that blood pressure, 6MWT, LVEF and LVEDD were
independent risk factors for the disease, consistent with the views
of Chambela et al (27).
Study subjects were screened in strict accordance
with the inclusion and exclusion criteria in this investigation,
and there were no differences between the experimental and control
groups in terms of sex, age and lifestyle, which improve the
authenticity and reliability of this study. In addition to
detecting the miR-21 and BNP expression levels in patients with PIH
complicated with heart failure, independent risk factors for this
disease were also found. All-round observations and statistics, as
well as the results were proven by the data, reflecting the rigor
of this experiment. The experimental research is to extend the
results obtained to a larger overall, so as to contribute to
understanding the group and society. However, due to limited
medical resources in Affiliated Hospital of Chengde Medical
University, the number of enrolled patients is insufficient, so the
results may lack extensive representation, and a future
confirmation study is necessary.
In summary, miR-21 and BNP, highly expressed in
patients with PIH complicated with heart failure, are expected to
become important biomarkers for diagnosing PIH complicated with
heart failure and judging the degree of heart failure in the
patients, and worthy of clinical popularization and
application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding a uthor on reasonable
request.
Authors contributions
CK, JC and JH were responsible for the total RNA
extraction, cDNA synthesis and PCR. XJ, YZ and JZ collected and
interpreted the patient general data. YG and XC reviewed the
manuscript and contributed to performing the statistical analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Affiliated Hospital of Chengde Medical University
(Chengde, China). Patients who participated in this research,
signed an informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abalos E, Duley L, Steyn DW and Gialdini
C: Antihypertensive drug therapy for mild to moderate hypertension
during pregnancy. Cochrane Database Syst Rev.
10:CD0022522018.PubMed/NCBI
|
|
2
|
Magee LA, Pels A, Helewa M, Rey E, von
Dadelszen P, Magee LA, Audibert F, Bujold E, Côté A-M, Douglas MJ,
et al Canadian Hypertensive Disorders of Pregnancy Working Group, :
Diagnosis, evaluation, and management of the hypertensive disorders
of pregnancy: Executive summary. J Obstet Gynaecol Can. 36:416–441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bramham K, Parnell B, Nelson-Piercy C,
Seed PT, Poston L and Chappell LC: Chronic hypertension and
pregnancy outcomes: Systematic review and meta-analysis. BMJ.
348:g23012014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gini R, Schuemie MJ, Mazzaglia G, Lapi F,
Francesconi P, Pasqua A, Bianchini E, Montalbano C, Roberto G,
Barletta V, et al: Automatic identification of type 2 diabetes,
hypertension, ischaemic heart disease, heart failure and their
levels of severity from Italian General Practitioners electronic
medical records: A validation study. BMJ Open. 6:e0124132016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z and Rana TM: Therapeutic targeting of
microRNAs: Current status and future challenges. Nat Rev Drug
Discov. 13:622–638. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng CJ, Bahal R, Babar IA, Pincus Z,
Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM,
et al: MicroRNA silencing for cancer therapy targeted to the tumour
microenvironment. Nature. 518:107–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muñoz-Culla M, Irizar H, Castillo-Triviño
T, Sáenz-Cuesta M, Sepúlveda L, Lopetegi I, López de Munain A,
Olascoaga J, Baranzini SE and Otaegui D: Blood miRNA expression
pattern is a possible risk marker for natalizumab-associated
progressive multifocal leukoencephalopathy in multiple sclerosis
patients. Mult Scler. 20:1851–1859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kukreja RC, Yin C and Salloum FN:
MicroRNAs: New players in cardiac injury and protection. Mol
Pharmacol. 80:558–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong X, Liu S, Zhang L, Yu S, Huo L, Qile
M, Liu L, Yang B and Yu J: Downregulation of miR-21 is involved in
direct actions of ursolic acid on the heart: Implications for
cardiac fibrosis and hypertrophy. Cardiovasc Ther. 33:161–167.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitchell A, Misialek JR, Folsom AR, Duprez
D, Alonso A, Jerosch-Herold M, Sanchez OA, Watson KE, Sallam T and
Konety SH: Usefulness of N-terminal pro-brain natriuretic peptide
and myocardial perfusion in asymptomatic adults (from the
multi-ethnic study of atherosclerosis). Am J Cardiol.
115:1341–1345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams KF Jr, Giblin EM, Pearce N and
Patterson JH: Integrating new pharmacologic agents into heart
failure care: Role of heart failure practice guidelines in meeting
this challenge. Pharmacotherapy. 37:645–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McMurray JJ, Adamopoulos S, Anker SD,
Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C,
Gomez-Sanchez MA, et al Task Force for the Diagnosis and Treatment
of Acute and Chronic Heart Failure 2012 of the European Society of
Cardiology; ESC Committee for Practice Guidelines, : ESC guidelines
for the diagnosis and treatment of acute and chronic heart failure
2012: The Task Force for the Diagnosis and Treatment of Acute and
Chronic Heart Failure 2012 of the European Society of Cardiology.
Developed in collaboration with the Heart Failure Association (HFA)
of the ESC. Eur J Heart Fail. 14:803–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bredy C, Ministeri M, Kempny A,
Alonso-Gonzalez R, Swan L, Uebing A, Diller GP, Gatzoulis MA and
Dimopoulos K: New York Heart Association (NYHA) classification in
adults with congenital heart disease: Relation to objective
measures of exercise and outcome. Eur Heart J Qual Care Clin
Outcomes. 4:51–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown MA, Magee LA, Kenny LC, Karumanchi
SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G and Ishaku S:
Hypertensive disorders of pregnancy: ISSHP classification,
diagnosis, and management recommendations for international
practice. Hypertension. 72:24–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barasa A, Rosengren A, Sandstrom TZ,
Ladfors L and Schaufelberger M: Heart failure in late pregnancy and
postpartum: Incidence and long-term mortality in Sweden from 1997
to 2010. J Card Fail. 23:370–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Theilen LH, Fraser A, Hollingshaus MS,
Schliep KC, Varner MW, Smith KR and Esplin MS: All-cause and
cause-specific mortality after hypertensive disease of pregnancy.
Obstet Gynecol. 128:238–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berger R, Moertl D, Peter S, Ahmadi R,
Huelsmann M, Yamuti S, Wagner B and Pacher R: N-terminal pro-B-type
natriuretic peptide-guided, intensive patient management in
addition to multidisciplinary care in chronic heart failure a
3-arm, prospective, randomized pilot study. J Am Coll Cardiol.
55:645–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du Y, Fu J, Yao L, Qiao L, Liu N, Xing Y
and Xue X: Altered expression of PPAR-γ and TRPC in neonatal rats
with persistent pulmonary hypertension. Mol Med Rep. 16:1117–1124.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patrick DM, Montgomery RL, Qi X, Obad S,
Kauppinen S, Hill JA, van Rooij E and Olson EN: Stress-dependent
cardiac remodeling occurs in the absence of microRNA-21 in mice. J
Clin Invest. 120:3912–3916. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu H, Wang J, Ma H, Xiao Z and Dong X:
MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid.
Oncotarget. 8:92914–92925. 2017.PubMed/NCBI
|
|
23
|
Stokes MB, Bergin P and McGiffin D: Role
of long-term mechanical circulatory support in patients with
advanced heart failure. Intern Med J. 46:530–540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kovács Á, Alogna A, Post H and Hamdani N:
Is enhancing cGMP-PKG signalling a promising therapeutic target for
heart failure with preserved ejection fraction? Neth Heart J.
24:268–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cavagna L, Caporali R, Klersy C, Ghio S,
Albertini R, Scelsi L, Moratti R, Bonino C and Montecucco C:
Comparison of brain natriuretic peptide (BNP) and NT-proBNP in
screening for pulmonary arterial hypertension in patients with
systemic sclerosis. J Rheumatol. 37:2064–2070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cengiz M, Yavuzer S, Kılıçkıran Avcı B,
Yürüyen M, Yavuzer H, Dikici SA, Karataş OF, Özen M, Uzun H and
Öngen Z: Circulating miR-21 and eNOS in subclinical atherosclerosis
in patients with hypertension. Clin Exp Hypertens. 37:643–649.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chambela MC, Mediano MFF, Ferreira RR,
Japiassú AM, Waghabi MC, da Silva GMS and Saraiva RM: Correlation
of 6-min walk test with left ventricular function and quality of
life in heart failure due to Chagas disease. Trop Med Int Health.
22:1314–1321. 2017. View Article : Google Scholar : PubMed/NCBI
|