Introduction
Changes in lifestyle, particularly in developing
countries has caused an increase in the incidence of diabetes and
it is expected to increase significantly in the future (1). Diabetes is often accompanied by the
development of complications that affect most major organ systems
(2). The most common
diabetes-related complications include blindness, renal failure and
cardiovascular disease (2). However,
one of the more serious complications associated with diabetes,
diabetic nephropathy, can cause long-term kidney disease and
end-stage renal disease and is a leading cause of death among
patients with diabetes (3). Although
there have been significant improvements in the treatment and
prevention of renal damage in patients with diabetes, patient
outcomes remain poor (4). Current
treatment is focused on the prevention of disease progression
(5). Therefore, identifying novel
therapeutic targets may improve the survival of patients with
diabetic nephropathy.
Smad7, which is associated with apoptosis in kidney
(6), is considered to be a
therapeutic target for the treatment of diabetic nephropathy
(7). Several studies have
demonstrated that under certain pathological conditions Smad7 can
interact with several long non-coding RNAs (lncRNAs), a subgroup of
non-coding RNAs whose aberrant expression is closely related to
human diseases, to exert its biological functions (8–10).
LncRNA psoriasis-susceptibility-related RNA gene induced by stress
(PRINS) is associated with several different types of human
disease, including kidney ischemia reperfusion injury and psoriasis
(11,12). In the current study, PRINS may be
involved in the development of nephropathy in patients with
diabetes via interactions with Smad7.
Materials and methods
Patients
Blood samples were collected from patients with
diabetes, including patients without obvious complications (n=43),
patients with diabetic nephropathy (n=33), diabetic retinopathy
(n=37), diabetic cardiomyopathy (n=29) and diabetic lung disease
(n=38) who were diagnosed and treated at the Peace Hospital of
Changzhi Medical College (Changzhi, China) from January 2015 to
January 2018. All patients were diagnosed according to the standard
established by the Chinese Medical Association (2014) (13). All patients were diagnosed and
treated for the first time. Patients with other more severe
diseases were excluded. In addition, blood samples from 48 healthy
control patients were also included as a control group. No
significant differences in age and sex were found between the
groups (Table I).
 | Table I.Participant information. |
Table I.
Participant information.
|
| Sex |
|
|
|---|
|
|
|
|
|
|---|
| Group | Male (n) | Female (n) | Age range
(years) | Mean age (years) |
|---|
| C | 27 | 21 | 33–67 | 48.1±6.2 |
| D | 24 | 19 | 29–69 | 48.4±7.1 |
| DN | 18 | 15 | 30–69 | 47.5±5.8 |
| DR | 19 | 18 | 35–69 | 49.4±6.6 |
| DC | 14 | 15 | 31–70 | 48.8±7.4 |
| DL | 20 | 18 | 31–66 | 47.9±6.7 |
The current study was approved by the Ethics
Committee at the Peace Hospital of Changzhi Medical College
(Changzhi, China), and all the participants provided written
informed consent.
Blood sample collection, RNA
extraction and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
Blood samples (20 ml) were collected from each
participant 24 h after admission. Total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
Total RNA concentration was measured using a NanoDrop™ 2000
Spectrophotometer (Thermo Fisher Scientific, Inc.) and RNA samples
with a A260/A280 ratio of 1.8–2.0 were reverse transcribed into
cDNA using SuperScript IV Reverse Transcriptase (Thermo Fisher
Scientific, Inc.) through the following thermocycling conditions:
25°C for 5 min; 50°C for 20 min and 80°C for 20 min. qPCR reaction
systems were prepared using SYBR® Green master mix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The following
primer pairs were used for the qPCR: lncRNA PRINS forward,
5′-TCCACATCCGGATTTACCTAAAC-3′ and reverse,
5′-CTGACGCACCCCTGACAGTCAG-3′; Smad7 forward,
5′-AAGTGTTCAGGTGGCCGGATCTCAG-3′ and reverse,
5′-ACAGCATCTGGACAGCCTGCAGTTG-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The thermocycling conditions used for the qPCR were: Initial
denaturation at 95°C for 52 sec; 40 cycles of 95°C for 18 sec and
57.5°C for 40 sec. lncRNA PRINS and Smad7 mRNA expression levels
were quantified using the 2−ΔΔCq method (14) and normalized to the internal control
β-actin.
Cell culture and transfection
Mouse podocyte cells were purchased from PrimCells,
LLC. (San Diego, CA, USA) and cultured with Roswell Park Memorial
Institute 1640 medium (Thermo Fisher Scientific, lnc.) supplemented
with 100 µg/ml streptomycin, 100 U/ml penicillin and 10% fetal calf
serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C in a
5% CO2 incubator. The PRINS cDNA fragment was obtained
by PCR amplification and cloned into the pEGFPC3 vector (Clontech
Laboratories, Inc., Mountainview, CA, USA) to generate a PRINS
expression vector. Cells at a cell density of 4×103/ml
were cultured in a six-well plate overnight before transfection.
Lipofectamine® 2000 reagent (Thermo Fisher Scientific.,
Inc.) was initially mixed with expression vectors (10 nM) to form
transfection reagent-vector complexes, prior to transfection. Cells
were transfected with transfection reagent-vector complexes at 37°C
for 6 h. PRINS overexpression was confirmed by RT-qPCR prior to
subsequent experimentation. Cells were harvested at 24 h after
transfection for subsequent experiments. In cases of D-glucose
treatment, mouse podocyte cells were treated with 5 mM (control),
10, 20 and 30 mM D-glucose (Sigma-Aldrich; Merck KGaA) for 6, 12
and 18 h, respectively, before use.
MTT assay
Cell viability was analyzed using the MTT assay.
Briefly, mouse podocoyte cells were collected and single-cell
suspensions at a density of 4×104 cells/ml were prepared
using Roswell Park Memorial Institute 1640 medium. Subsequently,
cells were seeded into 96-well plates at a density of
4×103 cells/well and 30 mM D-glucose was added to each
well and cells were cultured for 6 h at 37°C. Following incubation,
10 µl MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added
to each well and incubated for a further 4 h at 37°C. Cell
proliferation was determined by measuring the optical density (OD)
at a wavelength of 570 nm using a Fisherbrand™ accuSkan™ GO UV/Vis
microplate spectrophotometer (Thermo Fisher Scientific., Inc.) and
normalized to control cells (without transfection).
Western blot analysis
Total protein was extracted from cells using
xTractor™ buffer (Clontech Laboratories, Inc.) and total protein
was quantified using a bicinchoninic acid assay. Proteins were
mixed with loading buffer and denatured at 95°C for 5 min. In
total, 40 µg protein/lane was separated via SDS-PAGE on a 10% gel
(10%) and the separated proteins were transferred onto
polyvinylidene difluoride membranes. Membranes were blocked for 1 h
at room temperature with 5% skimmed milk. Subsequently, the
membranes were incubated with rabbit anti human primary antibodies
against Smad7 (1:2,000; cat. no. ab90086) and GAPDH (1:1,000; cat.
no. ab9485; both Abcam, Cambridge, UK) overnight at 4°C. Following
primary incubation, the membranes were incubated with anti-rabbit
IgG horseradish peroxidase-labeled secondary antibody (1:1,000;
cat. no. MBS435036; MyBioSource, Inc., San Diego, CA, USA) for 2 h
at room temperature. Protein bands were visualized using ECL™
Detection Reagent (cat. no. GERPN2105; Sigma-Aldrich; Merck KGaA).
Smad7 protein expression was quantified using ImageJ software
(version 1.47; National Institutes of Health, Bethesda, MD, USA)
and normalized to the internal control, GAPDH.
Statistical analysis
Data were presented as the mean ± standard
deviation. All statistical analyses were performed using SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). One-way
analysis of variance followed by the least significant difference
test was used to analyze comparisons among multiple groups.
Correlation analyses were performed using Pearson's correlation
coefficient analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
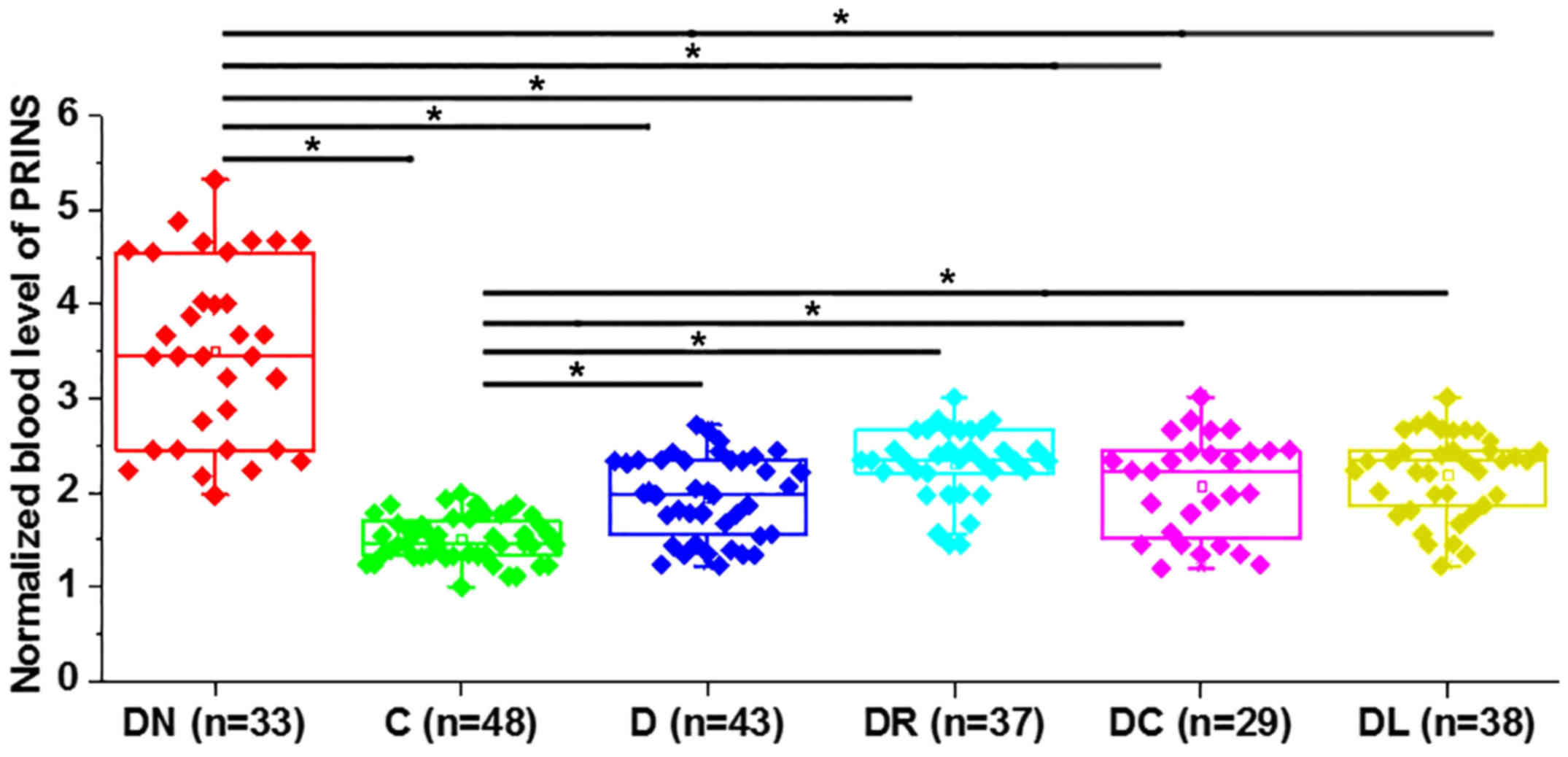
Expression level of PRINS increases in
patients with diabetes
Differential gene expression indicates whether a
specific gene is involved in a particular biological process or
disease. Initially the expression level of PRINS was detected by
RT-qPCR in blood samples from patients with diabetes and healthy
controls. The expression level of PRINS was significantly increased
in all patient groups compared with healthy controls (P<0.05;
Fig. 1). In addition, the expression
level of PRINS was significantly increased in patients with
diabetic nephropathy compared with other diabetes-related
complications (P<0.05).
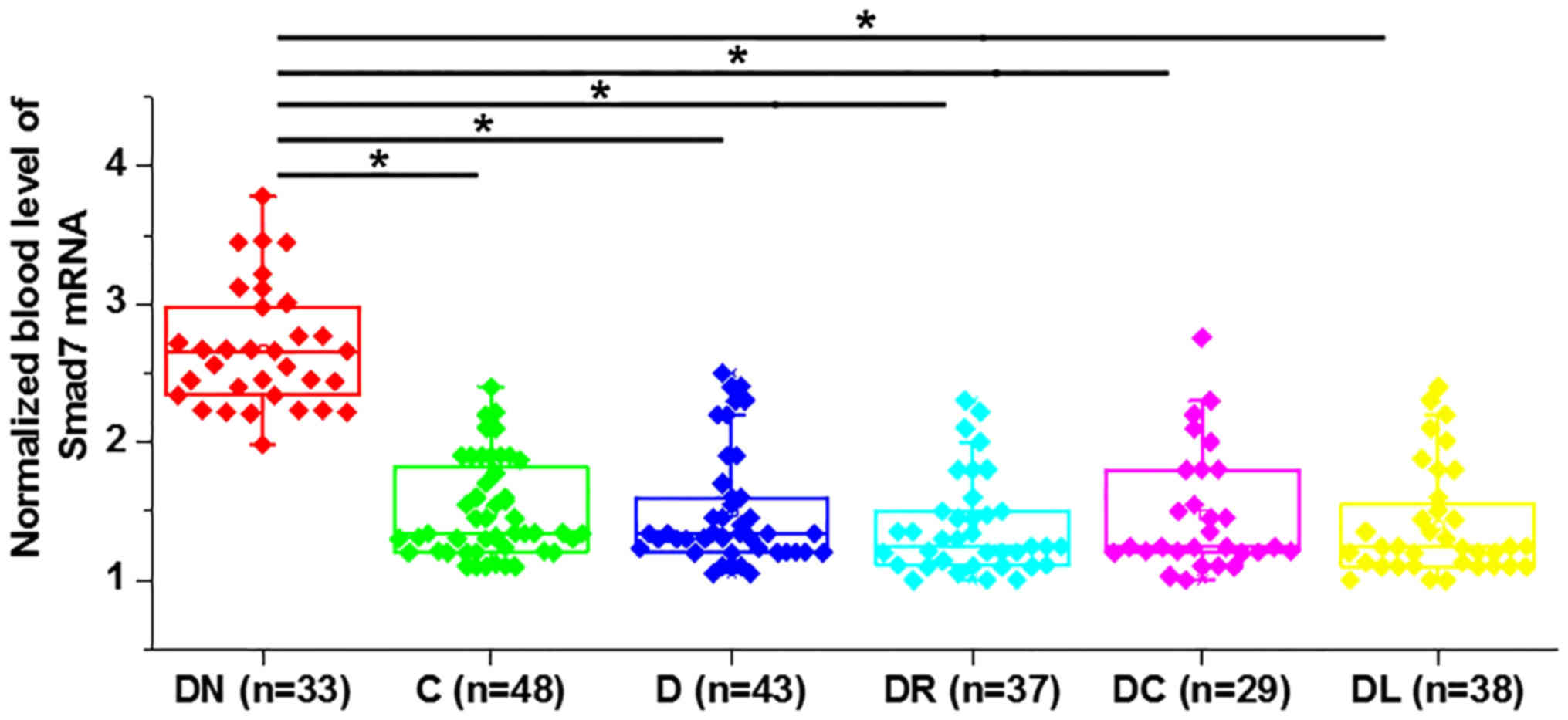
Expression level of Smad7 increases in
patients with diabetic nephropathy
The expression level of Smad7 was detected by
RT-qPCR in blood samples from patients with diabetes and healthy
controls. The expression level of Smad7 was significantly increased
in patients with diabetic nephropathy compared with other
diabetes-related complications (P<0.05; Fig. 2). However, no significant difference
in the expression level of Smad7 was observed in patients with
diabetes with other diabetes-related complications (P>0.05).
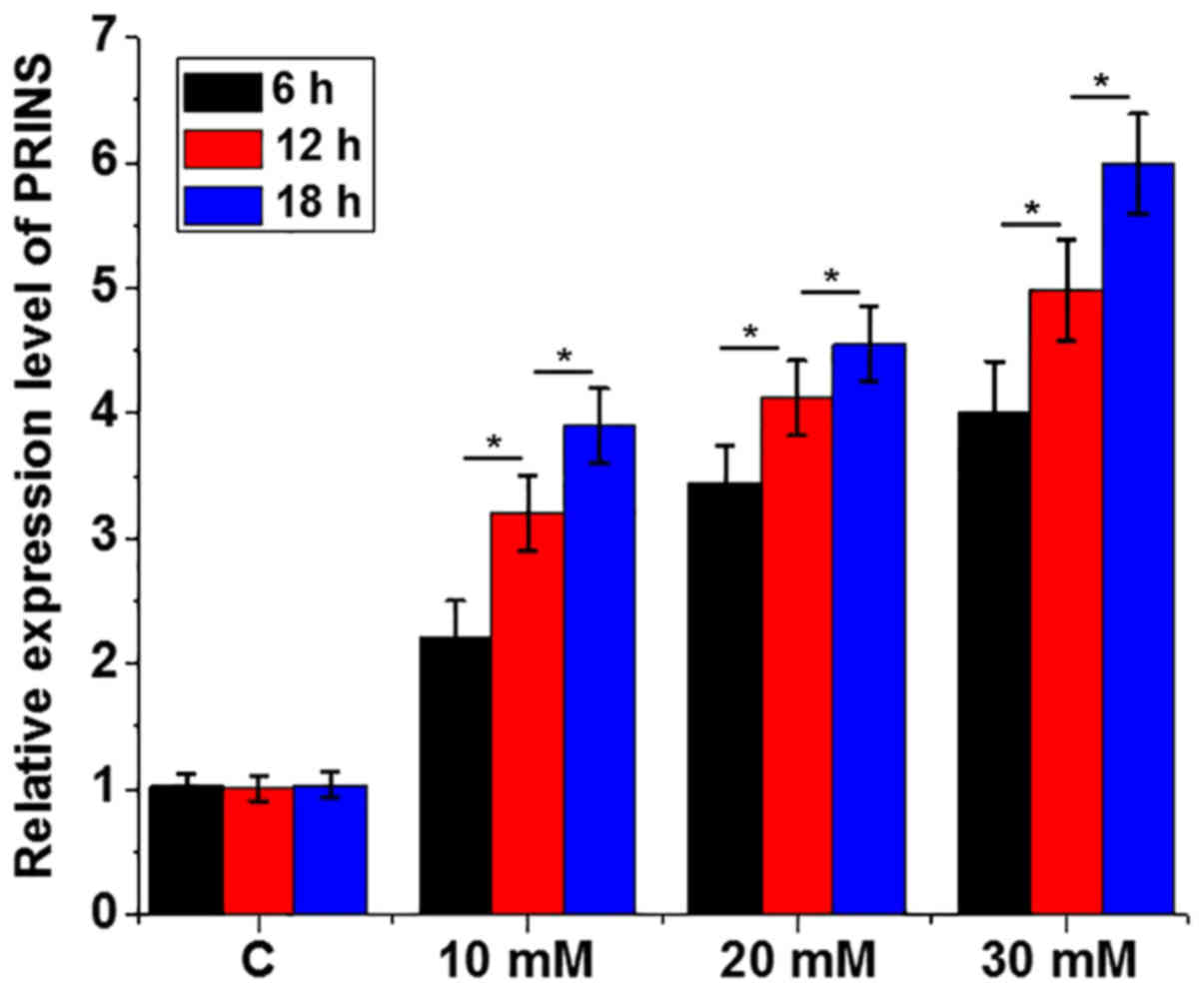
Treatment with high glucose
upregulates the expression level of PRINS in mouse podocyte
cells
Mouse podocyte cells derived from the renal
glomerular basement membrane are essential for normal renal
function and therefore these were used for all subsequent in
vitro experiments. To further confirm the effects of high
glucose on the expression of PRINS, mouse podocyte cells were
treated with 5 mM (control), 10, 20 and 30 mM D-glucose for 6, 12
and 18 h, respectively. Treatment with high glucose significantly
upregulated the expression level of PRINS (P<0.05; Fig. 3).
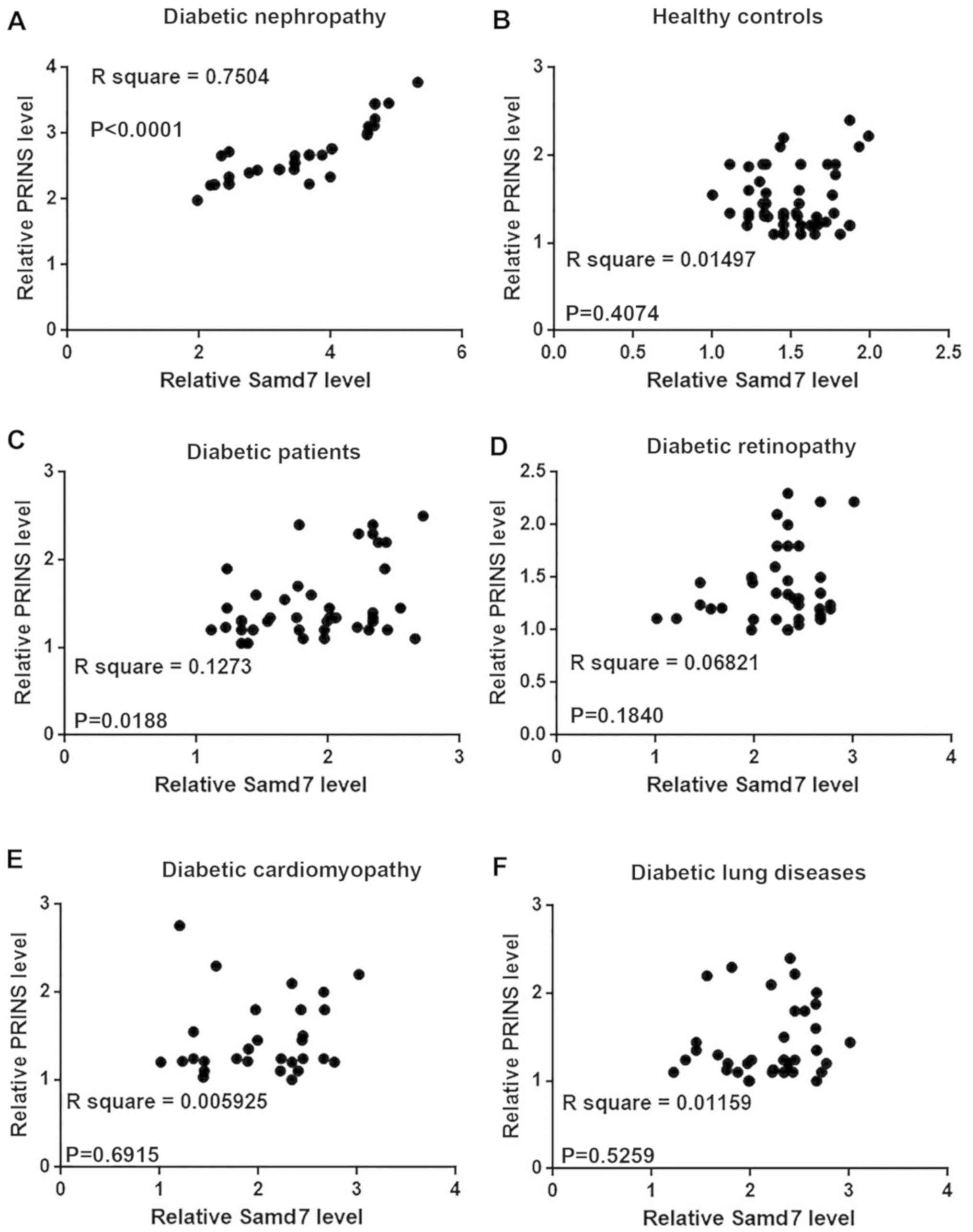
Serum PRINS and Smad7 levels are
significantly associated with diabetic nephropathy in patients with
diabetes
Correlation analysis between the expression level of
PRINS and Smad7 in blood samples from patients with diabetes was
analyzed by Pearson's correlation coefficient analysis. A
significant positive correlation between the relative mRNA
expression level of PRINS and Smad7 was observed in patients with
diabetic nephropathy (P<0.0001; Fig.
4A). However, no correlation was observed between the
expression level of PRINS and Smad7 mRNA in healthy controls
(Fig. 4B), patients without
complications (Fig. 4C), patients
with diabetic retinopathy (Fig. 4D),
diabetic cardiomyopathy (Fig. 4E) or
diabetic lung diseases (Fig.
4F).
Overexpression of PRINS reduces cell
viability and enhances Smad7 expression in mouse podocytes under
higher glucose treatment
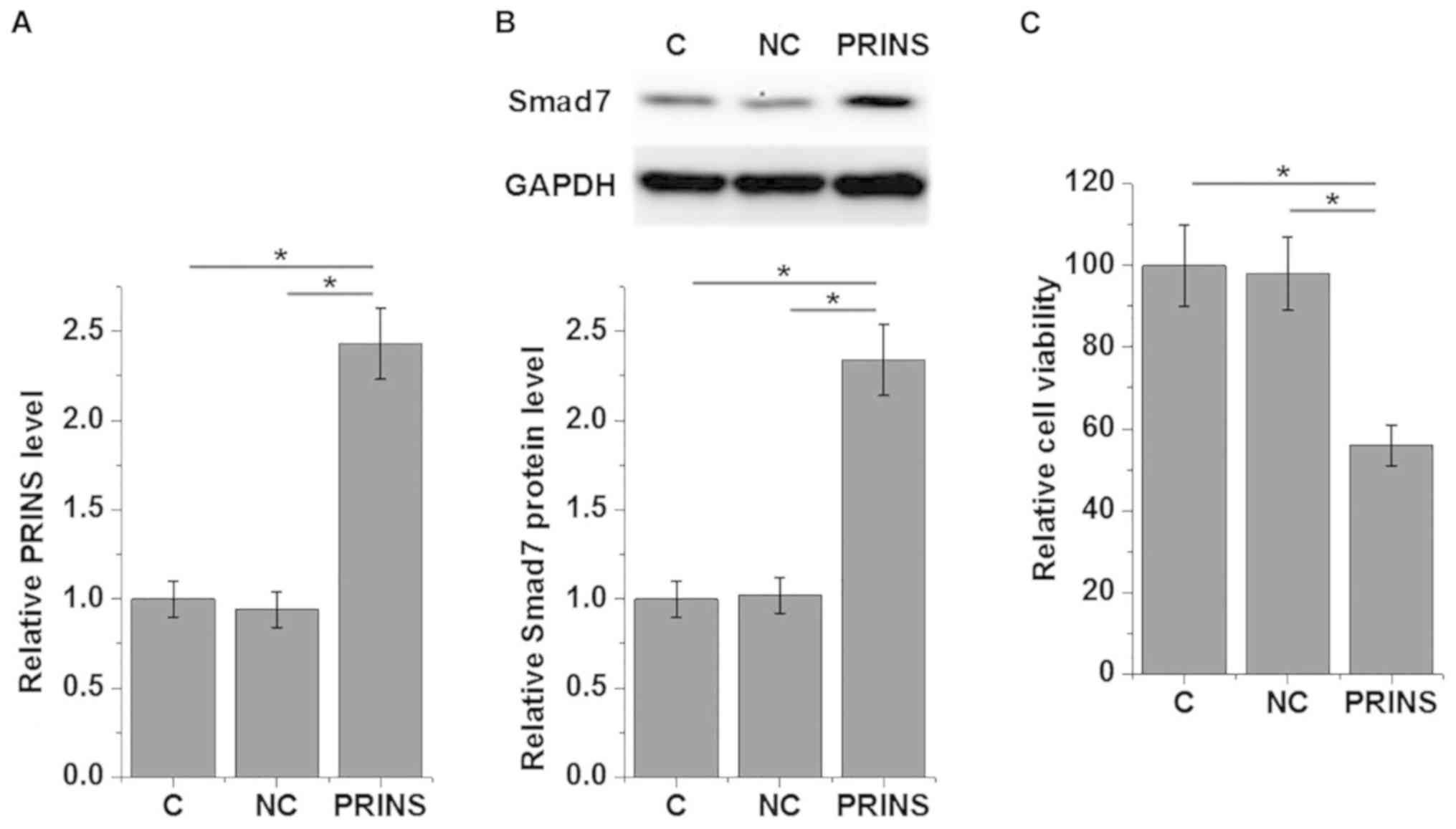
Overexpression of PRINS in mouse podocyte cells was
confirmed by RT-qPCR following transfection with PRINS expression
construct (Fig. 5A). Overexpression
of PRINS significantly enhanced the expression of Smad7 protein in
mouse podocyte cells (P<0.05; Fig.
5B). In addition, Mouse podocyte cell viability under 30 mM
D-glucose was examined by MTT assay and the viability of mouse
podocyte cells significantly decreased following overexpression of
PRINS compared with control and negative control (P<0.05;
Fig. 5C).
Discussion
Previous findings demonstrated that PRINS is
involved in kidney ischemia reperfusion injury (11) and allograft rejection in rat kidney
transplantation (15), and therefore
PRINS serves important roles in renal damage. In addition, the
current study demonstrated that PRINS may be involved in the
development of nephropathy in patients with diabetes, and PRINS may
exert its biological function by upregulating Smad7 expression.
Treatment with high glucose can affect normal
metabolism in patients with diabetes, which can directly or
indirectly affect the expression of a large number of lncRNAs
(16,17). Expression patterns of lncRNAs can
change depending on whether they accelerate or inhibit disease
progression (16,17). Circulating lncRNAs can serve as
signaling molecules under pathological conditions to induce
systemic reactions in patients with diabetes, including insulin
resistance and accelerated senescence (18). Upregulation of PRINS was observed
following acute tubule damage in rat kidney transplantation
(15). The current study
demonstrated that PRINS was significantly upregulated in patients
with diabetes compared with healthy controls. In addition, PRINS
expression was significantly upregulated in patients with diabetic
nephropathy. Furthermore, treatment with high glucose significantly
upregulated the expression level of PRINS in a time- and
dose-dependent manner in mouse podocyte cells. Therefore, the
upregulation of PRINS expression may be induced by both high
glucose and renal damage. In addition, the level of serum PRINS may
be used to distinguish patients with diabetes with diabetic
nephropathy from those without renal damage.
A previous study reported that Smad7 overexpression
is involved in the apoptosis of renal cells (6), and inhibition of renal apoptosis is
considered to be a potential therapeutic target in diabetic
nephropathy (7). However, Smad7 can
also inhibit renal fibrosis in animal models of chronic kidney
disease (19). Furthermore, Smad7
can serve a protective role in the mouse model of chronic
aristolochic acid nephropathy (20).
In the current study, the expression level of Smad7 was
significantly upregulated in patients with diabetes compared with
healthy controls, and in particular patients with diabetic
nephropathy. Previous findings demonstrated that Smad7-deficient
mice developed more severe diabetic kidney injury compared with
wild-type mice under high glucose conditions, and although renal
Smad7 had no effect on blood glucose, it significantly inhibited
TGF-β/Smad3-mediated renal fibrosis and the development of
microalbuminuria in the rat diabetic model (21), indicating the protective role of
Smad7 in diabetic nephropathy. The current study demonstrated that
overexpression of PRINS decreased the viability of mouse podocyte
cells, which are critical for renal function (22), and enhanced Smad7 protein expression.
In addition, a significantly positive correlation between the
expression of PRINS and Smad7 was observed in patients with
diabetic nephropathy, however, no correlation was observed in
patients with ther diabetes-related complications. PRINS may
therefore serve different roles in diabetic nephropathy; it may
promote the apoptosis of podocyte cells and upregulate Smad7
protein expression to inhibit GF-β/Smad3-mediated renal fibrosis.
However, further studies are required to understand more about this
complex regulatory network.
In conclusion, the expression level of PRINS was
significantly upregulated in patients with diabetes, and in
particular patients with diabetic nephropathy. PRINS may be
involved in diabetic nephropathy by enhancing Smad7 expression and
renal apoptosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ designed the experiments. HJ performed the
experiments. DX analyzed data. YQ drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee at the Peace Hospital of Changzhi Medical College
(Changzhi, China) and all participants provided written informed
consent.
Patient consent for publication
The study followed the tenets of the Declaration of
Helsinki and informed written consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahlqvist E, Van Zuydam NR, Groop LC and
McCarthy MI: The genetics of diabetic complications. Nat Rev
Nephrol. 11:277–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donate-Correa J, Martín-Núñez E,
Muros-de-Fuentes M, Mora-Fernández C and Navarro-González JF:
Inflammatory cytokines in diabetic nephropathy. Zhong Hua Tang Niao
Bing Za Zhi. 2015:9484172015.
|
|
4
|
Lim AKH: Diabetic
nephropathy-complications and treatment. Int J Nephrol Renovasc
Dis. 7:361–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
You H, Gao T, Cooper TK, Morris SM Jr and
Awad AS: Arginase inhibition: A new treatment for preventing
progression of established diabetic nephropathy. Am J Physiol Renal
Physiol. 309:F447–F455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeung ML, Yao Y, Jia L, Chan JF, Chan KH,
Cheung KF, Chen H, Poon VK, Tsang AK, To KK, et al: MERS
coronavirus induces apoptosis in kidney and lung by upregulating
Smad7 and FGF2. Nat Microbiol. 1:160042016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isermann B, Vinnikov IA, Madhusudhan T,
Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh
J, et al: Activated protein C protects against diabetic nephropathy
by inhibiting endothelial and podocyte apoptosis. Nat Med.
13:1349–1358. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nong Q, Li S, Wu Y and Liu D: LncRNA
COL1A2-AS1 inhibits the scar fibroblasts proliferation via
regulating miR-21/Smad7 pathway. Biochem Biophys Res Commun.
495:319–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu J and Xu Y: The lncRNA MEG3
downregulation leads to osteoarthritis progression via miR-16/SMAD7
axis. Cell Biosci. 7:692017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu TM, Palanisamy K, Sun KT, Day YJ, Shu
KH, Wang IK, Shyu WC, Chen P, Chen YL and Li CY: RANTES mediates
kidney ischemia reperfusion injury through a possible role of
HIF-1α and LncRNA PRINS. Sci Rep. 6:184242016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szegedi K, Sonkoly E, Nagy N, Németh IB,
Bata-Csörgo Z, Kemény L, Dobozy A and Széll M: The anti-apoptotic
protein G1P3 is overexpressed in psoriasis and regulated by the
non-coding RNA, PRINS. Exp Dermatol. 19:269–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chinese Medical Association for Diabetes
Mellitus, a Group of Microvascular Complications, . China expert
consensus for the prevention and treatment of diabetic nephropathy
(2014 edition). Chin J Diabetes. 6:792–801. 2014.(In Chinese).
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou XF, Song B, Duan JH, Hu ZD, Cui ZL and
Yang T: PRINS Long Noncoding RNA involved in IP-10 mediated
allograft rejection in rat kidney transplant. Transplant Proc.
50:1558–1565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao XY and Lin JD: Long noncoding RNAs: A
new regulatory code in metabolic control. Trends Biochem Sci.
40:586–596. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kornfeld JW and Brüning JC: Regulation of
metabolism by long, non-coding RNAs. Front Genet. 5:572014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carter G, Miladinovic B, Patel AA, Deland
L, Mastorides S and Patel NA: Circulating long noncoding RNA GAS5
levels are correlated to prevalence of type 2 diabetes mellitus.
BBA Clin. 4:102–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lan HY: Smad7 as a therapeutic agent for
chronic kidney diseases. Front Biosci. 13:4984–4992. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai XY, Zhou L, Huang XR, Fu P and Lan HY:
Smad7 protects against chronic aristolochic acid nephropathy in
mice. Oncotarget. 6:11930–11944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HY, Huang XR, Wang W, Li JH, Heuchel
RL, Chung AC and Lan HY: The protective role of Smad7 in diabetic
kidney disease: Mechanism and therapeutic potential. Diabetes.
60:590–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rubio-Navarro A, Sanchez-Niño MD,
Guerrero-Hue M, García-Caballero C, Gutiérrez E, Yuste C, Sevillano
Á, Praga M, Egea J, Román E, et al: Podocytes are new cellular
targets of haemoglobin-mediated renal damage. J Pathol.
244:296–310. 2018. View Article : Google Scholar : PubMed/NCBI
|