Introduction
Nephrotic syndrome (NS) is the most common type of
glomerulopathy in pediatric patients (1). Although the majority of patients with
pediatric NS have steroid-sensitive NS (SSNS), ~20% of them do not
achieve complete remission and the condition develops into
steroid-resistant NS (SRNS) (2). An
estimated 80–90% of the pediatric patients with SSNS undergo
relapse, and among those, 50% relapse frequently; thereby, the
condition turns into steroid-dependent NS (SDNS) (3–5). Hence,
suitable therapies for pediatric refractory NS (PRNS), which
includes SDNS and SRNS, represent a challenge.
Cyclosporin therapy for patients with PRNS has been
described in previous studies (6–8).
However, cyclosporin has a narrow therapeutic window, and also
displays considerable inter- and intra-individual variabilities in
terms of its pharmacokinetics (9),
making it difficult to define an optimal dose and regimen for PRNS
treatment.
It is encouraging that, with population
pharmacokinetics (PPK), PK information may be acquired from sparse
data pooled into a large group of subjects. In addition, population
analysis methodology differentiates between inter-individual
variability and residual unexplained variability, having greater
statistical power to verify the effects of multiple factors on PK
behavior, thereby making it possible to formulate an optimal dosing
and administration schedule (10).
At present, a small number of cyclosporin PPK models have been
established in various populations (9,11,12);
however, the cyclosporin PPK model in patients with PRNS has yet to
be constructed. The present study aimed to establish a cyclosporin
PPK model for patients with PRNS, and to distinguish factors that
may explain for any variability identified.
Materials and methods
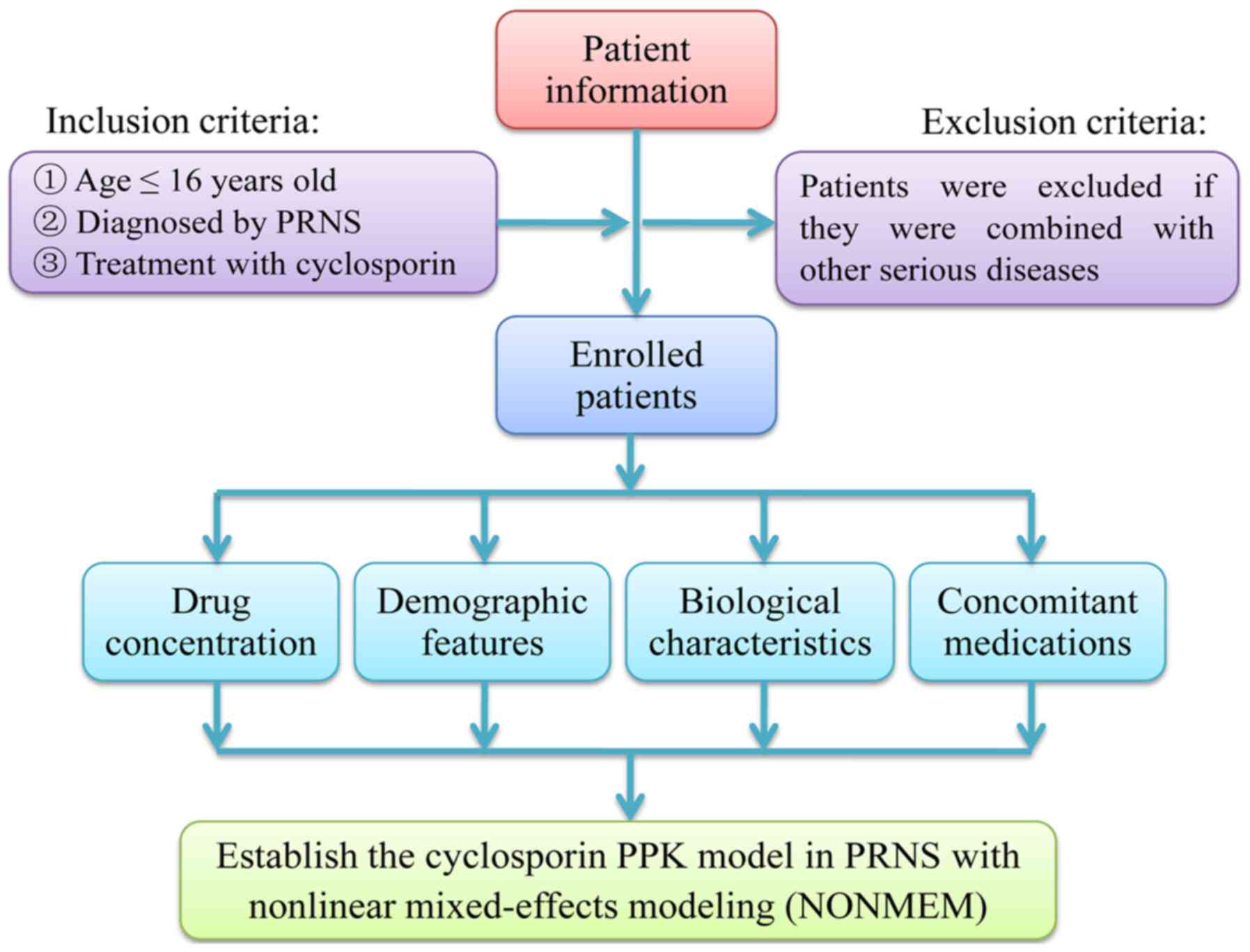
Patients and data collection
All data of the present study were derived from
real-world studies between June 2014 and June 2018 from the
Children's Hospital of Fudan University (Shanghai, China). Patients
aged <16 years who were diagnosed with PRNS and who were
receiving cyclosporin therapy were selected for the present study.
Patients were excluded if they had also been diagnosed with any
other serious disease, including kidney failure (Fig. 1). Relevant clinical information and
drug concentration data were collected from medical records and
therapeutic drug monitoring (TDM) records, respectively. The
present retrospective analysis was approved by the ethics committee
of the Children's Hospital of Fudan University (Shanghai, China)
without the requirement for written informed consent, since the
data were collected without patient identifiers (13,14).
Information extracted from the medical records
included the following: Sex, age, body weight (WT), body height,
levels of albumin, globulin, albumin/globulin, alanine
transaminase, aspartate transaminase, creatinine, urea, total
protein and total bile acid, direct bilirubin, total bilirubin,
hematocrit, hemoglobin count, mean corpuscular hemoglobin count,
mean corpuscular hemoglobin concentration and drugs that the
patients may have been receiving concomitantly with cyclosporin
(namely, diltiazem, dipyridamole, felodipine, fosinopril,
methylprednisolone, nifedipine, piperazine ferulate, prednisolone
or spirolactone).
Drug administration
All patients were orally administered cyclosporin
(in liquid solution). The dose of cyclosporin administered was
initially 25–80 mg daily, although the cyclosporin dose was later
adjusted according to the clinical efficacy and adverse events
experienced by the patient, as well as its trough concentration in
TDM.
Analytical method
Whole-blood concentrations of cyclosporin were
measured using an Emit® 2000 Cyclosporine Specific assay
(6R079UL; Siemens Healthcare Diagnostics, Inc., Newark, NJ, USA),
according to the manufacturer's protocol.
PPK modeling
Data were analyzed according to the non-linear
mixed-effects model (NONMEM) computer program (version VII; ICON
Development Solutions, Elicott City, MD, USA). The ‘first-order
conditional estimation method with interaction’ option was used to
estimate the PK parameters and their variability. A one-compartment
model with first-order elimination was used to describe the
absorption phase, as all the cyclosporin concentrations featured in
the present analysis were trough concentrations. It was not
possible to estimate the bioavailability (F) and absorption with a
lag time, since cyclosporin was orally administered and its
concentration data were insufficient. Therefore, the PK parameters
studied were the apparent oral clearance (CL/F) and the apparent
volume of distribution (V/F). The absorption rate constant (Ka) of
the model was fixed to 0.68 1/h according to a previous study
(9).
Random-effects model
The interindividual variability in the PK parameters
was explored using an exponential error model: Pi=T (P)
× exp (ηi), where Pi is the individual
parameter value, and T(P) is the typical individual parameter
value. ηi is the symmetrical distribution, which are
zero-mean chance variables with the variance term. The residual
error variability was evaluated with the mixed-error model, OB=IP ×
(1 + ε1) + ε2, where OB is the observation,
and IP represents the individual predicted concentration.
εn represents the symmetrical distribution, which
are zero-mean chance variables with a variance.
Covariate model
The association between WT and the PK parameters was
modeled according to the following equation:
Pi=Pstd ×
(WTi/WTstd)PWR, where
Pi is the PK parameter of the ith individual,
WTi is the body weight of the ith individual and
Pstd is the parameter of an individual with a WT,
WTstd, of 70 kg. PWR is the allometric coefficient: 0.75
for the CL/F and 1 for the V/F (15).
To explain the variability of the PK parameters, the
potential covariates included sex, age, WT, body height, levels of
albumin, globulin, albumin/globulin, alanine transaminase,
aspartate transaminase, creatinine, urea, total protein, total bile
acid, direct bilirubin, total bilirubin, the hematocrit, hemoglobin
count, mean corpuscular hemoglobin count, mean corpuscular
hemoglobin concentration and concomitant medications. The covariate
model was established in a stepwise fashion. To compare
hierarchical models, a likelihood ratio test was adopted. Changes
in the objective function values (OFVs) caused by the inclusion of
a covariate were proportional to twice the negative log likelihood
of the data and approximated to a Chi-square distribution. In the
univariate analysis, a decrease in the OFV >3.84 (P<0.05,
degrees of freedom=1) was used as a criterion for inclusion of the
covariate in the base model. The significant covariate-parameter
associations were reserved in the model. When a full regression
model was built, the model was further refined by eliminating the
covariate from each parameter one at a time in order to acquire the
final model. An increase in the OFV >6.64 (P<0.01, degrees of
freedom=1) was used as a criterion for retaining significant
covariate-parameter associations in the model (13).
Model validation
The goodness-of-fit plots of the final model was
generated by the statistics package of R version 3.4.2 (https://www.r-project.org/). An internal validation
method of bootstrap was used to evaluate the reliability and
stability of the final parameter estimates. Bootstrap was generated
by repeated random sampling, with replacement of the original data.
This procedure was performed with the software package Wings for
NONMEM and repeated 1,000 times with different random draws. The
medians and 2.5–97.5% percentiles of the bootstrap result set
parameters were compared with the final PK parameter estimates. The
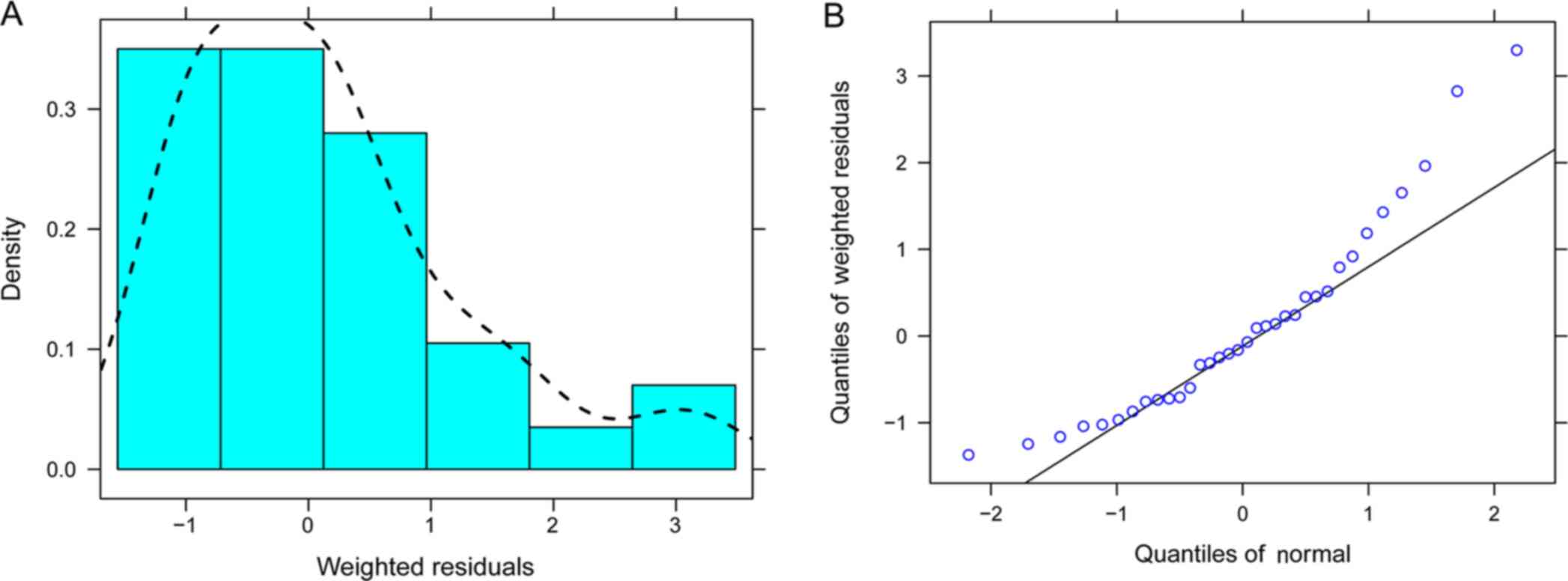
distribution of weighted residuals for the final model was
evaluated by histograms and quantile-quantile plots (13).
Results
Data collection from real-world
studies
A total of 18 Chinese pediatric patients with PRNS
(13 males and 5 females) were available for population modeling.
The patient characteristics and drug combinations are summarized in
Tables I and II, respectively.
 | Table I.Demographic and laboratory data of the
subjects. |
Table I.
Demographic and laboratory data of the
subjects.
| Parameter | Mean ± standard
deviation | Median (range) |
|---|
| Sex
(male/female) | 13/5 | / |
| Age (years) | 2.79±0.90 | 2.75 (1.18–4.57) |
| Weight (kg) | 15.28±2.95 | 15 (10–23) |
| Height (cm) | 91.28±8.23 | 94 (77–105) |
| Albumin (g/l) | 19.68±6.82 | 20.1 (10.1–33.2) |
| Globulin (g/l) | 24.69±3.18 | 24.3 (19.5–31.4) |
| Albumin/globulin | 0.83±0.34 | 0.75 (0.4–1.7) |
| Alanine transaminase
(IU/l) | 12.12±12.71 | 9 (1–79) |
| Aspartate
transaminase (IU/l) | 26.35±15.84 | 20 (11–75) |
| Creatinine
(µmol/l) | 25.47±23.72 | 20.5 (7–152) |
| Urea (µmol/l) | 4.19±2.05 | 3.65 (1.4–11.5) |
| Total protein
(g/l) | 45.16±7.41 | 43.55
(33.9–66.0) |
| Total bile acid
(µmol/l) | 4.79±3.85 | 3.6 (0.3–15.6) |
| Direct bilirubin
(µmol/l) | 0.81±0.83 | 0.6 (0.1–4.8) |
| Total bilirubin
(µmol/l) | 4.24±2.54 | 3.5 (1.3–12.1) |
| Hematocrit (%) | 39.64±5.17 | 39 (30.22–51.9) |
| Hemoglobin (g/l) | 130.34±17.53 | 127.5 (97–173.1) |
| Mean corpuscular
hemoglobin (pg) | 25.66±3.31 | 26.95 (18–30) |
| Mean corpuscular
hemoglobin concentration (g/l) | 330±13.94 | 333 (301–353) |
 | Table II.Drug combinations with
cyclosporin. |
Table II.
Drug combinations with
cyclosporin.
| Drug | N |
|---|
| Diltiazem |
|
| No | 15 |
| Yes | 3 |
| Dipyridamole |
|
| No | 16 |
| Yes | 2 |
| Felodipine |
|
| No | 17 |
| Yes | 1 |
| Fosinopril |
|
| No | 15 |
| Yes | 3 |
|
Methylprednisolone |
|
| No | 14 |
| Yes | 4 |
| Nifedipine |
|
| No | 17 |
| Yes | 1 |
| Piperazine
ferulate |
|
| No | 13 |
| Yes | 5 |
| Prednisolone |
|
| No | 5 |
| Yes | 13 |
| Spirolactone |
|
| No | 7 |
| Yes | 11 |
Modeling
A one-compartment model with first-order absorption
and elimination was determined to be the most suitable model for
the data. Ka was fixed to 0.68/h, in accordance with a previously
published study (9). The PK
parameters of cyclosporin, CL/F and V/F were estimated using
NONMEM. The final covariate models were as follows:
CL/F=θCL/F × (WT/70)0.75 × (1+spirolactone ×
θspirolactone). Spirolactone may be 1 or 0 for yes and
no, respectively regarding concomitant spirolactone administration;
and V/F=θV/F × (WT/70), where θCL/F and
θV/F are the typical population values for CL/F and V/F,
respectively, and θspirolactone is the coefficient of
spirolactone. The coefficient of spirolactone was negative.
Validation
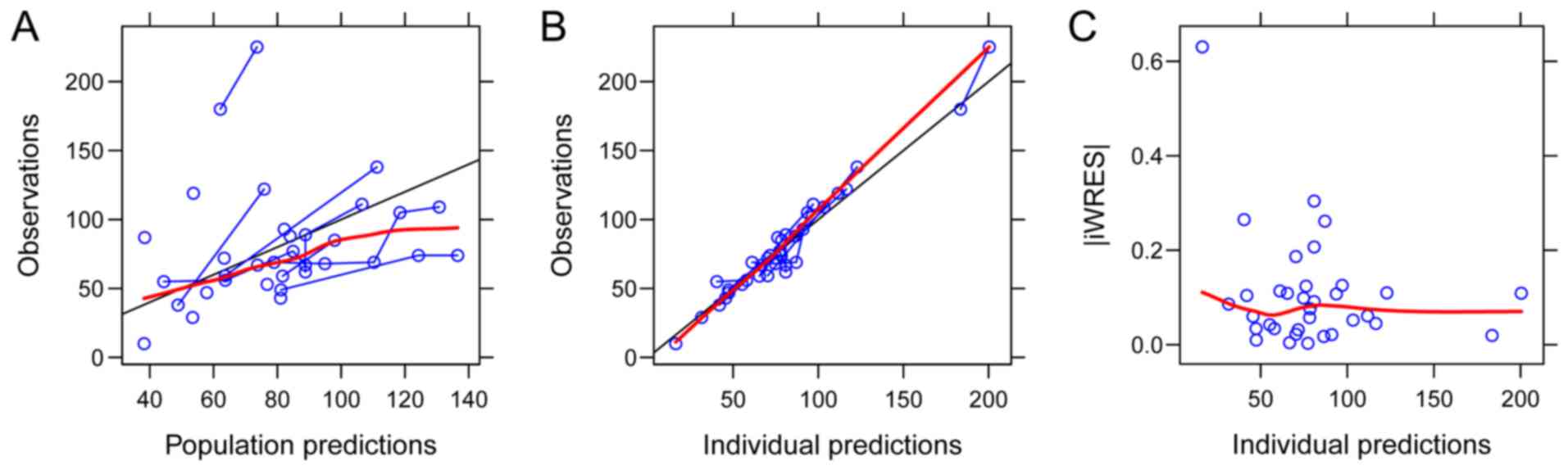
The goodness-of-fit plots of the final model are
provided in Fig. 2. Parameter
estimates of the final model and bootstrap validation are provided
in Table III. The median values of
the parameter estimate from bootstraps were close to the respective
values from the final population model, demonstrating that the
estimates for the PK parameters in the final population model were
accurate and that the model was reliable. The distribution of
weighted residuals in the final model is presented in Fig. 3.
 | Table III.Parameter estimates of final model and
bootstrap validation. |
Table III.
Parameter estimates of final model and
bootstrap validation.
|
|
|
| Bootstrap |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | Estimate | SE (%) | Median | 95% Confidence
interval | Bias (%) |
|---|
| CL/F (L/h) | 80.7 | 19.8 | 79.900 | (53.1, 106.5) | −0.991 |
| V/F (L) | 2030 | 75.4 | 2340 | (687, 3747.5) | 15.271 |
| Ka
(h−1) | 0.68 (fixed) | – | – | – | – |
|
θspirolactone | −0.265 | 33.4 | −0.266 | (−0.448,
−0.066) | 0.377 |
|
ωCL/F | 0.446 | 18.7 | 0.445 | (0.201, 0.569) | −0.224 |
|
ωV/F | 0.531 | 30.1 | 0.513 | (0.005, 0.749) | −3.390 |
| σ1 | 0.117 | 18.4 | 0.115 | (0.047, 0.149) | −1.710 |
| σ2 | 8.062 | 32.6 | 7.490 | (0.212,
11.843) | −7.095 |
Discussion
The traditional therapy for treatment of PRNS has
been steroids, although its long-term use may cause adverse effects
on the growth and development of pediatric patients. Patients with
PRNS undergo prolonged and repeated steroid therapy, which
increases the risk of obesity, cushingoid appearance, osteoporosis,
hypertension, infections, growth retardation and psychological
problems (16). Cyclosporin has been
widely used for patients with PRNS to improve treatment responses
and to reduce the adverse reactions of steroid therapy (6–8). Since
cyclosporin exhibits only a poor correlation between drug dosage
and blood concentrations, PPK studies of cyclosporin have proven to
be indispensable (9,11,12).
To the best of our knowledge, the present study may
be the first to provide a PPK model of cyclosporin in pediatric
patients with PRNS. A cyclosporin PPK analysis was performed in
Chinese PRNS patients using a population modeling approach, which
was particularly suitable as a means of investigation, since
logistical and ethical restrictions involved in studying pediatric
patients prohibit excessive blood sampling compared with
traditional PK studies (17). A
cyclosporin PPK model with the ability to predict PK processes in
individual patients with PRNS is of great value from a clinical
perspective.
In the construction of the present model, a
one-compartment model with first-order elimination was used to
describe the absorption phase, since all the cyclosporin
concentrations featured in the study were trough concentrations,
and the Ka of the model was fixed to the value of 0.68 1/h,
according to that of a previous study (9). Typical values of CL/F and V/F in the
final model were 80.7 l/h and 2,030 l, respectively. The
inter-individual variabilities in CL/F and V/F were 44.6 and 53.1%.
An allometry-based population PK model was developed for
cyclosporin: WT was incorporated, with allometric coefficients of
0.75 for CL/F and 1 for V/F (15).
The present model also incorporated various covariates on different
parameters, and the following covariate was determined to be
meaningful: ‘Spirolactone’ on CL/F. The coefficient of spirolactone
was negative, indicating that concomitant medication with
spirolactone was able to reduce cyclosporin clearance in the
patients with PRNS.
Of note, the present study had a number of
limitations. First, pharmacogenomic considerations of spirolactone
have not been verified in clinical use. Furthermore, the present
study was a retrospective analysis based on real-world studies,
which means that genotyping was not routinely performed in the
study population. Whether the inclusion of genotyping in the
present model may provide any improved explanation of the
variabilities of spirolactone in PRNS should be studied in the
future. In addition, it would be useful to evaluate the levels of T
lymphocytes and/or the corresponding cytokines produced, indicating
the release and concentration levels of the drug. However, this is
not within the domain of PPK; such a study would belong to the
domain of PK and pharmacodynamics (PD), and population PD/PK of
cyclosporin in patients with pediatric refractory nephrotic
syndrome will be established in future studies.
In conclusion, to the best of our knowledge, the
present study was the first to assess the PPK of cyclosporin in
patients with PRNS, and the presence of a clinically significant
interaction between spirolactone and cyclosporin in PRNS patients
based on real-world studies was also investigated. The coefficient
of spirolactone was negative, indicating that concomitant
medication with spirolactone was able to reduce cyclosporin
clearance in the patients with PRNS.
Acknowledgements
Not applicable.
Funding
This work was supported by the Clinical Pharmacy Key
Specialty Construction Project of Shanghai (grant no. YZ2017/5),
the Young Medical Talents of Wuxi (grant no. QNRC020), the Young
Project of Wuxi Health and Family Planning Research (grant no.
Q201706), AOSAIKANG pharmaceutical foundation (grant no. A201826,
the Wuxi Science and Technology Development Guidance Plan (Medical
and Healthcare; grant. no. CSZON1744).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and DW conceived and designed the study. DW and
XC collected and analyzed data. DW and XC wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The retrospective study was approved by the Research
Ethics Committee of Children's Hospital of Fudan University
(Shanghai, China). Written informed consent was not required, since
the data were collected without patient identifiers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eddy AA and Symons JM: Nephrotic syndrome
in childhood. Lancet. 362:629–639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McKinney PA, Feltbower RG, Brocklebank JT
and Fitzpatrick MM: Time trends and ethnic patterns of childhood
nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol. 16:1040–1044.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koskimies O, Vilska J, Rapola J and
Hallman N: Long-term outcome of primary nephrotic syndrome. Arch
Dis Child. 57:544–548. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lombel RM, Gipson DS and Hodson EM; Kidney
Disease, : Improving Global Outcomes: Treatment of
steroid-sensitive nephrotic syndrome: New guidelines from KDIGO.
Pediatr Nephrol. 28:415–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tarshish P, Tobin JN, Bernstein J and
Edelmann CM Jr: Prognostic significance of the early course of
minimal change nephrotic syndrome: Report of the international
study of kidney disease in children. J Am Soc Nephrol. 8:769–776.
1997.PubMed/NCBI
|
|
6
|
Goumenos DS, Katopodis KP, Passadakis P,
Vardaki E, Liakopoulos V, Dafnis E, Stefanidis I, Vargemezis V,
Vlachojannis JG and Siamopoulos KC: Corticosteroids and ciclosporin
A in idiopathic membranous nephropathy: Higher remission rates of
nephrotic syndrome and less adverse reactions than after
traditional treatment with cytotoxic drugs. Am J Nephrol.
27:226–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitano Y, Yoshikawa N, Tanaka R, Nakamura
H, Ninomiya M and Ito H: Ciclosporin treatment in children with
steroid-dependent nephrotic syndrome. Pediatr Nephrol. 4:474–477.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka R, Yoshikawa N, Kitano Y, Ito H and
Nakamura H: Long-term ciclosporin treatment in children with
steroid-dependent nephrotic syndrome. Pediatr Nephrol. 7:249–252.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni SQ, Zhao W, Wang J, Zeng S, Chen SQ,
Jacqz-Aigrain E and Zhao ZY: Population pharmacokinetics of
ciclosporin in Chinese children with aplastic anemia: Effects of
weight, renal function and stanozolol administration. Acta
Pharmacol Sin. 34:969–975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vadcharavivad S, Praisuwan S,
Techawathanawanna N, Treyaprasert W and Avihingsanon Y: Population
pharmacokinetics of tacrolimus in Thai kidney transplant patients:
Comparison with similar data from other populations. J Clin Pharm
Ther. 41:310–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fruit D, Rousseau A, Amrein C, Rollé F,
Kamar N, Sebbag L, Redonnet M, Epailly E, Marquet P and Prémaud A:
Ciclosporin population pharmacokinetics and Bayesian estimation in
thoracic transplant recipients. Clin Pharmacokinet. 52:277–288.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilhelm AJ, de Graaf P, Veldkamp AI,
Janssen JJ, Huijgens PC and Swart EL: Population pharmacokinetics
of ciclosporin in haematopoietic allogeneic stem cell
transplantation with emphasis on limited sampling strategy. Br J
Clin Pharmacol. 73:553–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang DD, Lu JM, Li Q and Li ZP: Population
pharmacokinetics of tacrolimus in paediatric systemic lupus
erythematosus based on real-world study. J Clin Pharm Ther.
43:476–483. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Wu D, Dong M, Zhu Y, Lu J, Li X,
Chen C and Li Z: Population pharmacokinetics of vancomycin and
AUC-guided dosing in Chinese neonates and young infants. Eur J Clin
Pharmacol. 74:921–930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anderson BJ and Holford NH:
Mechanism-based concepts of size and maturity in pharmacokinetics.
Annu Rev Pharmacol Toxicol. 48:303–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahn D, Hodson EM, Willis NS and Craig JC:
Corticosteroid therapy for nephrotic syndrome in children. Cochrane
Database Syst Rev. CD0015332015.PubMed/NCBI
|
|
17
|
Kauffman RE and Kearns GL: Pharmacokinetic
studies in paediatric patients. Clinical and ethical
considerations. Clin Pharmacokinet. 23:10–29. 1992. View Article : Google Scholar : PubMed/NCBI
|