Introduction
Primary percutaneous coronary intervention (PCI)
rapidly and completely restores blood flow in occluded coronary
arteries in patients with acute myocardial infarction (AMI), thus
effectively enabling the reperfusion of the infarcted myocardium,
reducing the mortality rate and the incidence of cardiac endpoint
events in AMI patients (1,2). However, for diabetic and non-diabetic
patients, a high blood glucose level during AMI is associated with
a poor AMI prognosis and somewhat affects the benefits of primary
PCI (3–6). A random high blood glucose level at
admission in non-diabetic patients with AMI is known as stress
hyperglycemia (3).
It is not fully understood how stress hyperglycemia
affects the prognosis of primary PCI (4,6,7). Previous studies have demonstrated that
inflammation serves an important role in this process (8–10).
Monocyte chemoattractant protein 1 (MCP-1) is an important
inflammatory cytokine during the inflammatory response. MCP-1
serves roles in chemotaxis and the activation of
monocytes/macrophages, the upregulation of the expression of
monocyte/macrophage adhesion molecules and the production of
inflammatory cytokines including interleukin (IL) 1 and 6,
chemotaxis and the activation of basophils (11–13).
These result in the release of histamine and inflammatory responses
that may be associated with vascular injury, the development of
atherosclerosis, plaque instability and restenosis following
coronary stenting (14,15). MCP-1 may serve an important role in
the mechanism by which stress hyperglycemia affects the prognosis
of AMI, but limited data are available regarding these associations
and further studies are required to confirm this.
A prospective study was conducted to investigate
MCP-1 levels at different time points before and after primary PCI,
and the prognosis of patients with acute ST-segment elevation
myocardial infarction (ASTEMI) 1-year post-PCI. Blood glucose
levels were also measured at admission to explore how stress
hyperglycemia affects the prognosis of primary PCI.
Materials and methods
Subjects
A total of 146 patients (age range, 35–80 years;
average age, 60.1 years) with ASTEMI who successfully underwent
primary PCI within 12 h of onset at the Second Affiliated Hospital
of Anhui Medical University between December 2014 and December 2016
were included in the present study. Each patient and/or his/her
family provided informed consent for PCI and written informed
consent for the present study. Ethics approval for the study was
granted from the Second Affiliated Hospital of Anhui Medical
University Ethics Committee. ASTEMI was diagnosed according to the
Third Universal Definition of the myocardial infarction document,
as described previously (16).
ASTEMI was defined as complaints of chest pain with ECG signs
compatible with AMI (ST-segment elevation >2 mm in precordial
leads and >1 mm in limb leads). Patients were included in the
current study if they were diagnosed with ASTEMI, over the age of
18 years and were successfully treated with PCI. Patients with the
following conditions were excluded: Severe peripheral vascular
disease, peptic ulcer, coagulation disorders, severe infections,
tumors, and connective tissue diseases, along with patients who
succumbed within 48 h of admission or did not successfully undergo
PCI (as the study required monitoring of MCP-1 levels for 48 h
post-operatively).
Group assignment
Eligible patients with ASTEMI were divided into
three groups (groups 1, 2 and 3), according to history of diabetes,
blood glucose level at admission, and glycated hemoglobin A1c
(HbA1c) level. The glucose oxidase method by automated analyzer was
utilized to measure blood glucose levels (17). Group 1 was the non-diabetic,
non-hyperglycemic group (blood glucose at admission <8.0
mmol/l); group 2 was the stress hyperglycemia group (non-diabetic,
hyperglycemic group; blood glucose at admission ≥8.0 mmol/l); and
group 3 was the diabetic group. Non-diabetic patients were defined
by having no history of diabetes, with fasting blood glucose levels
24 h after admission, 2-h postprandial blood glucose levels and
HbA1c levels that were incompatible with the diagnostic criteria of
diabetes. Diabetes was diagnosed according to the Standards of
Medical Care in Diabetes (2014) from the American Diabetes
Association (18).
Perioperative medications
Prior to PCI, patients were routinely given 300 mg
aspirin (Bayer AG, Leverkusen, Germany) and 300 mg clopidogrel
(Sanofi S.A., Paris, France) by oral administration. Upon
successful puncture, 3,000 IU unfractionated heparin was given via
an arterial sheath. After coronary angiography and before PCI,
additional unfractionated heparin was given via an arterial sheath
(until 100 IU/kg). Additionally, 2,000 IU heparin was given each
additional hour during PCI to maintain an activated clotting time
≥300 sec. Following PCI, aspirin, clopidogrel,
angiotensin-converting enzyme inhibitors (Perindopril, 2–8 mg daily
according to patient blood pressure; Servier, Suresnes, France) and
β-blockers (Metoprolol succinate, 23.75–95 mg daily according to
patient heart rate; Astrazeneca, Cambridge, UK) were routinely
administered, unless otherwise contraindicated.
Cardiovascular events
The incidence of cardiovascular events during
hospitalization (severe arrhythmias intra-PCI, severe heart failure
and mortality) and of major adverse cardiovascular events (MACEs),
including cardiogenic death, non-fatal myocardial infarction,
target vessel revascularization, and severe heart failure occurring
within one year after PCI were observed. Severe arrhythmia was
defined as sinus arrest for ≥3 sec, grade 2 (or above) type II
atrioventricular block, ventricular tachycardia and ventricular
fibrillation. Severe heart failure was defined as class IV,
according to the criteria of the New York Heart Association
(19).
MCP-1 sample collection and
testing
Blood samples (3 ml) were collected from the cubital
vein prior to PCI (at admission), 24 and 48 h after PCI into a
standard serum tube (no anticoagulant), followed by centrifugation
at 1,006.2 × g for 10 min at room temperature. The upper layer of
serum was collected into a test tube, which was sealed and stored
at −80°C for later use. The MCP-1 level was measured by ELISA (cat.
no. SEA087Hu; Cloud-Clone Corp., Wuhan, China).
Statistical analysis
Data are expressed as the mean ± standard deviation.
A Kolmogorov-Smirnov test was performed to verify the normality of
the distribution. For normally distributed data, one-way analysis
of variance was performed to analyze differences among the groups
and the least significant difference was calculated to analyze
between-group differences. Non-normally distributed measurement
data or measurement data with heterogeneous variance were analyzed
using the K independent samples method. Count data were analyzed
with a χ2 test. Correlations of measurement data with a
normal distribution were analyzed with Pearson's correlation
analysis. For non-normally distributed measurement data, Spearman's
rank correlation analysis was used. Partial correlation analysis
was applied to eliminate the influence of certain factors.
P<0.05 was considered to indicate a statistically significant
difference. Risk factors for MACEs 1-year post-PCI were analyzed
using binary logistic regression analysis. SPSS V19.0 (IBM Corp.,
Armonk, NY, USA) was used for statistical analysis.
Results
General information
A total of 146 patients with ASTEMI were eligible to
participate, of which 128 were men and 18 women, with an average
age of 60.1±11.0 years. Groups 1, 2, and 3 included 56, 50 and 40
patients, respectively. No significant differences among the groups
were observed in age, sex, peak creatine kinase level, blood lipid
profile, smoking history, history of hypertension or history of
cerebrovascular disease. Blood glucose level at admission was
significantly higher in group 3 (diabetic group) compared with
groups 1 and 2 (P<0.05; Table
I).
 | Table I.Baseline clinical
characteristics. |
Table I.
Baseline clinical
characteristics.
| Variables | Group 1 (n=56) | Group 2 (n=50) | Group 3 (n=40) |
|---|
| Male sex (%) | 52 (92.9) | 40 (80.0) | 36 (90.0) |
| Female sex (%) | 4 (7.1) | 10 (20) | 4 (10) |
| Age (years) | 58.9±11.0 | 61.2±10.1 | 60.5±12.0 |
| Smoking history
(%) | 26 (46.4) | 26 (52.0) | 20 (50.0) |
| Hypertensive
disease (%) | 22 (39.3) | 24 (48.0) | 24 (60.0) |
| Cerebrovascular
disease (%) | 6
(10.7) | 6
(12.0) | 4
(10.0) |
| Blood glucose on
admission (mmol/l) | 6.73±0.92 |
9.61±1.40a |
12.78±3.32a,b |
| Total cholesterol
(mmol/l) | 4.31±0.90 | 4.59±0.96 | 4.52±0.85 |
| TG (mmol/l) | 1.52±0.70 | 1.61±0.91 | 1.79±0.78 |
| HDL (mmol/l) | 1.05±0.35 | 0.99±0.25 | 1.02±0.30 |
| LDL (mmol/l) | 2.66±0.70 | 2.89±0.72 | 2.73±0.65 |
| VLDL (mmol/l) | 0.42±0.27 | 0.44±0.27 | 0.46±0.21 |
| LP-a (mmol/l) | 0.19±0.16 | 0.17±0.11 | 0.18±0.09 |
| CK (U/l) | 2699±1419 | 2744±1510 | 2866±1323 |
Coronary intervention-associated
data
The comparison of coronary angiography results of
each group during primary PCI reveled that more patients had
multivessel lesions in group 3 (diabetic group) compared with
groups 1 and 2 (P<0.05). No significant among-group differences
were observed in infarction-related artery, from AMI onset to
reperfusion treatment, or maximum dilating pressure during PCI
(P<0.05; Table II). This
indicates that these factors do not affect the differences in MCP-1
levels among groups.
 | Table II.Characteristics of primary PCI. |
Table II.
Characteristics of primary PCI.
|
|
| Infarction-related
artery |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | n | LAD | RCA | LCX | Multivessel disease
(%) | Time of onset to
reperfusion (h) | Maximum pressure of
expansion (kPa) |
|---|
| Group 1 | 56 | 26 | 6 | 24 | 30 (53.6) | 5.61±2.01 |
1,432.75±256.32 |
| Group 2 | 50 | 30 | 6 | 14 | 28 (56.0) | 5.83±1.61 |
1,408.25±262.38 |
| Group 3 | 40 | 20 | 2 | 18 | 36
(90.0)a,b | 5.97±1.79 | 1,418.4±256.26 |
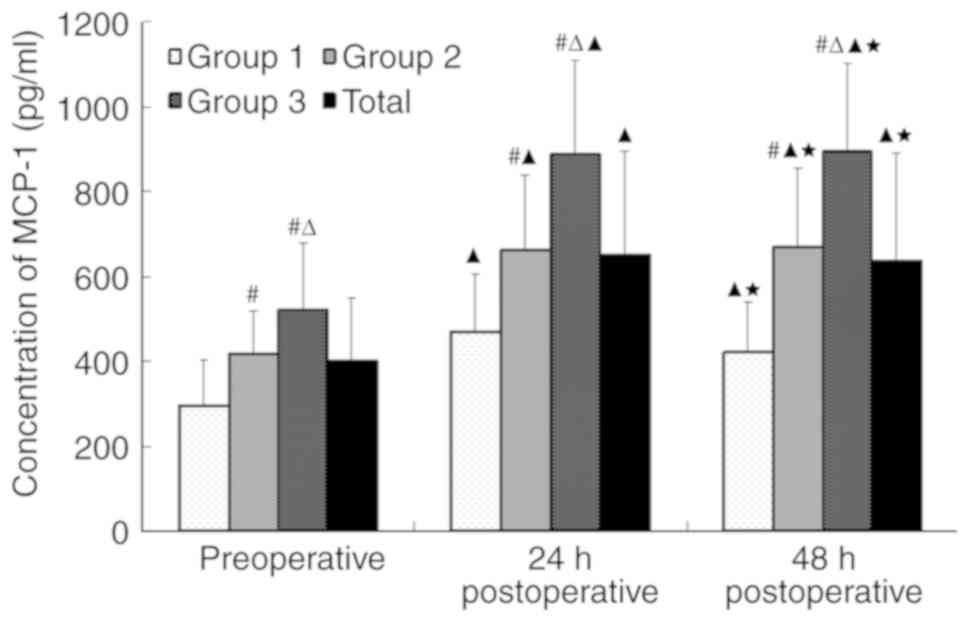
MCP-1 levels
The MCP-1 levels were higher 24 h following PCI
compared with prior to PCI in all three groups (P<0.05),
particularly in group 2 (stress hyperglycaemia group) and group 3
(diabetic group; P<0.001; Fig.
1). High MCP-1 levels 48 h after PCI were sustained, whereas
the MCP-1 levels significantly decreased 48 h after PCI compared
with 24 h after PCI in group 1 (non-diabetic, non-hyperglycemic
group; P<0.05; Fig. 1).
Significant differences were observed in MCP-1
levels at different time points: MCP-1 levels were significantly
higher in groups 2 and 3 compared with group 1, both before PCI and
24 and 48 h after PCI (P<0.05; Fig.
1).
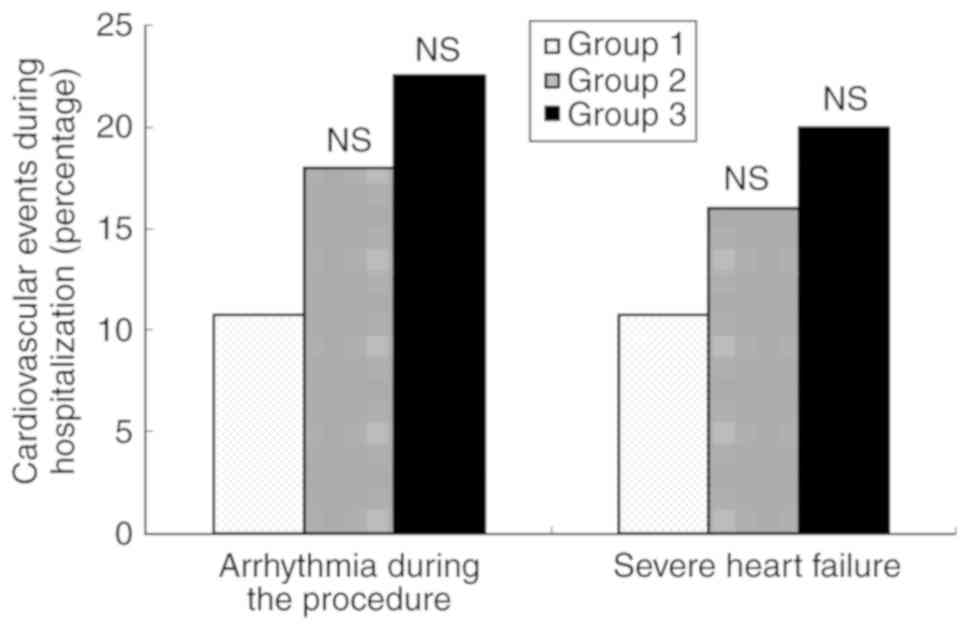
Cardiovascular events during
hospitalization
No patients with ASTEMI succumbed during
hospitalization. The incidences of intraoperative severe
arrhythmias and severe heart failure during hospitalization were
higher in groups 2 and 3 compared with group 1, but the differences
did not reach statistical significance (P>0.05; Fig. 2).
One-year post-PCI MACEs
The 1-year postoperative MACE rate was higher in
groups 2 and 3 compared with group 1 (P<0.05), with no
significant difference between groups 2 and 3 (Table III). Variables including blood
glucose level at admission, age, diabetes, hypertension, smoking
history, history of cerebrovascular diseases, infarction-related
artery, multivessel lesions, time from AMI onset to reperfusion
treatment, MCP-1 levels before PCI and 24 and 48 h after PCI, and
blood lipids were incorporated into binary logistic regression
analysis. The results demonstrated that blood glucose level at
admission (Wald=4.286, β=2.146, P=0.038), diabetes (Wald=9.165,
β=58.086, P=0.002), and preoperative MCP-1 levels (Wald=15.991,
β=1.024, P<0.001) were risk factors for MACEs occurring within 1
year after PCI. This indicates that the increased level of blood
glucose and MCP-1 at admission, as well as diabetes, were the risk
factors for the occurrence of MACE 1 year following primary PCI in
patients with ASTEMI.
 | Table III.MACE in the 1-year postoperative
period. |
Table III.
MACE in the 1-year postoperative
period.
| Group | n | MACE (%) | Cardiac deaths
(%) | Non-death AMI
(%) | Target vessel
revascularization (%) | Severe heart
failure (%) |
|---|
| Group 1 | 56 | 14 (25.0) | 5 (8.9) | 6 (10.7) | 4 (7.1) | 9 (16.1) |
| Group 2 | 50 | 22
(44.0)a | 6 (12.0) | 8 (16.0) | 8 (16.0) | 12 (24.0) |
| Group 3 | 40 | 20
(50.0)a | 5 (12.5) | 8 (20.0) | 6 (15.0) | 14 (35.0) |
Discussion
The present study demonstrated that the blood
glucose level can be higher at admission not only for patients with
ASTEMI with diabetes, but also for certain patients with ASTEMI
without diabetes. The latter condition is called stress
hyperglycaemia (3), which is defined
as the first random blood glucose level tested post-admission being
no less than 8.0 mmol/l. Among non-diabetic patients, patients with
stress hyperglycaemia had higher preoperative and postoperative
MCP-1 levels compared with those without hyperglycaemia at
admission. Furthermore, patients with stress hyperglycaemia and
diabetes had a higher rate of MACEs occurring within 1 year after
PCI compared with non-hyperglycemic patients. This result indicated
that for patients with ASTEMI, stress hyperglycaemia and diabetes
are associated with a poor prognosis post-PCI.
Diabetes is an independent risk factor for coronary
heart disease (20). Patients with
coronary heart disease and diabetes frequently have multiple,
severe and complicated coronary artery lesions (21). The present study also demonstrated
that the incidence of multivessel lesions was significantly higher
in diabetic patients with AMI compared with non-diabetic patients
with AMI. Furthermore, patients with diabetes are characterized by
chronic hyperglycaemia; during AMI, glucose metabolism disorders
may worsen, resulting in a further increase in the blood glucose
level at admission (3,4). It is known that patients with AMI and
diabetes have a poor prognosis following PCI (22–24).
However, in the case of AMI, not only patients with diabetes have
elevated blood glucose, but patients who have not been previously
diagnosed with diabetes may have elevated blood glucose at
admission (4–6). This is termed stress
hyperglycaemia.
During AMI, this stress hyperglycaemia is usually
transient and is eliminated as AMI is stabilized. However, certain
patients may develop diabetes in the future due to insulin
resistance (25,26), while a previous study reported that
high blood glucose at admission is unrelated to diabetes in the
future (27). Regardless of the
association between stress hyperglycaemia and diabetes, a number of
studies have demonstrated that stress hyperglycaemia is associated
with a poor prognosis in AMI (4,6,7).
Researchers continue to debate whether stress
hyperglycaemia indicates the severity of the condition of patients
with AMI or if hyperglycaemia itself may damage cardiac function
(28,29). The mechanism by which stress
hyperglycaemia affects the prognosis of AMI is not fully understood
(6,7). Studies have shown that inflammation
serves an important role in this process (8–10,30,31).
The study of Marfella et al (31) demonstrated that during AMI,
hyperglycaemia was associated with increased levels of inflammatory
markers, enhanced expression of cytotoxic T-cells, and reduced
expression of suppressor T-cells. There was a positive correlation
between stress hyperglycaemia and poor cardiac outcomes in patients
with AMI (31). The study primarily
confirmed the involvement of lymphocytes in the effects of stress
hyperglycaemia on cardiac function in AMI. However,
monocytes/macrophages are important immune cells in the body,
similar to lymphocytes. Following AMI, monocytes/macrophages are
rapidly recruited to the infarct zone, where they promote wound
healing and ventricular remodeling (32,33).
MCP-1 is an important inflammatory cytokine during the process of
monocyte/macrophage activation. To the best of our knowledge, there
is a lack of studies investigating the effect of stress
hyperglycaemia on perioperative MCP-1 levels in patients with AMI
undergoing PCI and the associated dynamic changes, thus the present
study focused on this.
Patients with diabetes frequently have high baseline
levels of MCP-1 (34,35). The present study also demonstrated
that for patients with ASTEMI, the MCP-1 levels pre-PCI were
significantly higher in patients with diabetes compared with
non-diabetic patients. Furthermore, due to AMI and PCI, the MCP-1
levels increased more significantly following PCI in patients with
diabetes, indicating that patients with diabetes were more
sensitive to certain inflammatory stimuli.
In addition, the present study indicated that MCP-1
levels at different time points before and after PCI were higher.
It also demonstrated that high MCP-1 levels lasted longer
(maintained for 48 h after PCI) in patients with stress
hyperglycaemia compared with non-diabetic and non-hyperglycemic
patients, with no significant difference from the trend observed in
patients with diabetes. Stress hyperglycaemia is usually transient;
the blood glucose level usually returns to normal at discharge,
indicating that stress hyperglycaemia is unrelated to the sustained
expression of inflammatory cytokines due to chronic hyperglycaemia
(36). El-Osta et al
(37) described that transient
hyperglycaemia induces long-lasting activating epigenetic
alterations in the promoter of nuclear factor-κ B subunit p65 in
aortic endothelial cells, which causes p65 gene expression to
increase. The epigenetic and gene expression alterations persist
for at least 6 days after normal physiological glucose levels are
restored, inducing increases in MCP-1 and vascular cell adhesion
molecule 1 expression. The study of El-Osta et al (37) reported that hyperglycaemia in
patients with stress hyperglycaemia, although transient, may still
result in days of activation of the upstream signaling pathway of
MCP-1 expression. This observation was confirmed by the serum MCP-1
levels in the patients with ASTEMI in the present study.
Furthermore, the present study demonstrated that for
non-diabetic patients, the increase in MCP-1 levels after PCI
compared with those prior to PCI was significantly associated with
blood glucose level at admission in a dose-dependent manner,
indicating that hyperglycaemia itself may be associated with
elevated chemokine levels and may prolong the effects of
chemokines. Hyperglycaemia may increase the expression levels of
MCP-1 and MCP-1-induced protein, thereby enhancing the effects of
reactive oxygen species, endoplasmic reticulum and autophagy,
resulting in myocardial apoptosis (30). This enhancement may increase the
incidence of no-reperfusion during primary PCI in patients with
AMI, resulting in myocardial microcirculation thrombosis (38–40), an
increase in the area of AMI (41).
It could also have an effect on the post-PCI cardiac recovery of
patients with AMI (42), and
subsequently higher post-PCI MACE rate in patients with
hyperglycemia compared with non-hyperglycemic patients. These
effect of hyperglycaemia on the expression of MCP-1 may represent
one of the mechanisms by which stress hyperglycaemia affects the
prognosis of AMI.
In conclusion, the present study demonstrated that
stress hyperglycaemia was associated with elevated serum MCP-1
levels in patients with ASTEMI undergoing primary PCI and may
enhance and prolong MCP-1-associated inflammatory responses
resulting in a poor prognosis post-PCI. This indicates that stress
hyperglycaemia may affect the prognosis of patients with ASTEMI
undergoing primary PCI via elevated MCP-1 levels. Thus, blocking
excessively high MCP-1 levels may become a potential option for
improving the prognosis of patients with ASTEMI following primary
PCI, though further research is required to validate these
results.
In the present study, patients with unsuccessful PCI
or those who succumbed within 48 h of admission were excluded, as
this study required monitoring of MCP-1 levels for 48 h after PCI;
this exclusion may result in selection bias. As for ethical
considerations, no interventions were given for MCP-1 levels to
further verify the association between MCP-1 levels and poor
prognosis. In addition, the sample size was small, and the study
period was short. Thus, the long-term prognoses of the subjects in
this study should be monitored and large multicenter studies are
required to further validate these results. The current study
revealed that stress hyperglycemia and high monocyte
chemoattractant protein-1 levels at admission are risk factors for
the adverse prognosis of patients with ASTEMI undergoing primary
PCI. Therefore, patients with ASTEMI exhibiting such biochemical
abnormalities should have more medical attention paid to them.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW conceived and designed the current study, and
revised the manuscript critically for important intellectual
content. NL and JS collected, analyzed and interpreted the data. NL
wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Ethics approval for the study was granted from the
Second Affiliated Hospital of Anhui Medical University Ethics
Committee. Each patient and/or his/her family provided written
informed consent for the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Task Force on Myocardial Revascularization
of The European Society of Cardiology (ESC), the European
Association for Cardio-Thoracic Surgery (EACTS)1; European
Association for Percutaneous Cardiovascular Interventions (EAPCI),
; Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg
S, Huber K, et al: Guidelines on myocardial revascularization. Eur
Heart J. 31:2501–2555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keeley EC, Boura JA and Grines CL: Primary
angioplasty versus intravenous thrombolytic therapy for acute
myocardial infarction: A quantitative review of 23 randomised
trials. Lancet. 361:13–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capes SE, Hunt D, Malmberg K and Gerstein
HC: Stress hyperglycaemia and increased risk of death after
myocardial infarction in patients with and without diabetes: A
systematic overview. Lancet. 355:773–778. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Straumann E, Kurz DJ, Muntwyler J,
Stettler I, Furrer M, Naegeli B, Frielingsdorf J, Schuiki E, Mury
R, Bertel O and Spinas GA: Admission glucose concentrations
independently predict early and late mortality in patients with
acute myocardial infarction treated by primary or rescue
percutaneous coronary intervention. Am Heart J. 150:1000–1006.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Timmer JR, Hoekstra M, Nijsten MW, van der
Horst IC, Ottervanger JP, Slingerland RJ, Dambrink JH, Bilo HJ,
Zijlstra F and van't Hof AW: Prognostic value of admission
glycosylated hemoglobin and glucose in nondiabetic patients with
ST-segment-elevation myocardial infarction treated with
percutaneous coronary intervention. Circulation. 124:704–711. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y,
Jeong MH, Seung KB, Lee SH and Chang K: The stress hyperglycaemia
ratio, an index of relative hyperglycaemia, as a predictor of
clinical outcomes after percutaneous coronary intervention. Int J
Cardiol. 241:57–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EJ, Jeong MH, Kim JH, Ahn TH, Seung
KB, Oh DJ, Kim HS, Gwon HC, Seong IW, Hwang KK, et al: KAMIR-NIH
registry investigators: Clinical impact of admission hyperglycaemia
on in-hospital mortality in acute myocardial infarction patients.
Int J Cardiol. 236:9–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kosuge M, Kimura K, Kojima S, Sakamoto T,
Matsui K, Ishihara M, Asada Y, Tei C, Miyazaki S, Sonoda M, et al:
Effects of glucose abnormalities on in-hospital outcome after
coronary intervention for acute myocardial infarction. Circ J.
69:375–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Worthley MI, Holmes AS, Willoughby SR,
Kucia AM, Heresztyn T, Stewart S, Chirkov YY, Zeitz CJ and Horowitz
JD: The deleterious effects of hyperglycaemia on platelet function
in diabetic patients with acute coronary syndromes mediation by
superoxide production, resolution with intensive insulin
administration. J Am Coll Cardiol. 49:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ray KK, Cannon CP, Morrow DA, Kirtane AJ,
Buros J, Rifai N, McCabe CH, Gibson CM and Braunwald E: Synergistic
relationship between hyperglycaemia and inflammation with respect
to clinical outcomes in non-ST-elevation acute coronary syndromes:
Analyses from OPUS-TIMI 16 and TACTICS-TIMI 18. Eur Heart J.
28:806–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikeda U, Matsui K, Murakami Y and Shimada
K: Monocyte chemoattractant protein-1 and coronary artery disease.
Clin Cardiol. 25:143–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Léséleuc L, Orlova M, Cobat A, Girard
M, Huong NT, Ba NN, Thuc NV, Truman R, Spencer JS, Adams L, et al:
PARK2 mediates interleukin 6 and monocyte chemoattractant protein 1
production by human macrophages. PLoS Negl Trop Dis. 7:e20152013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Gao XJ, Ren CG, Hu JH, Liu XW,
Zhang P, Zhang ZW and Fu ZJ: Monocyte chemoattractant protein-1
contributes to morphine tolerance in rats with cancer-induced bone
pain. Exp Ther Med. 13:461–466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cipollone F, Marini M, Fazia M, Pini B,
Iezzi A, Reale M, Paloscia L, Materazzo G, D'Annunzio E, Conti P,
et al: Elevated circulating levels of monocyte chemoattractant
protein-1 in patients with restenosis after coronary angioplasty.
Arterioscler Thromb Vasc Biol. 21:327–334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heil M, Ziegelhoeffer T, Wagner S,
Fernández B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann
G and Schaper W: Collateral artery growth (arteriogenesis) after
experimental arterial occlusion is impaired in mice lacking
CC-chemokine receptor-2. Circ Res. 94:671–677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD; Writing
Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal
Definition of Myocardial Infarction, ; et al: Third universal
definition of myocardial infarction. Eur Heart J. 33:2551–2567.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuen VG and McNeill JH: Comparison of the
glucose oxidase method for glucose determination by manual assay
and automated analyzer. J Pharmacol Toxicol Methods. 44:543–546.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
American Diabetes Association: Standards
of medical care in diabetes-2014. Diabetes Care. 37 (Suppl
1):S14–S80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JGF, Coats AJS, Falk V, González-Juanateyr JR, Harjola VP,
Jankowska EA, et al: 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure: The Task Force for
the diagnosis and treatment of acute and chronic heart failure of
the European Society of Cardiology (ESC). Developed with the
special contribution of the Heart Failure Association (HFA) of the
ESC. Eur J Heart Fail. 18:891–975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Li F, Guo J, Li C, Xu D and Wang
B: Insulin resistance, blood glucose and inflammatory cytokine
levels are risk factors for cardiovascular events in diabetic
patients complicated with coronary heart disease. Exp Ther Med.
15:1515–1519. 2018.PubMed/NCBI
|
|
21
|
Xu W, Tian M and Zhou Y: The relationship
between insulin resistance, adiponectin and C-reactive protein and
vascular endothelial injury in diabetic patients with coronary
heart disease. Exp Ther Med. 16:2022–2026. 2018.PubMed/NCBI
|
|
22
|
Antoniucci D, Valenti R, Migliorini A,
Parodi G, Moschi G, Memisha G, Santoro GM and Cerisano G: Impact of
insulin-requiring diabetes mellitus on effectiveness of reperfusion
and outcome of patients undergoing primary percutaneous coronary
intervention for acute myocardial infarction. Am J Cardiol.
93:1170–1172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Timmer JR, van der Horst IC, de Luca G,
Ottervanger JP, Hoorntje JC, de Boer MJ, Suryapranata H, Dambrink
JH, Gosselink M, Zijlstra F, et al: Zwolle Myocardial Infarction
Study Group: Comparison of myocardial perfusion after successful
primary percutaneous coronary intervention in patients with
ST-elevation myocardial infarction with versus without diabetes
mellitus. Am J Cardiol. 95:1375–1377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prasad A, Stone GW, Stuckey TD, Costantini
CO, Zimetbaum PJ, McLaughlin M, Mehran R, Garcia E, Tcheng JE, Cox
DA, et al: Impact of diabetes mellitus on myocardial perfusion
after primary angioplasty in patients with acute myocardial
infarction. J Am Coll Cardiol. 45:508–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knudsen EC, Seljeflot I, Abdelnoor M,
Eritsland J, Mangschau A, Arnesen H and Andersen GO: Abnormal
glucose regulation in patients with acute ST-elevation myocardial
infarction-a cohort study on 224 patients. Cardiovasc Diabetol.
8:62009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terlecki M, Bryniarski L, Bednarek A,
Kocowska M, Kawecka-Jaszcz K and Czarnecka D: The risk of diabetes
development in long-term observation of patients with acute
hyperglycaemia during myocardial infarction. Kardiol Pol.
73:606–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishihara M, Inoue I, Kawagoe T, Shimatani
Y, Kurisu S, Hata T, Nakama Y, Kijima Y and Kagawa E: Is admission
hyperglycaemia in non-diabetic patients with acute myocardial
infarction a surrogate for previously undiagnosed abnormal glucose
tolerance? Eur Heart J. 27:2413–2419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishihara M: Acute hyperglycemia in
patients with acute myocardial infarction. Circ J. 76:563–571.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roberts GW, Quinn SJ, Valentine N,
Alhawassi T, O'Dea H, Stranks SN, Burt MG and Doogue MP: Relative
hyperglycemia, a marker of critical illness: Introducing the stress
hyperglycemia ratio. J Clin Endocrinol Metab. 100:4490–4497. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Younce CW, Wang K and Kolattukudy PE:
Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1
production and induction of a novel zinc-finger protein MCPIP.
Cardiovasc Res. 87:665–674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marfella R, Siniscalchi M, Esposito K,
Sellitto A, De Fanis U, Romano C, Portoghese M, Siciliano S, Nappo
F, Sasso FC, et al: Effects of stress hyperglycemia on acute
myocardial infarction: Role of inflammatory immune process in
functional cardiac outcome. Diabetes Care. 26:3129–3135. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Birdsall HH, Green DM, Trial J, Youker KA,
Burns AR, MacKay CR, LaRosa GJ, Hawkins HK, Smith CW, Michael LH,
et al: Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce
migration of monocytes into ischemic canine myocardium within the
first one to five hours after reperfusion. Circulation. 95:684–692.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niu J, Jin Z, Kim H and Kolattukudy PE:
MCP-1-induced protein attenuates post-infarct cardiac remodeling
and dysfunction through mitigating NF-κB activation and suppressing
inflammation-associated microRNA expression. Basic Res Cardiol.
110:262015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zietz B, Büchler C, Herfarth H,
Müller-Ladner U, Spiegel D, Schölmerich J and Schäffler A:
Caucasian patients with type 2 diabetes mellitus have elevated
levels of monocyte chemoattractant protein-1 that are not
influenced by the −2518 A->G promoter polymorphism. Diabetes
Obes Metab. 7:570–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Piemonti L, Calori G, Lattuada G, Mercalli
A, Ragogna F, Garancini MP, Ruotolo G, Luzi L and Perseghin G:
Association between plasma monocyte chemoattractant protein-1
concentration and cardiovascular disease mortality in middle-aged
diabetic and nondiabetic individuals. Diabetes Care. 32:2105–2110.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tenerz A, Norhammar A, Silveira A, Hamsten
A, Nilsson G, Rydén L and Malmberg K: Diabetes, insulin resistance,
and the metabolic syndrome in patients with acute myocardial
infarction without previously known diabetes. Diabetes Care.
26:2770–2776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El-Osta A, Brasacchio D, Yao D, Pocai A,
Jones PL, Roeder RG, Cooper ME and Brownlee M: Transient high
glucose causes persistent epigenetic changes and altered gene
expression during subsequent normoglycemia. J Exp Med.
205:2409–2417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishihara M, Kojima S, Sakamoto T, Asada Y,
Tei C, Kimura K, Miyazaki S, Sonoda M, Tsuchihashi K, Yamagishi M,
et al: Acute hyperglycemia is associated with adverse outcome after
acute myocardial infarction in the coronary intervention era. Am
Heart J. 150:814–820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iwakura K, Ito H, Ikushima M, Kawano S,
Okamura A, Asano K, Kuroda T, Tanaka K, Masuyama T, Hori M and
Fujii K: Association between hyperglycemia and the no-reflow
phenomenon in patients with acute myocardial infarction. J Am Coll
Cardiol. 41:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ota S, Tanimoto T, Orii M, Hirata K,
Shiono Y, Shimamura K, Matsuo Y, Yamano T, Ino Y, Kitabata H, et
al: Association between hyperglycemia at admission and
microvascular obstruction in patients with ST-segment elevation
myocardial infarction. J Cardiol. 65:272–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pak S, Yatsynovich Y and Markovic JP: A
meta-analysis on the correlation between admission hyperglycemia
and myocardial infarct size on CMRI. Hellenic J Cardiol.
59:174–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ishihara M, Inoue I, Kawagoe T, Shimatani
Y, Kurisu S, Nishioka K, Umemura T, Nakamura S and Yoshida M:
Impact of acute hyperglycemia on left ventricular function after
reperfusion therapy in patients with a first anterior wall acute
myocardial infarction. Am Heart J. 146:674–678. 2003. View Article : Google Scholar : PubMed/NCBI
|