Introduction
Osteoporosis (OP), a disease in which the strength
of bones decreases and the risk of fracture increases, has become a
common public health problem (1). In
China, there are >80 million cases of primary OP every year
(2). Hip fracture induced by OP is
one of the most severe problems of aging persons, particularly in
women, as 10–24% women will succumb within one year following hip
fracture (3). With an increasing
elderly population worldwide, healthcare expenditures, particularly
those used to treat OP and associated fractures, are growing
annually (4). In order to develop
better drug treatments and cell therapies for osteoporosis, it is
highly desirable to establish an animal model of OP with a genetic
makeup closer to humans for preclinical research (5).
Animals including rats, rabbits, sheep and dogs have
been previously used to establish a variety of models for the study
of OP (6). Although they have been
widely used, they have their own disadvantages. For instance, rats
have not truly achieved skeletal maturity at the time-point of use;
rabbits have relatively less cancellous bone resulting in
inconvenience for bone densitometry; sheep have been used less
frequently due to the high cost and time consumption; the
osteoporosis model in sheep was established by ovariectomy for ≥12
months post-operatively (7).
Furthermore, they are not similar to humans in terms of their
living environment and social psychology, and there are significant
genetic differences between rodents and humans, meaning that these
models are unsatisfactory for certain diseases, such as hepatitis
and AIDS (8). Non-human primates,
including monkeys, are genetically similar to humans compared to
other experimental animals, making the simulation of human
pathology and physiology relatively accurate. However, their high
cost, low reproducibility and ethical concerns severely limit their
use as experimental models (9,10).
The tree shrew (Tupaia belangeri), is widely
distributed in South Asia, Southeast Asia and Southwest China
(11). It is characterized by short
reproductive and life cycles, high reproductivity, moderate size
and easy feeding. For decades, tree shrews have been increasingly
used as models of human disease as they are close relatives to
primate and have many human-like characteristics (12–18).
Whole-genome sequencing revealed that tree shrews have a higher
homology with humans than mice, rats and dogs (19). Tree shrews have been reported as
models of hepatitis virus infection, myopia, social stress and
depression (20). The present group
has previously reported that tree shrews are suitable to establish
an osteoporosis model using bilateral ovariectomy (21). However, the model remains to be
deemed suitable for the detection of OP occurrence and requires a
comprehensive evaluation and analysis compared with human
osteoporosis. In the present study the physical and molecular
changes of tree shrews suffering from OP were examined and further
compared with human patients with OP. Furthermore, a method to
evaluate the OP model in tree shrews was established, which may be
used to investigate the therapeutic response of OP as an
experimental model.
Materials and methods
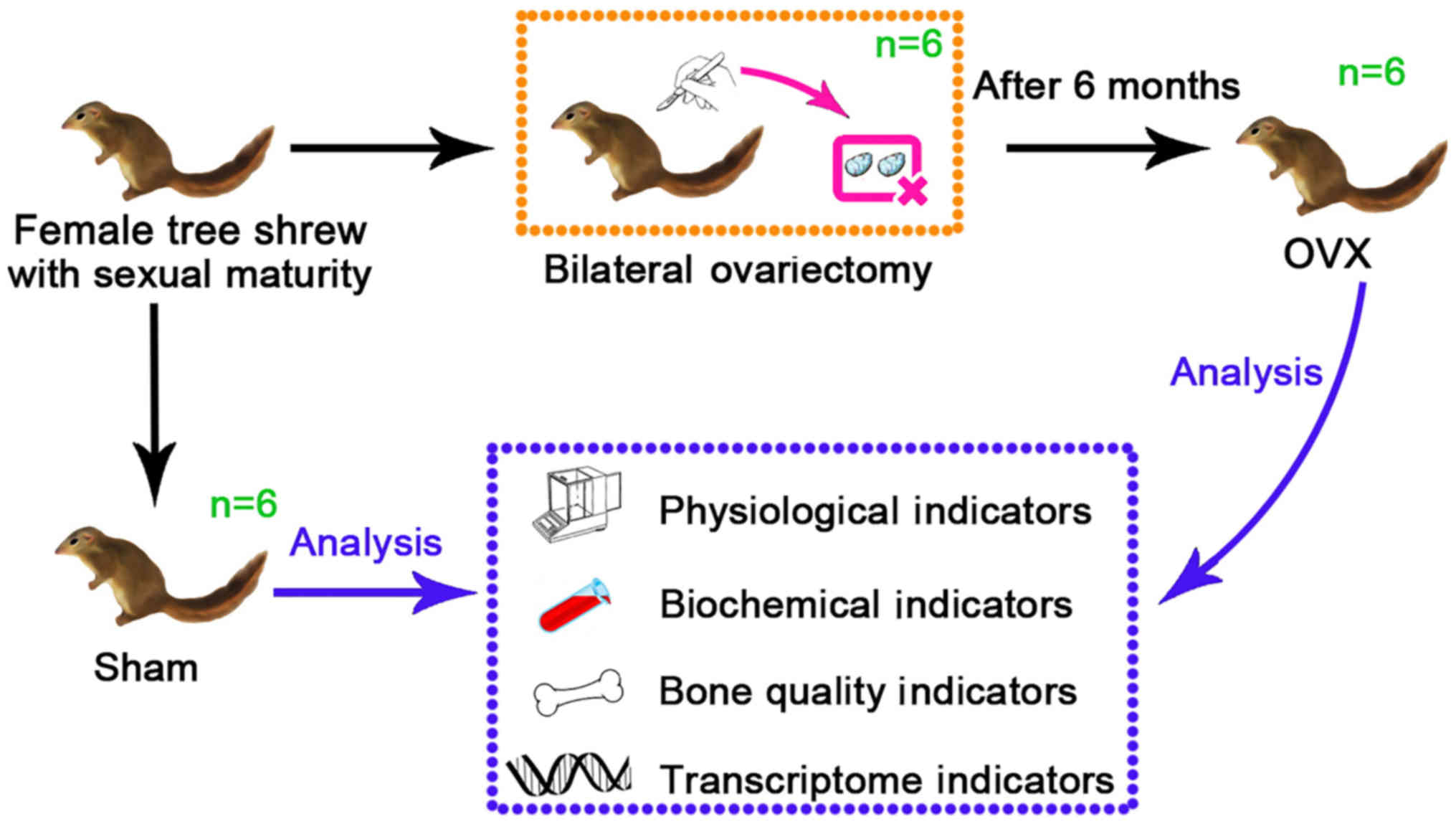
Animals, grouping and treatment
A total of 12 healthy female tree shrews (age, 6
months; weight, 127±15 g) were used for the present study. They
were purchased from the Institute of Medical Biology of the Chinese
Academy of Medical Sciences (Kunming, China; cat. no. SCXK-K
2013-0003), kept under standard conditions (all tree shrews were
housed in the same room under a 12-h light/dark cycle with 60±10%
relative humidity and temperature of 25±2°C, with ad libitum
access to water and food) for two weeks and were randomly divided
into an experimental group (OVX group, n=6) and a Control group
(Sham group, n=6). In the present study, the tree shrews of the two
groups were anesthetized by administering pentobarbital sodium via
intraperitoneal injection (dose, 40 mg/kg) (22). After anesthesia, an incision was made
in the middle of the abdomen into the abdominal cavity under
aseptic conditions, the bilateral ovaries were removed in the
ovariectomy (OVX) group, and the same amount of greater omentum was
removed in the Sham group. A diagram of the experimental design of
the study is presented in Fig. 1.
The animals were kept warm, and their feeding behavior and activity
were closely observed and recorded. After the surgery, bone mineral
density (BMD) analysis was performed every month. After 6 months,
the BMD was reduced in the OVX group compared with that in the Sham
group, and the tree shrews were euthanized for subsequent analysis
(21). All experimental procedures
were performed in accordance with the guidelines of the Kunming
University Committee for Care and Use of Laboratory Animals, which
followed the NIH's Guide for the Care and Use of Experimental
Animals, (Kunming, China) and were approved by the Animal
Experiments Ethics Committee of Kunming University (Kunming,
China).
Biochemical parameter analysis of
blood samples
During euthanasia, the blood was immediately
extracted and centrifuged at 3,000 × g for 10 min at 4°C after
incubation at room temperature for 2 h. The serum was collected and
stored at −20°C for further biochemical analysis. According to the
manufacturer's protocols (Shanghai Enzyme-linked, Shanghai, China),
bone alkaline phosphatase (BALP; cat. no. ml627904), osteocalcin
(BGP; cat. no. ml625695), procollagen type I N-terminal propeptide
(PINP; cat. no. ml6038002), procollagen type I C-terminal
propeptide (PICP; cat. no. ml6036832), cross-linked N-telopeptide
of type I collagen (NTXI; cat. no. ml0281751), cross-linked
N-telopeptides of type I collagen (CTXI; cat. no. ml0263011),
tartrate-resistant acid phosphatase (TRAP; cat. no. ml0259071),
calcium (Ca; cat. no. ml058009), phosphorus (P; cat. no. ml058011)
and estradiol (E2; cat. no. ml0216351) in tree shrew
serum were determined.
Determination of uterus
coefficients
Prior to sacrifice, the tree shrews were weighed,
and after sacrifice, the uterus of each tree shrew was completely
separated and weighed. Subsequently, the uterus coefficients were
calculated as follows: Uterus coefficient = wet weight of the
uterus/body weight.
Micro-CT scanning and analysis
Directly after euthanasia, the complete third lumbar
vertebrae were collected from the tree shrews of the OVX and Sham
groups. The muscles and connective tissues were peeled off and then
taken analyzed by micro-computed tomography (CT) scanning (Skyscan
1272; Bruker Corp., Billerica, MA, USA). Micro-CT analysis was
performed at the National & Regional Engineering Laboratory of
Tissue Engineering, Third Military Medical University (Chongqing,
China).
BMD, bone tissue volume fraction (BV/TV), bone
surface/volume ratio (BS/BV), trabecular number (Tb.N), trabecular
thickness (Tb.Th) and trabecular separation (Tb.Sp) were measured
separately using the CT analyser.
Histological analysis
After micro-CT scanning, the third lumbar vertebrae
were fixed in 4% paraformaldehyde for 72 h, and then decalcified by
soaking in 25% formic acid for 3 days. For paraffin-embedded
samples, the cross section was sliced for hematoxylin-eosin (HE),
ALP and TRAP staining. A HE staining kit was purchased from Beijing
Solarbio Sciences & Technology Co., Ltd (Beijing, China; cat.
no. G 1120). In brief, the sections were dewaxed, washed for 2 min
and stained with hematoxylin for 1 min. Subsequently, the samples
were washed with water and differentiation solution for 6 sec at
room temperature. The sections were counterstained with eosin for 1
min and washed with absolute ethanol, sealed with neutral gum and
then examined by microscopy. ALP staining was performed with a
5-bromo-4-chloro-3′-indolyphosphate (BCIP)/(nitro-blue tetrazolium)
NBT alkaline phosphatase color development kit (cat. no. C3206;
Beyotime Institute of Biotechnology, Shanghai, China). In brief,
the sections were dewaxed for 10 min, washed with distilled water
for 2 min, air-dried and placed into BCIP/NBT dye working buffer
containing 3 ml color buffer, 10 µl BCIP solution and 20 µl NBT
solution for 3 h at room temperature, then washed with water,
air-dried, and subjected to microscopic examination. The TRAP
staining kit was purchased from Beijing Solarbio Sciences &
Technology Co., Ltd (cat. no. G 1492). In brief, the sections were
dewaxed for 10 min, washed with distilled water for 2 min,
air-dried and placed into TRAP fixing solution for 1 min. The
sections were incubated in TRAP incubation buffer containing 1 ml
β-naphthol AS-BI buffer, 0.1 ml fast garnet GBC dye solution and 9
ml TRAP buffer for 1.5 h at 37°C and then washed with distilled
water. The sections were counterstained in hematoxylin for 5 min at
room temperature, washed, air-dried and microscopically examined.
ALP and TRAP staining of lumbar spines in the sham group and OVX
group (n=6) were visualized under a light microscope (Nikon Eclipse
Ci and NIS-Element 4.30; Nikon, Tokyo, Japan) without any further
histomorphometrical analysis of the number of cells and colour
intensity, as the intensity of ALP and TRAP staining was obviously
different between the two groups on visual inspection.
Scanning electron microscopy (SEM)
observation
The tibias were cut from tree shrews of two groups
and the bone tissues were fixed with 2.5% glutaraldehyde for 48 h.
Dehydration was performed with an ethanol gradient, and the samples
were dried with tert-butanol, vacuum-plated and observed by SEM
(S-3400N; Hitachi, Tokyo, Japan).
Calculation of ash weight
coefficient
The right humerus was cut from tree shrews of two
groups and dried in an oven at 110°C for 2 h. Subsequently, the dry
weight of the bones was determined. The dried right humerus was put
in a muffle furnace at 650°C for 8 h, and the ashes were then
weighed. The coefficient of ash weight was determined as follows:
Coefficient = ash weight/dry weight.
Biomechanical testing
The biomechanical properties of the left humerus
were evaluated by the three-point bending flexural test method. In
brief, the left humerus was placed in a biomechanical testing
instrument (Instron 5565; Instron, Norwood, MA, USA). The
conditions were as follows: Stride distance, 20 mm; and loading
velocity, 5 mm/sec. The data were recorded with a computer, and the
maximum load, maximum displacement, structural stiffness and
elastic modulus were calculated.
Transcriptome analysis
Total RNA was extracted and purified from the
tissues of the first lumbar vertebra of each animal using TRIzol
(Takara, Dalian, China) according to the manufacturer's protocols.
Total RNA was then quantified using the BioSpec-nano nucleic
acid-protein quantitative instrument (Shimadzu, Tokyo, Japan). The
RNA samples from the Sham and OVX groups were packed in dry ice and
sent to Novogene Bioinformatics Technology Co. Ltd (Beijing, China)
for further library preparation using the Agilent Bioanalyzer 2100
system (Agilent Technologies, Inc., Santa Clara, CA, USA). Library
preparations were then sequenced on an Illumina Hiseq platform
(Illumina, Inc., San Diego, CA, USA) and 125/150 bp paired-end
reads were generated.
Specifically, DESeq R software package (1.18.0) was
used to analyse the differential expression between the two groups
(three biological replicates in each group). DESeq provides a model
based on the negative binomial distribution to determine
statistical routines for differential expression in digital gene
expression data. Benjamini and Hochberg methods were used to
control the error discovery rate and adjust the resulting P-value.
Genes with a P-value <0.05 as determined by DESeq were regarded
as differentially expressed. The Gene Ontology (GO) seq R package
was used for enrichment analysis of differentially expressed genes
and correction of gene length deviation. After correction, GO with
P<0.05 was considered to be significantly enriched with
differentially expressed genes. The database of Kyoto Encyclopedia
of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) was used in bioinformatics
analysis. KOBAS 3.0 software (http://kobas.cbi.pku.edu.cn/) was used to test the
statistical enrichment of differentially expressed genes in KEGG
pathways. Next, the gene expression in healthy vs. OP patients was
compared with that of tree shrews in the Sham vs. OVX group. The
bone tissue samples were obtained from iliac bone in humans without
OP and femoral neck in patients with OP from January 2016 to
December 2018 from the first affiliated hospital of Kunming Medical
University (Kunming, China). As for the tree shrew samples, RNAs
from the bone tissues of humans were extracted and subjected to
sequencing analysis. The sequencing results of bone tissues from
healthy individuals and OS patients were compared with those of
tree shrews, and genes associated with bone formation were selected
for analysis. Based on the RNA-seq analysis, count per million
reads was used to denote the gene expression levels.
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed using SPSS 17.0 software for Windows (SPSS,
Inc., Chicago, IL, USA). The statistical differences among groups
were analyzed by using the Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
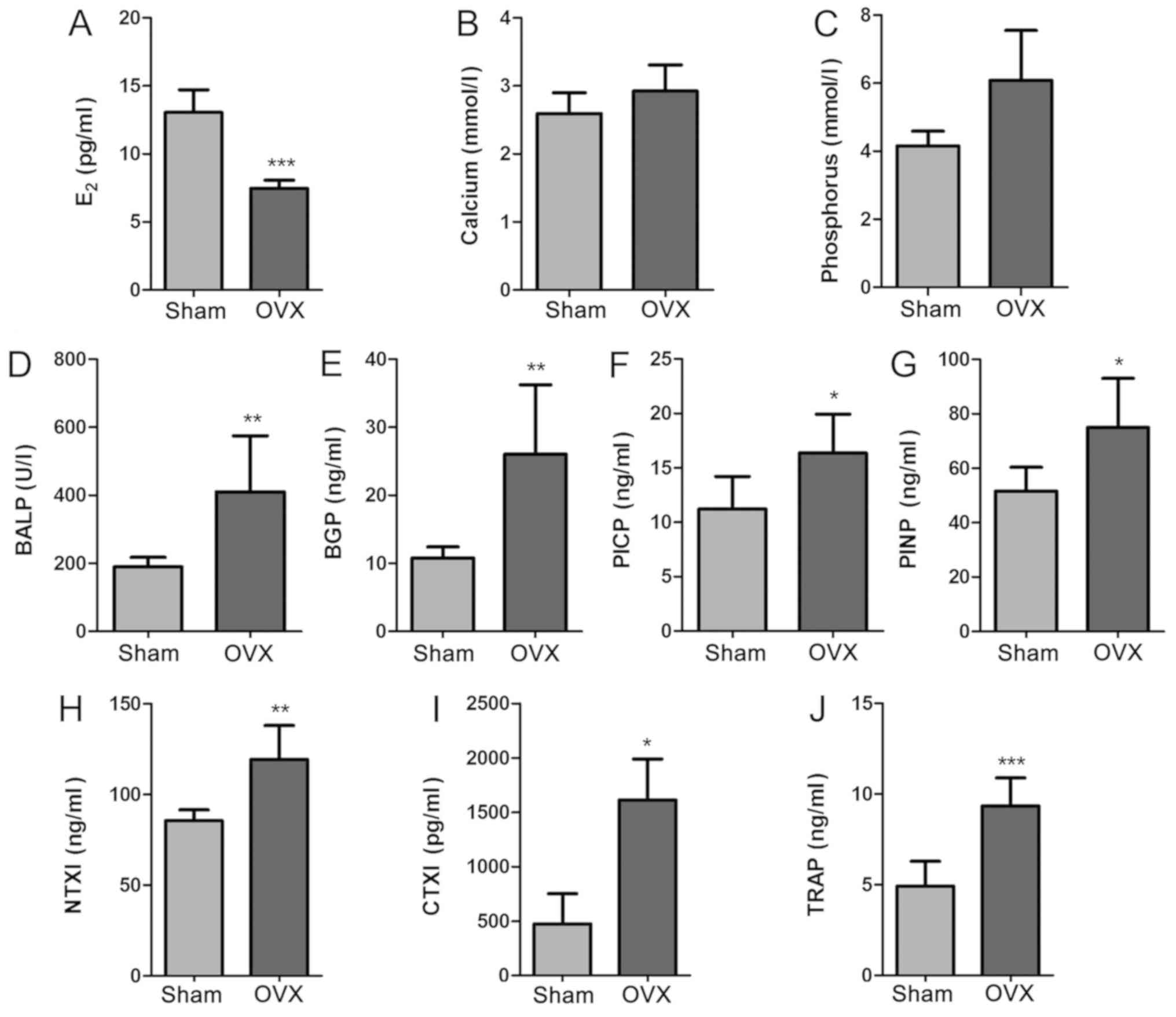
Blood biochemical indicator analysis
indicates high bone turnover and bone loss rate after OVX
In order to detect the changes of blood biochemical
indicators in tree shrews, the levels of E2, Ca, P,
BALP, BGP, PINP, PICP, NTXI, CTXI and TRAP in serum samples from
the two groups were determined using the appropriate assay kits
(Fig. 2). The levels of serum
E2 in the OVX group were significantly lower than those
in the Sham group (P<0.001; Fig.
2A). The Sham group appeared to have decreased Ca and P levels
compared with the OVX group, but the changes were not significant
(P>0.05; Fig. 2B and C). In
addition, the serum biomarkers of bone formation (BALP, BGP, PINP
and PICP) and bone resorption (NTXI, CTXI and TRAP) were evaluated.
These parameters were significantly increased in the OVX group
compared with those in the Sham group (P<0.01; Fig. 2D-J). These results demonstrated that
there was a significant difference between the Sham group and the
OVX group in certain aspects of blood biochemical indicators, and
the increase of bone metabolism markers reflects the high bone
turnover and bone loss rate in OVX group. The bilateral OVX
significantly reduced the E2 levels.
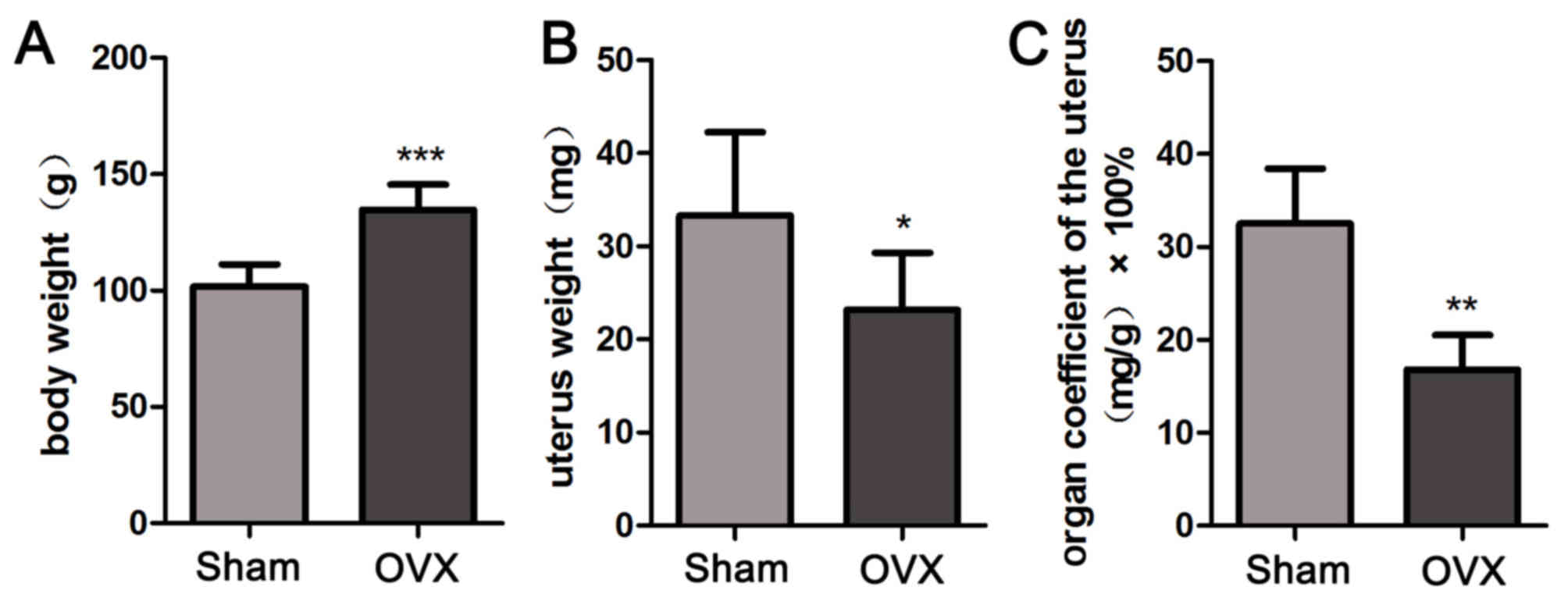
OVX reduces the uterus coefficient in
tree shrews
In order to calculate the uterus coefficients in the
Sham group and OVX group, the body weight and uterus weight of tree
shrews from the two groups were determined. As presented in
Fig. 3, the body weight in the OVX
group was significantly increased throughout the treatment period
compared with that in the Sham group (P<0.001; Fig. 3A), despite the same amount of food
provided to all animals. The OVX group appeared to have a
significantly decreased uterus weight compared with that in the
Sham group (P<0.05; Fig. 3B). The
uterus coefficient in the OVX group was significantly decreased
compared with that in the Sham group (P<0.01; Fig. 3C). The above results suggested that
bilateral OVX significantly reduces the uterus coefficient.
Micro-CT and histochemical
analysis
The results of the micro-CT imaging revealed that
the number and connections of trabeculae in the third lumbar
vertebra of OVX tree shrews were obviously decreased compared with
the sham group (Fig. 4A). The
results of the HE staining indicated that the trabecular bone was
compact, intact and continuous in the Sham group compared with that
in the OVX group (Fig. 4B). The
results of the microarchitectural analysis by micro-CT were then
quantified for statistical comparison between the groups. The BMD
of the third lumbar vertebra from the OVX group was significantly
decreased compared with that in the Sham group (P<0.01; Fig. 4C). The BS/BV in the OVX group was
significantly increased compared with that in the Sham group
(P<0.05; Fig. 4D). Furthermore,
the BV/TV and the Tb.Th in the OVX group were significantly
decreased compared with those in the Sham group (P<0.05;
Fig. 4E and G). The Tb.N determined
for the OVX group appeared to be decreased compared with that for
the Sham group, but the difference was not significant (P>0.05;
Fig. 4F). In addition, the Tb.Sp in
the OVX group appeared to be increased compared with that in the
Sham group, but the difference was also not significant (P>0.05;
Fig. 4H). ALP and TRAP staining
respectively revealed fewer osteoblasts and more osteoclasts in the
OVX compared with the Sham group (Fig.
4I and J). The above results indicated the successful
establishment of the experimental model.
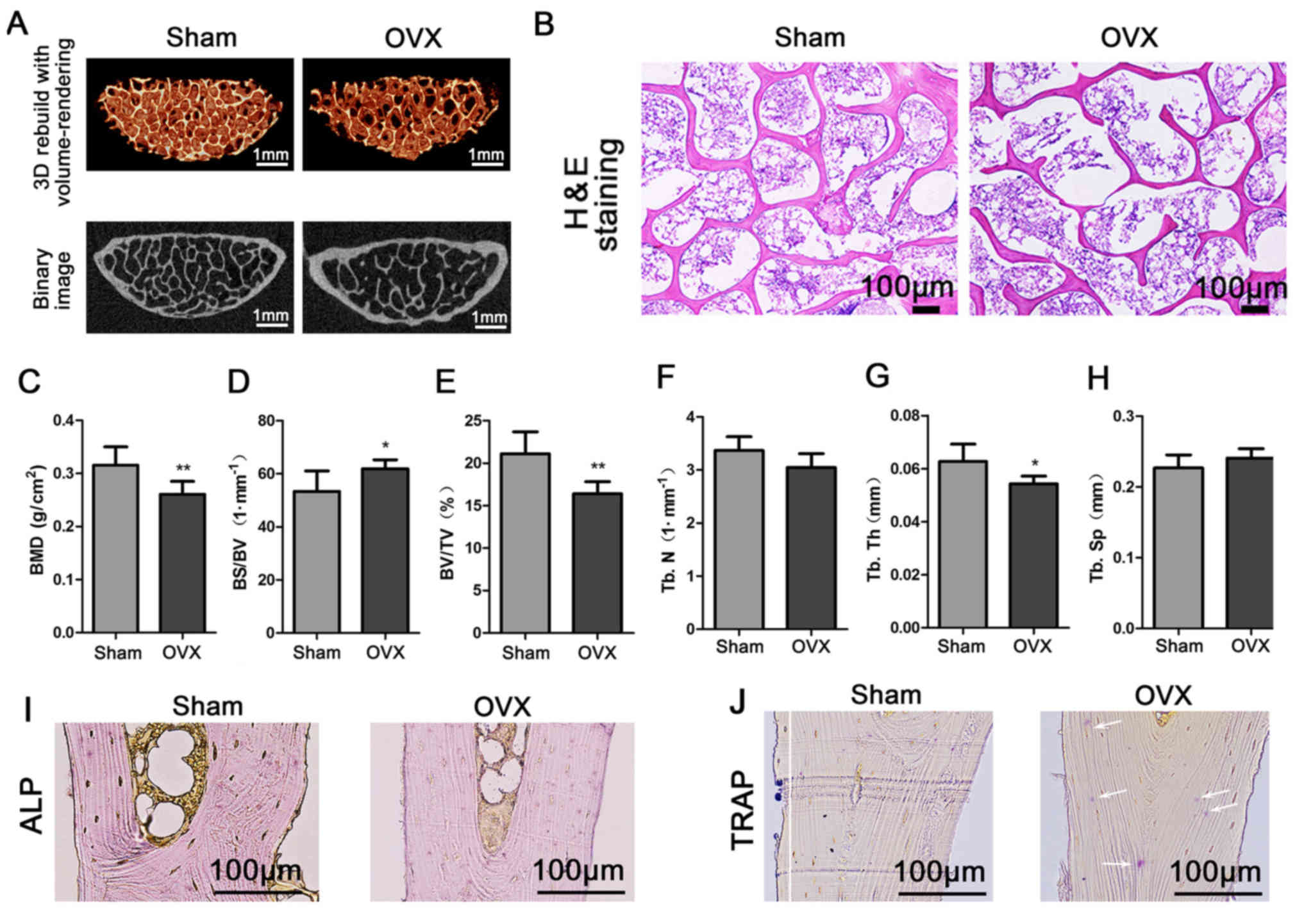 | Figure 4.Third lumbar vertebra quality in the
Sham and OVX groups. (A) Third lumbar vertebras were examined to
micro-computed tomography imaging. (B) Third lumbar vertebras were
stained with H&E. (C-H) Evaluation of bone quality parameters
of the third lumbar vertebra, including (C) the BMD, (D) BS/BV, (E)
BV/TV, (F) Tb.N, (G) Tb.Th and (H) Tb.Sp. Values are expressed as
the mean ± standard deviation (n=6). *P<0.05 and **P<0.01 vs.
the Sham group. (I) ALP staining revealed the osteoblasts. (J) TRAP
staining was employed to visualize the osteoclast (marked by white
arrows; scale bar, 100 µm). OVX, ovariectomy; 3D, 3-dimensional;
TRAP, tartrate-resistant acid phosphatase; BMD, bone mineral
density; BV/TV, bone tissue volume fraction; BS/BV, bone
surface/volume ratio; Tb.N, trabecular number; Tb.Th, trabecular
thickness; Tb.Sp, trabecular separation; ALP, alkaline
phosphatase. |
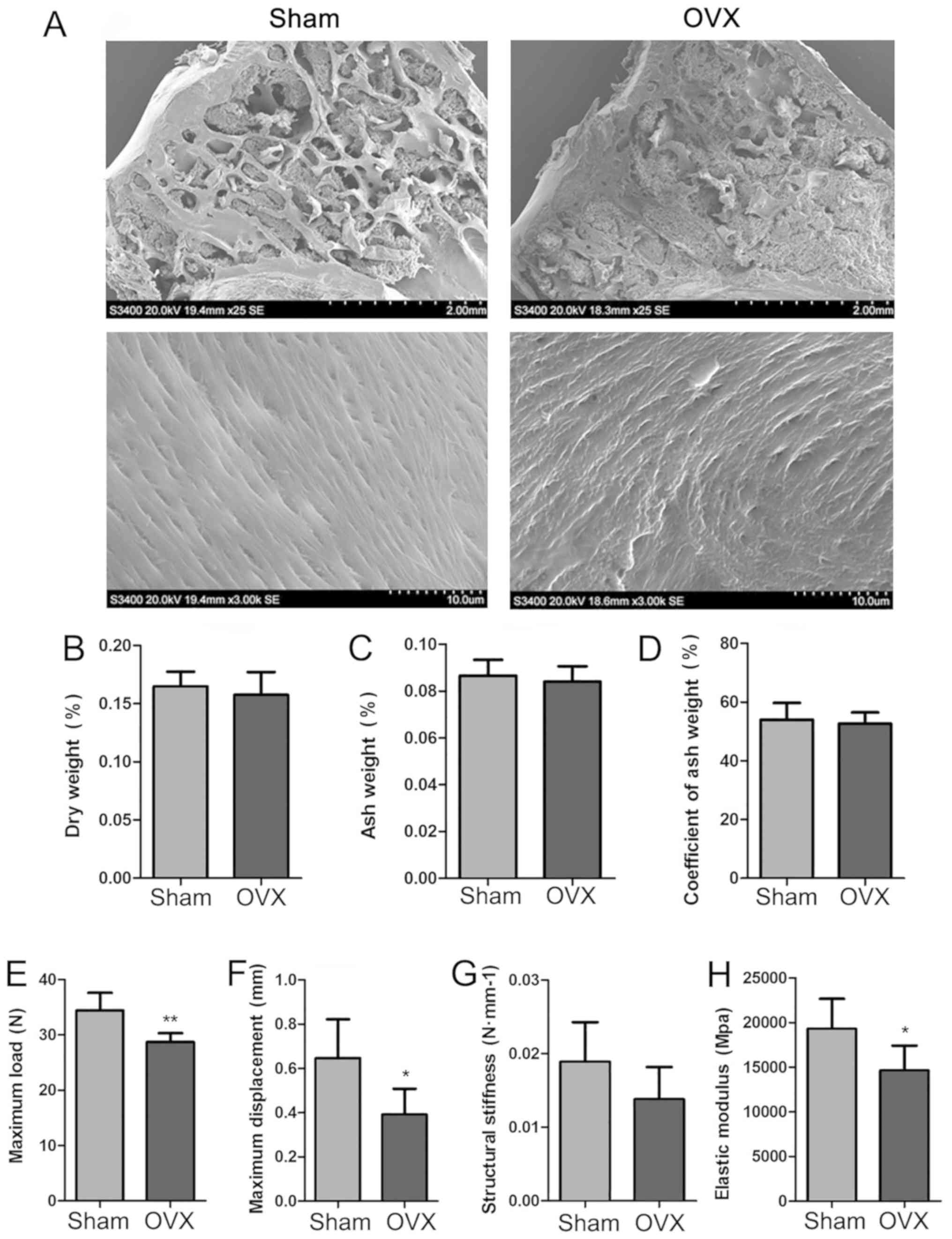
SEM imaging, biomechanical properties
and the coefficient of ash weight
In order to further determine the features of the
new experimental model of OP, SEM images of the proximal epiphysis
of the tibia were recorded, and the biomechanical properties of the
left humerus and the coefficient of ash weight of the right humerus
were determined for the Sham and OVX tree shrews (Fig. 5). SEM imaging under low magnification
indicated that the meshwork structure of the trabecular bone was
destroyed, while under high magnification, the collagen fibrils
were regularly arranged in the OVX group. By contrast, in the Sham
group, the trabecular reticular formation was observed to be
structurally complete on low magnification and the collagen fibrils
were regularly arranged, as identified under high magnification
(Fig. 5A). The dry weight, ash
weight and the coefficient of ash weight of the right humerus were
determined, and it appeared that those in the OVX group were
slightly decreased compared with those in the Sham group, but the
differences were not significant (P>0.05; Fig. 5B-D). The biomechanical properties
(maximum load, maximum displacement, structural stiffness and
elastic modulus formation) of the left humerus in the two groups
were assessed. Compared with the Sham group, the OVX group featured
a decreased maximum load, a decreased maximum displacement and
decreased elastic modulus formation, and these differences were
statistically significantly (P<0.05; Fig. 5E, F and H). The OVX group exhibited a
decreased structural stiffness compared with that in the Sham
group, but no significant difference was obtained (P>0.05;
Fig. 5G).
Bioinformatics analysis of
differentially expressed genes in the first lumbar vertebra
To explore potential underlying molecular mechanisms
involved in OP development in tree shrews subjected to OVX,
transcriptome analysis was performed. The results indicated that a
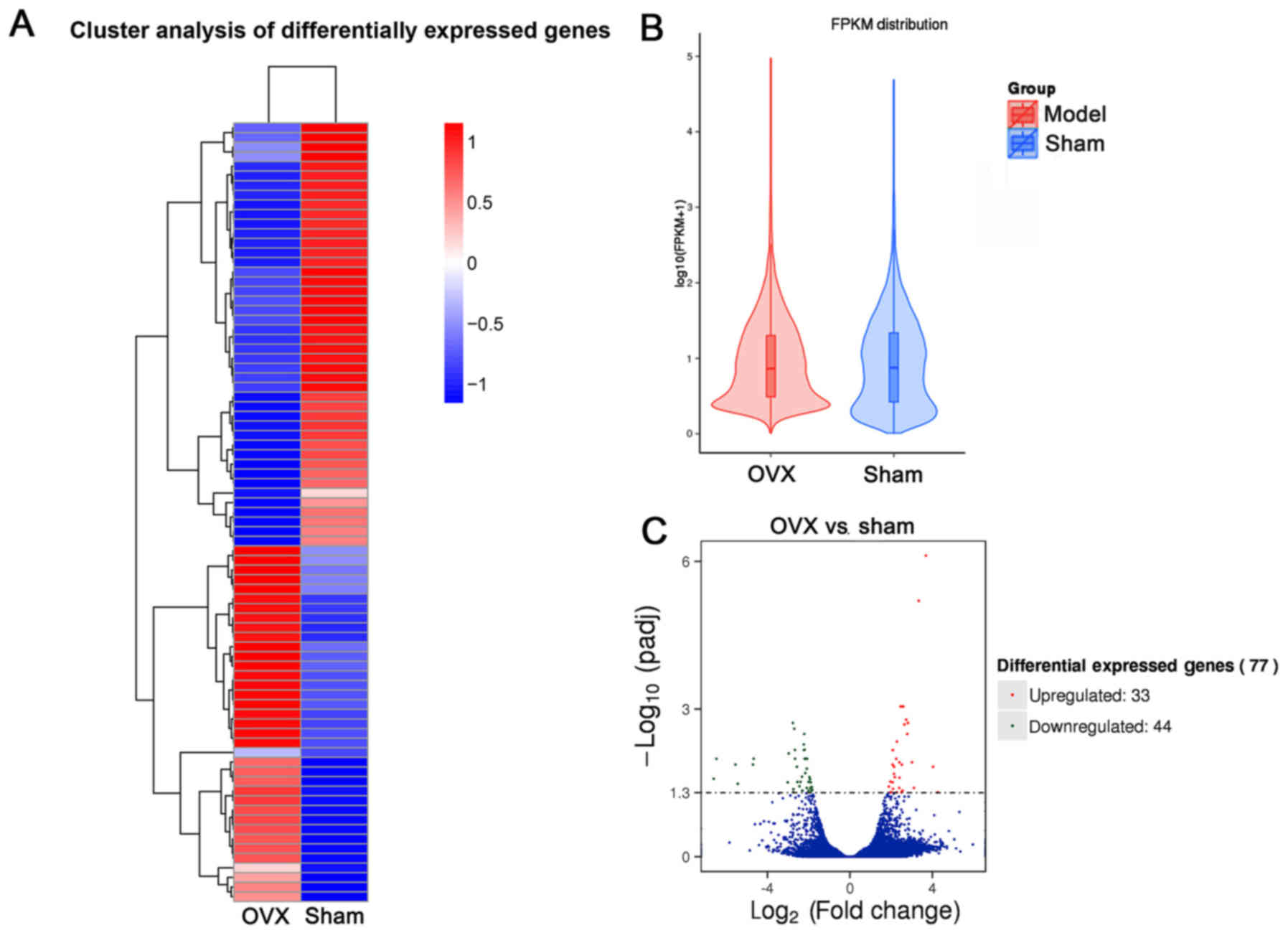
total of 81 gene modules were identified (Fig. 6A). By using this unbiased, objective
‘big-data’ processing of all genes, the Fragments Per Kilobase of
transcript per Million mapped reads density distribution was
revealed to be different between the OVX and Sham groups (Fig. 6B). 77 gene modules were significantly
differentially expressed between the two groups (Fig. 6C). Based on the ‘module-trait’
association plot, 33 genes were upregulated in the OVX group
compared with those in the Sham group, while 44 genes were
downregulated (Fig. 6C). Pathway
enrichment analysis showed the upregulated genes (Table SI) in OVX group were mainly
distributed in 15 pathways in which ‘metabolic pathways’ enriched 2
genes and the others had one gene (Fig.
7A). Among them, ‘Non-homologous end-joining’ had the largest
enrichment factor, which was associated with DNA repair in
osteoblasts (23). Among the KEGG
pathways enriched by the significantly downregulated genes
(Table SII) in the OVX group, ‘Drug
metabolism-cytochrome P450’ and ‘ABC transporters’ had the largest
enrichment factors (Fig. 7B), all of
which were associated with osteoporosis in postmenopausal woman or
in aging (24,25). The majority of upregulated genes were
accumulated in the GO terms of ‘peptidase activity’, ‘endopeptidase
activity’ and ‘proteolysis’ (Fig.
7C). The top 3 GO terms for downregulated genes were
‘transferase activity’, ‘ATPase activity’ and ‘single-organism
biosynthetic process’ (Fig. 7D) in
the OP model of tree shrews.
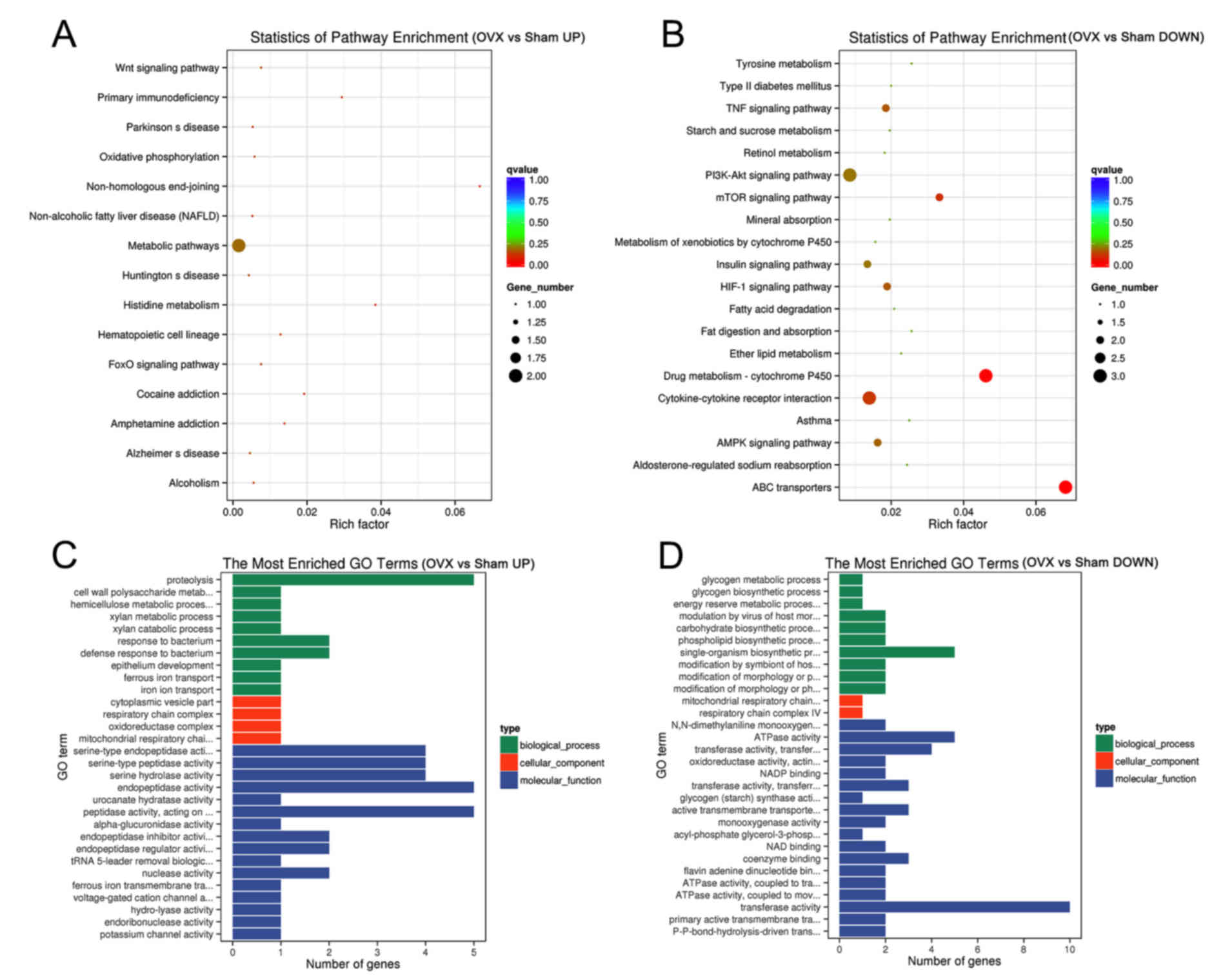 | Figure 7.Bioinformatics analysis of the
genetic profile of the first lumbar vertebra from the Sham and OVX
groups. (A and B) Statistics of enriched pathways by (A) the
upregulated and (B) the downregulated differentially expressed
genes in the OVX vs. Sham group. (C and D) The most enriched GO
terms (OVX vs. Sham) by (C) the upregulated and (D) the
downregulated differentially expressed genes. UP, upregulated;
DOWN, downregulated; GO, gene ontology; Fox, forkhead box; PI3K,
phosphoinositide-3 kinase; mTOR, mammalian target of rapamycin;
TNF, tumor necrosis factor; HIF, hypoxia-inducible factor; AMPK, 5′
AMP-activated protein kinase; OVX, ovariectomy. |
The differential expression of certain
osteogenesis-associated genes in OP was then compared between human
patients and the tree shrew model by analyzing their gene
expression profiles. The expression of the genes bone
γ-caroboxyglutamate protein (BGLAP), integrin binding sialoprotein
(IBSP), osteoprotegerin and Sp7 transcription factor (SP7)
exhibited a downward trend without statistically significance
between healthy and OP individuals in humans and tree shrews.
Alkaline phosphatase, biomineralization associated was
significantly decreased in patients with OP, and exhibited similar
trends without significance in the tree shrew OVX group. For the
gene of runt-related transcription factor 2 (RUNX2), a
significantly decreased expression level was observed both in
humans and tree shrews while the expression of sclerostin was not
obviously changed (Fig. S1).
Discussion
As the bones of mice are small, it is difficult to
appreciate the OP macroscopic features, including reduced amounts
and porosity of the cancellous bone (26). Although rats are widely used to model
OP, they have certain disadvantages. The major drawback of the rat
skeleton is that certain bones retain lifelong growth and do not
fuse epiphyses (27). The overall
skeletal morphology of the tree shrew is highly similar to that of
primates and the skeletal growth and bone metabolism are also much
closer to humans than those of rats (28). The CT 3-dimensional visualization of
tree shrews indicates that the tips of the fingers have the
characteristics of claws. The length of the fingers and toes are
closer to those of humans, which is suitable for grasping. At the
same time, the tree shrew's brain is relatively developed, the
volume of the skull cavity is large, there is a bone bridge behind
the orbit and the formation of the bone orbit, the thumb and other
fingers are separated, thereby having similar features to those of
humans (29). In the present study,
the tree shrew was selected as an experimental animal to induce OP
and the established model was evaluated by multiple approaches.
In OP, bone loss and a high bone turnover rate are
consistent; the biochemical markers of bone metabolism reflect the
overall bone turnover, and may be determined to reveal the bone
loss situation. The increase of bone metabolism markers reflects
the high bone turnover and bone loss rate. Of note, OP results from
an imbalance of bone resorption and bone formation. Therefore, the
biochemical markers for bone formation and resorption in blood
samples were investigated in the present study. As biochemical
markers of bone formation, BALP, BGP, PINP, PICP may be used for
detecting OP (30). BALP is a
phosphatase that is elevated in OP patients with high bone turnover
(31). The level of BGP, reflecting
bone formation, is elevated in postmenopausal OP (32). PINP and PICP have a similar
association, in terms of the occurrence of OP being accompanied by
an increase in their activity (33).
NTXI, CTXI and TRAP are biochemical markers of bone resorption, and
may also be used for detection of OP. CTXI and NTXI are the
internationally recognized biochemical markers of bone resorption,
whose levels are obviously increased in patients with OP (34). The levels of TRAP reflect the
activity of osteoclasts and the bone resorption status (35). In addition, the elements Ca and P are
considered to be phenotypic markers of bone formation (36); in the present study, the levels of
these substances were increased in the OVX group, but the changes
were not significant. The menopause is closely associated with
estrogen deficiency that may accelerate the pathogenesis of OP
(37). In the present study, the
serum levels of E2 significantly decreased in the OVX
group compared with those in the Sham group. The present study
demonstrated that the changes in the plasma levels of certain
biochemical markers of bone formation and resorption in OVX tree
shrews were similar to those in OP patients, indicating that the OP
model in tree shrews closely resembles OP in human patients, which
would make it suitable for experimental studies.
In the present study, the tree shrews from the OVX
group exhibited an increase in body weight compared with the
Sham-operated animals, which is in agreement with the body weight
changes of post-menopausal women (38). Beyond that, the uterus weight and
organ coefficient of the uterus of the tree shrews in the OVX group
were significantly reduced compared with those in the Sham group.
This indicated that OVX tree shrews have similar physiological
characteristics to post-menopausal women.
The primary objective of establishing animal models
is to assess potential pharmacological and non-pharmacological
approaches for the prevention and treatment of OP and associated
fractures, it is desirable to establish suitable monitoring
methods. As previously described, OP is a systemic skeletal disease
characterized by bone loss and microstructure degradation of bone
tissue, and is accompanied by increased susceptibility to bone
fragility and fracture (39). BMD,
biomechanical properties and bone microstructure are commonly used
to evaluate bone quality (40). The
present results indicated that the BMD of the third lumbar vertebra
in the OVX group was significantly decreased compared with that in
the Sham group. The biomechanical properties (maximum deflection
and maximum load) were significantly decreased in the OVX group.
For the bone microarchitecture analysis, although trabecular number
and trabecular separation had no significant changes, the Tb.Th and
BV/TV, whose value decreased when OP occurred, were observed to be
significantly decreased in the OP model in tree shrews. The BS/BV,
which was negatively associated with bone mass, was markedly
increased in the model. These results implied that the bone quality
of tree shrews in the OVX group was significantly lower than of
those in the Sham group.
In the present study, a significantly altered
transcription of a large number of genes in the OVX vs. the Sham
group was observed. A total of 81 differentially expressed mRNAs in
the first lumbar vertebra of the Sham and OVX tree shrews were
identified by RNA sequencing using the Illumina HiSeq 4000
platform. A total of 33 mRNAs were significantly upregulated in the
OVX vs. the Sham group (Table SI)
and 44 mRNAs were significantly downregulated (Table SII). Among them, 5 genes named MyoD
family inhibitor (MDFI), secreted frizzled-related protein 4
(SFRP4), calcyphosine like (CAPSL), oncomodulin (ONCM) and matrix
extracellular phosphoglycoprotein (MEPE) are highly relevant to OP.
MDFI has an important role in chondrogenesis (41). SFRP4 is involved in bone
morphogenesis (42). CAPSL has a
role in calcium ion binding (43).
ONCM is a high-affinity calcium ion-binding protein that belongs to
the superfamily of calmodulin proteins (44). MEPE regulates bone mineralization and
mice lacking this gene are resistant to aging-associated trabecular
bone loss (45). Genes involved in
DNA repair or cell survival, including DDIAS (46), were decreased in the OVX group.
The possible biological progression, cellular
functions and molecular pathways in OP were evaluated by GO and
KEGG analyses on the coding genes. The results indicated that
bone-associated pathways were affected in the OVX group. For
instance, the WNT signaling pathway, the suppression of which may
lead to OP (47), and the tumor
necrosis factor signaling pathway, which is the major signaling
pathway leading to bone loss (48).
The upregulation of phosphoinositide-3 kinase/Akt signaling may
enhance osteogenesis (49) and
activation of mammalian target of rapamycin signaling pathways
enhances osteogenic differentiation (50). Furthermore, the insulin signaling
pathway may enhance osteogenesis (51) and hypoxia-inducible factor-1
signaling may promote osteoblast proliferation (52). These signaling pathways were affected
in the OVX group.
In addition, the expression level of RUNX2 decreased
significantly in OP patients. RUNX2 is upregulated in immature
osteoblasts and is the first transcription factor required for the
osteoblast differentiation (53). It
regulates the expression of SP7, IBSP and BGLAP, which are
associated with bone forming (54).
RUNX2 was markedly changed between the Sham and OVX groups in tree
shrews, which was consistent with the reduction of bone formation
in OP disease.
In summary, the present study provided an
experimental method to establish and evaluate an OP tree shrew
model. The procedures of OVX were described in detail. The tests to
evaluate the OP model in tree shrews and their results were also
comprehensively outlined, and prove the successful establishment of
OP in tree shrews by ovariectomy.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Qijie Dai for
Micro-CT Scanning at the National & Regional Engineering
Laboratory of Tissue Engineering, The Third Military Medical
University (Chongqing, China).
Funding
The present study was supported by the National
Science and Key Technology Support Program (grant no.
2014BAI01B01-07).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MH and RPZ conceived and designed the experiments;
YLW, ZXM, YYZ and BLL wrote the manuscript; JJD, PFB, XFW, SPY and
ZQW performed the animal experiments; JXL, CEL, DPM and TKM
performed other experiments; CTY, YJL, NL and HBZ analyzed the
data; HYW, JL and YY revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Medical Ethics Committee of Medicine Department
of Kunming University (Kunming, China) approved the use of the
human tissues for scientific research purposes (permit code, FL No.
2-Ethical Review 2016). All donors provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jackson RD and Mysiw WJ: Insights into the
epidemiology of postmenopausal osteoporosis: The women's health
initiative. Semin Reprod Med. 32:454–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Z, Piao J, Pang L, Qing X, Nan S, Pan
Z, Guo Y, Wang X, Li F, Liu J and Cheng X: The diagnostic criteria
for primary osteoporosis and the incidence of osteoporosis in
China. J Bone Miner Metab. 20:181–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Black DM, Arden NK, Palermo L, Pearson J
and Cummings SR: Prevalent vertebral deformities predict hip
fractures and new vertebral deformities but not wrist fractures.
study of osteoporotic fractures research group. J Bone Miner Res.
14:821–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dolan P and Torgerson DJ: The cost of
treating osteoporotic fractures in the United Kingdom female
population. Osteoporos Int. 8:611–617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graham SM, Howgate D, Anderson W, Howes C,
Heliotis M, Mantalaris A, Tsiridis E and Tsapakis E: Risk of
osteoporosis and fracture incidence in patients on antipsychotic
medication. Expert Opin Drug Saf. 10:575–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Komori T: Animal models for osteoporosis.
Eur J Pharmacol. 759:287–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dias IR, Camassa JA, Bordelo JA, Babo PS,
Viegas CA, Dourado N, Reis RL and Gomes ME: Preclinical and
translational studies in small ruminants (sheep and goat) as models
for osteoporosis research. Curr Osteoporos Rep. 16:182–197. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glebe D and Bremer CM: The molecular
virology of hepatitis B virus. Semin Liver Dis. 33:103–112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao J, Liu R and Chen CS: Tree shrew
(Tupaia belangeri) as a novel laboratory disease animal model. Zool
Res. 38:127–137. 2017.PubMed/NCBI
|
|
10
|
Moore E: Medical relevance of UK-funded
non-human primateresearch published from January 1997 to July 2012.
J R Soc Med. 107:264–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Chen SY, Nie WH, Jiang XL and Yao
YG: Evaluating the phylogenetic position of Chinese tree shrew
(Tupaia belangeri chinensis) based on complete mitochondrial
genome: Implication for using tree shrew as an alternative
experimental animal to primates in biomedical research. J Genet
Genomics. 39:131–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Zhang Y, Liang B, Lü LB, Chen CS,
Chen YB, Zhou JM and Yao YG: Tree shrews under the spot light:
Emerging model of human diseases. Dongwuxue Yanjiu. 34:59–69.
2013.(In Chinese). PubMed/NCBI
|
|
13
|
Baldwin MK, Wei H, Reed JL, Bickford ME,
Petry HM and Kaas JH: Cortical projections to the superior
colliculus in tree shrews (Tupaia belangeri). J Comp Neurol.
521:1614–1632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petruzziello F, Fouillen L, Wadensten H,
Kretz R, Andren PE, Rainer G and Zhang X: Extensive
characterization of Tupaia belangeri neuropeptidome using an
integrated mass spectrometric approach. J Proteome Res. 11:886–896.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XH, Dai ZX, Zhang GH, Han JB and
Zheng YT: Molecular characterization, balancing selection, and
genomic organization of the tree shrew (Tupaia belangeri) MHC class
I gene. Gene. 522:147–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Chang Q, Zhang Y, Zou X, Chen L,
Zhang L, Lv L and Liang B: Relationships between body weight,
fasting blood glucose concentration, sex and age in tree shrews
(Tupaia belangeri chinensis). J Anim Physiol Anim Nutr (Berl).
7:1179–1188. 2013. View Article : Google Scholar
|
|
17
|
Xing HJ, Jia K, He J, Shi C, Fang M, Song
L, Zhang P, Zhao Y, Fu J and Li S: Establishment of the tree shrew
as an alcohol-induced fatty liver model for the study of alcoholic
liver diseases. PLoS One. 10:e01282532015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Young PA: Genomic evidence supported tree
shrew is closely related to primates. Int J Mol Evol Biodivers.
3:1–4. 2013.
|
|
19
|
Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX,
Fan DD, He J, Hou HL, Hu L, Hu XT, et al: Genome of the Chinese
tree shrew. Nat Commun. 4:14262013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye LH, Meng HE, Huang YC, Zhao GQ, Lei YJ,
Zhou YC and Chen XB: Tree shrew as a new animal model for the study
of lung cancer. Oncol Lett. 11:2091–2095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang F, Li YJ, Wang YL, Wang YJ, Dai JJ
and Hu M: Establishment of tree shrew osteoporosis model by
bilateral ovariectomy. J Kunming Medical Univ. 1:24–27. 2016.(In
Chinese).
|
|
22
|
Zhu MC, Li H, Gyanwali B, He GY, Qi CL,
Yang XM, Li ZH, Yao ZX, Wang Z and Tang A: Auditory brainstem
responses after electrolytic lesions in bilateral subdivisions of
the medial geniculate body of tree shrews. Neurol Sci.
38:1617–1628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chandra A, Wang L, Young T, Zhong L, Tseng
WJ, Levine MA, Cengel K, Liu XS, Zhang Y, Pignolo RJ and Qin L:
Proteasome inhibitor bortezomib is a novel therapeutic agent for
focal radiation-induced osteoporosis. FASEB J. 32:52–62. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka S, Haji M, Takayanagi R, Tanaka S,
Sugioka Y and Nawata H: 1,25-Dihydroxyvitamin D3 enhances the
enzymatic activity and expression of the messenger ribonucleic acid
for aromatase cytochrome P450 synergistically with dexamethasone
depending on the vitamin D receptor level in cultured human
osteoblasts. Endocrinology. 37:1860–1869. 1996. View Article : Google Scholar
|
|
25
|
Noronha-Matos JB and Correia-de-Sá P:
Mesenchymal stem cells ageing: Targeting the ‘purinome’ to promote
osteogenic differentiation and bone repair. J Cell Physiol.
231:1852–1861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harkema L, Youssef SA and de Bruin A:
Pathology of mouse models of accelerated aging. Vet Pathol.
53:366–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jee WS and Yao W: Overview: Animal models
of osteopenia and osteoporosis. J Musculoskel Neuron Interact.
1:193–207. 2001.
|
|
28
|
Li B, Zhang RP, Li JT, He BL, Zhen H, Wang
LM and Jiao JL: Measurement and analysis of anatomical parameter
values in tree shrews. Dongwuxue Yanjiu. 34:132–138. 2013.(In
Chinese). PubMed/NCBI
|
|
29
|
Han YY, Xu X, Lu CX, Kuang DX, Quan PF,
Wang WG, Sun XM, Li N and Dai JJ: Establishment of CT 3 D
visualization models and analysis of the skeletal system in adult
tree shrews. Acta Lab Anim Sci Sin. 25:153–159. 2017.(In
Chinese).
|
|
30
|
Kobayashi Y and Tokue A: Clinical
usefulness of blood PICP, PINP and ICTP concentrations as bone
metastasis markers in prostate cancer patients. Nihon Rinsho.
56:2072–2076. 1998.(In Japanese). PubMed/NCBI
|
|
31
|
Haarhaus M, Monier-Faugere MC, Magnusson P
and Malluche HH: Bone alkaline phosphatase isoforms in hemodialysis
patients with low versus non-low bone turnover: A diagnostic test
study. Am J Kidney Dis. 66:99–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao D, Wang J, Liu Y and Liu X:
Expressions and clinical significance of serum bone Gla-protein,
bone alkaline phosphatase and C-terminal telopeptide of type I
collagen in bone metabolism of patients with osteoporosis. Pak J
Med Sci. 31:91–94. 2015.PubMed/NCBI
|
|
33
|
Funck-Brentano T, Biver E, Chopin F,
Bouvard B, Coiffier G, Souberbielle JC, Garnero P and Roux C:
Clinical utility of serum bone turnover markers in postmenopausal
osteoporosis therapy monitoring: A systematic review. Semin
Arthritis Rheum. 41:157–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li XP, Liu XY, Fan B and Li XY: Early
changes in bone specific turnover markers during the healing
process of vertebral fracture. Chin J Osteoporosis Bone Miner Res.
8:305–311. 2015.(In Chinese).
|
|
35
|
Azuma Y, Kaji K, Katogi R, Takeshita S and
Kudo A: Tumor necrosis factor-alpha induces differentiation of and
bone resorption by osteoclasts. J Biol Chem. 275:4858–4864. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang MY, Shen C, An MF, Xie CQ, Wu X, Zhu
QQ, Sun B, Huang YP, Zhao YL, Wang XJ and Sheng J: Combined
treatment with Dendrobium candidum and black tea extract promotes
osteoprotective activity in ovariectomized estrogen deficient rats
and osteoclast formation. Life Sci. 220:31–41. 2018. View Article : Google Scholar
|
|
37
|
Khosla S, Melton LJ III and Riggs BL: The
unitary model for estrogen deficiency and the pathogenesis of
osteoporosis: Is a revision needed? J Bone Miner Res. 26:441–451.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jensen LB, Vestergaard P, Hermann AP, Gram
J, Eiken P, Abrahamsen B, Brot C, Kolthoff N, Sørensen OH,
Beck-Nielsen H, et al: Hormone replacement therapy dissociates fat
mass and bone mass, and tends to reduce weight gain in early
postmenopausal women: A randomized controlled 5-year clinical trial
of the danish osteoporosis prevention study. J Bone Miner Res.
18:333–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kanis JA, Brazier JE, Stevenson M, Calvert
NW and Lloyd JM: Treatment of established osteoporosis: A
systematic review and cost-utility analysis. Health Technol Asses.
6:1–146. 2002. View Article : Google Scholar
|
|
40
|
Tang XL, Zhu TY, Hung VW, Qin L, Wong CK,
Kun EW, Tam LS and Li EK: Increased organ damage associated with
deterioration in volumetric bone density and bone microarchitecture
in patients with systemic lupus erythematosus on longterm
glucocorticoid therapy. J Rheumatol. 39:1955–1963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Chen C, Bi X, Zhou C, Tao H, Ni C,
Yang P, Chen S, Ye M and Duan S: DNA methylation of CMTM3, SSTR2,
and MDFI genes in colorectal cancer. Gene. 630:1–7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakanishi R, Akiyama H, Kimura H, Otsuki
B, Shimizu M, Tsuboyama T and Nakamura T: Osteoblast-targeted
expression of Sfrp4 in mice results in low bone mass. J Bone Miner
Res. 23:271–277. 2010. View Article : Google Scholar
|
|
43
|
Santiago JL, Alizadeh BZ, Martínez A,
Espino L, de la Calle H, Fernández-Arquero M, Figueredo MA, de la
Concha EG, Roep BO, Koeleman BP and Urcelay E: Study of the
association between the CAPSL-IL7R locus and type 1 diabetes.
Diabetologia. 51:1653–1658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sporeno E, Barbato G, Graziani R, Pucci P,
Nitti G and Paonessa G: Production and structural characterization
of amino terminally histidine tagged human oncostatin M in E. coli.
Cytokine. 6:255–264. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nampei A, Hashimoto J, Hayashida K, Tsuboi
H, Shi K, Tsuji I, Miyashita H, Yamada T, Matsukawa N, Matsumoto M,
et al: Matrix extracellular phosphoglycoprotein (MEPE) is highly
expressed in osteocytes in human bone. J Bone Miner Metab.
22:176–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Im JY, Lee KW, Won KJ, Kim BK, Ban HS,
Yoon SH, Lee YJ, Kim YJ, Song KB and Won M: DNA damage-induced
apoptosis suppressor (DDIAS), a novel target of NFATc1, is
associated with cisplatin resistance in lung cancer. Biochim
Biophys Acta. 1863:40–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Krishnan V, Bryant HU and MacDougald OA:
Regulation of bone mass by Wnt signaling. J Clin Invest.
116:1202–1209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bin G, Cuifang W, Bo Z, Jing W, Jin J,
Xiaoyi T, Cong C, Yonggang C, Liping A, Jinglin M and Yayi X: Fluid
shear stress inhibits TNF-α-induced osteoblast apoptosis via ERK5
signaling pathway. Biochem Biophys Res Commun. 466:117–123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu SS, Liang QH, Liu Y, Cui RR, Yuan LQ
and Liao EY: Omentin-1 stimulates human osteoblast proliferation
through PI3K/Akt signal pathway. Int J Endocrinol. 2013:3689702013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shen C, Kim MR, Noh JM, Kim SJ, Ka SO, Kim
JH, Park BH and Park JH: Erratum to: Glucocorticoid suppresses
connexin 43 expression by inhibiting the Akt/mTORsignaling pathway
in osteoblasts. Calcif Tissue Int. 99:982016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li BX, Wang Y, Liu Y, Ma JX and Li YK:
Altered gene expression involved in insulin signaling pathway in
type II diabetic osteoporosis rats model. Endocrine. 43:136–146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rankin EB, Wu C, Khatri R, Wilson TL,
Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E and
Giaccia AJ: The HIF signaling pathway in osteoblasts directly
modulates erythropoiesis through the production of EPO. Cell.
149:63–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Komori T: Regulation of osteoblast
differentiation by Runx2. Adv Exp Med Biol. 658:43–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Komori T: Roles of Runx2 in Skeletal
Development. Adv Exp Med Biol. 962:83–93. 2017. View Article : Google Scholar : PubMed/NCBI
|