Introduction
Liver cirrhosis, the histological development of
regenerative nodules surrounded by fibrous bands after chronic
liver injury, can lead to portal hypertension and liver cancer,
with high incidence in developed countries (1). Liver cirrhosis in the long-term can
cause increased portal pressure, and cause the rupture of blood
vessels in the upper digestive tract, especially esophageal gastric
varices, resulting in acute bleeding, which is fierce and has fast
disease progression as well as high mortality (2,3).
Therefore, early local hemostasis treatment and lower portal
pressure are very important for patients. In the past, the most
common method for the treatment of this disease in clinical
practice was to use the three-chamber two-capsule tube to stop
bleeding, but the possibility to bleed again, and the high
incidence of adverse reactions and complications have limited its
use in clinical practice (4). At
present, the treatment of liver cirrhosis with gastrointestinal
hemorrhage mainly adopts emergency endoscopic therapies and drug
treatments. Due to the severity and rapid development of the
disease, finding suitable and effective treatment methods is very
important for patients (5).
According to the study of D'Amico et al
(6), which compared the efficacy and
safety between the emergency endoscopic therapy and the drug
treatment, the drug treatment has similar efficacy as the emergency
endoscopic therapy with fewer side-effects. Octreotide acetate, an
octapeptide derivative of synthetic natural somatostatin, is widely
used in clinical practice because of its certain control of the
blood flow of portal vein and liver, its low incidence of adverse
reactions, and because it is usually combined with other drugs due
to its short onset time (7).
Thrombin, a somatostatin analogue that directly participates in the
blood coagulation and promotes the conversion of soluble fibrinogen
in the plasma into insoluble fibrin for quick hemostasis, can
promote the production of epithelial cells and the healing of body
wound, working as a quick-acting local hemostatic (8,9). So far,
only a few clinical studies have been reported on the combination
of octreotide acetate and thrombin in the treatment of patients
with liver cirrhosis and gastrointestinal hemorrhage (10,11).
Therefore, this study investigated the clinical efficacy of
octreotide acetate combined with thrombin.
Patients and methods
Patient data
A retrospective analysis of 157 patients with liver
cirrhosis complicated with gastrointestinal hemorrhage, admitted to
the Weifang People's Hospital (Weifang, China), from March 2012 to
September 2014, was performed. Seventy-four patients treated with
octreotide acetate were set as the octreotide group, aged from 38
to 71 years, with a mean age of 57.37±3.83 years, and an average
bleeding volume of 643.14±40.71 ml; while 83 patients treated with
octreotide acetate combined with thrombin were set as the
combination group, aged from 35 to 73 years, with a mean age of
56.18±4.04 years, and an average bleeding volume of 636.32±41.84
ml. Inclusion criteria: patients diagnosed with liver cirrhosis
with upper gastrointestinal hemorrhage; patients with a complete
medical record; patients receiving no relevant treatment in other
hospitals. Exclusion criteria: patients with allergic reactions and
contraindications for the drugs used in this study; patients who
were transferred or died during hospitalization; patients during
pregnancy or lactation; patients with gastrointestinal bleeding
caused by other diseases; patients with blood diseases or tumors;
patients with a communication disorder or cognitive disorder. The
study was approved by the Ethics Committee of Weifang People's
Hospital. Patients who participated in this research had complete
clinical data and cooperated with the medical staff to complete the
relevant medical treatment. Signed informed consents were obtained
from the patients and/or their guardians.
Methods
Patients were given blood supplement immediately
after admission and were asked to follow routine fasting. Then,
conventional symptomatic supportive treatments, such as, treatment
for acid suppression, liver protection, anti-shock, and maintenance
of water and electrolyte balance, were performed to maintain normal
body consumption. Patients in the octreotide group were given 0.1
mg octreotide acetate by injection (Chengdu Shengnuo Biopharm Co.,
Ltd., Chengdu, China; medical product permitted by the China Food
and Drug Administration: H20153159), combined with 20 ml of 0.9%
sodium chloride, continuously pumped intravenously at the speed of
0.025–0.05 mg/h (the injection was continuously pumped until 48–72
h after the hemostasis); in the combination group, patients were
injected with a mixture of 0.1 mg octreotide acetate and 2,000
units of thrombin (Penglai Nuokang Pharmaceutical Co., Ltd.,
Penglai, China; medical product permitted by the China Food and
Drug Administration: H20051840) and 20 ml saline through gastric
tube bolus or oral administration once every 4–6 h. The treatment
for both groups continued for 3 days.
Regular basic nursing was performed and the fasting
time and bedtime of patients were carefully guided to prevent
hemorrhoids. Vital signs, such as, the patient's blood pressure,
pulse, and breathing were closely observed and recorded.
Conditions, such as, nausea, color-change of vomit from brown to
bright red, and increased incidence of black and thin stool, it was
required to be reported to the nurse immediately, in order to get
adjusted treatment and nursing measures. Patients and their family
members were informed in detail about the drug name, drug function,
dosage, time and method of administration, and effective
communication with the patient was required to reduce patients'
anxiety and depression and to improve patients' satisfaction toward
nursing. All patients were directed to avoid irritating food and to
attend follow-up regularly.
Observation indicators and standards
Observation indicators
Factors, including the average hemostasis time (the
time from the start of medication to successful hemostasis), the
hospitalization time, the amount of blood transfusion during
hospitalization, the efficacy of hemostasis at 24 h after
treatment, the visual analog scale (VAS) score before treatment,
and at 24 and 72 h after treatment, were compared between the
octreotide and the combination group.
Evaluation of the treatment efficacy
(12), and the VAS scoring standard
(13)
Significant efficacy: no hematemesis, black stools,
or abnormal blood routine examination results within 24 h after
treatment was considered as successful hemostasis; certain
efficacy: relieved hematemesis, black stools, and
not-obviously-abnormal blood routine examination results within 24
h after treatment was considered as effective hemostasis; no
efficacy: aggravated or not-relieved hematemesis, black stools, and
abnormal blood routine examination results within 24 h after
treatment was considered as ineffective hemostasis. Overall
effective rate = (number of patients with significant efficacy +
number of patients with certain efficacy)/total patient number of
the group ×100%. VAS score: the score ranges from 0 to 10 points,
and the higher the score, the higher the patient's pain index
is.
Statistical methods
Statistical analysis was carried out using SPSS 17.1
software (Shanghai Yuchuang Network Technology Co., Ltd., Shanghai,
China). Count data were expressed as a percentage [n (%)], and
their difference between the two groups was measured by Chi-square
test. Measurement data were expressed as the mean ± standard
deviation and their difference between the two groups was compared
by t-test. Analysis of variance of repeated measures, with Least
Significant Difference test, was used to compare the differences
between different time-points. P<0.05 was considered to indicate
a statistically significant difference.
Results
Comparison of clinical data of
patients
Considering the accuracy and credibility of the
experimental results, factors such as, sex, age, weight, anemia,
hematemesis, alcohol abuse, and bleeding location, were compared
between the two groups. The difference between the two groups was
not found to be statistically significant (P>0.05), indicating
that the two groups are comparable to each other. Table I shows the basic information of the
patients.
 | Table I.Basic information of patients in the
octreotide and the combination group. |
Table I.
Basic information of patients in the
octreotide and the combination group.
| Characteristics | Octreotide group
(n=74) | Combination group
(n=83) | Chi-square test | P-value |
|---|
| Sex |
|
| 0.083 | 0.773 |
| Male | 48 (64.86) | 52 (62.65) |
|
|
|
Female | 26 (35.14) | 31 (37.35) |
|
|
| Age (years) |
|
| 2.412 | 0.120 |
| ≤45 | 33 (44.59) | 27 (32.53) |
|
|
|
>45 | 41 (55.41) | 56 (67.47) |
|
|
| Weight (kg) |
|
| 0.004 | 0.947 |
| ≤60 | 29 (39.19) | 39 (46.99) |
|
|
|
>60 | 45 (60.81) | 44 (53.01) |
|
|
| Anemia |
|
| 2.575 | 0.109 |
| Yes | 56 (75.68) | 53 (63.86) |
|
|
| No | 18 (24.32) | 30 (36.14) |
|
|
| Hematemesis |
|
| 0.716 | 0.398 |
| Yes | 27 (36.49) | 25 (30.12) |
|
|
| No | 47 (63.51) | 58 (69.88) |
|
|
| Alcohol abuse |
|
| 0.801 | 0.371 |
| Yes | 31 (41.89) | 29 (34.94) |
|
|
| No | 43 (58.11) | 54 (65.06) |
|
|
| Bleeding
location |
|
| 0.117 | 0.733 |
|
Esophageal variceal | 35 (47.30) | 37 (44.58) |
|
|
| Gastric
fundus varices | 39 (52.70) | 46 (55.42) |
|
|
Comparison of mean hemostasis time and
hospitalization time between the octreotide and the combination
group
The hemostasis time of patients in the octreotide
group was significantly higher than that in the combination group,
and the difference was statistically significant (t=17.340,
P<0.001). The hospitalization time of the octreotide group was
significantly higher than that of the combination group, and the
difference was statistically significant (t=8.100, P<0.001)
(Table II).
 | Table II.Comparison of mean hemostasis time and
hospitalization time between the octreotide and the combination
group. |
Table II.
Comparison of mean hemostasis time and
hospitalization time between the octreotide and the combination
group.
| Index | Octreotide group
(n=74) | Combination group
(n=83) | t value | P-value |
|---|
| Mean hemostasis time
(h) | 29.54±3.28 | 21.18±2.76 | 17.340 | <0.001 |
| Hospitalization time
(days) | 11.02±3.13 |
7.27±2.67 |
8.100 | <0.001 |
Comparison of blood transfusion during
hospitalization between the octreotide and the combination
group
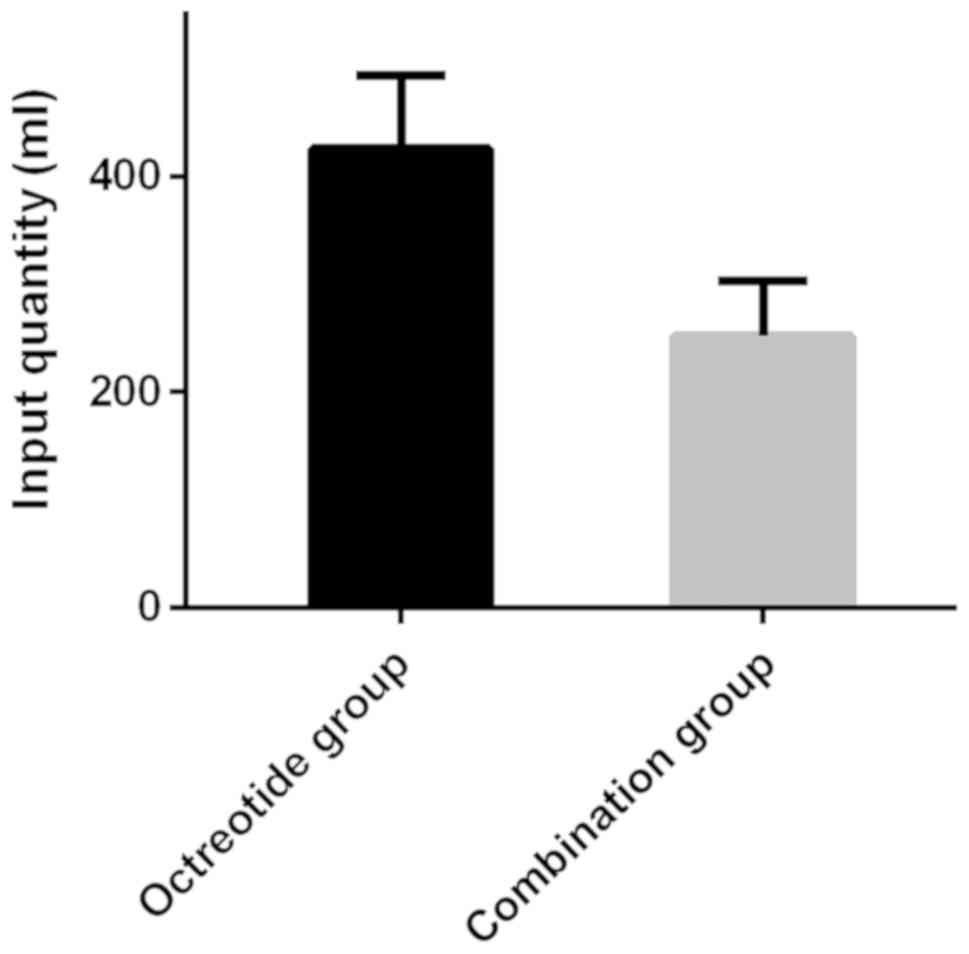
The blood transfusion volume of the octreotide group
(425.68±68.35 ml) was significantly higher than that of the
combination group (251.41±51.23 ml), and the difference was
statistically significant (t=18.200, P<0.001) (Fig. 1).
Comparison of hemostatic efficacy
between the octreotide and the combination group after
treatment
The overall effective rate of the combination group
was 97.59% (58 patients with significant efficacy, 23 patients with
certain efficacy, 2 patients with no efficacy); and the overall
effective rate of the octreotide group was 89.19% (47 patients with
significant efficacy, 19 patients with certain efficacy, 8 patients
with no efficacy. The difference in the overall effective rate
between the two groups was statistically significant (P<0.05)
(Table III).
 | Table III.Comparison of the overall effective
rate between the octreotide and the combination group after
treatment. |
Table III.
Comparison of the overall effective
rate between the octreotide and the combination group after
treatment.
| Groups | Significant
efficacy | Certain efficacy | No efficacy | Overall effective
rate |
|---|
| Octreotide group
(n=74) | 47 (63.51) | 19 (25.68) | 8 (10.81) | 66 (89.19) |
| Combination group
(n=83) | 58 (69.88) | 23 (27.71) | 2 (2.41) | 81 (97.59) |
| Chi-square
test | – | – | – | 4.630 |
| P-value | – | – | – | 0.031 |
Comparison of VAS scores between the
octreotide and the combination group before and after
treatment
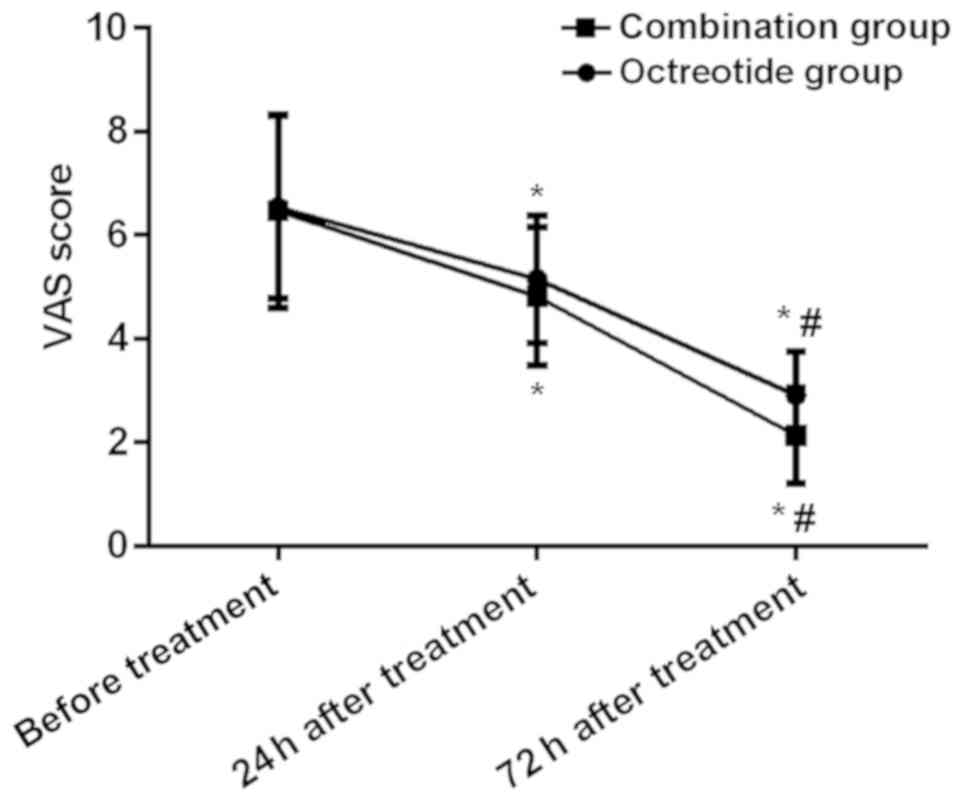
The VAS scores of the combination group at 24 and 72
h after treatment were lower than those of the octreotide group,
and the differences were statistically significant (P<0.05); the
VAS scores of both the octreotide and the combination group at 24
and 72 h after treatment were significantly lower than those before
treatment, and the VAS scores at 72 h after treatment were
significantly lower than that at 24 h after treatment. The
differences were statistically significant (P<0.05) (Table IV and Fig. 2).
 | Table IV.Comparison of VAS scores between the
octreotide and the combination group before and after
treatment. |
Table IV.
Comparison of VAS scores between the
octreotide and the combination group before and after
treatment.
| Groups | Octreotide group
(n=74) | Combination group
(n=83) | t value | P-value |
|---|
| Before
treatment | 6.54±1.76 | 6.47±1.87 | 0.241 | 0.810 |
| 24 h after
treatment |
5.15±1.24a |
4.82±1.33a | 2.087 | 0.039 |
| 72 h after
treatment |
2.91±0.84a,b |
2.14±0.92a,b | 2.054 | <0.001 |
| F value | 139.400 | 194.600 |
|
|
| P-value | <0.001 | <0.001 |
|
|
Discussion
Liver cirrhosis is a diffuse liver damage caused by
repeated incidence of liver disease due to one or more etiologies,
leading to dysfunction of normal liver lobules and vascular
structures, and causing liver fibrosis (14). Patients with liver cirrhosis, which
is a common and serious disease in the clinic, have a very high
risk of liver cancer, with liver cancer incidence of 2–5%/year
(15,16). As the society is becoming more
modernized, the cause of liver cirrhosis has increased, leading to
an increasing trend in the number of patients with liver cirrhosis.
As a serious complication of liver cirrhosis, portal hypertension
with symptoms, such as, blocked blood flow or increased blood flow,
and increased hydrostatic pressure in the portal vein, may cause
esophageal and gastric varices bleeding, with an incidence of
~23.13% (17–19). According to the statistics by Lyles
et al (20), liver cirrhosis
with gastrointestinal hemorrhage has a mortality rate of ~8.4%,
which seriously jeopardizes the life of patients.
This study retrospectively analyzed 157 patients
with liver cirrhosis complicated with gastrointestinal hemorrhage
that were admitted to Weifang People's Hospital, from March 2012 to
September 2014. Seventy-four patients were treated with octreotide
acetate and 83 patients were treated with octreotide acetate and
thrombin, and then comprehensively compared factors between the two
groups, such as, the treatment efficacy, mean hemostasis time,
hospitalization time, blood transfusion volume during
hospitalization, and VAS score, were investigated. The results
revealed that the mean hemostasis time and hospitalization time of
the combination group were significantly shorter than the that of
the octreotide group. Octreotide and thrombin can increase the
contractile force of the low esophageal sphincter, can assist to
reduce the reflux of gastric contents, protect the esophageal
mucosa, promote platelet aggregation, and increase the coagulation
rate (21).
DeLaney and Greene (22), in their study on the efficacy of
octreotide and thrombin treatment in patients with upper
gastrointestinal bleeding, have pointed out that the average
hemostasis time and symptom-relieving time of patients treated with
octreotide acetate and thrombin are significantly reduced, which
further corroborates our research. The present study found that the
patients in the octreotide group have significantly more blood
transfusion during hospitalization than those in the combination
group; the overall effective rate of combination group (97.59%),
was found to be significantly greater than that of the octreotide
group (89.19%). The above differences were all statistically
significant. Since octreotide acetate can reduce the production and
release of vasodilators (such as, glucagon, vasoactive intestinal
peptide), it can reduce the total blood flow to the liver, reduce
the blood flow of the portal vein, and keep the human hemodynamics
relatively stable (23).
The efficacy of octreotide was discovered by Zhou
et al (24) who reported on
the hemostasis efficacy of lansoprazole combined with different
dosages of octreotide in the treatment of liver cirrhosis with
upper gastrointestinal bleeding. However, due to the
pharmacological properties of octreotide acetate, such as, the
short onset time and some adverse reactions, octreotide was not
recommended to be used alone. Thrombin, a somatostatin analogue
that can quickly convert the blood acting on the surface of the
lesion into a stable blood clot after local application and have a
relatively small incidence of adverse reactions, is suitable for
the hemostasis of small blood vessels, capillaries and parenchymal
viscera in which it is difficult to achieve hemostasis by ligation
(25).
Friedlander et al (26) have reported that the combination of
octreotide acetate and somatostatin significantly improves the
recovery of white blood cells, blood glucose and blood calcium in
patients with pancreatitis, compared to the single use of
octreotide acetate or somatostatin, and achieves a reduction in the
incidence of complications, which is supportive of the results of
the present study. No literature exists on lower VAS scores of
patients treated with octreotide acetate combined with somatostatin
compared to those of patients treated with octreotide acetate
alone, which is therefore of research value to be further studied.
The severe liver damage and obvious systemic symptoms, such as,
jaundice, fatigue and poor coagulation function may worsen the
depression of patients with liver cirrhosis, so, medical staff
should promptly give a psychological guide to patients and
patiently respond to questions from patients and/or their families
(27). Since upper gastrointestinal
bleeding may cause shock or even death of the patient, the medical
staff need to pay attention to the patient's vital signs in a
timely manner, strengthen the basic nursing, implement predictive
care, and prepare a series of nursing interventions, such as,
emergency care for possible bleeding (28). Good communication between nurses and
patients and nursing interventions, if combined with drug
treatment, will benefit patients' compliance and rehabilitation,
worthy of clinical promotion and application.
In this experiment, the sample size was relatively
small due to the small number of patients with liver cirrhosis
complicated with upper gastrointestinal hemorrhage in the above
hospital, and therefore, the results might have some contingency.
Thus, the authors will continue the follow-up for a longer period
of time.
In summary, octreotide acetate and thrombin in the
treatment of liver cirrhosis complicated with gastrointestinal
bleeding can relieve the clinical symptoms of patients and achieve
good efficacy and high safety, worthy of promotion in clinical
practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW was responsible for the writing of the
manuscript. XW and JZhang recorded and analyzed the observation
indicators. YL and JZhao acquired and interpreted the patients'
general data. XW assisted with the evaluation of the treatment
efficacy and the VAS scoring. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China). Patients who
participated in this research had complete clinical data and
cooperated with the medical staff to complete the relevant medical
treatment. Signed informed consents were obtained from the patients
and/or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fukui H, Saito H, Ueno Y, Uto H, Obara K,
Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, et al:
Evidence-based clinical practice guidelines for liver cirrhosis
2015. J Gastroenterol. 51:629–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancela E, Ministro P, Peixoto P, Sadio A,
Castanheira A, Silva A and Caldas A: Concomitant life threatening
lesions in a cirrhotic patient: The value of endoscopic treatment.
Rev Esp Enferm Dig. 102:617–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abid S, Jafri W, Hamid S, Salih M, Azam Z,
Mumtaz K, Shah HA and Abbas Z: Terlipressin vs. octreotide in
bleeding esophageal varices as an adjuvant therapy with endoscopic
band ligation: A randomized double-blind placebo-controlled trial.
Am J Gastroenterol. 104:617–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escorsell À, Pavel O, Cárdenas A, Morillas
R, Llop E, Villanueva C, Garcia-Pagán JC and Bosch J; Variceal
Bleeding Study Group, : Esophageal balloon tamponade versus
esophageal stent in controlling acute refractory variceal bleeding:
A multicenter randomized, controlled trial. Hepatology.
63:1957–1967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drastich P, Lata J, Petrtyl J, Bruha R,
Prochazka V, Vanasek T, Zdenek P, Skibova J, Hucl T and Spicak J:
Endoscopic variceal band ligation compared with propranolol for
prophylaxis of first variceal bleeding. Ann Hepatol. 10:142–149.
2011.PubMed/NCBI
|
|
6
|
D'Amico G, Pietrosi G, Tarantino I and
Pagliaro L: Emergency sclerotherapy versus vasoactive drugs for
variceal bleeding in cirrhosis: A Cochrane meta-analysis.
Gastroenterology. 124:1277–1291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garbuzenko DV: Current approaches to the
management of patients with liver cirrhosis who have acute
esophageal variceal bleeding. Curr Med Res Opin. 32:467–475. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crean S, Michels SL, Moschella K and
Reynolds MW: Bovine thrombin safety reporting: An example of study
design and publication bias. J Surg Res. 158:77–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ballard JL, Weaver FA, Singla NK, Chapman
WC and Alexander WA: Safety and immunogenicity observations pooled
from eight clinical trials of recombinant human thrombin. J Am Coll
Surg. 210:199–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ludwig D, Schädel S, Brüning A, Schiefer B
and Stange EF: 48-hour hemodynamic effects of octreotide on
postprandial splanchnic hyperemia in patients with liver cirrhosis
and portal hypertension: Double-blind, placebo-controlled study.
Dig Dis Sci. 45:1019–1027. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaireti R, Rajani R, Bergquist A, Melin
T, Friis-Liby IL, Kapraali M, Kechagias S, Lindahl TL and Almer S:
Increased thrombin generation in splanchnic vein thrombosis is
related to the presence of liver cirrhosis and not to the
thrombotic event. Thromb Res. 134:455–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanai M, Hamada A, Endo Y, Takeda Y,
Yamakawa M, Nishikawa H and Torii A: Efficacy of argon plasma
coagulation in nonvariceal upper gastrointestinal bleeding.
Endoscopy. 36:1085–1088. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boonstra AM, Schiphorst Preuper HR, Balk
GA and Stewart RE: Cut-off points for mild, moderate, and severe
pain on the visual analogue scale for pain in patients with chronic
musculoskeletal pain. Pain. 155:2545–2550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge PS and Runyon BA: Treatment of patients
with cirrhosis. N Engl J Med. 375:767–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Liu W, Chen YH, Fan CL, Dong PL, Wei
FL, Li B, Chen DX and Ding HG: Antiviral drug resistance increases
hepatocellular carcinoma: A prospective decompensated cirrhosis
cohort study. World J Gastroenterol. 19:8373–8381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo LH and Xu HX: Contrast-enhanced
ultrasound in the diagnosis of hepatocellular carcinoma and
intrahepatic cholangiocarcinoma: Controversy over the ASSLD
guideline. BioMed Res Int. 2015:3491722015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schuppan D and Afdhal NH: Liver cirrhosis.
Lancet. 371:838–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J,
Peng Y, Li J, Deng H and Guo X: Albumin-bilirubin score for
predicting the in-hospital mortality of acute upper
gastrointestinal bleeding in liver cirrhosis: A retrospective
study. Turk J Gastroenterol. 27:180–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romcea AA, Tanţău M, Seicean A and Pascu
O: The etiology of upper gastrointestinal bleeding in cirrhotic
patients. Clujul Med. 86:21–23. 2013.PubMed/NCBI
|
|
20
|
Lyles T, Elliott A and Rockey DC: A risk
scoring system to predict in-hospital mortality in patients with
cirrhosis presenting with upper gastrointestinal bleeding. J Clin
Gastroenterol. 48:712–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tripathi D, Stanley AJ, Hayes PC, Patch D,
Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M,
et al Clinical Services and Standards Committee of the British
Society of Gastroenterology, : U.K. guidelines on the management of
variceal haemorrhage in cirrhotic patients. Gut. 64:1680–1704.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DeLaney M and Greene CJ: Emergency
department evaluation and management of patients with upper
gastrointestinal bleeding. Emerg Med Pract. 17:1–19.
2015.PubMed/NCBI
|
|
23
|
Mocarzel LO, Bicca J, Jarske L, Oliveira
T, Lanzieri P, Gismondi R and Ribeiro ML: Cirrhotic cardiomyopathy:
Another case of a successful approach to treatment of hepatorenal
syndrome. Case Rep Gastroenterol. 10:531–537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou DX, Zhou HB, Wang Q, Zou SS, Wang H
and Hu HP: The effectiveness of the treatment of octreotide on
chylous ascites after liver cirrhosis. Dig Dis Sci. 54:1783–1788.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu X, Tian P, Xu GJ, Sun XL and Ma XL:
Thrombin-based hemostatic agent in primary total knee arthroplasty.
J Knee Surg. 30:121–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Friedlander TW, Weinberg VK, Small EJ,
Sharib J, Harzstark AL, Lin AM, Fong L and Ryan CJ: Effect of the
somatostatin analog octreotide acetate on circulating insulin-like
growth factor-1 and related peptides in patients with
non-metastatic castration-resistant prostate cancer: Results of a
phase II study. Urol Oncol. 30:408–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SH, Oh EG and Lee WH: Symptom
experience, psychological distress, and quality of life in Korean
patients with liver cirrhosis: A cross-sectional survey. Int J Nurs
Stud. 43:1047–1056. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang F, Xiang ML and Liu YM: Thrombin in
combination with intensive nursing in treating upper
gastrointestinal bleeding in children. J Biol Regul Homeost Agents.
30:491–495. 2016.PubMed/NCBI
|