Introduction
Esophageal cancer (EC) is one of the most common
cancer types of the digestive tract worldwide and remains one of
the fourth leading causes of cancer-associated mortality in China
(1,2). Esophageal squamous cell carcinoma
(ESCC) and esophageal adenocarcinoma (EA) are the two major
histological subtypes of EC. In China, ESCC accounts for ~90% of
all cases of EC, whereas EA is the predominant subtype in Western
countries (3–5). Surgery is considered to be the standard
treatment for this localized disease and is the best
single-modality therapy for potentially this resectable disease
(2). However, most patients with EC
already have locally advanced or metastatic disease at the time of
diagnosis. Radiotherapy (RT) combined with chemotherapy, with or
without surgery, has become the major treatment (2).
Cigarette smoking is well known to promote the
development of EC, irrespective of the pathological type (6,7). Most
studies on the subject have revealed that smoking is a risk factor
for the occurrence of ESCC (8–12). A
review indicated that cigarette smoking induces a more malignant
tumor phenotype by increasing the cell proliferation, migration and
invasion, as well as angiogenesis, and by activating cellular
pro-survival pathways (13).
However, few studies have focused on the effect of smoking on EC
patient survival outcomes. In a study by Wang et al
(14), the patients underwent
esophagectomy without any pre-operative therapy, and smoking was
identified to be an independent prognostic factor for overall
survival (OS) [hazard ratio (HR)=2.186; 95% confidence interval
(CI), 1.309–3.650; P=0.003] and disease-free survival (DFS)
(HR=2.471; 95% CI, 1.467–4.163; P=0.001). However, another study
from Southern China indicated that smoking history only affected
treatment outcomes in those ESCC patients receiving surgery plus
chemotherapy, and not in those receiving surgery alone (15). Furthermore, one study from Shandong
province reported a negative result, namely that neither smoking
nor drinking affected the 2-year OS or DFS of ESCC patients
(16). Considering these
inconsistent results, the impact of smoking on the survival of ESCC
remains elusive and the patients mainly received surgery in
previous studies (14–16).
Smoking was reported as an independent predictor of
a pathological complete response to neoadjuvant chemoradiotherapy
in patients with ESCC (17). That
study performed no further analysis of the impact of smoking on
long-term survival. A recent study identified cigarette smoking as
a significant and independent poor prognostic risk factor for OS
among those patients with ESCC receiving definitive RT or
concurrent chemoradiotherapy, with or without esophagectomy
(18). In addition, smoking was
demonstrated to have an unfavorable impact on tumor control by
irradiation in animal models, by exacerbating tissue hypoxia
(19,20). Tumor hypoxia is well known to
influence the reaction to radiation and chemotherapy (21,22). In
the present study, it was speculated that smoking not only induces
malignant transformation of normal cells, but may also change
tumor-associated genes or associated metabolic activity, thus
making tumor cells more aggressive and less sensitive to RT and
chemotherapy. Therefore, RT with or without chemotherapy, as the
major treatment for those patients with local advanced ESCC, is
probably affected by smoking to a greater extent than by surgery.
Therefore, the aim of the present study was to elucidate the effect
of a history of cigarette smoking on the survival of patients with
ESCC receiving RT, with or without chemotherapy.
Patients and methods
Patients
The medical records of the eligible patients, who
were hospitalized at the Sun Yat-sen University Cancer Center
(Guangzhou, China) between January 2007 and December 2013, were
retrospectively reviewed. Patients were eligible if their biopsy
specimens were histologically confirmed as ESCC and if they had no
distant metastasis, were previously untreated and received RT with
or without chemotherapy after diagnosis. Essential pre-treatment
assessments were the review of the complete patient history,
including a family history of cancer and lifestyle behavior;
physical examination; hematology and biochemistry profiles;
computed tomography of the neck, chest and upper abdomen; and
endoscopic ultrasound. Patients who had distant metastasis,
received surgery or had incomplete data were excluded.
The information retrieved from the medical records
included age, sex, pathological type, smoking status at diagnosis
(never/current/former smoker), number of cigarettes smoked per day,
number of years of smoking and alcohol drinking status at diagnosis
(yes vs. no). The patients' smoking status was defined as follows:
Never smokers, patients who had never smoked prior to treatment;
current smokers, patients who smoked prior to treatment or had
stopped for <1 year; former smokers, patients who had stopped
smoking for at least 1 year prior to treatment. The tumor locations
included cervical, upper third of thoracic esophagus, middle third
of thoracic esophagus and lower third of thoracic esophagus. All
patients were re-staged according to the sixth edition of the Union
for International Cancer Control (UICC)/American Joint Committee on
Cancer (AJCC) staging system for ESCC (23).
Treatment
The treatment strategy for the patients with ESCC
was discussed by a multidisciplinary team, which included surgeons,
a physician, a radiation specialist, a radiologist and a
pathologist. The final treatment choice was made according to the
National Comprehensive Cancer Network (NCCN) guidelines and the
overall condition of the patient, which included the physical
performance and the economic status (2). All of the patients included received
RT. The radiation techniques and dose prescriptions were in
accordance with those described previously (24,25). The
chemotherapy consisted of fluoropyrimidine- or taxane-based
regimens [cisplatin combined with 5-fluorouracil (5-FU) or
cisplatin combined with docetaxel] every 3 weeks or weekly
(26,27). A total of 423 out of 479 (88.3%)
patients received RT plus chemotherapy. Among the patients who
received chemotherapy, 110 received chemotherapy containing 5-FU,
while 323 received chemotherapy containing docetaxel.
Follow-up
Patients were followed up at regular intervals after
completing their treatment. The specific follow-up intervals were
one month after completion of treatment, then every 2 months during
the first 6 months, every 3 months for the next 6 months, every 4
months during the second year and every 6 months thereafter.
Study endpoints
The endpoint of the present study was the OS,
defined as the time from treatment to death resulting from any
cause. First, the association between survival and the smoking
status at diagnosis (never smokers, former smokers and current
smokers) was assessed. Second, the cumulative effects of smoking in
terms of pack-years (PYs) were assessed. The PYs were calculated by
multiplying the number of packs of cigarettes smoked per day by the
number of years the patient had smoked.
Statistical analysis
Survivals rates were estimated using the
Kaplan-Meier method and compared between subgroups using the
log-rank test. Univariate analyses were performed to determine
variables associated with OS. Multivariate analyses were performed
using the Cox proportional hazards model. Comparisons of
demographic, clinical and pathological variables between subgroups
were performed using χ2 statistics, Fisher's exact test
or the Kruskal-Wallis test. Continuous variables were assessed
using restricted cubic splines (RCS) nested with Cox models using
the RCS macro of the SAS software 9.1 (SAS Institute, Cary, NC,
USA) and the cutoff scores of the continuous variables were
subsequently selected based on receiver operating characteristic
curve analyses. A two-sided P<0.05 was considered to indicate
statistical significance. Statistical analyses were performed using
SPSS 22.0 (IBM Corp., Armonk, NY, USA) and SAS software 9.1.
Results
Patient characteristics, treatment and
outcomes
A total of 479 patients with ESCC were included. The
clinical stage distribution according to the sixth edition of the
UICC/AJCC staging system for the 479 patients was as follows: Stage
II, n=75 (15.6%); stage III, n=227 (47.4%); and stage IV, n=177
(37.0%). Overall, 56/479 patients (11.7%) were treated with RT
alone and 423/479 (88.3%) received RT plus chemotherapy. Of these
423 patients, 336 (70.1%) received concurrent chemotherapy, 52
(10.9%) received a combination of induction and concurrent
chemotherapy, and 35 patients (7.3%) received a combination of
concurrent and adjuvant chemotherapy. With respect to RT, 72
patients (15.0%) were treated with two-dimensional RT (2DRT), 298
patients (62.2%) with three-dimensional conformal RT (3DCRT) and
109 patients (22.8%) with intensity-modulated RT (IMRT).
Within a median follow-up duration of 27.89 months
(range, 0.8–116.3 months), 286 patients died. The 1-, 2-, 3- and
5-year survival rates were 73.3, 56.0, 47.4 and 39.5%,
respectively.
Patient characteristics
The percentage of never smokers, former smokers and
current smokers in the entire cohort was 35.7% (171/479), 9.0%
(43/479) and 55.3% (265/479), respectively. When the entire
population was stratified by the smoking status, no significant
differences were identified in terms of the T-stage, N-stage,
M-stage, clinical stage and RT techniques between the different
groups. However, significant differences were observed in terms of
age, sex, drinking status, tumor grade, tumor location and
chemotherapy approach. Male patients were more frequent among the
former and current smokers (Table
I).
 | Table I.Demographic and clinicopathological
characteristics of patients with esophageal squamous cell carcinoma
by status of smoking. |
Table I.
Demographic and clinicopathological
characteristics of patients with esophageal squamous cell carcinoma
by status of smoking.
|
Characteristics | All (n=479) | Never smoker
(n=171) | Former smoker
(n=43) | Current smoker
(n=265) | P-value |
|---|
| Age (years) |
|
|
|
| 0.002 |
|
<60 | 234 (48.9) | 79 (46.2) | 11 (25.6) | 144 (54.3) |
|
|
≥60 | 245 (51.1) | 92 (53.8) | 32 (74.4) | 121 (45.7) |
|
| Sex |
|
|
|
| <0.001 |
|
Male | 379 (79.1) | 75 (43.9) | 42 (97.7) | 262 (98.9) |
|
|
Female | 100 (20.9) | 96 (56.1) | 1 (2.3) | 3 (1.1) |
|
| Drinking |
|
|
|
| <0.001 |
| No | 294 (61.4) | 156 (91.2) | 22 (51.2) | 116 (43.8) |
|
|
Yes | 185 (38.6) | 15 (8.8) | 21 (48.8) | 149 (56.2) |
|
| Tumor grade |
|
|
|
| 0.020 |
|
High | 124 (25.9) | 59 (34.5) | 11 (25.6) | 54 (20.4) |
|
|
Intermediate | 278 (58.0) | 86 (50.3) | 27 (62.8) | 165 (62.3) |
|
|
Low | 77 (16.1) | 26 (15.2) | 5 (11.6) | 46 (17.3) |
|
| Tumor location |
|
|
|
| 0.040 |
|
Cervical | 63 (13.2) | 27 (15.8) | 4 (9.3) | 32 (12.1) |
|
|
Uppera | 140 (29.2) | 55 (32.2) | 12 (27.9) | 73 (27.5) |
|
|
Middleb | 242 (50.5) | 80 (46.7) | 18 (41.9) | 144 (54.4) |
|
|
Lowerc | 34 (7.1) | 9 (5.3) | 9 (20.9) | 16 (6.0) |
|
| T-stage |
|
|
|
| 0.768 |
| T2 | 82 (17.1) | 31 (18.1) | 6 (14.0) | 45 (17.0) |
|
| T3 | 263 (54.9) | 95 (55.6) | 27 (62.8) | 141 (53.2) |
|
| T4 | 134 (28.0) | 45 (26.3) | 10 (23.2) | 79 (29.8) |
|
| N-stage |
|
|
|
| 0.162 |
| N0 | 53 (11.1) | 25 (14.6) | 3 (7.0) | 25 (9.4) |
|
| N1 | 426 (88.9) | 146 (85.4) | 40 (93.0) | 240 (90.6) |
|
| M-stage |
|
|
|
| 0.797 |
| M0 | 303 (63.3) | 109 (63.7) | 29 (67.4) | 165 (62.3) |
|
|
M1a | 176 (36.7) | 62 (36.3) | 14 (32.6) | 100 (37.7) |
|
| Clinical stage |
|
|
|
| 0.629 |
| II | 75 (15.6) | 32 (18.7) | 7 (16.3) | 36 (13.6) |
|
|
III | 227 (47.4) | 79 (46.2) | 22 (51.2) | 126 (47.5) |
|
| IV | 177 (37.0) | 60 (35.1) | 14 (32.5) | 103 (38.9) |
|
| Treatment |
|
|
|
| 0.007 |
| RT
alone | 56 (11.7) | 27 (15.8) | 11 (25.6) | 18 (6.8) |
|
|
CCRT | 336 (70.1) | 114 (66.7) | 27 (62.8) | 195 (73.6) |
|
|
IC+CCRT | 52 (10.9) | 19 (11.1) | 3 (7.0) | 30 (11.3) |
|
|
CCRT+AC | 35 (7.3) | 11 (6.4) | 2 (4.6) | 22 (8.3) |
|
| RT technique |
|
|
|
| 0.151 |
| 2D
RT | 72 (15.0) | 17 (9.9) | 7 (16.3) | 48 (18.1) |
|
|
3DCRT | 298 (62.2) | 108 (63.2) | 27 (62.8) | 163 (61.5) |
|
|
IMRT | 109 (22.8) | 46 (26.9) | 9 (20.9) | 54 (20.4) |
|
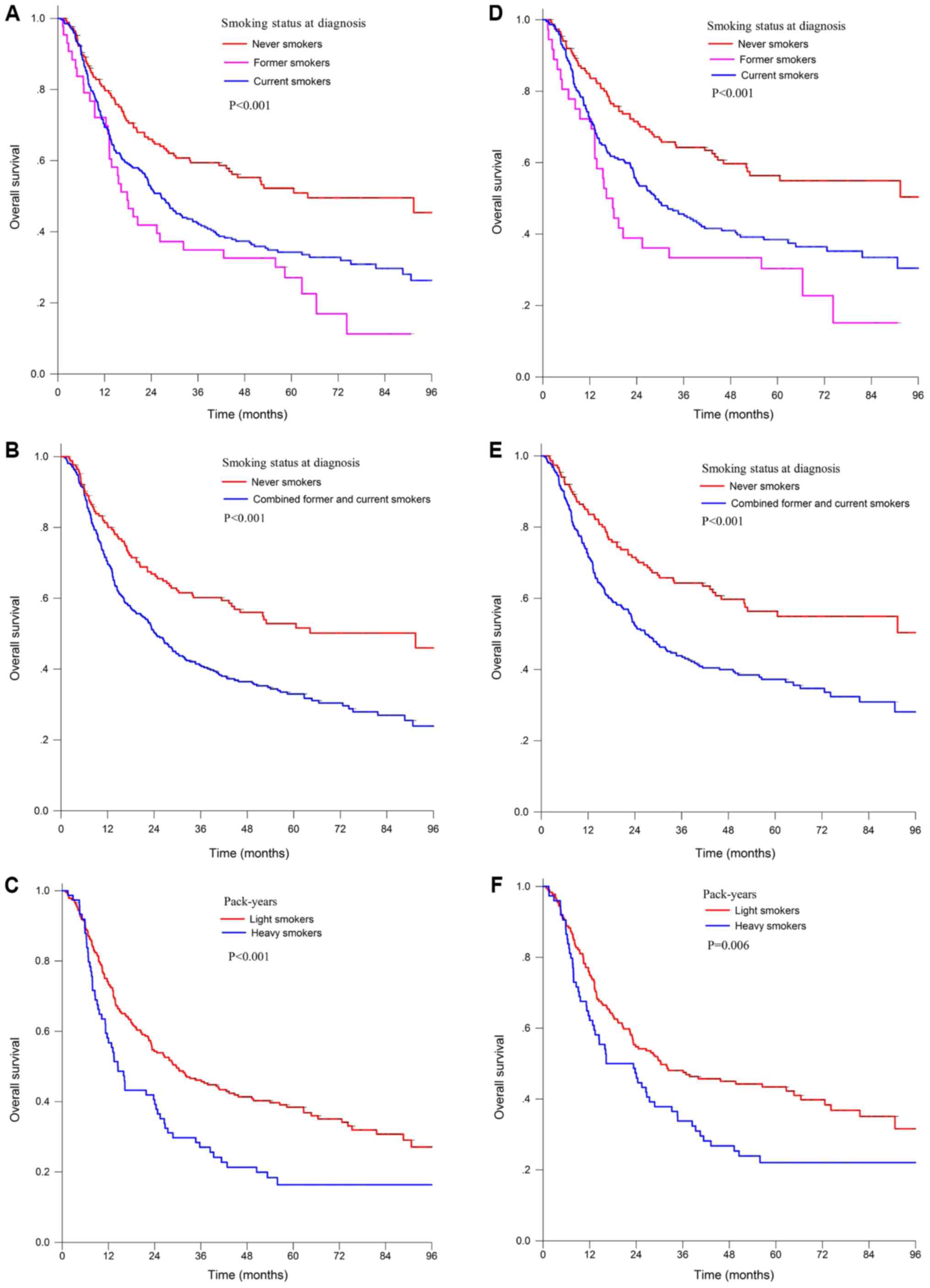
Kaplan-Meier analysis of the impact of
smoking on survival
Former and current smokers had a poorer OS than
never smokers in the entire population (Fig. 1A). The 5-year OS was 27.0% for former
smokers vs. 34.3% for current smokers (P<0.001), and vs. 50.9%
for never smokers (P<0.001). No significant difference in OS was
identified between the former and current smokers (P=0.129). The
small sample and no significant difference in survival (P=0.129)
prompted us to combine the former and current smokers into a single
group, whose 5-year survival rate was 32.3%, which was
significantly poorer than that of the never smokers (P<0.001;
Fig. 1B).
The cumulative effect of smoking also had a
significant effect on the survival of patients with ESCC. In
patients with a history of smoking, 47.5 PYs was identified as the
cutoff value for heavy and light smokers associated with OS. Heavy
smokers had a poorer 5-year OS of 16.3% compared with that of light
smokers, with a 5-year OS of 38.4% (log-rank test, P<0.001;
Fig. 1C).
Among the patients treated with IMRT/3DCRT, current
smokers or former smokers also had a poorer OS than never smokers
[5-year OS, 56.4% for never smokers vs. 38.4% for current smokers
(P<0.001) and vs. 30.3% for former smokers (P<0.001);
Fig. 1D]. The significant difference
compared with the never smokers still remained when current smokers
and former smokers were combined (5-year OS, 36.1 vs. 58.2%;
log-rank test, P<0.001; Fig. 1E).
No significant difference was observed between the former and
current smokers (P=0.101). Among those patients with a smoking
history, heavy smokers with >42.5 PYs of cigarettes had a poorer
5-year OS of 22.1% compared with light smokers, with a 5-year OS of
43.4% (P=0.006; Fig. 1F).
Univariate analysis of the impact of
cigarette smoking on survival
Among the patients with ESCC, univariate analyses
identified drinking history (HR 1.57; 95% CI 1.25–1.98;
P<0.001), advanced T stage (HR 1.40; 95% CI 1.17–1.67;
P<0.001), advanced M stage (HR 1.54; 95% CI 1.21–1.95;
P<0.001), advanced clinical stage (HR 1.62; 95%CI 1.28–2.05;
P<0.001) and smoking history (HR 1.86; 95% CI 1.42–2.44;
P<0.001) as significant risk factors for shorter OS (Table II). Female patients had a longer OS
than male patients (HR 0.57; 95% CI 0.41–0.79; P<0.001).
Restricted to those patients with a smoking history, Higher PYs
(>47.5) was a significant risk factor for shorter OS comparing
to low PYs (≤47.5) (HR 1.70; 95% CI 1.26–2.30; P=0.001). Similar
results were found in those patients receiving 3DRT/IMRT.
 | Table II.Univariate analysis in patients with
esophageal squamous cell carcinoma. |
Table II.
Univariate analysis in patients with
esophageal squamous cell carcinoma.
|
| Entire
population | IMRT/3DRT
cohort |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥60 vs. <60
years) | 1.12
(0.88–1.41) | 0.360 | 1.21
(0.93–1.57) | 0.165 |
| Sex (female vs.
male) | 0.57
(0.41–0.79) | 0.001 | 0.49
(0.34–0.72) | <0.001 |
| Drinking (yes vs.
no) | 1.57
(1.25–1.98) | <0.001 | 1.56
(1.20–2.03) | 0.001 |
| Tumor grade
(high/intermediate vs. low) | 1.02
(0.75–1.39) | 0.895 | 0.96
(0.67–1.39) | 0.847 |
| Tumor location
(cervical/upper vs. middle/lower) | 1.16
(0.92–1.47) | 0.216 | 1.15
(0.88–1.51) | 0.289 |
| T-stage (T4 vs. T3
/T2) | 1.40
(1.17–1.67) | <0.001 | 1.31
(1.07–1.59) | 0.009 |
| N-stage (N1 vs.
N0) | 1.48
(0.98–2.24) | 0.060 | 2.10
(1.22–3.61) | 0.008 |
| M-stage (M1a vs.
M0) | 1.54
(1.21–1.95) | <0.001 | 1.55
(1.18–2.02) | 0.001 |
| Clinical stage (IV
vs. II/III) | 1.62
(1.28–2.05) | <0.001 | 1.62
(1.24–2.12) | <0.001 |
| Chemotherapy (yes
vs. no) | 0.64
(0.45–0.89) | 0.009 | 0.69
(0.47–1.02) | 0.065 |
| RT technology
(IMRT/3DRT vs. 2DRT) | 0.46
(0.35–0.62) | <0.001 | – | – |
| Smoking
history | 1.86
(1.42–2.44) | <0.001 | 2.07
(1.53–2.82) | <0.001 |
| PYs | 1.70
(1.26–2.30)a | 0.001 | 1.56
(1.13–2.16)b | 0.007 |
Multivariate analysis of the impact of
cigarette smoking on survival
The number of PYs had linear effects on OS in most
cases, which was proven by the analysis using RCS nested within Cox
modes (data not shown). In the multivariate analysis, the smoking
status (former and current smokers vs. never smokers), T-stage and
M-stage were identified as significant and independent prognostic
factors for OS for the entire population and the patients treated
with IMRT/3DCRT (Table III). PYs
(heavy vs. light smokers) were had similar results (Table III).
 | Table III.Multivariate regression analysis in
patients with esophageal squamous cell carcinoma using the Cox
proportional hazards model for overall survival in terms of smoking
history (smoking history vs. no smoking history) and PYs (heavy vs.
light smokers). |
Table III.
Multivariate regression analysis in
patients with esophageal squamous cell carcinoma using the Cox
proportional hazards model for overall survival in terms of smoking
history (smoking history vs. no smoking history) and PYs (heavy vs.
light smokers).
|
| Entire
population | IMRT/3DRT
cohort |
|---|
|
|
|
|
|---|
|
| Smoking history
(n=479) | PYsa,c
(n=308) | Smoking history
(n=407) | PYsb,c
(n=253) |
|---|
|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥60 vs. <60
years) | 1.27
(0.99–1.62) | 0.06 | 1.05
(0.78–1.41) | 0.766 | 1.29
(0.98–1.69) | 0.07 | 1.11
(0.81–1.53) | 0.52 |
| Sex (female vs.
male) | 0.92
(0.59–1.44) | 0.71 | 1.05
(0.32–3.45) | 0.933 | 0.84
(0.511.41) | 0.52 | 1.17
(0.28–4.94) | 0.82 |
| Drinking (yes vs.
no) | 1.17
(0.89–1.53) | 0.24 | 1.05
(0.79–1.41) | 0.725 | 1.13
(0.84–1.52) | 0.42 | 1.02
(0.74–1.41) | 0.78 |
| Tumor grade
(high/intermediate vs. low) | 1.00
(0.83–1.20) | 0.99 | 1.05
(0.84–1.31) | 0.657 | 0.98
(0.79–1.21) | 0.83 | 1.02
(0.78–1.32) | 0.92 |
| Tumor location
(cervical/upper vs. middle/lower) | 1.09
(0.93–1.26) | 0.28 | 1.09
(0.91–1.31) | 0.333 | 1.08
(0.91–1.28) | 0.35 | 1.04
(0.85–1.27) | 0.74 |
| T-stage (T4 vs. T3
/T2) | 1.45
(1.21–1.75) | <0.001 | 1.59
(1.28–1.99) | <0.001 | 1.34
(1.09–1.65) | 0.01 | 1.41
(1.11–1.81) | 0.01 |
| N-stage (N1 vs.
N0) | 1.18
(0.78–1.80) | 0.43 | 0.95
(0.59–1.55) | 0.854 | 1.54
(0.89–2.69) | 0.13 | 1.34
(0.67–2.66) | 0.40 |
| M-stage (M1a vs.
M0) | 1.69
(1.32–2.16) | <0.001 | 1.75
(1.31–2.34) | <0.001 | 1.53
(1.17–2.02) | 0.01 | 1.69
(1.23–2.34) | 0.001 |
| Chemotherapy (yes
vs. no) | 0.92
(0.83–1.03) | 0.14 | 0.89
(0.78–1.01) | 0.069 | 0.97
(0.86–1.09) | 0.62 | 0.93
(0.81–1.07) | 0.32 |
| RT technology
(IMRT/3DRT vs. 2DRT) | 0.76
(0.61–0.93) | 0.01 | 0.86
(0.68–1.09) | 0.220 | – | – | – | – |
| Smoking
history | 1.57
(1.06–2.31) | 0.02 | – | – | 1.74
(1.12–2.68) | 0.01 | – | – |
| PYs | – | – | 1.75
(1.28–2.41)a | <0.001 | – | – | 1.55
(1.11–2.16)b | 0.01 |
In addition, the authors of the current study also
assessed the association between smoking history and OS across
strata of other potential predictors of patient outcome in the
entire population (Table IV). The
impact of the age, drinking status, tumor location or chemotherapy
on the risk of death was not significantly affected by the smoking
history. The effect of a history of smoking to increase the risk of
death was restricted to male patients (adjusted HR=1.63; 95% CI,
1.08–2.45; P=0.020), as well as patients with a low degree of
differentiation (adjusted HR=3.47; 95% CI, 1.08–11.19; P=0.037), a
clinical stage of II/III (adjusted HR=1.44; 95% CI, 1.02–2.04;
P=0.039) and treatment by 3DCRT/IMRT (adjusted HR=1.74; 95% CI,
1.12–2.68; P=0.013). No significant impact was observed among
female patients, possibly due to small sample sizes. Of note, a
different result was obtained for patients with 2DRT: A smoking
history had a positive impact on OS (HR=0.34; 95% CI 0.12–0.91;
P=0.033). This result is unexpected as it was hypothesized that a
history of smoking would negatively impact OS; this notable result
may be due to the small sample size, as only 72 patients received
2DRT, and among them, 17 were never smokers, 7 were former smokers
and 48 were current smokers.
 | Table IV.Impact of clinicopathological
characteristics on 5-year OS in the entire population and effect of
the smoking history on the survival of patients with esophageal
squamous cell carcinoma in subgroups by clinicopathological
characteristics. |
Table IV.
Impact of clinicopathological
characteristics on 5-year OS in the entire population and effect of
the smoking history on the survival of patients with esophageal
squamous cell carcinoma in subgroups by clinicopathological
characteristics.
|
|
|
| No. of deaths/total
no. of patients in the group |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Factor | 5-year OS (%) |
P-valuea | No smoking
history | Smoking
history | Adjusted HR of
mortality (95%CI) |
P-valueb |
|---|
| Total | 39.5 |
| 68/164 | 218/315 | 1.57
(1.06–2.31) | 0.025 |
| Age (years) |
| 0.359 |
|
|
|
|
|
<60 | 41.6 |
| 30/77 | 107/157 | 1.54
(0.86–2.75) | 0.139 |
|
≥60 | 37.5 |
| 38/87 | 111/158 | 1.57
(0.91–2.70) | 0.102 |
| Sex |
| 0.001 |
|
|
|
|
|
Male | 35.7 |
| 29/68 | 215/311 | 1.63
(1.08–2.45) | 0.020 |
|
Female | 55.0 |
| 39/96 | 3/4 | 0.40
(0.04–3.63) | 0.400 |
| Drinking |
| <0.001 |
|
|
|
|
| No | 47.1 |
| 61/152 | 91/142 | 1.56
(1.00–2.43) | 0.052 |
|
Yes | 28.2 |
| 7/12 | 127/173 | 1.19
(0.54–2.64) | 0.663 |
| Degree of
differentiation |
| 0.895 |
|
|
|
|
|
High/intermediate | 39.6 |
| 59/140 | 179/261 | 1.38
(0.90–2.09) | 0.136 |
|
Low | 38.9 |
| 24/96 | 53/97 | 3.47
(1.08–11.19) | 0.037 |
| Tumor location |
| 0.215 |
|
|
|
|
|
Cervical/upperc | 42.0 |
| 32/79 | 86/123 | 1.70
(0.94–3.07) | 0.077 |
|
Middled/lowere | 37.7 |
| 35/84 | 132/192 | 1.61
(0.93–1.79) | 0.090 |
| Clinical stage |
| <0.001 |
|
|
|
|
|
II+III | 46.0 |
| 39/108 | 123/194 | 1.44
(1.02–2.04) | 0.039 |
| IV | 28.1 |
| 29/56 | 95/121 | 1.57
(0.85–2.87) | 0.146 |
| Chemotherapy |
| 0.008 |
|
|
|
|
| No | 21.3 |
| 14/26 | 25/30 | 2.04
(0.69–6.05) | 0.197 |
|
Yes | 41.7 |
| 54/138 | 193/285 | 1.44
(0.95–2.20) | 0.090 |
| Radiation
technique |
| <0.001 |
|
|
|
|
|
2DRT | 15.0 |
| 14/17 | 48/55 | 0.34
(0.12–0.91) | 0.033 |
|
3DCRT/IMRT | 44.1 |
| 54/147 | 170/260 | 1.74
(1.12–2.68) | 0.013 |
The impact of smoking on survival was then further
assessed in detail (Table V). A
smoking history (HR=1.57; 95% CI, 1.06–2.32; P=0.025) and current
smoking (HR=3.01; 95% CI, 1.15–7.86; P=0.025) as opposed to a
never-smoking status, was associated with a higher of risk of
death. The risk of death for heavy smokers was higher than that for
light smokers, with an HR of 1.75 (95% CI, 1.28–2.41; P<0.001).
When the PYs were evaluated as a continuous variable, the HR for
death increased by 1% per pack-year (HR=1.01; 95% CI, 1.003–1.011;
P=0.004).
 | Table V.Effect of smoking history on overall
survival in patients with esophageal squamous cell carcinoma after
adjustment for potential prognostic factors.a |
Table V.
Effect of smoking history on overall
survival in patients with esophageal squamous cell carcinoma after
adjustment for potential prognostic factors.a
|
| Entire
population | IMRT/3DCRT
cohort |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Smoking status at
diagnosis |
|
|
|
|
| Former
vs. never | 1.57
(1.06–2.32) | 0.025 | 1.53
(1.02–2.30) | 0.039 |
| Current
vs. never | 3.01
(1.15–7.86) | 0.025 | 3.00
(1.14–7.86) | 0.025 |
| PYs |
|
|
|
|
| Heavy
vs. light | 1.75
(1.28–2.41)b | <0.001 | 1.55
(1.11–2.16)c | 0.011 |
|
Continuous PYs | 1.01
(1.003–1.011) | 0.004 | 1.01
(1.004–1.012) | 0.016 |
In the subgroup of patients treated with IMRT/3DCRT,
the HR for death was 1.53 (95% CI, 1.02–2.30; P=0.039) for former
smokers and 3.00 (95% CI, 1.14–7.86; P=0.025) for current smokers,
compared with that for never smokers. Heavy smokers had a higher
risk of death than light smokers, with an HR of 1.55 (95% CI,
1.11–2.16; P=0.011). Similarly, when the pack-years was evaluated
as a continuous variable, the HR for death increased by 1% per
pack-year (HR=1.01; 95% CI, 1.004–1.012; P=0.016).
Discussion
The present study on 479 patients with ESCC
receiving RT with or without chemotherapy indicated that smoking
was an independent prognostic factor for poor survival after
adjustment for other known prognostic factors, including age, sex,
drinking status, degree of differentiation, tumor location,
T-stage, N-Stage, M-stage, clinical stage, chemotherapy
administration and radiation technique. The risk of death was also
identified to be increased depending on the PYs of cigarettes. A
specific analysis of the cohort of 407 patients treated with
3DCRT/IMRT was also performed to account for the heterogeneity of
RT techniques. Considering that virtually all current smoking
patients were male, an analysis based on sex was also performed
revealing that smoking had a significant impact on death among male
patients (adjusted HR=1.63; 95% CI, 1.08–2.45; P=0.020) but in not
female patients (adjusted HR=0.40; 95% CI, 0.04–3.63; P=0.400),
possibly due to small sample sizes. Finally, the impact of
long-term smoking on OS was assessed by treating the number of PYs
as a continuous variable. The resulting HRs for death increased by
a small but significant value between 0.003 and 0.011. A previous
study reported a similar result, namely that smoking decreased the
OS of patients with oropharyngeal cancer by 1% per PY of smoking
(28). The small sample size may be
one of the reasons for the unsatisfactory interval between 0.003
and 0.011 obtained in the present study.
According to the 2014 Surgeon General's Report on
smoking and tobacco use, there is sufficient evidence to infer a
causal association between cigarette smoking and increased
all-cause mortality and cancer-specific mortality, but is not
sufficient to infer a causal association between cigarette smoking
and the risk of recurrence, poorer response to treatment and
increased treatment-associated toxicity (29). A review discussing the known
biological effects of smoking on cancer cell biology emphasized the
clinical effects of continued smoking in patients with cancer
treated with chemotherapy or RT (30). Smoking causes adverse outcomes in
patients with cancer, leading to complications associated with
cancer treatment and continued development of comorbid disease
(30). The two aforementioned
studies considered lung cancer, prostate cancer, head and neck
cancer, breast cancer, cervical cancer, Hodgkin's disease, colon
cancer and male cancer patients (29,30).
However, few studies focused on patients with ESCC receiving RT
with or without chemotherapy. The study by Shitara et al
(31) indicated that heavy cigarette
smoking (cumulative smoking of >20 PYs) was a poor prognostic
factor in patients with ESCC who had been treated by
chemoradiotherapy. However, in their analysis, non-smokers and
light smokers were combined into a group of non-heavy smokers
(cumulative smoking of up to 20 PY), which may have introduced bias
and only provides limited information on the effect of smoking
behavior (31). An analysis of 1,084
patients with ESCC revealed a significant association between OS
and smoking history in the group treated with chemotherapy plus
surgery, but not in that treated with surgery alone (15). That study indicated that smoking
affected the outcome of chemotherapy. In a recent study, which
focused on patients receiving definitive RT or concurrent
chemoradiotherapy, cigarette smoking was identified as a
significant and independent poor prognostic risk factor for OS by a
multivariate Cox regression analysis (18). Two groups of patients received
esophagectomy after chemoradiotherapy, which may have caused bias.
The study by Zhang et al (16) reported a negative result, namely that
smoking was not a prognostic factor for survival of patients with
ESCC who received definitive RT. However, the cohort comprised only
79 patients. In the present study, all of the patients received RT
and 70.1% of patients received chemotherapy. Therefore, the present
results more strongly support the view that smoking is an
independent predictor of poorer survival for patients with ESCC who
received RT with or without chemotherapy.
In the present study, smoking had a significant
impact on the risk of death among male but not female patients,
possibly due to small sample sizes. No impact of smoking on
survival was observed in breast cancer patients (32,33). In
addition, no significant impact of smoking on OS was obtained in
female patients with nasopharyngeal carcinoma (34). The reason may be that smoking in
quantity and intensity is less frequent among women than among men.
However, with the amount of women actively and passively smoking
increasing, this association may change (35). In the present study, 100 female
patients were included, of which only four of had a history of
smoking. Thus, more samples of female patients are required to
evaluate the impact of smoking on survival of patients with ESCC
receiving RT with or without chemotherapy.
No significant difference of OS was identified
between former and current smokers, and the two groups had a
similarly poor survival compared with never smokers (P<0.001).
The relatively poor survival for smokers with a higher number of
PYs compared with those with a low number of PYs demonstrated the
unfavorable cumulative effects of long-term, heavy smoking. The
negative influence of smoking on survival was still maintained in
the former smokers. Despite the cessation of smoking for >1
year, the possible impact of smoking on exacerbating tissue
hypoxia, which induces the expression of a variety of genes
associated with an aggressive malignant phenotype, and promoting
chemoradioresistance and tumor progression may have already
occurred and remains in these former smokers (21,36,37).
Furthermore, continued smoking after diagnosis may reduce the
efficacy of anti-cancer treatment and increase the proportion of
cancer stem-like cells, resulting in a poor outcome; in addition,
increased higher rates of treatment complications and side effects,
such as higher treatment-associated weight loss, lead to a poorer
quality of life (38–43). A study reported that 60% of patients
smoked during the week prior to surgery and 13% who were abstinent
prior to surgery had resumed smoking (44). Their relapse of smoking was probably
associated with a higher perceived difficulty in quitting, higher
tendency toward depression, greater fears regarding cancer
recurrence, a lower quitting self-efficacy and a lower perception
regarding their cancer-associated risk. In addition, cessation of
smoking after a cancer diagnosis was reported to significantly
reduce the risk of death compared with persistent smoking (29). For the cohort of the present study,
data on the smoking status during treatment or follow-up are
lacking; therefore, the possibility that certain former smokers
resumed smoking during treatment or follow-up cannot be excluded.
Evidence-supported measures that increase chances of cessation
include direct physician advice, approved pharmacotherapy,
structured counseling and a follow-up plan (45). Individual behavioral counseling, a
combination of pharmacological and behavioral interventions for
smoking cessation, are effective in assisting smokers to quit
(46).
Of note, the present study has certain limitations.
First, there was an inherent bias owing to the study's
retrospective design. The smoking and drinking status at diagnosis
were based on the medical records, rather than standardized
questionnaires at enrollment. Furthermore, in the present cohort,
males accounted for the majority and few female patients had a
smoking history (four smokers among 100 female patients),
indicating that the present results may only apply to males. The
patients included were all from Southern China, and the
applicability of the present results to patients from other
geographical areas remains elusive. In addition, the patients were
re-staged using the sixth AJCC/UICC staging system and not the most
recent staging system according to which the N-stage is based on
the number of positive lymph nodes. In the sixth AJCC/UICC staging
system, N-stage was defined as with or without regional lymph node.
The difference in N-stage may change the treatment strategies.
Furthermore, there was heterogeneity in the chemotherapy regimens,
administration schedules and prescription doses, which ranged from
50.4 to 66.0 Gy or even higher doses (7 patients received >66.0
Gy; the maximum dose received by any one patients was 70 Gy).
Finally, the treatment strategies were not entirely
consistent with the latest NCCN guidelines. For instance, the T2
patients did not receive surgery due to rejection or intolerance of
surgery, a group for whom surgery is the primary treatment, and
some T3-T4 patients did not receive induction chemoradiotherapy
followed by surgery. There were various reasons why those patients
did not receive surgery. The patients with a location of the tumor
in the lower esophagus, for whom surgery is the preferred
treatment, only accounted for 7.1%. Certain patients had
comorbidities based on which they were not able to tolerate
surgery. Certain patients refused surgery considering the
associated complications and cost. The cohort was restricted to
those subjects who received RT, with or without chemotherapy, not
those who received surgery. Furthermore, induction chemotherapy
followed by surgery is the standard treatment for EC according to
the NCCN guidelines. The evidence that these guidelines are based
on mostly comes from Western countries, in which EA is the
pre-dominant subtype; however, in China, the most prevalent subtype
is ESCC.
The most common location of the tumor in Western
countries is the lower esophagus, for which surgery is the
preferred choice. In China, the tumor is located in the cervical
and upper esophagus for most cases, for which surgery is more
difficult. At our institution, the treatment strategies for the
patients with ESCC were discussed by a multidisciplinary team,
according to the NCCN guidelines and the status of the patients.
The final treatment plan was based on the NCCN guidelines, the
specific situation and the choice of each patient. Among the T2
patients in the present study, 44% (36/82) had a tumor located in
the cervical and upper esophagus, and 81.7% of cases (67/82) were
N1. To reduce the bias caused by the treatment strategy and
selection, sub-group analyses were performed to evaluate the
association between the smoking history and OS. In addition to an
analysis of the entire population, those patients who received
3DCRT/IMRT were also assessed separately, and similarly significant
results were obtained. It is important to validate the present
results in a prospective study with an independent cohort.
In conclusion, the present study indicated that a
smoking history at diagnosis was an independent prognostic factor
for poor survival among patients with ESCC. This result may help to
manage the tobacco use among patients with ESCC. The smoking status
should be taken into consideration in prospective studies on ESCC.
The present results require to be validated in future studies and
the molecular/genetic mechanism of the effect of smoking on ESCC
should be further elucidated and interpreted.
Acknowledgements
The authors greatly thank Dr. Hui He (Intensive Care
Unit, Panyu Central Hospital, Cancer Institute of Panyu, Guangzhou)
for his assistance in revising the figures.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
All the authors were involved in conceiving and
designing the study. GRZ, ZS and JYL collected the data. GRZ
performed the statistical analysis. GRZ and ZS drafted and wrote
the manuscript. FYX and QL gave advice on the study design,
interpreted the results and critically revised the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethical approval and consent to
participate
This study was approved by the Ethics Committee of
Sun Yat-sen University Cancer Center (Guangzhou, China) and Panyu
Central Hospital (Guangzhou, China). All procedures performed in
studies involving human participants were in accordance with the
ethical standards of the institutional and/or national research
committee and with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards. Patient records were
anonymized and de-identified prior to analysis. Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EC
|
esophageal cancer
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
EA
|
esophageal adenocarcinoma
|
|
OS
|
overall survival
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
DFS
|
disease-free survival
|
|
RT
|
radiotherapy
|
|
UICC
|
Union for International Cancer
Control
|
|
AJCC
|
American Joint Committee on Cancer
|
|
2DRT
|
two-dimensional RT
|
|
3DCRT
|
three-dimensional conformal RT
|
|
IMRT
|
intensity modulated RT
|
|
PY
|
pack year
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Besh S, Chao J, Das P, Denlinger C, Fanta P, Fuchs CS, et al:
Esophageal and esophagogastric junction cancers, version 1.2015. J
Natl Compr Canc Netw. 13:194–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H
and Zou X: Report of cancer incidence and mortality in China, 2010.
Ann Transl Med. 2:612014.PubMed/NCBI
|
|
4
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang HZ, Jin GF and Shen HB:
Epidemiologic differences in esophageal cancer between Asian and
Western populations. Chin J Cancer. 31:281–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oze I, Matsuo K, Ito H, Wakai K, Nagata C,
Mizoue T, Tanaka K, Tsuji I, Tamakoshi A, Sasazuki S, et al:
Cigarette smoking and esophageal cancer risk: An evaluation based
on a systematic review of epidemiologic evidence among the Japanese
population. Jpn J Clin Oncol. 42:63–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castellsague X, Munoz N, De Stefani E,
Victora CG, Castelletto R, Rolón PA and Quintana MJ: Independent
and joint effects of tobacco smoking and alcohol drinking on the
risk of esophageal cancer in men and women. Int J Cancer.
82:657–664. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yaegashi Y, Onoda T, Morioka S, Hashimoto
T, Takeshita T, Sakata K and Tamakoshi A: Joint effects of smoking
and alcohol drinking on esophageal cancer mortality in Japanese
men: Findings from the Japan collaborative cohort study. Asian Pac
J Cancer Prev. 15:1023–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu CL, Lang HC, Luo JC, Liu CC, Lin HC,
Chang FY and Lee SD: Increasing trend of the incidence of
esophageal squamous cell carcinoma, but not adenocarcinoma, in
Taiwan. Cancer Causes Control. 21:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishiguro S, Sasazuki S, Inoue M, Kurahashi
N, Iwasaki M and Tsugane S: Effect of alcohol consumption,
cigarette smoking and flushing response on esophageal cancer risk:
A population-based cohort study (JPHC study). Cancer Lett.
275:240–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang JM, Zeng XJ, Chen JS, Ping-zhao, Li
JY, Zhang KL, Wu YP and Liu BQ: Smoking and mortality from
esophageal cancer in China: A large case-control study of 19,734
male esophageal cancer deaths and 104,846 living spouse controls.
Int J Cancer. 119:1427–1432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lagergren J, Bergstrom R, Lindgren A and
Nyren O: The role of tobacco, snuff and alcohol use in the
aetiology of cancer of the oesophagus and gastric cardia. Int J
Cancer. 85:340–346. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobus SL and Warren GW: The biologic
effects of cigarette smoke on cancer cells. Cancer Am Cancer Soc.
120:3617–3626. 2014.
|
|
14
|
Wang N, Tan B, Cao F, Song Q, Wang J, Jia
Y and Cheng Y: Prognostic influence of smoking on esophageal
squamous cell carcinoma. Int J Clin Exp Med. 8:18867–18872.
2015.PubMed/NCBI
|
|
15
|
Zheng Y, Cao X, Wen J, Yang H, Luo K, Liu
Q, Huang Q, Chen J and Fu J: Smoking affects treatment outcome in
patients with resected esophageal squamous cell carcinoma who
received chemotherapy. PLoS One. 10:e1232462015.
|
|
16
|
Zhang F, Han H, Wang C, Wang J, Zhang G,
Cao F and Cheng Y: A retrospective study: The prognostic value of
anemia, smoking and drinking in esophageal squamous cell carcinoma
with primary radiotherapy. World J Surg Oncol. 11:2492013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang RW, Chao YK, Wen YW, Chang HK, Tseng
CK, Chan SC and Liu YH: Predictors of pathological complete
response to neoadjuvant chemoradiotherapy for esophageal squamous
cell carcinoma. World J Surg Oncol. 12:1702014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yen YC, Chang JH, Lin WC, Chiou JF, Chang
YC, Chang CL, Hsu HL, Chow JM, Yuan KS, Wu ATH and Wu SY:
Effectiveness of esophagectomy in patients with thoracic esophageal
squamous cell carcinoma receiving definitive radiotherapy or
concurrent chemoradiotherapy through intensity-modulated radiation
therapy techniques. Cancer Am Cancer Soc. 123:2043–2053. 2017.
|
|
19
|
Jensen JA, Goodson WH, Hopf HW and Hunt
TK: Cigarette smoking decreases tissue oxygen. Arch Surg.
126:1131–1134. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grau C, Nordsmark M, Khalil AA, Horsman MR
and Overgaard J: Effect of carbon monoxide breathing on hypoxia and
radiation response in the SCCVII tumor in vivo. Int J Radiat Oncol
Biol Phys. 29:449–454. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cosse JP and Michiels C: Tumour hypoxia
affects the responsiveness of cancer cells to chemotherapy and
promotes cancer progression. Anticancer Agents Med Chem. 8:790–797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nordsmark M, Bentzen SM, Rudat V, Brizel
D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, et
al: Prognostic value of tumor oxygenation in 397 head and neck
tumors after primary radiation therapy. An international
multi-center study. Radiother Oncol. 77:18–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fi G, Di P and Id F: AJCC cancer staging
manual. (6th). Springer-Verlag. (New York). 2002.
|
|
24
|
Zhang P, Xi M, Li QQ, Hu YH, Guo X, Zhao
L, Liu H, Liu SL, Luo LL, Liu Q and Liu MZ: Concurrent cisplatin
and 5-fluorouracil versus concurrent cisplatin and docetaxel with
radiotherapy for esophageal squamous cell carcinoma: A propensity
score-matched analysis. Oncotarget. 7:44686–44694. 2016.PubMed/NCBI
|
|
25
|
Liu H, Lu L, Zhu Q, Hao Y, Mo Y, Liu M, Hu
Y, Cui N and Rong T: Cervical nodal metastases of unresectable
thoracic esophageal squamous cell carcinoma: Characteristics of
long-term survivors after concurrent chemoradiotherapy. Radiother
Oncol. 99:181–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xi M, Zhang P, Zhang L, Yang YD, Liu SL,
Li Y, Fu JH and Liu MZ: Comparing docetaxel plus cisplatin versus
fluorouracil plus cisplatin in esophageal squamous cell carcinoma
treated with neoadjuvant chemoradiotherapy. Jpn J Clin Oncol.
47:683–689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo LL, Xi M, Yang YD, Li QQ, Zhao L,
Zhang P, Liu SL and Liu MZ: Comparative outcomes of induction
chemotherapy followed by definitive chemoradiotherapy versus
chemoradiotherapy alone in esophageal squamous cell carcinoma. J
Cancer. 8:3441–3447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gillison ML, Zhang Q, Jordan R, Xiao W,
Westra WH, Trotti A, Spencer S, Harris J, Chung CH and Ang KK:
Tobacco smoking and increased risk of death and progression for
patients with p16-positive and p16-negative oropharyngeal cancer. J
Clin Oncol. 30:2102–2111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Warren GW, Alberg AJ, Kraft AS and
Cummings KM: The 2014 surgeon General's report: ‘The health
consequences of smoking-50 years of progress’: A paradigm shift in
cancer care. Cancer. 120:1914–1916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Warren GW, Sobus S and Gritz ER: The
biological and clinical effects of smoking by patients with cancer
and strategies to implement evidence-based tobacco cessation
support. Lancet Oncol. 15:e568–e580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shitara K, Matsuo K, Hatooka S, Ura T,
Takahari D, Yokota T, Abe T, Kawai H, Tajika M, Kodaira T, et al:
Heavy smoking history interacts with chemoradiotherapy for
esophageal cancer prognosis: A retrospective study. Cancer Sci.
101:1001–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vatten LJ, Foss OP and Kvinnsland S:
Overall survival of breast cancer patients in relation to
preclinically determined total serum cholesterol, body mass index,
height and cigarette smoking: A population-based study. Eur J
Cancer. 27:641–646. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Terry PD and Rohan TE: Cigarette smoking
and the risk of breast cancer in women: A review of the literature.
Cancer Epidemiol Biomarkers Prev. 11:953–971. 2002.PubMed/NCBI
|
|
34
|
Ouyang PY, Su Z, Mao YP, Liang XX, Liu Q,
Deng W and Xie FY: Prognostic impact of cigarette smoking on the
survival of patients with established nasopharyngeal carcinoma.
Cancer Epidemiol Biomarkers Prev. 22:2285–2294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johnson KC, Miller AB, Collishaw NE,
Palmer JR, Hammond SK, Salmon AG, Cantor KP, Miller MD, Boyd NF,
Millar J and Turcotte F: Active smoking and secondhand smoke
increase breast cancer risk: The report of the Canadian expert
panel on tobacco smoke and breast cancer risk (2009). Tob Control.
20:e22011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brizel DM, Sibley GS, Prosnitz LR, Scher
RL and Dewhirst MW: Tumor hypoxia adversely affects the prognosis
of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys.
38:285–289. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
An Y, Kiang A, Lopez JP, Kuo SZ, Yu MA,
Abhold EL, Chen JS, Wang-Rodriguez J and Ongkeko WM: Cigarette
smoke promotes drug resistance and expansion of cancer stem
cell-like side population. PLoS One. 7:e479192012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zevallos JP, Mallen MJ, Lam CY, Karam-Hage
M, Blalock J, Wetter DW, Garden AS, Sturgis EM and Cinciripini PM:
Complications of radiotherapy in laryngopharyngeal cancer: Effects
of a prospective smoking cessation program. Cancer. 115:4636–4644.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arcavi L and Benowitz NL: Cigarette
smoking and infection. Arch Intern Med. 164:2206–2216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duffy SA, Terrell JE, Valenstein M, Ronis
DL, Copeland LA and Connors M: Effect of smoking, alcohol, and
depression on the quality of life of head and neck cancer patients.
Gen Hosp Psychiatry. 24:140–147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Browman GP, Wong G, Hodson I, Sathya J,
Russell R, McAlpine L, Skingley P and Levine MN: Influence of
cigarette smoking on the efficacy of radiation therapy in head and
neck cancer. N Engl J Med. 328:159–163. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gritz ER: Smoking and smoking cessation in
cancer patients. Br J Addict. 86:549–554. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simmons VN, Litvin EB, Jacobsen PB, Patel
RD, McCaffrey JC, Oliver JA, Sutton SK and Brandon TH: Predictors
of smoking relapse in patients with thoracic cancer or head and
neck cancer. Cancer. 119:1420–1427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Steliga MA: Smoking cessation in clinical
practice: How to get patients to stop. Semin Thorac Cardiovasc
Surg. 30:87–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lancaster T and Stead LF: Individual
behavioural counselling for smoking cessation. Cochrane Database
Syst Rev. 3:CD0012922017.PubMed/NCBI
|