Introduction
The human corneal endothelium represents the single
cell layer between the corneal stroma and anterior chamber, which
exhibits barrier and ‘pump’ functions to maintain corneal
transparency (1). Trauma, diseases,
surgery and aging may lead to a decrease in human corneal
endothelial cells (HCECs) (2).
Considering that HCECs do not proliferate in vivo, corneal
transplantation is required for patients with corneal endothelial
dysfunction in order to restore vision (3,4).
However, the shortage of cornea donors is problematic. Corneal
surgeons may now replace injured corneal endothelia with a thin,
transparent, biocompatible, tissue-engineered substratum exhibiting
corneal endothelial cells or other replaceable cells (5). Tissue-engineered corneas are currently
of interest, thus motivating researchers to develop an appropriate
biocompatible scaffold and determine replaceable cells.
It has been recently demonstrated that nanofibrous
structures formed by electrospinning may promote cell spreading,
proliferation, migration and attachment (6,7).
Nanofibrous scaffolds established by electrospinning may provide
cells with a growth environment that is similar to extracellular
matrices of native tissues. Nutrients and waste may be efficiently
exchanged, and the large surface provided by electrospinning may
contribute to the transduction of biochemical signals by cultured
cells (8,9). Natural polymers, such as collagen and
gelatin, have been electrospun into fibrous scaffolds for cornea
tissue engineering and endothelial cells (10–13).
Gelatin and collagen are natural components of the extracellular
matrix, which can provide an appropriate surface for cell
proliferation, adhesion and differentiation (14). However, the mechanical
characteristics of natural scaffolds are typically hard to
establish. For example, when placed in liquid solution, natural
scaffolds rarely retain their structural integrity (15,16).
However, the mechanical strength of synthetic electrospun
scaffolds, such as polycaprolactone (PCL) and
poly(L-lactide-co-glycolide), is usually satisfactory and their
biodegradability is strongly regulated (17,18).
Scaffolds combining natural and synthetic polymers may
simultaneously maintain the advantages and attenuate the
disadvantages of scaffolding (19).
In our previous study (20,21), it
was indicated that bone marrow endothelial progenitor cells (BEPCs)
have the potential to differentiate into corneal endothelial cells
(CECs). Induced BEPCs were revealed to resemble CECs regarding cell
shape, expression levels of aquaporin-1 and exhibition of tightly
opposed cell junctions. When transplanted into corneas with
defective CEC, induced BEPCs have been previously demonstrated to
maintain corneal transparency. The results of the previous study
suggest that BEPCs have the potential to be used for
tissue-engineered corneal endothelium.
The aim of the present study was to observe the
morphology, adherence, proliferation and stem cell markers of BEPCs
cultured on two hybrid scaffolds: Gelatin/PCL (70:30) and
collagen/PCL (70:30). This was performed to investigate the
potential value of nanofibrous structures in the culturing of BEPCs
and subsequently whether these structures may be used for
tissue-engineered corneal endothelium.
Materials and methods
Preparation of gelatin/PCL and
collagen/PCL scaffolds
A gelatin/PCL solution was established in accordance
with a previous study (22), gelatin
type A (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and poly
(ε-caprolactone) PCL (molecular weight, 80,000; Sigma-Aldrich;
Merck KGaA), with a mass ratio of 70:30, were dissolved in 10%
(w/v) 2,2,2-trifluoroethanol (TFE; purity ≥99%; Sigma-Aldrich;
Merck KGaA) under sufficient stirring at room temperature for 24 h.
Acetic acid (0.2% diluted in TFE) was added to the solutions to
establish a transparent solution. To prepare the collagen/PCL
solution, Type I collagen (Sichuan Mingrang Bio-Tech Co., Ltd.,
Chengdu, China) and PCL with a mass ratio of 70:30 were dissolved
in hexafluoroisopropanol (purity ≥99%; Sigma Aldrich; Merck KGaA)
at a concentration of 8% (w/v) and stirred vigorously at room
temperature for 48 h. The two solutions were subsequently
administered into a 10 ml plastic syringe and subjected to
electrospinning using the parameters presented in Table I. A KDS100 syringe pump (KD
Scientific, Inc., Holliston, MA, USA) and a high voltage power
supply (TXR1020N30-30; Teslaman Technology, Co., Ltd., Dalian,
China) were used to regulate the solution dispensing rate and
applied voltage, respectively. The obtained membranes were placed
in a vacuum oven for 1 week to remove the residual solvent prior to
further experimentation.
 | Table I.Parameters used for electrospinning
fibrous membrane. |
Table I.
Parameters used for electrospinning
fibrous membrane.
| Sample | Solvent | Concentration
(%) | Applied voltage
(kV) | Feed rate
(ml/h) | Collecting distance
(cm) | Temperature | Humidity (%) |
|---|
| Gelatin/PCL
(70:30) | TFE | 10 w/v | 9–10 | 1.5 | 13 | 20–25°C | 45–65 |
| Collagen/PCL
(70:30) |
HFIP | 8 w/v | 13–14 | 1 | 13 | 20–25°C | 45–65 |
Morphology and hydrophilicity of
scaffolds
The morphologies of the electrospun gelatin/PCL
fibers and collagen/PCL fibers were investigated via scanning
electron microscopy (SEM; JEOL JSM-5600LV Series Scanning Electron
Microscope; Japan Electron Optics Laboratory Co., Ltd., Tokyo,
Japan) at an acceleration voltage of 8–10 kV. Electrospun
gelatin/PCL fibers and collagen/PCL fibers were fixed with 2.5%
glutaraldehyde for 24 h at 4°C. Prior to imaging, samples were
coated with gold for 50 sec to increase conductivity. The average
diameters of the fibers were determined from the SEM-derived images
using ImageJ 1.40G software (National Institutes of Health,
Bethesda, MD, USA). In addition, a minimum of 100 nanofibers from
each sample obtained from different SEM images were manually
investigated and analyzed.
The hydrophilicities of the electrospun gelatin/PCL
and collagen/PCL fibrous membranes were determined following the
analysis of the contact angles of water droplets on membranes,
which were measured using a video contact angle instrument
(Attension Theta; BiolinScientific, Q-Sense AB, Gothenburg,
Sweden). Deionized water (3 µl) was automatically dropped onto the
surface of the membranes and the contact angle was then
determined.
Isolation and culture of BEPCs
Lower-limb bone marrow was obtained a total of 20
female Sprague Dawley rats (age, 4 weeks; weight, ~100–120 g),
which were purchased from the Suzhou Amufi Biological Science and
Technology Co., Ltd (Suzhou, China). Rats were bred in a
specific-pathogen-free environment with free access to water and
fodder. The temperature was kept constant at 25°C, with a humidity
of ~70% and a 12 h light/dark cycle. All experimental procedures
were approved by the Animal Research Committee of Ninth People's
Hospital, Shanghai Jiao Tong University School of Medicine
(Shanghai, China). Marrow was isolated by cutting off both ends of
the bone, then separated with a syringe. The marrow was washed with
phosphate-buffered saline (PBS; Sigma-Aldrich; Merck KGaA). In
order to isolate mononuclear cells, myeloid tissue was treated with
3 ml red blood cell lysis buffer (ammonium chloride solution;
Stemcell Technologies, Inc., Vancouver, BC, Canada) for 1 min and
then mixed with 7 ml PBS. Following centrifugation at 400 × g for
10 min at room temperature, cells were suspended in Endothelial
Cell Growth Medium-2 (EGM-2; CC-3162; Lonza Group, Ltd., Basel,
Switzerland), cultured on 10 cm culture dishes (Corning
Incorporated, Corning, NY, USA) at 37°C in 5% CO2
humidified atmosphere, and subsequently supplemented with
hydrocortisone, vascular endothelial growth factor, ascorbic acid,
gentamicin, amphotericin-B, recombinant insulin growth factor,
human epidermal growth factor, human fibroblast growth factor-B and
5% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Culturing media was replaced with fresh media a
total of 2 days post-seeding. Following this, media was replaced
every other day. When cells reached a confluence of 80–90%, BEPCs
were treated with 0.25% trypsin and 0.02% EDTA (Sigma-Aldrich;
Merck KGaA) and were recultured in a different 10 cm culture dish
at a ratio of 1:2. Following this, the collagen/PCL and gelatin/PCL
scaffolds were soaked in 75% ethanol for 10 min. The scaffolds were
washed three times with PBS for 15 min prior to further
experimentation.
Morphology and adherence of BEPCs on
scaffolds
BEPCs were seeded onto the two scaffolds in a
24-well plate at a density of 2×104 cells/well. At day 1
post-seeding, the morphology of BEPCs was investigated via SEM as
aforementioned. The adherence of BEPCs cultured on the scaffolds
was visualized using rhodamin-phalloidin (1:500; cat. no.
40734ES75; Yeasen, Shanghai, China). The F-actin cytoskeleton was
visualized using rhodamin-phalloidin to observe cellular adherence
via fluorescence staining. BEPCs that had been cultured for 24 h
were washed with PBS a total of three times for 10 min. BEPCs were
fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 10
min at room temperature. Following 10 min of washing with PBS three
times, BEPCs were permeabilized with 0.3% Triton X-100
(Sigma-Aldrich; Merck KGaA) in PBS. Subsequently, the cells were
washed three times with PBS for 10 min, and 200 µl
Tetramethylrhodamine B isothiocyanate Phalloidin (1:500; cat. no.
40734ES75; Yeasen) was added to each slide. Following 30 min of
incubation under light protection at room temperature, BEPCs were
washed for 5 min for a total of three times. Cell nuclei were then
counterstained with 4, 6-diamidino-2-phenylindole (DAPI;
Invitrogen; Thermo Fisher Scientific, Inc.). Fluorescent images
were subsequently captured using a Nikon eclipse 80i fluorescence
microscope at a magnification of ×3 (Nikon Corporation; Tokyo,
Japan).
Cell viability and proliferation
assays
Viability staining was performed using a calcein
acetoxymethy1 ester/ethidium homodimer 1 assay (LIVE/DEAD
Viability/Cytotoxicity Kit; cat. no. L3224; Thermo Fisher
Scientific, Inc.). BEPCs were seeded onto the scaffolds on glass
slides in a 24-well plate at a density of 5×104
cells/well. A total of 48 h post-seeding, the cells were incubated
in PBS containing 2 µl calcein acetoxymethy1 ester and 4 µl
ethidium homodimer 1 at 37°C for 45 min. Subsequently, the BEPCs
were washed twice with PBS, and fluorescent images were captured
using a Nikon eclipse 80i fluorescence microscope at a
magnification of ×3.
Cell proliferation on the scaffolds was determined
to investigate the biocompatibility of the scaffolds. Briefly,
BEPCs were seeded onto glass slides and scaffold membranes, as well
as the size of slides and membranes, were matched with the 24 well
plates. In each well, cells (5×104) were seeded in 1 ml
of EGM-2 with 5% FBS. A Cell Count-8 kit (CCK-8; Life, New York,
USA) was used to determine cell proliferation, which was performed
in accordance with the manufacturer's protocol. Subsequently, the
plates were examined with ELISA at a wavelength of 490 nm. At 0,
24, 48 and 72 h time intervals, the optical density (OD) values
from triplicates of each group were determined.
Immunocytochemistry of Ki-67
protein
BEPCs cultured on the two hybrid scaffolds and glass
slides were fixed with 4% paraformaldehyde for 10 min at room
temperature and then permeabilized with 0.3% Triton X-100 in PBS.
Blocking was performed using 10% normal goat serum (Gibco; Thermo
Fisher Scientific, Inc.). Cells were incubated with rabbit
monoclonal anti-Ki-67 antibodies (1:200; Abcam, Cambridge, UK) at
4°C overnight. Following incubation, the BEPCs were washed with PBS
for 10 min in triplicate, then incubated with fluorescent labeled
secondary antibodies (1:500; Alexa Fluor 546 goat anti-rabbit
immunoglobulin G; Invitrogen; Thermo Fisher Scientific, Inc.) in
PBS for 1 h at room temperature. Following three washing steps
(with PBS), cell nuclei were counterstained with DAPI for 5 min at
room temperature (Invitrogen; Thermo Fisher Scientific, Inc.).
Fluorescent images were captured using a Nikon eclipse 80i
fluorescence microscope. Ki-67 protein staining was investigated in
five visual fields at a magnification of 10×0.30, and the total
percentages of positive cells were calculated.
Immunocytochemistry staining for
incorporation of bromodeoxyuridine (BrdU)
BEPCs were cultured for 8 h in the presence of 1 µl
BrdU (Sigma-Aldrich; Merck KGaA) dissolved in 1 ml culture medium.
The cells were fixed in 4% paraformaldehyde for 15 min at room
temperature and subsequently incubated with 10% sheep serum (Gibco;
Thermo Fisher Scientific, Inc.) and 0.25% Triton X-100 in PBS for 1
h at room temperature. Following incubation, the BEPCs were washed
in PBS, incubated with 0.83% HCl for 30 min at room temperature,
washed in Hanks' Balanced Salt Solution and PBS at room
temperature. Following incubation with anti-BrdU antibodies (1:800;
cat. no. ab8152; Abcam) at 4°C overnight, BEPCs were washed in PBS
and incubated with fluorescent-conjugated secondary antibodies
(1:500; Alexa Fluor 546-goat anti-rabbit) for 1 h at room
temperature. Cell nuclei were subsequently stained with DAPI for 5
min at room temperature. Fluorescent images were obtained using a
Nikon eclipse 80i fluorescence microscope. BrdU protein staining
was investigated in five visual fields at a magnification of
10×0.30, and the total percentages of live and positive cells were
subsequently determined.
Investigation of stem cell
markers
The identification and characterization of BEPCs
were performed using rabbit monoclonal anti-CD34antibodies (1:200;
Abcam) and rabbit monoclonal anti-CD133antibodies (1:200; Abcam).
Following culturing of BEPCs on scaffolds and glass slides for 3
days, BEPCs were fixed with 4% paraformaldehyde, dissolved in PBS
for 10 min and permeabilized using 0.3% Triton X-100 in PBS. BEPCs
were then subjected to treatment with 10% normal goat serum (Gibco;
Thermo Fisher Scientific, Inc.). Following this, cells were
incubated with rabbit monoclonal anti-CD34 and rabbit monoclonal
anti-CD133 antibodies at 4°C for 12 h. The coverslips and two
hybrid scaffolds were subsequently washed three times with PBS for
10 min each. The two hybrid groups and the control group were
incubated with fluorescent labeled secondary antibodies (1:500;
Alexa Fluor 546 goat anti-rabbit immunoglobulin G; BD Biosciences,
Franklin Lakes, NJ, USA) in PBS for 1 h. Following three washes
with PBS, cell nuclei were counterstained with DAPI for 5 min at
room temperature. Fluorescent images were obtained using a Nikon
eclipse 80i fluorescence microscope. CD34 protein staining was
investigated in five visual fields at a magnification of 10×0.30,
and the total percentages of live and positive cells were
determined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression levels of BEPCs cultured on two
hybrid scaffolds and glass slides were analyzed via RT-qPCR. mRNA
was isolated from samples at the 7 day time interval using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A NanoDrop
ND1000 Spectrophotometer (Thermo Fisher Scientific, Inc.) was used
to quantify the extracted RNA samples. Samples with A260/A280
ratios between 1.9–2.1 were used for subsequent analysis. Total RNA
was subjected to RT using the PrimeScript RT reagent kit (Perfect
Real Time; Takara Biotechnology Co., Ltd., Dalian, China). RT-qPCR
was performed in a 10 µl reaction volume containing 1 µl of
complementary DNA, 3 µl of doubled-distilled H20, 5 µl
of reaction mixture and 1 µl of primers. The primer sequences used
are presented in Table II. In the
present study, seven primers [aquaporin 1 (AQP1), collagen type
VIII alpha 1 (COL8A1), ATP binding cassette subfamily G member 2
(ABCG2), leucine rich repeat containing G protein-coupled receptor
5 (LGR5), Apoptosis-Related Cysteine Peptidase (Caspase-3),
Activated Leukocyte Cell Adhesion Molecule (CD166) and interleukin
1 (IL-1)] were used to investigate proliferation, stem cell
characteristics, apoptosis and inflammatory reaction of BEPCs in
the scaffold groups and the control group. RT-qPCR was performed
using a Light Cycler 96 System (Roche Diagnostics, Basel,
Switzerland). Each assay was performed in triplicate. Relative gene
expression was analyzed by using the Pfaffl method (23).
 | Table II.Primers utilized for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primers utilized for reverse
transcription-quantitative polymerase chain reaction.
| Genes | Accession
number | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) | Product size
(bp) |
|---|
| AQP-1 | NM_012778.1 |
ACCTGCTGGCCATTGACTAC |
AGGGCACTCCCAATGAATGG | 127 |
| COL8A1 | NM_001107100.1 |
TTGCTTACCATGTTCACTGCAAGG |
AAAGCCCTTCTTGTACTCGTCGTA | 101 |
| ABCG2 | NM_181381.2 |
GTGTAGGTCGGTGTGCGAGT |
GATCTATGCCTTTCTAGCTGTCCC | 70 |
| LGR5 | NM_001106784.1 |
GCGTCTTCACCTCCTACCTG |
TTTCCAGCAAGACGCAACTC | 106 |
| CD166 | NM_031753.1 |
AGGGGACAACATCACCCTTC |
AGCCTGCCCTGGTAAGTAAA | 82 |
| Caspase-3 | NM_012922.2 |
GACCATGGAGAACAACAAAAC |
GGCAGGCCTGAATGATGAAG | 494 |
| IL-1 | NM_017019.1 |
TCGGGAGGAGACGACTCTAA |
GAAAGCTGCGGATGTGAAGT | 201 |
| GAPDH | NM_017008.4 |
CATGTTTGTGATGGGTGTGAACCA |
AAAGTTGTCATGGATGACCTTGGC | 115 |
Statistical analysis
Data in the present study were presented as the mean
± standard error of the mean. One-way analysis of variance with
Tukey's HSD post hoc test were used to investigate the statistical
significance between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
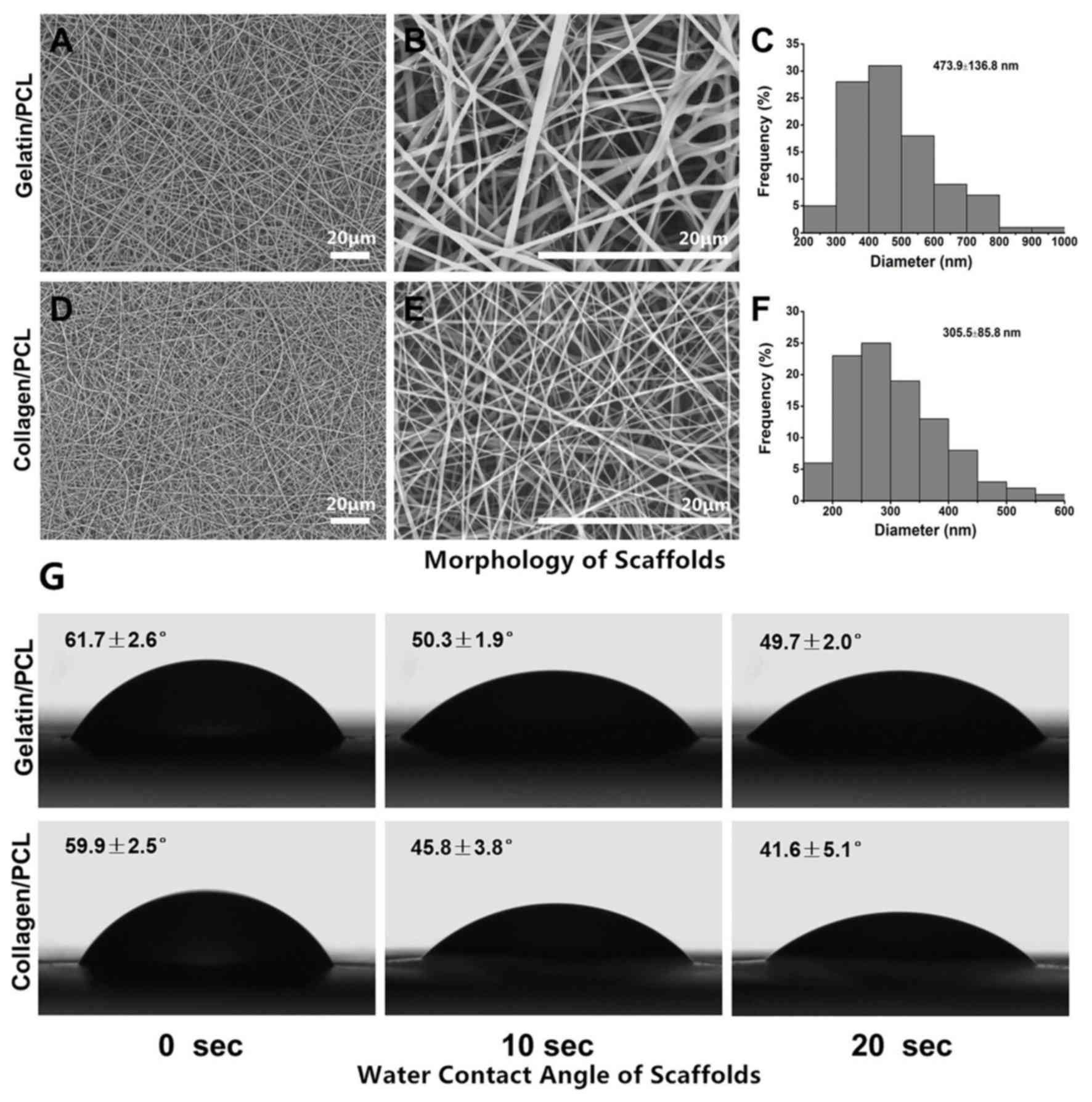
Morphology of scaffolds
The fiber morphology and dimension distribution of
the two variations of electrospun membranes were investigated via
SEM. As presented in Fig. 1A-C,
gelatin/PCL (70:30) fibers were demonstrated to be smooth and fine,
with an average fiber diameter of 473.9±136.8 nm. Electrospun
collagen/PCL fibrous membranes (70:30) were also revealed to be
smooth and uniform, with an average diameter of 305.5±85.8 nm
(Fig. 1D-F). According to the
results, the two samples presented with similar morphology and
microstructures despite varying materials and electrospinning
conditions.
Water contact angle of scaffolds
The hydrophilicity of the membranes, which can be
altered according to its composition, has marked effects on the
adhesion, proliferation and viability of cells (24). The water contact angle may be
determined to investigate wettability. As revealed in Fig. 1G, water droplets were rapidly
absorbed into the fibrous networks at the beginning, the contact
angle reached almost the same as that at 0 sec (61.7±2.6° vs.
59.9±2.5°). As the time increased, the exhibited contact angle
slowly decreased. Notably, the collagen/PCL (70:30) fibrous
membrane decreased at a faster rate compared with the gelatin/PCL
(70:30) fibrous membrane. These results suggested that the
gelatin/PCL and collagen/PCL electrospun membranes were
hydrophilic, and thus potentially suitable for cell adhesion.
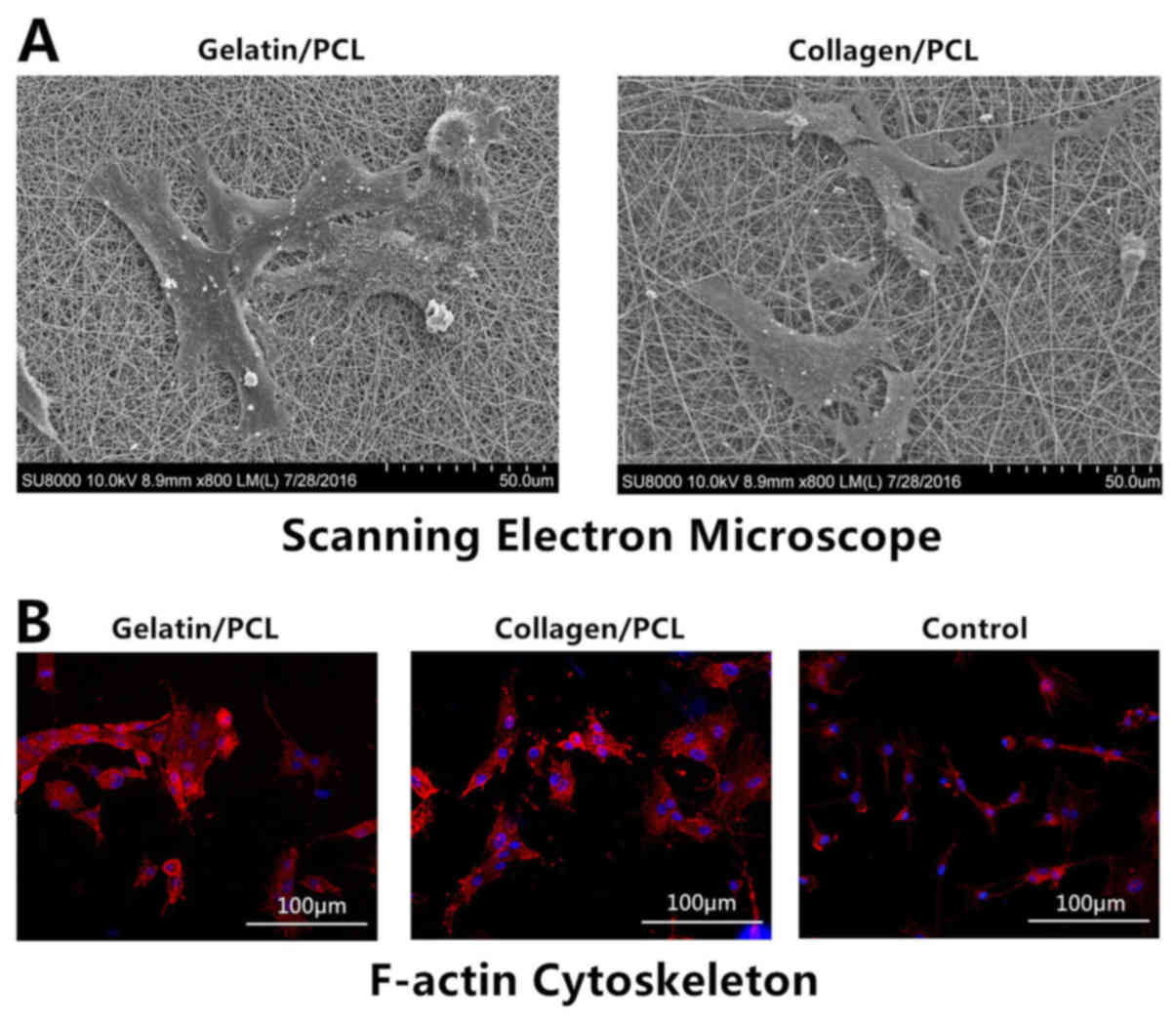
Cell morphology and adherence on
scaffolds
SEM was used to investigate cell morphology and
adherence on scaffolds. Following 24 h of cell seeding, BEPCs were
revealed to be well spread on and strongly adhered to both
scaffolds. BEPCs on the two hybrid scaffolds exhibited a
three-dimensional interdigitated structure and presented a
crossover growth trend. The results of SEM were indicated (Fig. 2A). To investigate the adherence of
BEPCs and the cellular compatibility on the two hybrid scaffolds,
F-actin cytoskeletons were visualized in the scaffold groups and
the control group using rhodamin-phalloidin. Cells in the hybrid
scaffold groups exhibited aggregation and increased levels of
F-actin bundles compared with cells in the control group (Fig. 2B). These results suggested that
collagen/PCL (70:30) and gelatin/PCL (70:30) scaffolds exhibited
cellular compatibility and benefited cell adherence.
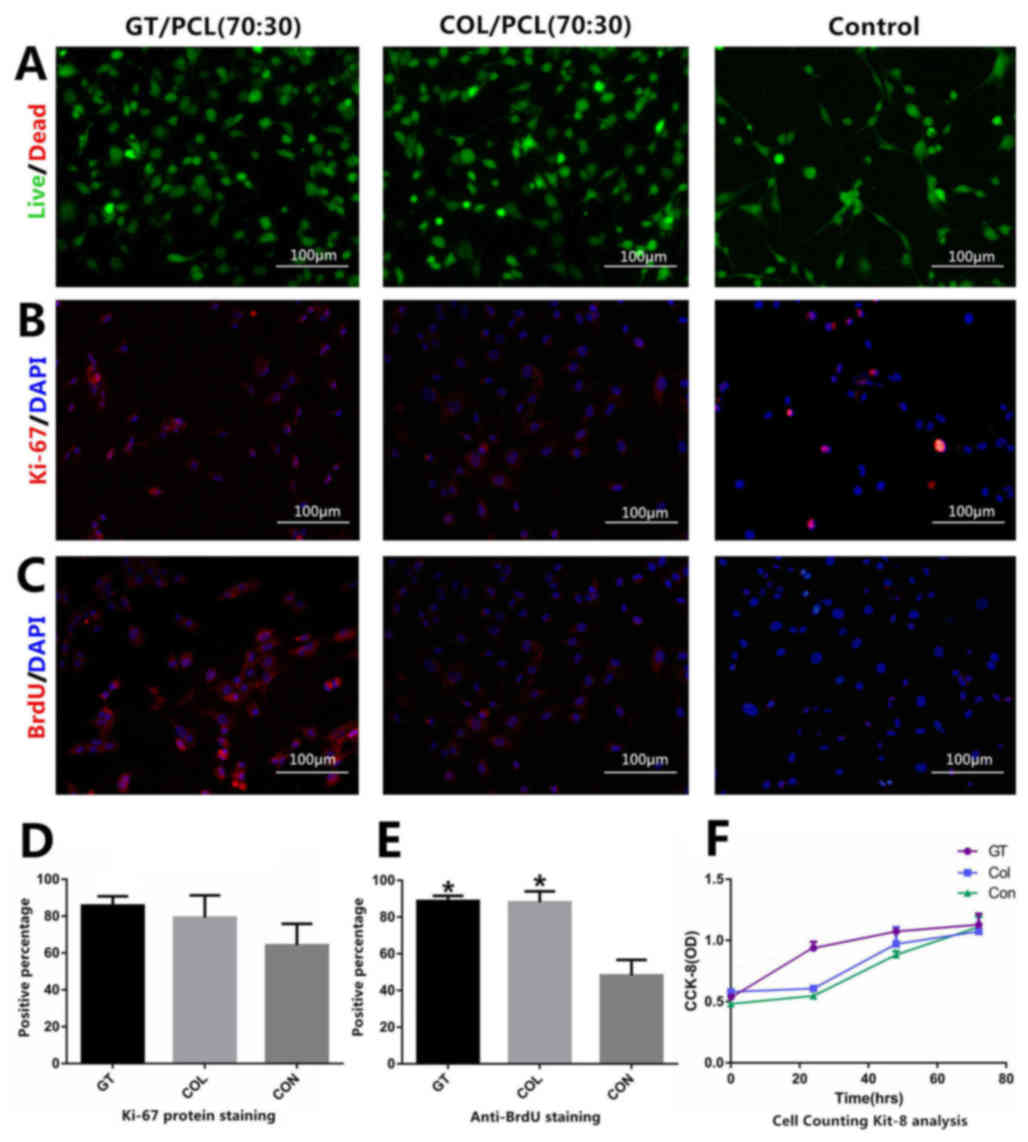
Cell proliferation and viability on
hybrid scaffolds
A live&dead Viability/Cytotoxicity kit was used
to investigate the toxicity of the materials. Apart from the
results regarding cell densities, no significant differences were
exhibited between any of the groups. Marked levels of dead cells
were not revealed in any groups (Fig.
3A). Immunocytochemistry staining of Ki-67 and BrdU was
performed to investigate levels of cell proliferation. The results
of Ki-67 and BrdU staining demonstrated that BEPCs on collagen/PCL
(70:30) and gelatin/PCL (70:30) exhibited greater levels of
proliferation compared with the control group (Fig. 3B and C)., D and E, compared with the
control group, both scaffold groups presented a statistically
significant increase following BrdU staining (P<0.05; Fig. 3E). The results of Ki-67 and BrdU
staining, which was investigated in five visual fields at a
magnification of 10×0.30, are presented in Fig. 3D and E. Furthermore, CCK-8 analysis
was performed to investigate the cell proliferation and cellular
compatibility of the two hybrid scaffolds. The results demonstrated
that the OD values were not significantly different between the
collagen/PCL (70:30) group and the control group. Notably, the OD
values exhibited by the gelatin/PC L (70:30) group were increased
compared with the two groups at 0, 24 and 48 h time intervals. At
the 72 h time interval, the OD values were not significantly
different among the three groups (Fig.
3F).
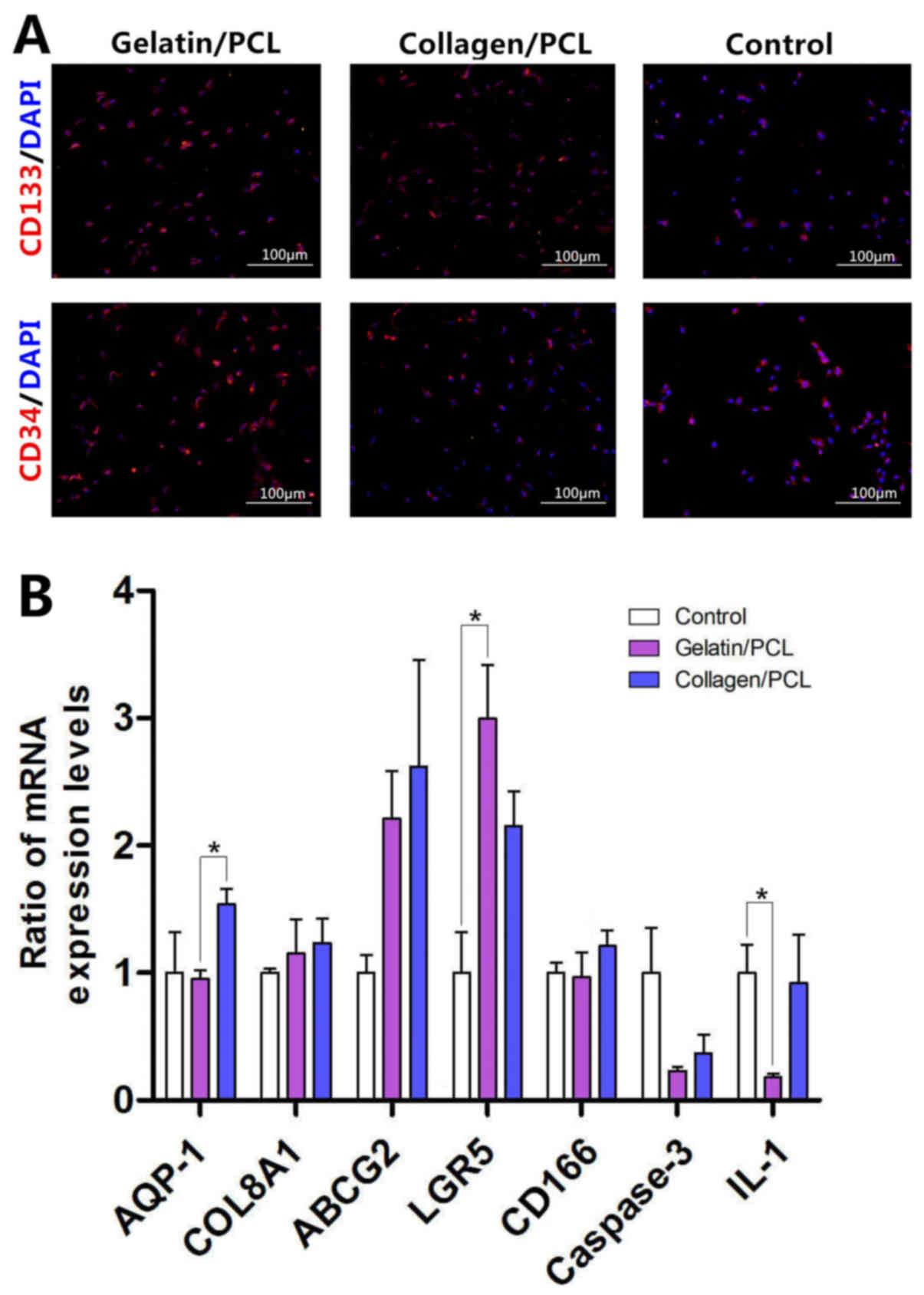
Stem cell characteristics of BEPCs on
two hybrid scaffolds
Immunocytochemistry analysis investigating the
expression levels of CD133 and CD34 proteins, which represent
biomarkers of stem cells, was performed to determine and evaluate
the stem cell characteristics of BEPCs on two hybrid scaffolds.
Cultured BEPCs on the two hybrid scaffolds demonstrated increased
levels of fluorescence intensity exhibited by the CD133 and CD34
proteins compared with the control group; however, this result was
not statistically significant (Fig.
4A). In addition, both scaffold groups exhibited increased
expression levels of ABCG2 and LGR5 compared with the control
group. Notably, the expression levels of other proteins (AQP-1,
COL8A1 and CD166) on both scaffold groups were not statistically
different compared with the control group (Fig. 4B).
Biocompatibility of two hybrid
scaffolds
The expression levels of caspase-3 and IL-1 were
investigated in the present study to determine the biocompatibility
of the two hybrid scaffolds. Apoptosis levels, as determined via
the investigation of caspase-3 expression levels, were revealed to
be decreased in the two scaffold groups compared with the control
group; however, this result was not statistically significant.
Furthermore, the results demonstrated that there were no
significant differences in the expression levels of IL-1 exhibited
in the collagen/PCL group and control group; whereas the expression
levels of IL-1 exhibited by the gelatin/PCL group were
significantly suppressed compared with the control group (Fig. 4B). These results suggested that the
two scaffolds exhibited biocompatibility.
Discussion
Tissue-engineering for corneal endothelium has been
heavily researched in recent years regarding the treatment for
patients with corneal endothelial decompensation. Establishing a
suitable carrier material is important for establishing
tissue-engineered endothelium. Natural polymers, such as collagen
and gelatin, have previously been electrospun into fibrous
scaffolds for tissue engineering of corneal epithelium (10). However, it remains unknown whether
collagen and gelatin are appropriate materials for
tissue-engineered endothelium. Scaffolds used in tissue-engineered
corneal endothelium are required to exhibit sufficient mechanical
strength and good biocompatibility, which may promote cell
proliferation and differentiation. Hybrid scaffolds could meet the
requirements by taking advantage of natural and synthetic material
properties (25,26). In the present study, gelatin/PCL and
collagen/PCL hybrid scaffolds were successfully established using
electrospun techniques, and the modulating responses of BEPCs
cultured on the scaffolds were investigated. This aimed to
elucidate the potential applicability of the scaffolds in
tissue-engineered endothelium.
The two hybrid scaffolds established in the present
study demonstrated expected hydrophilicity, wettability and
biocompatibility characteristics, which were most likely due to the
exhibition of beneficial characteristics associated with natural
and synthetic polymers. SEM analyses demonstrated that BEPCs
presented a crossover growth trend and exhibited a
three-dimensional interdigitated structure on the two scaffolds. As
presented in Fig. 2, BEPCs in the
two hybrid scaffold groups exhibited increased levels of
aggregation and F-actin bundles compared with the control group.
The cellular morphology and adhesion suggested that the electrospun
nanofibers established in the present study successfully emulated
the properties of the extracellular matrix. BEPCs were well spread
and strongly adhered to both scaffolds.
BEPCs cultured on both scaffolds exhibited greater
levels of proliferation and viability. Immunocytochemistry staining
of Ki-67 and BrdU proteins revealed that the two scaffolds provided
a suitable environment for cell growth, likely due to properties
exhibited by the scaffolds that are associated with the
extracellular matrix. However, CCK-8 analysis demonstrated that
there were no significant differences in the proliferation levels
exhibited by each group. At 24 and 48 h time intervals, the OD
values of gelatin/PCL were increased compared with the other two
groups, whereas the collagen/PCL group did not exhibit significant
differences in OD values compared with the control group. In
addition, the OD values exhibited at 0 and 72 h time intervals were
not significantly different between any of the groups. Therefore,
it may be suggested that such differences may be due to dissimilar
topography between scaffolds and glass slides, thus permitting
cells to attach and spread more easily from the outset. The growth
of BEPCs on scaffolds for increased durations of time requires
further study. In the present study, few dead cells on scaffolds
were observed, which suggested that the two hybrid nanofiber
scaffolds investigated in the present study were safe and nontoxic
for proliferating cells. Furthermore, the results revealed that the
mRNA expression levels of caspase-3 and IL-1 were decreased in
gelatin/PCL groups, which suggested that the scaffold did not
induce inflammatory responses or apoptosis. The collagen/PCL
scaffold exhibited decreased levels of caspase-3, while the
expression levels of IL-1 did not demonstrate a significant
difference compared with the control group. A previous study
revealed that, when cultured with NIH 3T3 cells, collagen exhibits
significantly enhanced levels of cytocompatibility compared with
gelatin (27). In the present study,
no significant differences between the two hybrid scaffolds were
demonstrated, which suggests that the two natural components may be
safely used when applied to the culturing BEPCs. The
biocompatibility of scaffolds used in tissue-engineered endothelium
is important to ensure cell density and to forma cell monolayer
(28–30).
Tissue-engineered endothelium requires a substratum
with an organized monolayer of cells. The morphology and cell
density of the newly formed cell monolayer depends on the
differentiation status of the transplanted cells (28–31). It
is important for scaffolds to enable cells to retain
characteristics associated with stem cells. BEPCs cultured on
hybrid nanofibers scaffolds demonstrated a marked increase in the
levels of stem cell markers. CD34 and CD133 protein fluorescence
intensities expressed by the scaffold groups were enhanced compared
with the control group; however, this result was not statistically
significant. This suggested that BEPCs cultured on the two hybrid
scaffolds demonstrated more avidity. PCR analyses of marker genes
and differentiation genes associated with stem cells, including
ABCG2 and LGR5 (32–34), exhibited increased expression levels
in the scaffold groups compared with the control group. Nanofibers
were revealed to simulate the three-dimensional extracellular
matrix, and thus it may be suggested that approximate
three-dimensional topography and chemical stimulation of hybrid
nanofiber scaffolds have important roles in pluripotency
maintaining of BEPC. However, expression levels of marker genes
associated with CECs in the scaffold groups, including AQP-1,
COL8A1 and CD166, were revealed to not be significantly different
compared with the control group in the present study. These results
suggested that the two scaffolds may enhance the pluripotency of
BEPCs via numerous mechanisms; however, culturing on the scaffolds
alone did not promote the differentiation of BEPCs into CECs.
Whether BEPCs cultured on the scaffolds may differentiate into CECs
requires further study.
In the present study, collagen/PCL (70:30) and
gelatin/PCL (70:30) combined scaffolds were successfully
established using electrospun techniques and exhibited sufficient
levels of hydrophilicity, wettability and biocompatibility. BEPCs
cultured on the two scaffolds exhibited greater levels of
proliferation and adhesion, and retained more characteristics
associated with stem cells. The two scaffolds exhibited the
possibility of enhancing the differentiation potential of BEPCs.
These results suggested that the two hybrid scaffolds maybe used to
culture BEPCs that exhibit the potential to differentiate into
corneal endothelial-like cells. By improving the manufacturing
method of these two hybrid scaffolds, novel strategies to enhance
the transparency and mechanical properties of scaffolds so that
they are more appropriate for tissue-engineered endothelium may be
established. The results of the present study may provide novel
ideas for the treatment of corneal endothelial dystrophy.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation (grant nos. 81370992, 81770332,
81570812, 81500765, 81600774 and 81601622), The Science and
Technology Commission of Shanghai (grant no. 17DZ2260100), National
High Technology Research and Development Program (863 Program;
grant no. 2015AA020311), Shanghai Municipal Education Commission
Gaofeng Clinical Medicine Grand Support (grant no. 20161421), Youth
Science and Technology Talent Sail Plan of Shanghai (grant no.
15YF1407400), Shanghai Municipal Commission of Health and Family
Planning Found (grant no. 20144Y0221) and the Shanghai Young Doctor
Training Program.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, BF, FL and YF conceived and designed the current
study. YH, BF, WZ, CY, FY, QY and CS performed the experiments. YH
and CS wrote the paper. BF, FL and YF reviewed and edited the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Research Committee of Ninth People's Hospital, Shanghai Jiao
Tong University School of Medicine (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DPAI
|
4,6-diamidino-2-phenylindole
|
|
PCL
|
polycaprolactone
|
|
AQP1
|
aquaporin 1
|
|
COL8A1
|
collagen type VIII alpha 1
|
|
ABCG2
|
ATP binding cassette subfamily G
member 2
|
|
LGR5
|
leucine rich repeat containing G
protein-coupled receptor 5
|
|
IL-1
|
interleukin 1
|
References
|
1
|
Bourne WM, Nelson LR and Hodge DO: Central
corneal endothelial cell changes over a ten-year period. Invest
Ophthalmol Vis Sci. 38:779–782. 1997.PubMed/NCBI
|
|
2
|
Peh GS, Beuerman RW, Colman A, Tan DT and
Mehta JS: Human corneal endothelial cell expansion for corneal
endothelium transplantation: An overview. Transplantation.
91:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Navaratnam J, Utheim TP, Rajasekhar VK and
Shahdadfar A: Substrates for expansion of corneal endothelial cells
towards bioengineering of human corneal endothelium. J Funct
Biomater. 6:917–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joyce NC: Proliferative capacity of the
corneal endothelium. Prog Retin Eye Res. 22:359–389. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Proulx S and Brunette I: Methods being
developed for preparation, delivery and transplantation of a
tissue-engineered corneal endothelium. Exp Eye Res. 95:68–75. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bashur CA, Dahlgren LA and Goldstein AS:
Effect of fiber diameter and orientation on fibroblast morphology
and proliferation on electrospun poly(D,L-lactic-co-glycolic acid)
meshes. Biomaterials. 27:5681–5688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu C, Inai R, Kotaki M and Ramakrishna S:
Electrospun nanofiber fabrication as synthetic extracellular matrix
and its potential for vascular tissue engineering. Tissue Eng.
10:1160–1168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li WJ, Laurencin CT, Caterson EJ, Tuan RS
and Ko FK: Electrospun nanofibrous structure: A novel scaffold for
tissue engineering. J Biomed Mater Res. 60:613–621. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chew SY, Wen J, Yim EK and Leong KW:
Sustained release of proteins from electrospun biodegradable
fibers. Biomacromolecules. 6:2017–2024. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Ren L and Wang Y: Crosslinked
collagen-gelatin-hyaluronic acid biomimetic film for cornea tissue
engineering applications. Mater Sci Eng C Mater Biol Appl.
33:196–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K, Chen X, Pan Y, Cui Y, Zhou X, Kong
D and Zhao Q: Enhanced vascularization in hybrid PCL/gelatin
fibrous scaffolds with sustained release of VEGF. Biomed Res Int.
2015:8650762015.PubMed/NCBI
|
|
12
|
Hosseini Y, Agah M and Verbridge SS:
Endothelial cell sensing, restructuring, and invasion in collagen
hydrogel structures. Integr Biol (Camb). 7:1432–1441. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye J, Wang J, Zhu Y, Wei Q, Wang X, Yang
J, Tang S, Liu H, Fan J, Zhang F, et al: A thermoresponsive
polydiolcitrate-gelatin scaffold and delivery system mediates
effective bone formation from BMP9-transduced mesenchymal stem
cells. Biomed Mater. 11:0250212016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Ouyang H, Lim CT, Ramakrishna S
and Huang ZM: Electrospinning of gelatin fibers and gelatin/PCL
composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater.
72:156–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tillman BW, Yazdani SK, Lee SJ, Geary RL,
Atala A and Yoo JJ: The in vivo stability of electrospun
polycaprolactone-collagen scaffolds in vascular reconstruction.
Biomaterials. 30:583–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McKenna KA, Hinds MT, Sarao RC, Wu PC,
Maslen CL, Glanville RW, Babcock D and Gregory KW: Mechanical
property characterization of electrospun recombinant human
tropoelastin for vascular graft biomaterials. Acta Biomater.
8:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han F, Jia X, Dai D, Yang X, Zhao J, Zhao
Y, Fan Y and Yuan X: Performance of a multilayered small-diameter
vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials.
34:7302–7313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin A, Zhang K, McClure MJ, Huang C, Wu J,
Fang J, Mo X, Bowlin GL, Al-Deyab SS and El-Newehy M:
Electrospinning collagen/chitosan/poly(L-lactic
acid-co-ε-caprolactone) to form a vascular graft: Mechanical and
biological characterization. J Biomed Mater Res A. 101:1292–1301.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Yan C, Zhu M, Yao Q, Shao C, Lu W,
Wang J, Mo X, Gu P, Fu Y and Fan X: Electrospun nanofibrous SF/P
(LLA-CL) membrane: A potential substratum for endothelial
keratoplasty. Int J Nanomedicine. 10:3337–3350. 2015.PubMed/NCBI
|
|
20
|
Shao C, Fu Y, Lu W and Fan X: Bone
marrow-derived endothelial progenitor cells: A promising
therapeutic alternative for corneal endothelial dysfunction. Cells
Tissues Organs. 193:253–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao C, Chen J, Chen P, Zhu M, Yao Q, Gu
P, Fu Y and Fan X: Targeted transplantation of human umbilical cord
blood endothelial progenitor cells with immunomagnetic
nanoparticles to repair corneal endothelium defect. Stem Cells Dev.
24:756–767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng B, Tu H, Yuan H, Peng H and Zhang Y:
Acetic-acid-mediated miscibility toward electrospinning homogeneous
composite nanofibers of GT/PCL. Biomacromolecules. 13:3917–3925.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoeruek E, Saygili O, Spitzer MS, Tatar O,
Bartz-Schmidt KU and Szurman P: Human anterior lens capsule as
carrier matrix for cultivated human corneal endothelial cells.
Cornea. 28:416–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goddard JM and Joseph JH: Polymer surface
modification for the attachment of bioactive compounds. Progr
Polymer Sci. 32:698–725. 2007. View Article : Google Scholar
|
|
25
|
Duan H, Feng B, Guo X, Wang J, Zhao L,
Zhou G, Liu W, Cao Y and Zhang WJ: Engineering of epidermis skin
grafts using electrospun nanofibrous gelatin/ polycaprolactone
membranes. Int J Nanomedicine. 8:2077–2084. 2013.PubMed/NCBI
|
|
26
|
Fu W, Liu Z, Feng B, Hu R, He X, Wang H,
Yin M, Huang H, Zhang H and Wang W: Electrospun gelatin/PCL and
collagen/PLCL scaffolds for vascular tissue engineering. Int J
Nanomedicine. 9:2335–2344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Zhang W, Yuan J and Shen J:
Differences in cytocompatibility between collagen, gelatin and
keratin. Mater Sci Eng C Mater Biol Appl. 59:30–34. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engelmann K, Drexler D and Böhnke M:
Transplantation of adult human or porcine corneal endothelial cells
onto human recipients in vitro. Part I: Cell culturing and
transplantation procedure. Cornea. 18:199–206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engelmann K, Bednarz J and Bohnke M:
Endothelial cell transplantation and growth behavior of the human
corneal endothelium. Ophthalmologe. 96:555–562. 1999.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Engelmann K, Bohnke M and Friedl P:
Isolation and long-term cultivation of human corneal endothelial
cells. Invest Ophthalmol Vis Sci. 29:1656–1662. 1988.PubMed/NCBI
|
|
31
|
Tan DT, Anshu A and Mehta JS: Paradigm
shifts in corneal transplantation. Ann Acad Med Singapore.
38:332–338. 2009.PubMed/NCBI
|
|
32
|
Hirata-Tominaga K, Nakamura T, Okumura N,
Kawasaki S, Kay EP, Barrandon Y, Koizumi N and Kinoshita S: Corneal
endothelial cell fate is maintained by LGR5 through the regulation
of hedgehog and Wnt pathway. Stem Cells. 31:1396–1407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okumura N, Nakamura T, Kay EP, Nakahara M,
Kinoshita S and Koizumi N: R-spondin1 regulates cell proliferation
of corneal endothelial cells via the Wnt3a/β-catenin pathway.
Invest Ophthalmol Vis Sci. 55:6861–6869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|