Introduction
Portal vein thrombosis (PVT) is a form of venous
thrombus, which develops into the trunk of the portal vein and may
extend to the splenic or superior mesenteric veins (1). PVT occurs in association with
pancreatitis, cirrhosis, diverticulitis and malignancy, and is
known as a serious complication of splenectomy (2,3). It has
been reported that the prevalence of PVT is 4.4–15% in patients
with cirrhosis, approximately 35% in cirrhotic patients with
hepatocellular carcinoma (4) and
37.5–43.5% in patients who have undergone a splenectomy (5). As PVT is the main cause of portal
hypertension, preventing PVT has become an important goal in
clinical practice (6).
P-selectin, a cell adhesion molecule with
procoagulant properties, serves an important role in thrombosis
(7). An interaction between
P-selectin and P-selectin glycoprotein ligand (PSGL) has been
demonstrated to induce the recruitment of neutrophils and
macrophages to promote the generation of procoagulant
microparticles (8). Recombinant
(r)PSGL immunoglobulin G (Ig) is an antibody of PSGL-1 and
antagonizes P-selectin by competing with PSGL-1 to inhibit
thrombosis (9). It has been reported
that following 14-day treatment with rPSGL-Ig, the percentage of
spontaneous vein reopening in the proximal iliac vein of baboons
with vein thrombi was significantly increased compared with that in
the control group (62% vs. 8%, respectively) (10). The occurrence of thrombi in the
jugular veins of cats was completely (4.0 mg/kg) or partially (1.0
mg/kg) prevented by rPSGL-Ig (11).
rPSGL-Ig inhibits the binding of circulating activated platelets
with neutrophils at damaged arterial surfaces (12). Although rPSGL-Ig is known to inhibit
thrombosis, the specific roles of rPSGL-Ig in preventing PVT remain
unclear.
Since the first vein thrombosis model was
established in rats in 1980 by ligation of the inferior vena cava
(13), additional models have been
constructed using various methods. Complete vein ligation is a
commonly used modeling method for vein thrombosis (14,15).
Although a stable thrombus can be formed, complete vein ligation is
not suitable for dynamic observation of antithrombotic drugs
because the patency of the venous proximal end cannot be guaranteed
(16). Additionally, foreign bodies
and electric stimulation cause great damage to veins, and the
injection of a thrombus-inducing agent may influence the evaluation
of antithrombotic drugs (17–19). As
studies using PVT models are limited, a suitable modeling method of
PVT is urgently needed.
In the current study, a novel modeling method,
intermittent portal vein obstruction (IPVO) combined with endangium
destruction, was used to construct a PVT model in rats. The effects
of rPSGL-Ig on PVT formation were evaluated by B-scan
ultrasonography, hematoxylin-eosin (HE) staining and transmission
electron microscopy (TEM). The authors of the current study
developed an effective method for constructing a PVT model in rats
and determined the inhibitory role of rPSGL-Ig in PVT
formation.
Materials and methods
Construction of the rat model of
PVT
A total of 90 Sprague Dawley specific pathogen-free
rats (weight, 200–300 g; age, 2–3 months; female, n=45; male, n=45)
were purchased from Shanghai Jiesijie Laboratory Animal Co. Ltd.
(Shanghai, China). The rats were housed at 26±1°C in a standalone
environment under an alternating day and night cycle of 12/12 h
with free access to water and food. Rats were made to fast for 24 h
prior to model construction and the PVT model was constructed
through IPVO combined with endangium destruction. The rats were
anesthetized by an intraperitoneal injection of 5% chloral hydrate
(350 mg/kg) and fixed on an operating table. The abdominal cavity
was opened by making a longitudinal incision (4–5 cm) along the
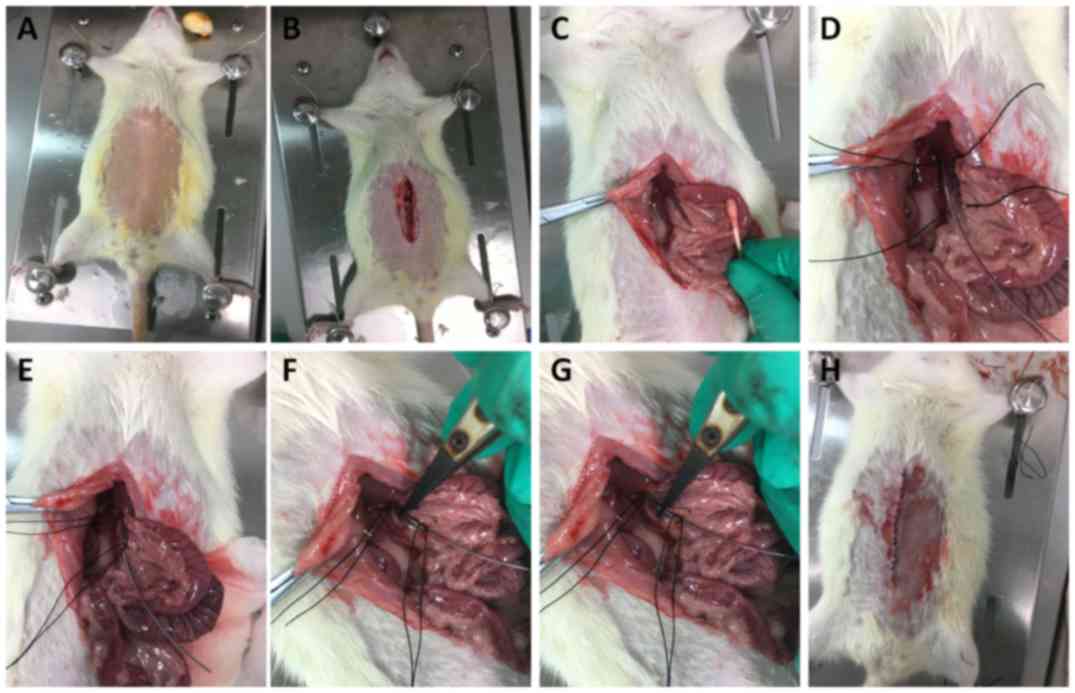
epigastric midline (Fig. 1A and B).
The intestinal canal was removed from the abdominal cavity with a
wet cotton swab and covered with gauze containing physiological
saline (Fig. 1C). Connective tissue
around the portal vein was bluntly separated and 1.5–2 cm of the
free portal vein was isolated (Fig.
1D). Next, the proximal and distal ends of the free portal vein
(including the epidural catheter) were ligated using a suture 4-0
(Fig. 1E). Toothless tweezers were
used to clamp the portal vein at the proximal and distal ends for 5
sec every 5 min (Fig. 1F and G).
Portal vein ligation was stopped after 20 min of treatment and then
blood flow was allowed for 10 min. These steps (ligation and clamp)
were repeated five times. Finally, 2 ml 3% ceftazidime solution was
injected into the abdominal cavity and the abdominal cavity was
sutured (Fig. 1H). The formation of
PVT (including the thrombus size and vessel diameter) was
continuously observed prior to and following the surgery using a
B-mode ultrasound instrument (Philips Healthcare, Amsterdam, The
Netherlands). The sham operation group was used as a control; in
this group the portal vein was only dissociated, and no ligation
and clamping was performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR analyses were performed to detect the mRNA
expression level of P-selectin, a biomarker of PVT (20) in rats of the model and control groups
24 h following surgery. Total RNA was extracted from blood samples
using RNA Extraction kit and reverse transcribed into cDNA using
the First Strand cDNA Synthesis kit (both Beyotime Institute of
Biotechnology, Shanghai, China). The following thermocycling
conditions were used for cDNA synthesis: 45°C for 60 min and 70°C
for 10 min. qPCR was subsequently performed using the
BeyoFast™ SYBR Green qPCR Mix (2X) kit (Beyotime
Institute of Biotechnology) using the following specific primer
pairs: P-selectin forward, 5′-GAGGCAGAGACCTCACAGCCAG-3′ and
reverse, 5′-GTCAGGTAAGTGGCCAATG-3′; and β-actin forward,
5′-ACACCTTCTACAATGAGCTG-3′ and reverse, 5′-CTGCTTGCTGATCCCATCT-3′.
The following thermocycling conditions were used for qPCR: Initial
denaturation at 94°C for 3 min; 30 cycles at 94°C for 30 sec, 62°C
for 30 sec and 72°C for 30 sec; and a final extension at 72°C for 5
min. The relative P-selectin mRNA levels were quantified using the
2−ΔΔCq method (21) and
normalized to the internal reference gene β-actin.
rPSGL-Ig intervention
Rats were divided into three groups (10 rats/group):
Model, Control and rPSGL-Ig. rPSGL-Ig was prepared by Hangzhou
S-Evans Biosciences Co., Ltd. (Hangzhou, China). A total of 4 mg/kg
rPSGL-Ig was intraperitoneally injected into PVT rats at 1 h after
model construction (the rPSGL-Ig group). The formation of PVT in
the rPSGL-Ig group was further evaluated by B-scan ultrasonography
6, 12 and 24 h after the surgeries, and by HE staining and TEM.
HE staining and TEM
HE staining was performed on the portal vein,
central hepatic vein and vasa intestinae tenuis of rats in
different groups 24 h following surgery. Tissue samples were fixed
in 2% paraformaldehyde overnight at 4°C. Tissue samples were
dehydrated by ascending ethanol series (50, 70, 80 and 90% ethanol
each for 15 min, 70% ethanol overnight, and 100% ethanol for 20
min), soaked in acetone twice for 15 min and embedded in Araldite.
Following 48-h polymerization at 65°C, the embedded tissue samples
were cut into ultrathin slices (70 nm) using an ultramicrotome (EM
UC7; Leica Microsystems GmbH, Wetzlar, Germany). Tissue sections
were stained with hematoxylene for 4 min at room temperature and
couter stained with eosin for 90 sec at room temperature, and
observed under a light microscope (YYS-190E; Shanghai Optical
Instrument, Shanghai, China) at a magnification ×100 and ×400.
TEM were performed on the portal vein of rats in
different groups. Tissue slices were prepared as described above.
Following staining with uranyl acetate and lead citrate (both
Sinopharm Chemical Reagent Co., Ltd., Beijing, China) each for 10
min at room temperature, the samples were observed by TEM
(JEM-1230; JEOL, Ltd., Tokyo, Japan) 6, 12 and 24 h after the
surgeries.
Evaluation of the thrombolytic effect
of rPSGL-Ig
PVT model SD rats were divided randomly into four
groups (n=6/group): Control, 4 mg/kg rPSGL-Ig, 6 mg/kg rPSGL-Ig and
8 mg/kg rPSGL-Ig. Whole blood was collected from the portal vein at
48 h to detect the mRNA changes in P-selectin by RT-qPCR. Next, the
PVT model SD rats (n=10/group) were injected with saline, 8 mg/kg
rPSGL-Ig or 2×104 U/kg urokinase (URO; Livzon
Pharmaceutical Group Inc., Zhuhai, China), a commonly used
thrombolytic drug (22). The
thrombus size pretherapy, and 12, 24, and 48 h after rPSGL-Ig
treatment were observed using a B-mode ultrasound instrument.
Statistical analysis
Quantitative data were expressed as mean ± standard
deviation. The quantitative data between groups were analyzed by a
one-way analysis of variance followed by Bonferroni post hoc tests,
which were analyzed with SPSS version 17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 indicated that the difference between groups
was statistically significant.
Results
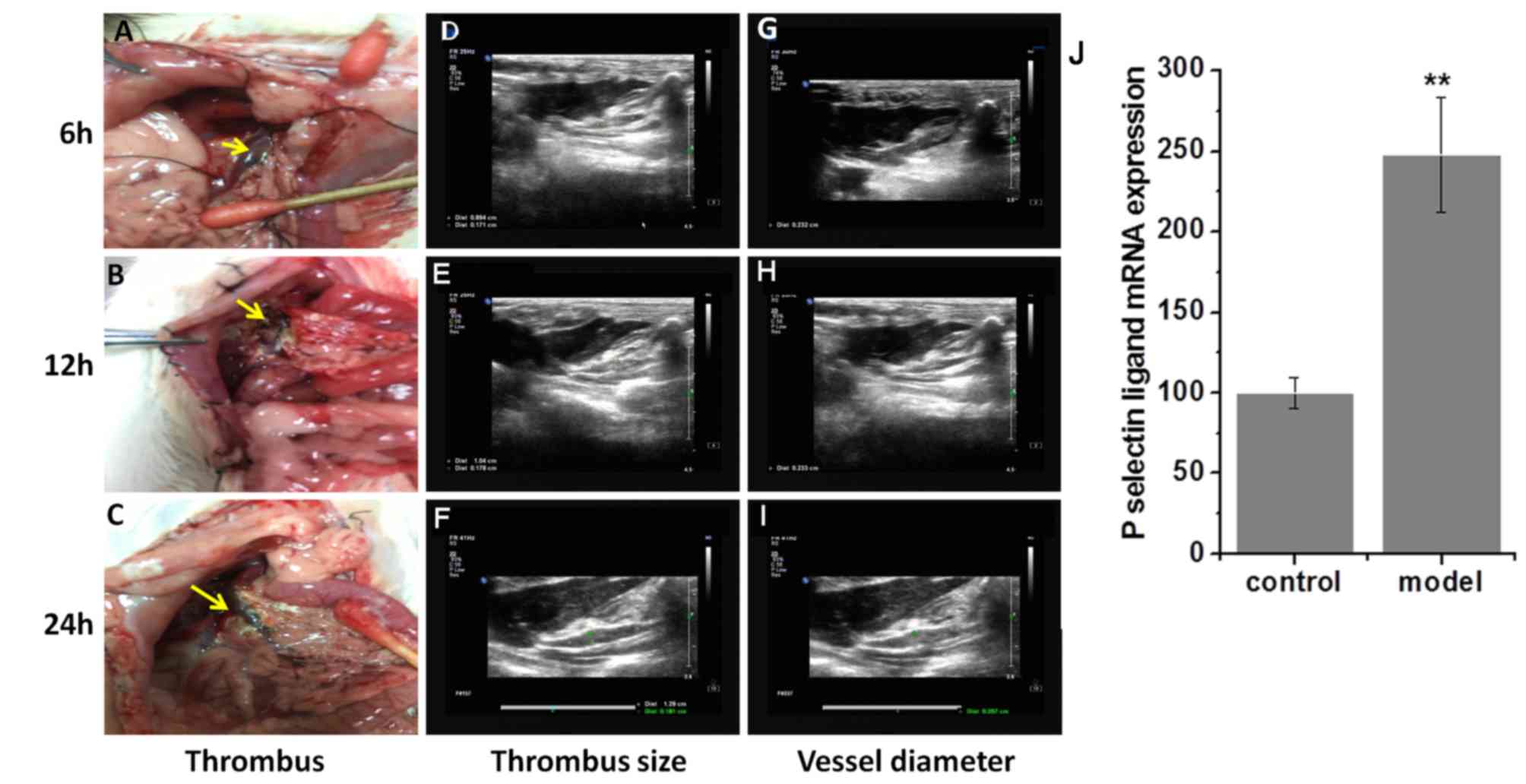
Thrombi and vessel diameters are
larger in the rat model of PVT
A rat model of PVT was successfully constructed
(Fig. 1). To examine the stability
of the thrombus, the vascular wall with visible thrombus was
punctured at 6, 12 and 24-h post-surgery. An unstable red thrombus,
which occurred following a hemorrhage, was formed 6 h after surgery
and became enlarged 12 h after surgery (Fig. 2A and B). At 24 h after surgery, a
stable black thrombus developed, exhibiting a hardened vascular
wall and venous stenosis (Fig. 2C).
B-Scan ultrasonography demonstrated that the thrombus size and
vessel diameter in the model group were significantly increased
starting 6 h post-surgery compared with the control group in what
appeared to be a time-dependent manner (all P<0.05; Table I; Fig.
2D-I). Additionally, a 2.5-fold higher expression of P-selectin
was observed in the model group compared with the control group
(P<0.01; Fig. 2J).
 | Table I.Portal vein thrombosis formation
detected by B-scan ultrasonography. |
Table I.
Portal vein thrombosis formation
detected by B-scan ultrasonography.
|
|
|
| Post-operation |
|---|
|
|
|
|
|
|---|
| Parameter | Group | 30 min
pre-operation | 30 min | 3 h | 6 h | 12 h | 24 h |
|---|
| Thrombus size
(cm2) | Control | 0 | 0 | 0 | 0 | 0 | 0 |
|
| Model | 0 | 0 | 0 |
0.079±0.037a |
0.138±0.035a |
0.204±0.039a |
|
| rPSGL-Ig | 0 | 0 | 0 |
0.049±0.006a,b |
0.062±0.022a,b |
0.096±0.025a,b |
| Vessel diameter
(cm) | Control | 0.134±0.009 | 0.135±0.011 | 0.134±0.009 | 0.142±0.009 | 0.147±0.020 | 0.148±0.010 |
|
| Model | 0.136±0.013 | 0.134±0.015 |
0.146±0.013a |
0.178±0.033a |
0.194±0.026a |
0.210±0.031a |
|
| rPSGL-Ig | 0.135±0.007 | 0.137±0.015 |
0.140±0.008b |
0.156±0.009b |
0.168±0.025a,b |
0.176±0.018a,b |
rPSGL-Ig inhibits PVT formation
The inhibitory effects of rPSGL-Ig on the formation
of PVT were evaluated by B-scan ultrasonography. Thrombus size and
vessel diameter were increased in the model group (Table I). Intervention with rPSGL-Ig
significantly inhibited PVT formation by lowering the thrombus size
and vessel diameter at 6–24 h post-surgery compared with the model
group (all P<0.05).
rPSGL-Ig relieves histopathological
changes in the portal vein, central hepatic vein, and vasa
intestinae tenuis
Histopathological changes in the portal vein,
central hepatic vein and vasa intestinae tenuis in rats of
different groups were evaluated by HE staining. A normal structure
of endangium with flattened endothelial cells and oriented typical
media smooth muscle cells in portal vein was presented in control
group (Fig. 3A and B). As shown in
Fig. 3C and D, the portal vein in
the model group exhibited a damaged endangium, exfoliated
endothelial cells and thickened media smooth muscle cells. A
thrombus containing an evident fibrin network, platelet trabeculae,
adipocytes and leukocytes was observed in the portal vein of the
model group. Following intervention with rPSGL-Ig, thrombus
formation in the portal vein was markedly inhibited, revealing an
imperceptible fibrin network and platelet trabeculae, and reduced
adipocytes (Fig. 3E and F).
Additionally, erythrocytes were not observed in the central hepatic
veins of the control group, which also presented with a compact
venous wall structure without cellular swelling (Fig. 4A and B). However, an expanded venous
wall, deposited erythrocytes and slightly swollen liver cells were
observed in the central hepatic veins of the model group (Fig. 4C and D). Although blood extravasation
was also observed in the rPSGL-Ig group, other histopathological
changes were markedly relieved (Fig. 4E
and F). Furthermore, erythrocytes were not present and normal
venous structure with compact intestinal wall structures was
observed in the vasa intestinae tenuis of the control group
(Fig. 5A and B). Similar
histopathological changes were observed in the vasa intestinae
tenuis of the model and rPSGL-Ig groups (Fig. 5C-F).
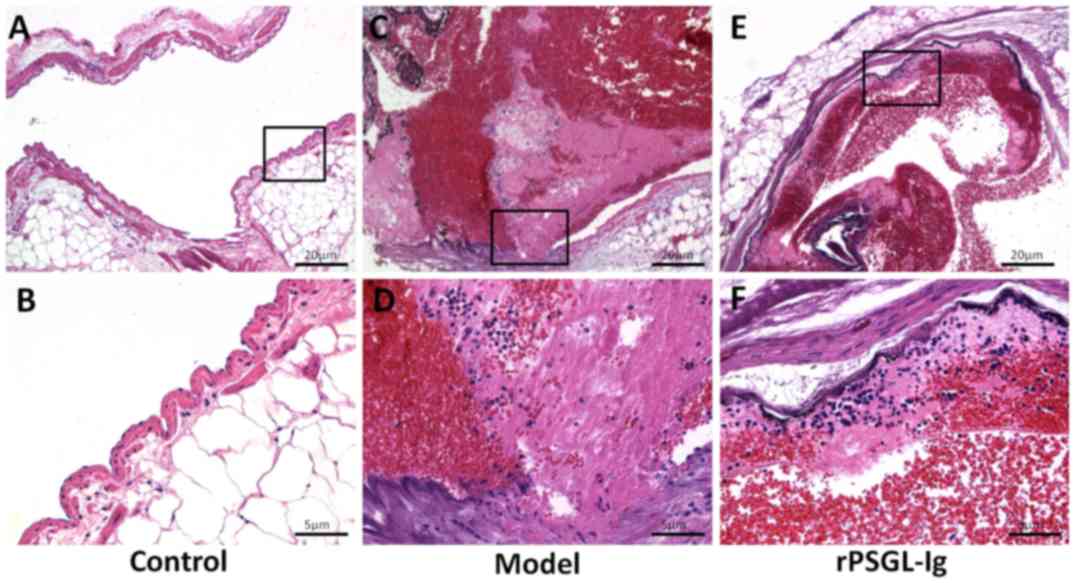 | Figure 3.Portal veins were stained with
hematoxylin and eosin in the control (A, magnification, ×100; B,
magnification, ×400), portal vein thrombosis model (C,
magnification, ×100; D, magnification, ×400) and rPSGL-Ig (E,
magnification, ×100; F, magnification, ×400) groups. |
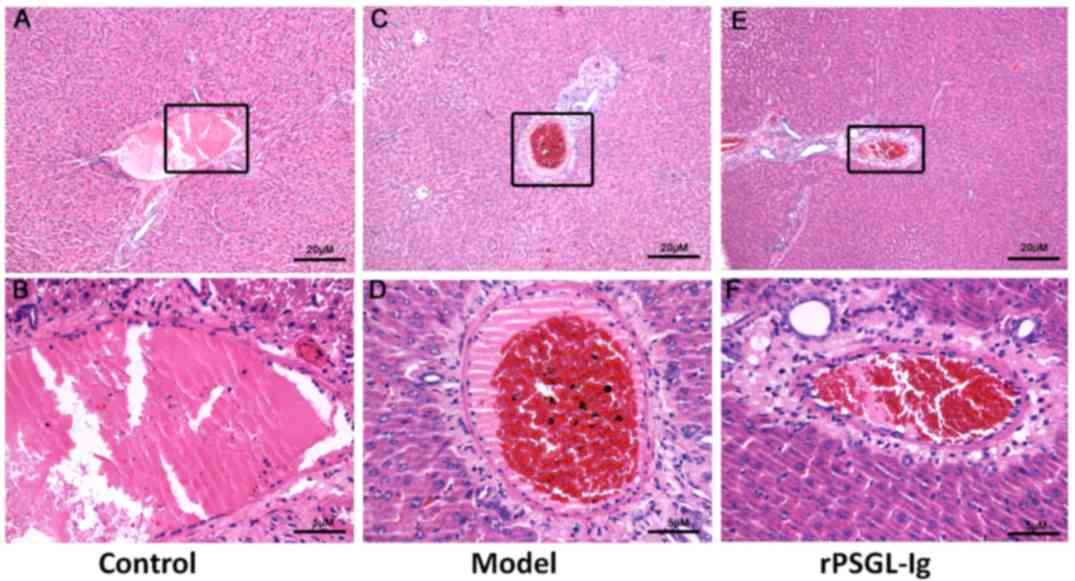 | Figure 4.Central hepatic veins were stained
with hematoxylin and eosin in the control (A, magnification, ×100;
B, magnification, ×400), portal vein thrombosis model (C,
magnification, ×100; D, magnification, ×400) and rPSGL-Ig (E,
magnification, ×100; F, magnification, ×400) groups. |
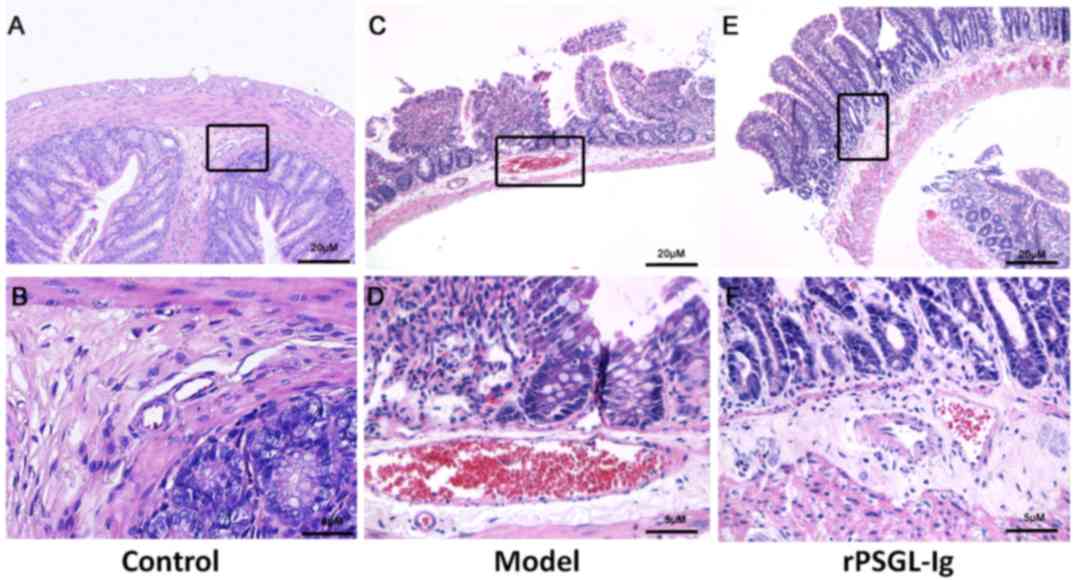 | Figure 5.Vasa intestinae tenuis were stained
with hematoxylin and eosin in the control (A, magnification, ×100;
B, magnification, ×400), portal vein thrombosis model (C,
magnification, ×100; D, magnification, ×400) and rPSGL-Ig (E,
magnification, ×100; F, magnification, ×400) groups. |
rPSGL-Ig relieves histopathological
changes in the portal vein
TEM was performed to evaluate histopathological
changes in the portal veins in rats of different groups. As shown
in Fig. 6A-C, a normal
ultrastructure of the portal vein was observed in the control group
with structurally complete granulocytes and vascular endothelial
cells. In the model group, massive red blood cell deposition along
with destruction of the surrounding histiocytic structure was
observed 6-h post-surgery (Fig. 6D).
The surrounding histiocytic structure was destroyed and necrosed 12
and 24-h post-surgery, respectively, with numerous cell fragments
and a small number of granulocytes remaining (Fig. 6E and F). Following treatment with
rPSGL-Ig, there were many deposited erythrocytes and slight
thrombosis in the portal veins at 6-h post-surgery, with partial
destruction of surrounding veins and histiocytic cell structures
(Fig. 6G). There were no obvious
changes at 12-h post-surgery (Fig.
6H), however, a reduction in the number of erythrocytes
deposited and the lack of thrombus in the portal vein, as well as
intact surrounding veins and histiocytic cell structures was
observed at 24-h post-surgery following treatment with rPSGL-Ig
(Fig. 6I).
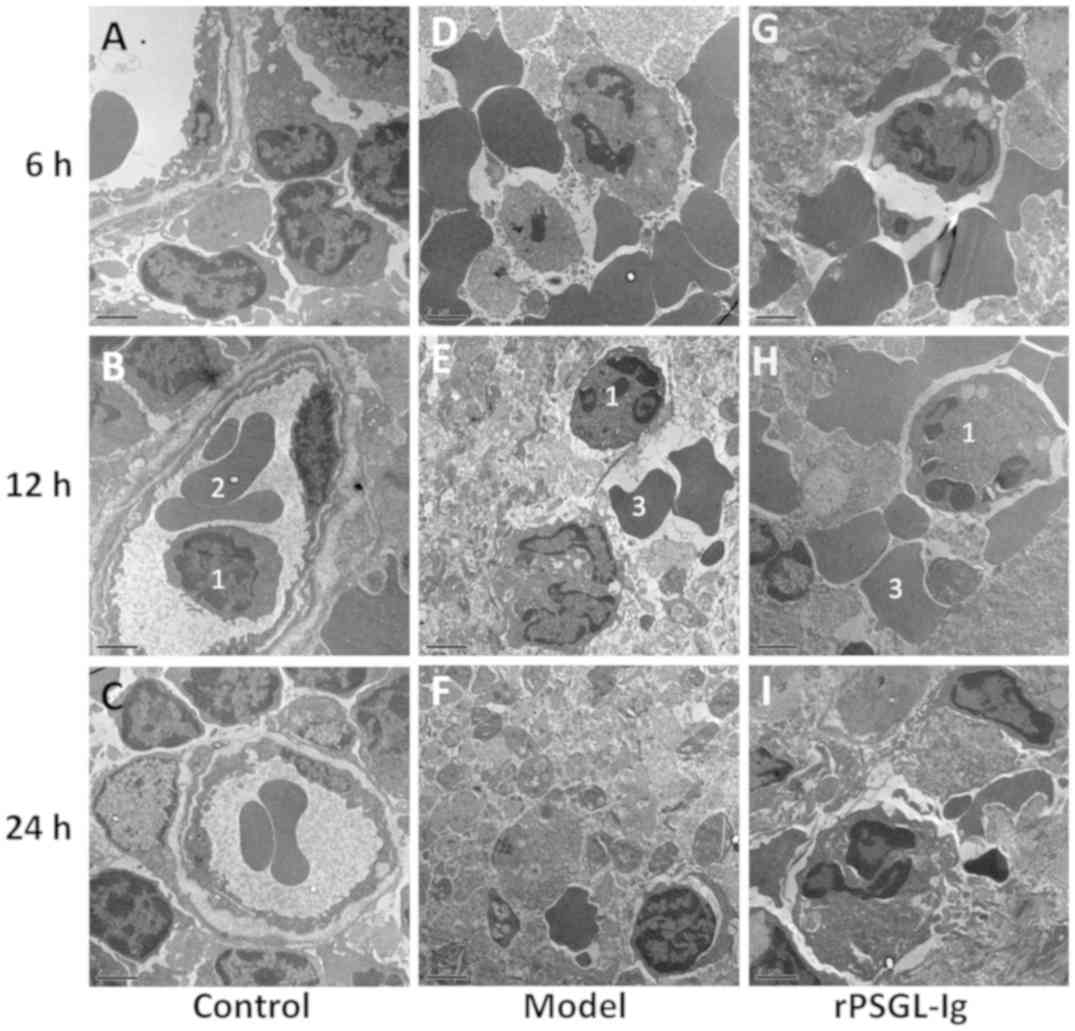 | Figure 6.Transmission electron microscope
observation of the portal vein in the control group at (A) 6, (B)
12 and (C) 24 h post-surgery, the portal vein thrombosis model at
(D) 6, (E) 12 and (F) 24 h post-surgery, and the rPSGL-Ig group at
(G) 6, (H) 12 and (I) 24 h post-surgery. Magnification, ×1,000. 1,
Granulocyte; 2, vascular endothelial cell; 2, deposited red blood
cell; rPSGL-Ig, recombinant P-selectin glycoprotein ligand
immunoglobulin G. |
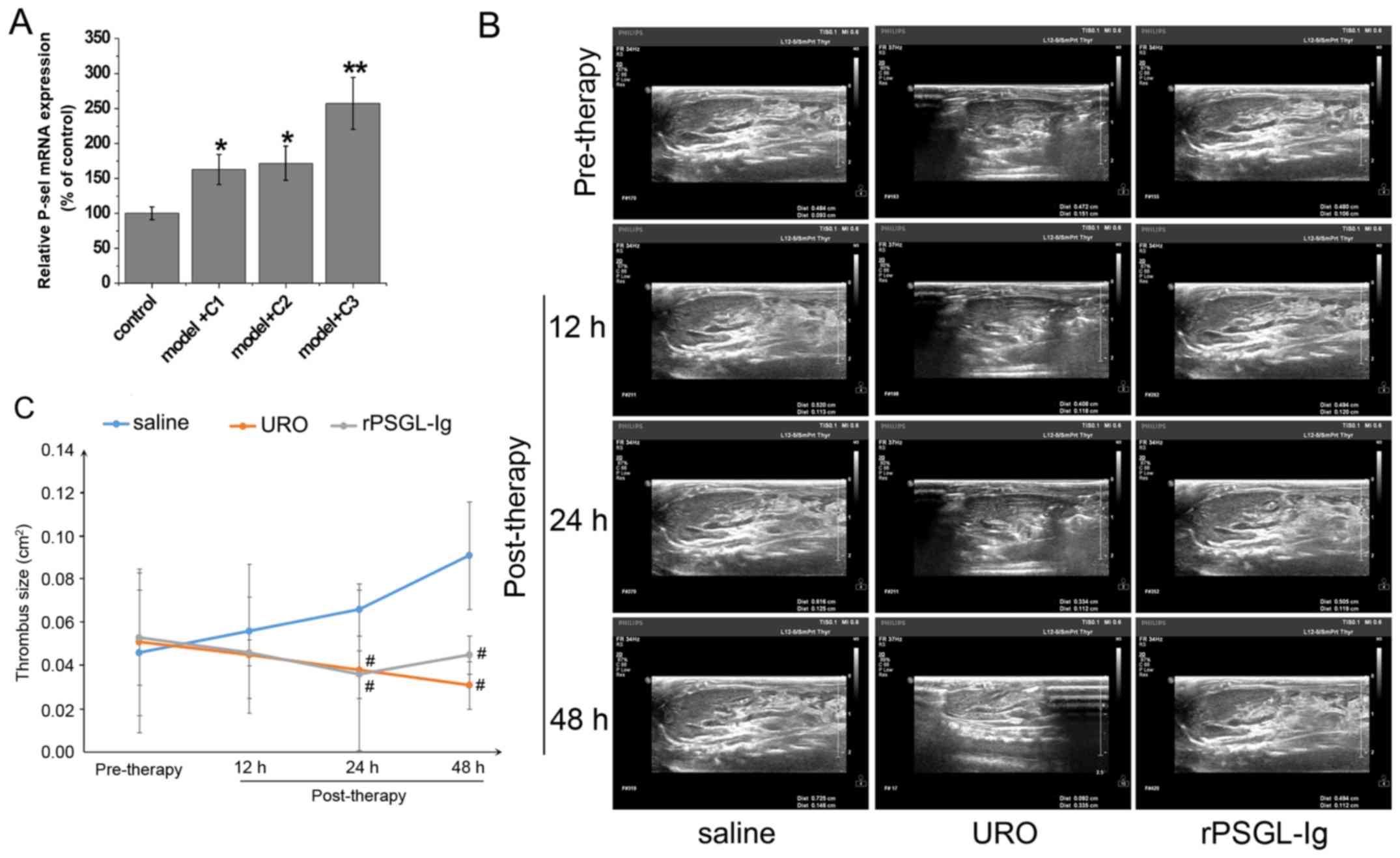
rPSGL-Ig has thrombolytic effects in
the portal vein of PVT rats
Evaluation of the thrombolytic effect of rPSGL-Ig
revealed a decreased thrombus length (Table II), while the level of P-selectin
mRNA was significantly upregulated compared with the control group
(P<0.01; Fig. 7A) at a dose of 8
mg/kg. Thus, 8 mg/kg rPSGL-Ig was used to detect thrombolytic
effects. No significant change in thrombus size in the rPSGL-Ig
group was observed, while thrombus size decreased gradually in the
URO group with time (Fig. 7B and C).
However, the saline group showed an increasing trend. Thrombus size
was significantly decreased in the URO and rPSGL-Ig groups compared
with the saline group at 24 and 48-h post-surgery (all P<0.05).
The best thrombolytic effect was observed in the URO group followed
by the rPSGL-Ig group, while the saline group showed the lowest
effects. Therefore, rPSGL-Ig intervention exhibited a thrombolytic
effect on PVT rats, however it was less effective than URO.
 | Table II.Thrombus length in rats following
treatment with rPSGL-Ig. |
Table II.
Thrombus length in rats following
treatment with rPSGL-Ig.
| Group | Thrombus length
(cm) |
|---|
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 mg/kg
rPSGL-Ig | 0.5 | 0.5 | 0.7 | 0.6 | 0.8 | 0.7 |
| 6 mg/kg
rPSGL-Ig | 0.2 | 0.3 | 0.1 | 0.3 | 0.4 | 0.3 |
| 8 mg/kg
rPSGL-Ig | 0.1 | 0 | 0 | 0 | 0.2 | 0.1 |
Discussion
The current study was initially designed to
establish a novel method for constructing a portal vein thrombosis
(PVT) model. The specific anatomical characteristics of PVT greatly
limit the application of traditional modeling methods. Complete
vein ligation is commonly conducted to construct vein thrombosis
models (23,24). However, complete ligation of the
portal vein may induce intestinal congestion, and even intestinal
necrosis and perforation. As the portal vein is the main blood
supply to the liver, complete ligation of the portal vein can also
lead to hepatic failure. These complications contribute to the
animal models succumbing to their injuries. In the current study, a
stable PVT model was constructed in rats by IPVO combined with
endangium destruction. The proximal and distal ends of the free
portal vein were ligated to block blood flow, and then the portal
vein was clamped from the proximal to the distal ends to damage the
intima. A total of 24 h post-surgery, stable black thrombi with
hardened vascular walls and venous stenosis were formed, supporting
the validity of the modeling method. Additionally, since the portal
vein is relatively thin, and has confluent intestinal and splenic
branches, puncture catheterization is difficult to perform
(25). In the current study, B-scan
ultrasonography was used to evaluate thrombus formation. The
results demonstrated that the thrombus size and vessel diameter in
the model group was significantly increased from 6 h post-surgery
in what appeared to be a time-dependent manner. These findings
further illustrate that the modeling method is feasible and
efficient.
PSGL-1, a high-affinity ligand of P-selectin, serves
an important role in thrombus formation (26). PSGL-1 is involved in
leukocyte-endothelial and leukocyte-platelet interactions, and
contributes to the development of a platelet-rich thrombus
following vessel wall injury (26).
In the current study, a 4.0-fold higher expression of P-selectin
was observed in the model group compared with the control group,
demonstrating the procoagulant role of P-selectin in PVT. Studies
have revealed that inhibition of the P-selectin signaling pathway
is an effective therapeutic target in the thrombus. For example,
the P-selectin small-molecule antagonist, PSI-697, has been
demonstrated to reduce the thrombus weight by 18% relative to
vehicle by inhibiting the binding of P-selectin to PSGL (27). A deficiency in PSGL protects mice
from thrombosis following collagen and epinephrine challenge,
resulting in mild thrombocytopenia, less fibrin deposition and a
low number of thrombosed blood vessels (28). As a specific antibody for PSGL,
rPSGL-Ig can also antagonize P-selectin by competing with PSGL-1 to
inhibit thrombosis. The authors of the current study demonstrated
that rPSGL-Ig induced upregulation of the P-selectin mRNA,
indicating that transcription of SELP was substantially
increased when P-selectin was antagonized by rPSGL-Ig. Furthermore,
it has been reported that rPSGL-Ig can be used to successfully
treat established vein thrombosis with no anticoagulation,
thrombocytopenia or wound complications (10). P-Selectin inhibition with rPSGL-Ig
decreased vein wall fibrosis and enhanced thrombus resolution in a
rat model of deep vein thrombosis (29). However, the specific roles of
rPSGL-Ig in PVT are not fully understood. In the current study, the
antithrombotic effects of rPSLG-Ig in PVT were evaluated. The
results revealed that the thrombus size and vessel diameter were
significantly decreased in the rPSGL-Ig group compared with the
model group. Additionally, histopathological changes in the portal
vein, central hepatic vein and vasa intestinae tenuis in the PVT
model were markedly relieved by rPSLG-Ig. These findings are
consistent with those of previous studies (30,31) and
further illustrate that rPSGL-Ig is an effective agent for
preventing PVT. In PVT rats, rPSLG-Ig may inhibit platelet
activation in an injured arterial circulation, and the combination
of activated platelets and neutrophils through the competitive
binding of PSGL with rPSLG-Ig prevent the formation of PVT.
Evaluation of the thrombolytic effect of rPSGL-Ig
demonstrated that it was more evident as the dosage increased. URO
is a commonly used thrombolytic drug in the clinic (32). In the current study, URO was used as
a positive control to evaluate the thrombolytic effect of rPSGL-Ig.
The results of the B-scan ultrasonography in portal vein revealed
that the thrombus in the rPSGL-Ig group was larger compared with
that in the URO group, indicating that the thrombolytic effect was
not as good as that of URO. However, compared with the saline
group, rPSGL-Ig exhibited a thrombolytic therapy function by
attenuating thrombus formation. Considering the small sample size
of the rats and the concentration range of rPSGL-Ig, the
thrombolysis effect of rPSGL-Ig requires further study in future
experiments. The results of the current study results suggest that
rPSGL-Ig prevents PVT formation and promotes thrombolysis.
In conclusion, a PVT model was successfully
constructed in rats by IPVO combined with endangium destruction.
Intervention with rPSGL-Ig significantly inhibited PVT formation,
the thrombus size and the vessel diameter, and markedly relieved
histopathological changes in the portal vein, central hepatic vein
and vasa intestinae tenuis. However, application of rPSGL-Ig for
preventing PVT is limited in clinical practice. Further studies on
the clinical effects of rPSGL-Ig are required.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Social Public
Technology Research and Development Program from the Science and
Technology Department of Hunan Province, China (grant no.
2014C33137), and the General Science and Research Project Program
(grant no. 2010YSB08) and the Social Public Research Program (grant
no. 2017GY47) from the Science and Technology Bureau of Huzhou
City, China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and JS participated in the design of this study
and performed statistical analysis. HS, YW, GC, and WC carried out
the study and collected important background information. JZ and LY
drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Huzhou Central Hospital (Huzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PVT
|
portal vein thrombosis
|
|
rPSGL-Ig
|
recombinant P-selectin glycoprotein
ligand immunoglobulin G
|
|
TEM
|
transmission electron microscope
|
|
URO
|
urokinase
|
References
|
1
|
Cohen R, Mallet T, Gale M, Soltys R and
Loarte P: Portal vein thrombosis. Case Rep Vasc Med.
2015:8230632015.PubMed/NCBI
|
|
2
|
Winslow ER, Brunt LM, Drebin JA, Soper NJ
and Klingensmith ME: Portal vein thrombosis after splenectomy. Am J
Surg. 184:631–636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manzano-Robleda Mdel C, Barranco-Fragoso
B, Uribe M and Méndez-Sánchez N: Portal vein thrombosis: What is
new? Ann Hepatol. 14:20–27. 2015.PubMed/NCBI
|
|
4
|
Amitrano L, Guardascione MA, Brancaccio V,
Margaglione M, Manguso F, Iannaccone L, Grandone E and Balzano A:
Risk factors and clinical presentation of portal vein thrombosis in
patients with liver cirrhosis. J Hepatol. 40:736–741. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lixue D, Wujun W, Zhang Y, Sun Z, Haitian
H and Liu Q: Clinical analysis of portal vein thrombosis after
splenocaval shunt plus devascularization in treatment of portal
hypertension. Chin J Hepatobiliary Surg. 16:353–355. 2010.(In
Chinese).
|
|
6
|
Harmanci O and Bayraktar Y: Portal
hypertension due to portal venous thrombosis: Etiology, clinical
outcomes. World J Gastroenterol. 13:2535–2540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng JG, Chen M and Chou KC: P-selectin
cell adhesion molecule in inflammation, thrombosis, cancer growth
and metastasis. Curr Med Chem. 11:2153–2160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
André P, Hartwell D, Hrachovinovã I,
Saffaripour S and Wagner DD: Pro-coagulant state resulting from
high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA.
97:13835–13840. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Myers DD Jr, Schaub R, Wrobleski SK, Londy
FJ III, Fex BA, Chapman AM, Greenfield LJ and Wakefield TW:
P-selectin antagonism causes dose-dependent venous thrombosis
inhibition. Thromb Haemost. 85:423–429. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Myers D, Wrobleski S, Londy F, Fex B,
Hawley A, Schaub R, Greenfield L and Wakefield T: New and effective
treatment of experimentally induced venous thrombosis with
anti-inflammatory rPSGL-Ig. Thromb Haemost. 87:374–382. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eppihimer MJ and Schaub RG:
P-Selectin-dependent inhibition of thrombosis during venous stasis.
Arterioscler Thromb Vasc Biol. 20:2483–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Théorêt JF, Bienvenu JG, Kumar A and Merhi
Y: P-selectin antagonism with recombinant p-selectin glycoprotein
ligand-1 (rPSGL-Ig) inhibits circulating activated platelet binding
to neutrophils induced by damaged arterial surfaces. J Pharmacol
Exp Ther. 298:658–664. 2001.PubMed/NCBI
|
|
13
|
Reyers I, Mussoni L, Donati MB and de
Gaetano G: Failure of aspirin at different doses to modify
experimental thrombosis in rats. Thromb Res. 18:669–674. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brill A, Fuchs TA, Chauhan AK, Yang JJ, De
Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S and
Wagner DD: von Willebrand factor-mediated platelet adhesion is
critical for deep vein thrombosis in mouse models. Blood.
117:1400–1407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakase H, Heimann A and Kempski O:
Alterations of regional cerebral blood flow and oxygen saturation
in a rat sinus-vein thrombosis model. Stroke. 27:720–728. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seyed Mortaz SS, Golfam F, Khalaj AR,
Taheri HR and Kholdi N: The effect of distal vein branch ligation
in side-to-side arterivenous fistula on the patency rate and
complication in one year. Daneshvar Medicine. 16:19–24. 2009.
|
|
17
|
Xu Z, Lioi J, Mu J, Kamocka MM, Liu X,
Chen DZ, Rosen ED and Alber M: A multiscale model of venous
thrombus formation with surface-mediated control of blood
coagulation cascade. Biophys J. 98:1723–1732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khalid A, Azeez EA, Bhatti TH and Eshak Y:
Transcutanous electric nerve stimulation and deep venous
thrombosis. Anesthesia & Analgesia. 86:S121998. View Article : Google Scholar
|
|
19
|
Nosaka M, Ishida Y, Kimura A and Kondo T:
Time-dependent appearance of intrathrombus neutrophils and
macrophages in a stasis-induced deep vein thrombosis model and its
application to thrombus age determination. Int J Legal Med.
123:235–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fei Y, Zong GQ, Chen J and Liu RM:
Evaluation of the value of d-dimer, P-selectin, and platelet count
for prediction of portal vein thrombosis after devascularization.
Clin Appl Thromb Hemost. 22:471–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bing Y, Zhao QH and Yu Z: Effect of
urokinase on vein wall remodeling after deep vein thrombosis in
rats. Di San Jun Yi Da Xue Xue Bao. 32:1970–1975. 2010.(In
Chinese).
|
|
23
|
Kyogashima M, Onaya J, Hara A and Taketomi
T: Sulfatide can markedly enhance thrombogenesis in rat deep vein
thrombosis model. Glycoconj J. 15:915–922. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nosaka M, Ishida Y, Kimura A and Kondo T:
Immunohistochemical detection of MMP-2 and MMP-9 in a
stasis-induced deep vein thrombosis model and its application to
thrombus age estimation. Int J Legal Med. 124:439–444. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burcharth F: Percutaneous transhepatic
catheterization of the portal venous system. Springer. (Japan).
1991. View Article : Google Scholar
|
|
26
|
Furie B and Furie BC: Role of platelet
P-selectin and microparticle PSGL-1 in thrombus formation. Trends
Mol Med. 10:171–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bedard PW, Clerin V, Sushkova N,
Tchernychev B, Antrilli T, Resmini C, Keith JC Jr, Hennan JK, Kaila
N, Debernardo S, et al: Characterization of the novel P-selectin
inhibitor PSI-697
[2-(4-chlorobenzyl)-3-hydroxy-7,8,9,10-tetrahydrobenzo[h]
quinoline-4-carboxylic acid] in vitro and in rodent models of
vascular inflammation and thrombosis. J Pharmacol Exp Ther.
324:497–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miszti-Blasius K, Debreceni IB, Felszeghy
S, Dezso B and Kappelmayer J: Lack of P-selectin glycoprotein
ligand-1 protects mice from thrombosis after collagen/epinephrine
challenge. Thromb Res. 127:228–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Myers DD Jr, Henke PK, Wrobleski SK,
Hawley AE, Farris DM, Chapman AM, Knipp BS, Thanaporn P, Schaub RG,
Greenfield LJ and Wakefield TW: P-selectin inhibition enhances
thrombus resolution and decreases vein wall fibrosis in a rat
model. J Vasc Surg. 36:928–938. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McEver RP: P-selectin and PSGL-1:
Exploiting connections between inflammation and venous thrombosis.
Thromb Haemost. 87:364–365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kneuer C, Ehrhardt C, Radomski MW and
Bakowsky U: Selectins-potential pharmacological targets? Drug
Discov Today. 11:1034–1040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marder VJ and Sherry S: Thrombolytic
therapy: Current status. N Engl J Med. 318:1512–1520. 1988.
View Article : Google Scholar : PubMed/NCBI
|