Introduction
Bone is a calcified connective tissue formed via the
differentiation of osteoprogenitor cells into mature osteoblasts.
Osteoblasts, the bone forming cells, are characterized by their
cuboidal appearance, and their association with bone matrix
formation (1). Several reports have
demonstrated the importance of bone marrow mesenchymal stem cells
(BMSCs) in cell-based therapy for bone tissue regeneration and the
treatment of skeletal tissue damage (2–5). BMSCs
have a number of advantages, such as their great potential for
proliferation and differentiation. BMSCs exhibit plasticity, and
the ability to differentiate into chondrocytes, osteocytes and
adipocytes in vitro and in vivo (6). In addition, BMSCs have the ability to
secrete biological factors with paracrine regenerative and
anti-inflammatory effects (7). The
differentiation of BMSCs in vitro is reliant on the culture
conditions; for example, osteogenic medium (OM) supplemented with
dexamethasone, ascorbic acid and β-glycerol phosphate induces the
differentiation of BMSCs into an osteogenic lineage (8).
All-trans retinoic acid (ATRA) is derivative of
vitamin A, which is vital for important physiological processes and
functions (9). A number of previous
studies have reported the key role of ATRA in the regulation of
bone cell function (10–12); ATRA may promote physiological bone
remodeling (11). The ability of
ATRA to influence osteoblast differentiation has been observed in
numerous cell systems (13–19). In addition, ATRA has been reported to
enhance in vitro osteogenesis in multiple cell types,
including preosteoblasts (20),
calvarial osteoblasts (16) and
mesenchymal stem cells (MSCs) (14,18,21,22).
However, ATRA does not induce the osteogenic differentiation of
BMSCs; dexamethasone is primarily used to initiate osteogenesis,
whereas ATRA inhibits osteoblast gene expression and mineralization
(23–25). The effect of ATRA on
osteoblastogenesis and osteoclastogenesis is dependent on the
differentiation marker examined, as well as the cell system
employed (26). In a previous study,
ATRA exerted divergent effects on osteoblastogenesis and
adipogenesis in MSCs (13). ATRA may
reduce the osteogenic differentiation capacity and promote the
adipogenesis of mouse embryonic palate mesenchymal cells via its
influence on bone morphogenetic protein (BMP) signaling (27). Studies performed in vitro and
in vivo have suggested that bone may be a main target of
retinoid action (10).
Curcumin has become a subject of scientific interest
as a potential therapeutic agent in orthopedic fields. Curcumin is
a phenolic natural product extracted from the rhizome of Curcuma
longa (turmeric). It has been widely used as a dietary spice
and traditional medicine for many centuries in Eastern populations
as a treatment for numerous diseases (28–30). In
addition, curcumin supplementation has been demonstrated to be
efficient in the prevention and management of osteopenia (31). Several studies have reported its
useful effects on bone health and fat metabolism (32–34). It
was previously demonstrated that curcumin protects against
ovariectomy-induced bone loss and decreased osteoclastogenesis in
animal models (35–38). In addition, curcumin improved bone
microarchitecture and increased mineral density in mice (39,40).
Furthermore, the therapeutic effect of curcumin has also been
reported in arthritis (41). Jain
et al (42) revealed that a
curcumin-eluting tissue scaffold increased the mRNA and protein
expression of known osteogenic markers.
At the cellular level, curcumin modulates important
molecular targets that participate in the regulation of bone
remodeling (43–46). The effects of curcumin on bone cells
in vitro have been reported (47–52). The
action of curcumin on osteoblast cells is controversial (49,53).
Curcumin enhanced the osteogenic differentiation of MSCs in
vitro (53). By contrast,
curcumin attenuated the osteogenic differentiation and
calcification of rat vascular smooth muscle cells (54). Curcumin has also been revealed to
activate the Wnt/β-catenin signaling pathway (55–57);
therefore, curcumin is considered an effective treatment for
osteoporosis. However, other studies have demonstrated that
curcumin suppresses this pathway (58,59).
Curcumin is able to improve bone health in patients with
osteoporosis by acting on multiple steps in the activation and
differentiation of osteoclasts, and improving mineral density and
mechanical properties. The potential mechanisms that have been
proposed include inhibition of nuclear factor (NF)-κB, receptor
activator of NF-κB ligand (RANKL), nitric oxide production, the
generation of reactive oxygen species and inflammatory cytokine
synthesis (38,39,47,50,51,60,61).
Transdifferentiation is a process in which adult,
mature and fully differentiated cells differentiate into another
specific terminal cell type via the induction of lineage-specific
transcription factors. Previous studies have revealed that
fibroblasts can be converted into several lineages, including
neurons (62), cardiomyocytes
(63), hepatocytes (64) and osteoblasts (65,66) via
the ectopic expression of multiple lineage-specific transcription
factors or microRNAs (67).
Importantly, this approach has also been applied in vivo
using a number of lineage-specific transcription factors (68). Several studies have reported that the
growth factor human lim mineralization protein (hLMP) is directly
associated with osteoblastic differentiation, and appears to be a
positive regulator of bone formation (69–72). The
production of multiple osteogenetic growth factors via the
administration of a single therapeutic molecule amplifies
osteoinductive signaling, and thus may be highly advantageous when
using LMP. The different types of secreted BMPs may be mixed and
potentially form heterodimeric BMPs that may have more potent
osteoinductivity than homodimeric BMPs (73).
The aim of the present study was to evaluate and
clarify the efficiency of the natural osteogenic modulators
curcumin and ATRA during the osteogenic differentiation of mouse
BMSCs, as there are numerous contradictory opinions regarding their
roles during this process. Therefore, the present study tested the
effect of curcumin on another osteogenic transdifferentiation model
to elucidate its wide range of effects on different cell types.
Mouse embryonic fibroblasts (MEFs), undergoing osteogenic
reprogramming using hLMP3, as a positive regulator of osteoblast
differentiation, were cultured in curcumin-enriched medium. The
results revealed a significant difference in bone markers between
the curcumin-enriched MEFs and the control MEFs. These findings
highlight the role of curcumin in osteogenic differentiation in
different cell lines.
Materials and methods
All reagents were purchased from Gibco; Thermo
Fisher Scientific, Inc., (Waltham, MA, USA) unless stated otherwise
in the text.
Experimental animals
A total of 25 male BALB/c mice (5–6 weeks old; body
weight, 15–21 g) and 2 pregnant female C57/BL mice (12–13 weeks
old; body weight, 23–26 g) at 13 days post-coitum were used
throughout the present study. Animals were obtained from the
Laboratory Animal Centre, Jiangsu University (Zhenjiang, China).
The animals were housed in cages in a temperature-controlled room
(20-25°C and 40-0% humidity), with a 12-h light/dark cycle and free
access to commercial food and water. All procedures involving
animals and their care conformed to the USA National Institutes of
Health guidelines (NIH Pub. No. 85-23, revised 1996). All animal
experiments were reviewed and approved by the Institutional Animal
Care and Use Committee of School of Animal Science and Technology,
Yangzhou University (Yangzhou, China; approval no.
YZUDWSY2017-0029). The procedures were performed in accordance with
the Regulations of the Administration of Affairs Concerning
Experimental Animals (China, 1988), and the Standards for the
Administration of Experimental Practices (Jiangsu, China;
2008).
Isolation and culture of mouse
BMSCs
The isolation and culture of BMSCs was conducted as
previously described (74). In
brief, 5–6-week-old male BALB/c mice were sacrificed via the
cervical dislocation method. The animals were then rinsed with 70%
ethanol for few min. The hind limbs were excised from the trunk of
the body, and the bones were kept in PBS for the subsequent steps
under a sterile hood. The bones were placed on sterile gauze and
were gently rubbed to remove any attached soft tissue. The
epiphysis was cut, a 26-gauge syringe needle was inserted into the
bone marrow cavity and the marrow was flushed out using Dulbecco's
modified Eagle's medium (DMEM); flushing was continued until pale
white bone was observed. The cell suspension was filtered through a
70-mm filter mesh to remove any bony spicules, muscle or cell
clumps. The number of viable cells was counted via trypan blue
staining. Cells were cultured in 95-mm dishes using 1 ml complete
culture medium (CCM) at a density 25×106 cells/ml, and
then kept at 37°C in a humidified atmosphere containing 95% air and
5% CO2. The CCM comprised DMEM containing 15% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA), 2 mM L-glutamine and 1% penicillin-streptomycin (Beyotime
Institute of Biotechnology, Haimen, China). Cells were cultured
with frequent medium changes as described previously (74). When primary cultures became nearly
confluent, the culture was treated with 0.5 ml 0.25% trypsin EDTA
for 2 min at room temperature. The cells that were lifted within 2
min were harvested and cultured in a 60-mm plate. Once the culture
reached 70–80% confluence, the cells were harvested for successive
passages. The subsequent experiments were performed with cells from
passages 3 and 4.
Isolation and culture of MEFs
Pregnant female mice (C57/BL) at 13 days post-coitum
was sacrificed via the cervical dislocation method. Uterine horns
were removed, washed with PBS and opened. Embryos were harvested
and the head and the visceral organs were removed. After washing
with PBS, the embryos were minced, and then incubated at 37°C for
15 min in 0.25% trypsin EDTA with gentle shaking. Trypsin was
neutralized with an equal amount of MEF medium and cells were
collected by centrifugation at 500 × g for 7 min at room
temperature (20-25°C). Cells were then resuspended and cultured on
gelatin-coated dishes with MEF growth medium containing high
glucose DMEM mixed with 10% FBS, 4 mM L-glutamine and 1:100
penicillin-streptomycin at 37°C with 5% CO2. The cells
were examined daily using an inverted microscope (Olympus TH4-200;
Olympus Corporation, Tokyo, Japan) to assess their general
appearance, and to identify any signs of microbial contamination.
Once confluent, cells were passaged 1:3 and the second passage
cells were trypsinized and frozen at −80°C. In the present
experiments, MEFs within three passages were used to avoid
replicative senescence.
Lentiviral vector
The codon-optimized hLMP-3 cDNA sequence obtained
from Pola et al (75), was
synthesized, and cloned into T-Vector pMD19 (Takara Biotechnology,
Co., Ltd., Dalian, China) by GenScript (Project ID. 7162905-1;
GenScript, Jiangsu, China). cDNA sequencing and the primer design
of hLMP-3 were also conducted by GenScript. The construction of the
viral expression vector containing the transcription factors hLMP-3
was performed by Genomeditech (Shanghai, China; Project ID.
GM-Lc-01147). The primers used for hLMP-3 (Accession number:
AAK30569.1) were as follows: Forward,
5′-CCCTCGAGGGCTTGGCCATGGATAGTTTCAAGGTGGTC-3′ and reverse,
5′-GCGTCGACGTTCAGCCACTTGAGGCGGGCATCTG-3′ (restriction recognition
sites are underlined).
Characterization of mouse BMSCs
BMSCs (passage 3) were used for MSC cell surface
marker experiments. The MSC surface markers cluster of
differentiation (CD)44 and CD90, and also the hematopoietic marker
CD45 were examined by flow cytometry as previously described
(76,77). Briefly, cells at passage 3 were
harvested using trypsin/EDTA. Following cell counting, cell
suspensions were stained with the following fluorescence-conjugated
antibodies: Fluorescein isothiocyanate (FITC)-anti-mouse/human CD44
antibody (1:100; cat. no. 103021; BioLegend, Inc., San Diego, CA,
USA), FITC anti-mouse CD45 monoclonal antibody (1:100; cat. no.
11-0451; eBioscience; Thermo Fisher Scientific, Inc.) and FITC
anti-mouse CD90 monoclonal antibody (1:100; cat. no. 11-0903;
eBioscience; Thermo Fisher Scientific, Inc.) for 1 h at 4°C. Cells
stained with FITC-labeled rat anti-mouse immunoglobulin G (IgG;
1:100; cat. no. 406001; BioLegend, Inc.) served as controls. Cells
were pelleted, washed twice with PBS and fixed with 1%
paraformaldehyde in PBS. Cell surface antigens were detected with a
flow cytometer using BD Accuri C6 Plus software (BD Biosciences,
Franklin Lakes, NJ, USA).
In vitro osteogenic induction of
BMSCs
BMSCs were seeded at a density of 50
cells/cm2 and cultured until they reached 70%
confluency. The CCM was then changed to OM (fresh complete medium
with 15% FBS) supplemented with 50 µg/ml ascorbic acid (Sigma
Aldrich; Merck KGaA, Darmstadt, Germany), 10 mM β-glycerol
phosphate disodium salt (Sigma Aldrich; Merck KGaA), and 100 nM
dexamethasone (Sigma Aldrich; Merck KGaA). Cells cultured in
osteogenic medium only comprised the OM group; while in the
curcumin group (CR group), 15 µM curcumin was added to the OM. This
concentration was selected based on previous studies that used
curcumin in cell culture to induce osteogenic differentiation with
minimal cytotoxicity (53,78,79).
Curcumin was prepared according to the method previously described
by Gu et al (53). Briefly,
curcumin (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) was dissolved in dimethyl sulfoxide and stored at
−20°C. In the ATRA group, 1 µM ATRA (Sigma-Aldrich; Merck KGaA) was
added to the OM. This concentration has repeatedly been used to
study the effects of ATRA on in vitro osteogenic
differentiation; since the normal physiological level of ATRA is
≤0.01 µM and the effective pharmacological concentration is >0.1
µM, the 1 µM concentration was selected to induce osteogenic
differentiation (27,80–82).
In vitro osteogenic induction of
MEFs
The protocol used was a modified version of that
described by Yamamoto et al (83); the preparation and dilution of the OM
components were conducted according to the protocol provided by the
supplier. MEFs were re-suspended in CCM, and seeded onto 35-mm
dishes (Corning®; 5×104 cells/dish) or a
24-well plate (Corning®; 1.2×104 cells/well)
on day 1. The next day, transduction of MEFs was performed using
the supernatant containing the pGMLVPE1-hLMP-3 expression vector
(multiplicity of infection=4), and supplemented with 4 µg/ml
polybrene (Genomeditech). Following culture for 24 h, the
virus-containing medium was replaced with an OM composed of fresh
complete culture medium (10% FBS) supplemented with 50 µg/ml
ascorbic acid, 10 mM β-glycerol phosphate disodium salt and 100 nM
dexamethasone. In the curcumin-supplemented group, 15 µM curcumin
was added to the medium.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cell samples using
TRIZOL® reagent (cat. no. 15596-026; Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Following isolation, 1 µg RNA was reverse-transcribed into cDNA
using FastQuant RT kits with gDNase (cat. no. KR106; Tiangen
Biotech Co., Ltd., Beijing, China). The reverse transcription
reaction was performed at 42°C for 15 min, followed by 95°C for 3
min. The cDNA samples were analyzed by RT-qPCR in a 7500 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using Super Real Premix plus (SYBR Green; cat. no. FP205; Tiangen
Biotech Co., Ltd.). The thermocycling conditions for qPCR were as
follows: 95°C for 15 min, followed by 40 amplification cycles of
95°C for 10 sec and 60°C for 32 sec. The sequences of the RT-qPCR
primers are listed in Table I. The
primers were designed and manufactured Takara Biotechnology Co.,
Ltd. Relative quantification was calculated with 2−ΔΔCq
(84) and normalized to β-actin.
Data are presented as levels relative to the expression level in
the control cells.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| β-actin |
CATCCGTAAAGACCTCTATGCCAAC |
ATGGAGCCACCGATCCACA |
| Runx2 |
TGCAAGCAGTATTTACAACAGAGG |
GGCTCACGTCGCTCATCTT |
| OSX |
GCGACCACTTGAGCAAACATC |
CGGCTGATTGGCTTCTTCTT |
| BMP-2 |
TGATGTGGGGTGGAATGACT |
CAGCAAGGGGAAAAGGACAC |
In vitro mineralization assay
Early osteogenic differentiation was evaluated by
alkaline phosphatase staining (ALP). Matrix mineralization was
evaluated by Alizarin red (ALZ) and von Kossa (VK) staining. The
staining intensity was quantified using Fiji in ImageJ software,
version 1 (National Institutes of Health, Bethesda, MD, USA)
(85).
ALP staining
Histochemical staining was performed using
5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium
(BCIP/NBT) ALP color development kits (cat. no. C3206; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. The cells were washed with PBS buffer, then fixed with 4%
formalin at room temperature for 2 min. The cells were then washed
3 times with PBS. Following the last wash, an appropriate amount of
BCIP/NBT staining solution was added, ensuring that the sample was
fully covered. After the addition of the working solution, cells
were incubated in the dark at 37°C for 5–30 min in an incubator
until the desired color developed. The BCIP/NBT stain working
solution was then removed, and cells were washed once or twice with
distilled water to stop the color reaction.
ALZ staining
The cells were washed with 1X PBS, and fixed in 10%
formaldehyde in 1X PBS for 15 min. Following fixation, the cells
washed with distilled water 2 or 3 times. Cells were incubated in
40 mM ALZ staining solution for 5 min in the dark. Finally, cells
were washed with distilled water 4 times to remove the excess
stain. The ALZ staining solution was prepared by diluting the ALZ
staining powder (cat. no. A5533; Sigma-Aldrich; Merck KGaA) in
distilled water. The pH was adjusted to 4.1–4.3 with 10% ammonium
hydroxide using a pH Meter (Mettler Toledo, Columbus, OH, USA).
VK staining
For VK staining, the cells were washed with PBS,
fixed with 10% formalin and stained with freshly prepared 5% silver
nitrate solution for 30 min with exposure to UV light. After
washing with distilled water 3 times, the cells were treated with
5% sodium thiosulfate solution for 3 min to remove any remaining
silver nitrate. Final washing with distilled water was repeated 3
times. The silver nitrate solution (5%) was prepared by diluting
silver nitrate powder (cat. no. GB12595-90; Beijing HenGye
Zhongyuan Chemical Co., Ltd., Beijing, China) in distilled water.
The same diluent was used for the preparation of a solution of
sodium thiosulfate (cat. no. 217263; Sigma-Aldrich; Merck
KGaA).
Fluorescent immunocytochemistry
The cells were rinsed briefly with PBS, and then
fixed for 20 min with 4% paraformaldehyde in 0.1 M phosphate buffer
(pH 7.4) at room temperature. The cells were permeabilized for 10
min with 0.1% TritonX-100 in PBS, to allow for specific antibody
entrance into the cells, and then blocked for 45–60 min with 4%
bovine serum albumin (BSA) in PBS at room temperature. Cells were
incubated overnight (16–18 h) at 4°C with mouse monoclonal OCN
antibody (1:500; cat. no. sc-376726; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The cells were then washed 3 times with
washing buffer. This was followed by 1 h incubation at room
temperature with the secondary antibody Alexa Fluor 647-labeled
goat anti-mouse IgG (1:500; cat. no. ab150115; Abcam, Cambridge,
MA, USA). Following a final round of 3 washes with the wash buffer,
nuclei were counterstained using DAPI at room temperature for 5 min
(1 mg/ml PBS; Invitrogen; Thermo Fisher Scientific, Inc.). Cells
were examined using inverted fluorescence microscopy (Olympus
TH4-200).
Western blot analysis
All steps were conducted according to the
manufacturer's protocol, using a Bio-Rad system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Cell lysates were collected
with RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) supplemented with phenyl methane sulfonyl fluoride
(Beyotime Institute of Biotechnology). Following centrifugation at
12,000 × g at 4°C for 10 min, the supernatant was collected and the
protein concentration was determined using a BCA protein assay kit
(Beyotime Institute of Biotechnology). Protein samples were heated
for 10 min at 95°C, and then separated by 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (20 µg protein/lane).
Samples were then blotted onto polyvinylidene fluoride membranes
(Beijing Solarbio Science & Technology Co., Ltd.) using a
Trans-Blot SD system (Bio-Rad Laboratories, Inc.). The membranes
were blocked with BSA blocking buffer (cat. no. CW2143S; CWBIO,
Beijing, China) at room temperature for 2 h, and then incubated
with the primary mouse monoclonal osteocalcin (OCN) antibody
(1:500; cat. no. sc-376726; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. After washing three times, the corresponding secondary
antibody horseradish peroxidase-labeled goat anti-mouse IgG
(1:2,000; cat. no. 665739; Merck KGaA) was added. The membranes
were incubated at 37°C for 2 h and then washed with TBST (cat. no.
CW0043S; CWBIO). Bands were visualized with enhanced
chemiluminescence method western blot kits (cat. no. CW0048M;
CWBIO) using the FluorChem Q system (ProteinSimple, San Jose, CA,
USA). β-actin (1:1,000; cat. no. AA128; Beyotime Institute of
Biotechnology) was used as a loading control with overnight
incubation at 4°C. The protein band intensity was quantified using
Fiji in ImageJ version 1 (85).
Statistical analysis
All quantitative data are expressed as the mean ±
standard deviation (n=3). Statistical analyses were performed using
SPSS software version 21 (IBM Corp., Armonk, NY, USA). Statistical
significance was determined using one-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. For some
experiments the statistical significance was determined using
paired sample t-tests (P<0.05 and P<0.01). GraphPad Prism
software version 6 (GraphPad Software, Inc., La Jolla, CA, USA) was
used to produce the graphs.
Results
Morphological and immunotyping
characterization of mouse BMSCs
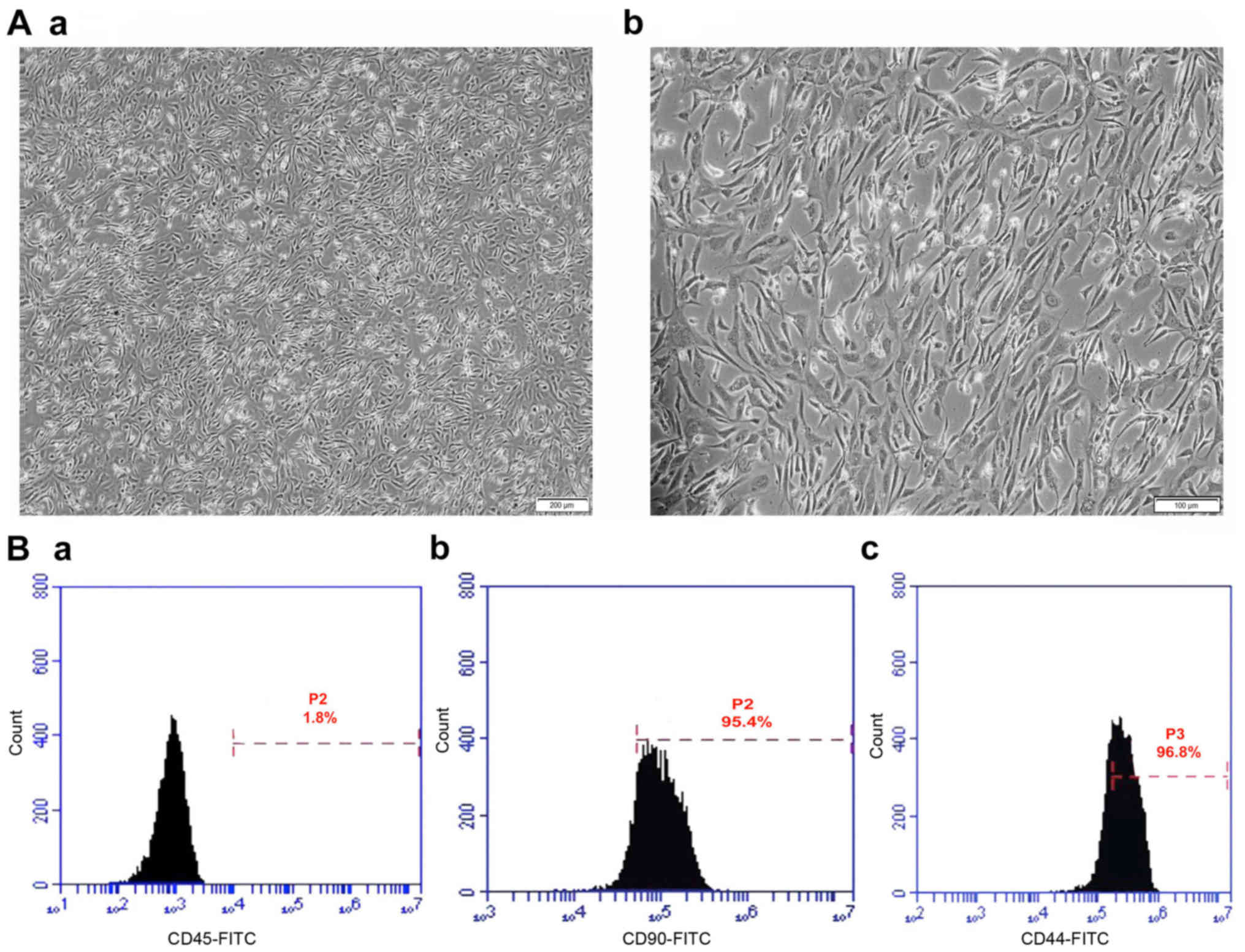
Bone marrow was harvested from BALB/c mice, and P3
cells were seeded into 35-mm culture dishes at a density of
25×106 cells/ml. BMSCs are recognized as the adherent
cells derived from bone marrow and are capable of extensive
proliferation, producing a fibroblastic shape. Following 15 days of
culture, almost homogeneous populations of fibroblast-like cells
were observed (Fig. 1A). To confirm
cell identity, immunofluorescent staining was used to identify the
BMSCs with the surface markers CD90 and CD44, while the surface
marker CD45 was used to detect hematopoietic cell contamination.
The cultured cells were positive for the MSC markers CD44 and CD90,
and negative for the hematopoietic cell marker CD45 (Fig. 1B).
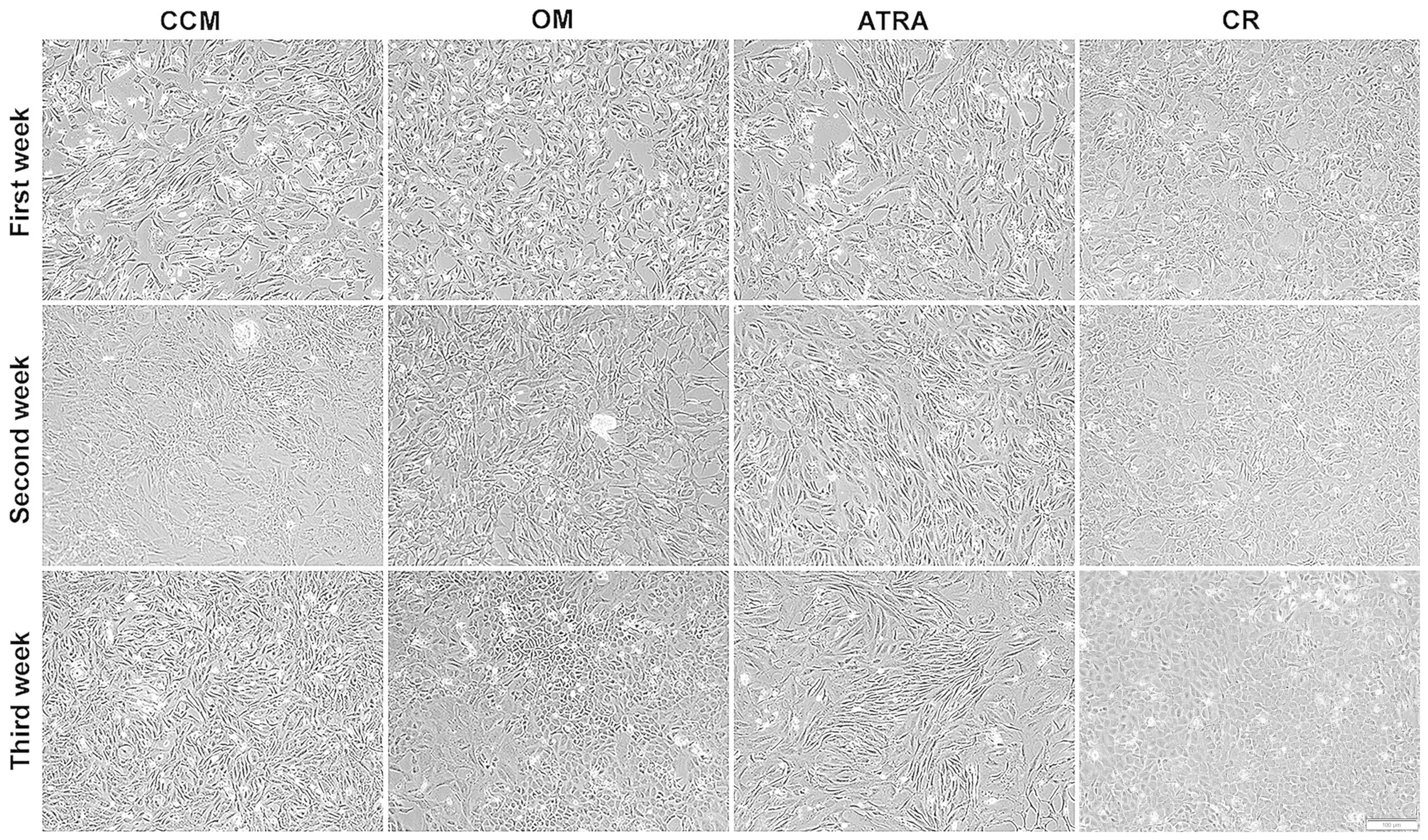
Morphological changes during the
osteogenic differentiation of BMSCs
BMSCs were induced to differentiate into an
osteogenic lineage, and were able to proliferate in vitro.
The morphology of BMSCs changed following the first week of culture
in OM (OM group) from their spindle-like fibroblast shape to the
characteristic cuboidal morphology of primary osteoblasts.
Comparable results were observed in the CR group. By contrast, the
BMSCs cultured in CCM did not differentiate into the osteoblast
lineage, and cells in the ATRA group displayed a more elongated
shape, and dendrites were clearly visible (Fig. 2).
Osteogenic differentiation capacity of
BMSCs following induction with curcumin and ATRA
The present study performed an in vitro
mineralization assay to determine the effects of curcumin and ATRA
on the onset of the osteogenic differentiation of BMSCs. ALP, ALZ
and VK staining were conducted during the differentiation period.
ALP activity was analyzed as an early indicator of osteogenic
differentiation. The results revealed that OM induced ALP activity
in the mouse BMSCs after 7 and 14 days. The ALP staining intensity
was significantly increased in the CR group (P<0.01 vs. the OM
and ATRA groups); while no significant difference was detected
between the OM and ATRA groups. Matrix mineralization was detected
by ALZ and VK staining. Mineralization was not clearly detected
after 7 days, and was slowly developing after 14 days. However,
after 21 and 28 days, the OM and CR groups exhibited positively
stained mineralized nodules. Mineralization was significantly
stronger in the CR group compared with the OM group. By contrast,
the ATRA group did not show any signs of mineralization (Fig. 3).
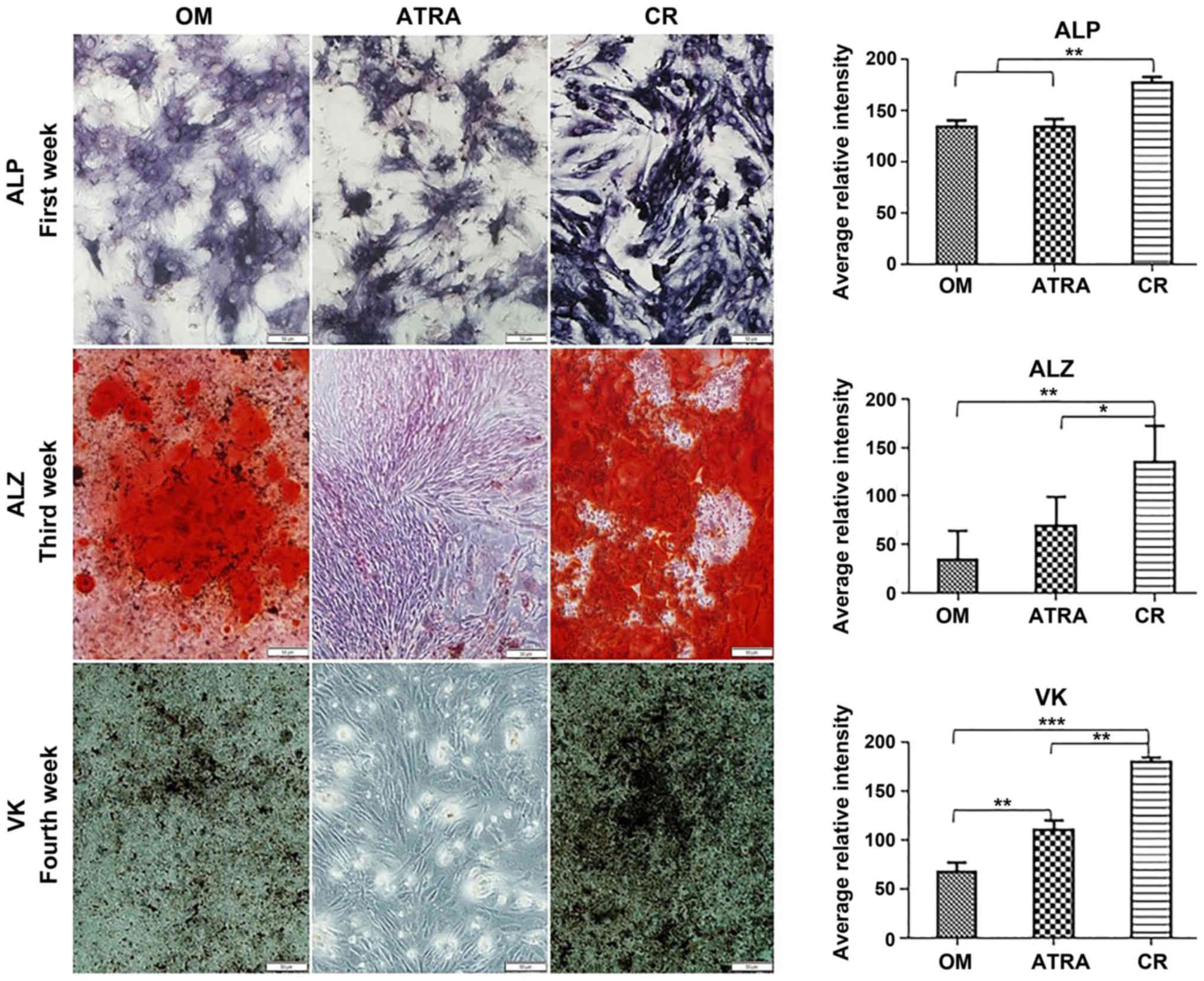 | Figure 3.In vitro mineralization assay
of BMSCs during osteogenic differentiation following induction with
curcumin and ATRA. ALP staining results were obtained 1 week
post-induction, ALZ staining was performed 3 weeks post-induction
and VK staining was conducted 4 weeks post-induction. The level of
calcium deposition and, in turn, the extent of mineralization was
higher in the CR group than in the OM and ATRA groups. By contrast,
the ATRA group did not present any symptoms of mineralization.
Quantification of ALP, ALZ and VK staining intensity was performed
with Fiji in ImageJ software. Quantitative data are expressed as
the mean ± standard deviation (n=3). Statistical significance was
determined using one-way analysis of variance, followed by Tukey's
post hoc test for multi-group comparisons. *P<0.05, **P<0.01
and ***P<0.001. BMSCs, bone marrow mesenchymal stem cells; OM
group, BMSCs in osteogenic medium; CR group, BMSCs in osteogenic
medium + curcumin; ATRA group, BMSCs in osteogenic medium + ATRA;
ATRA, all-trans retinoic acid; ALP, alkaline phosphatase; ALZ,
Alizarin red; VK, von Kossa. |
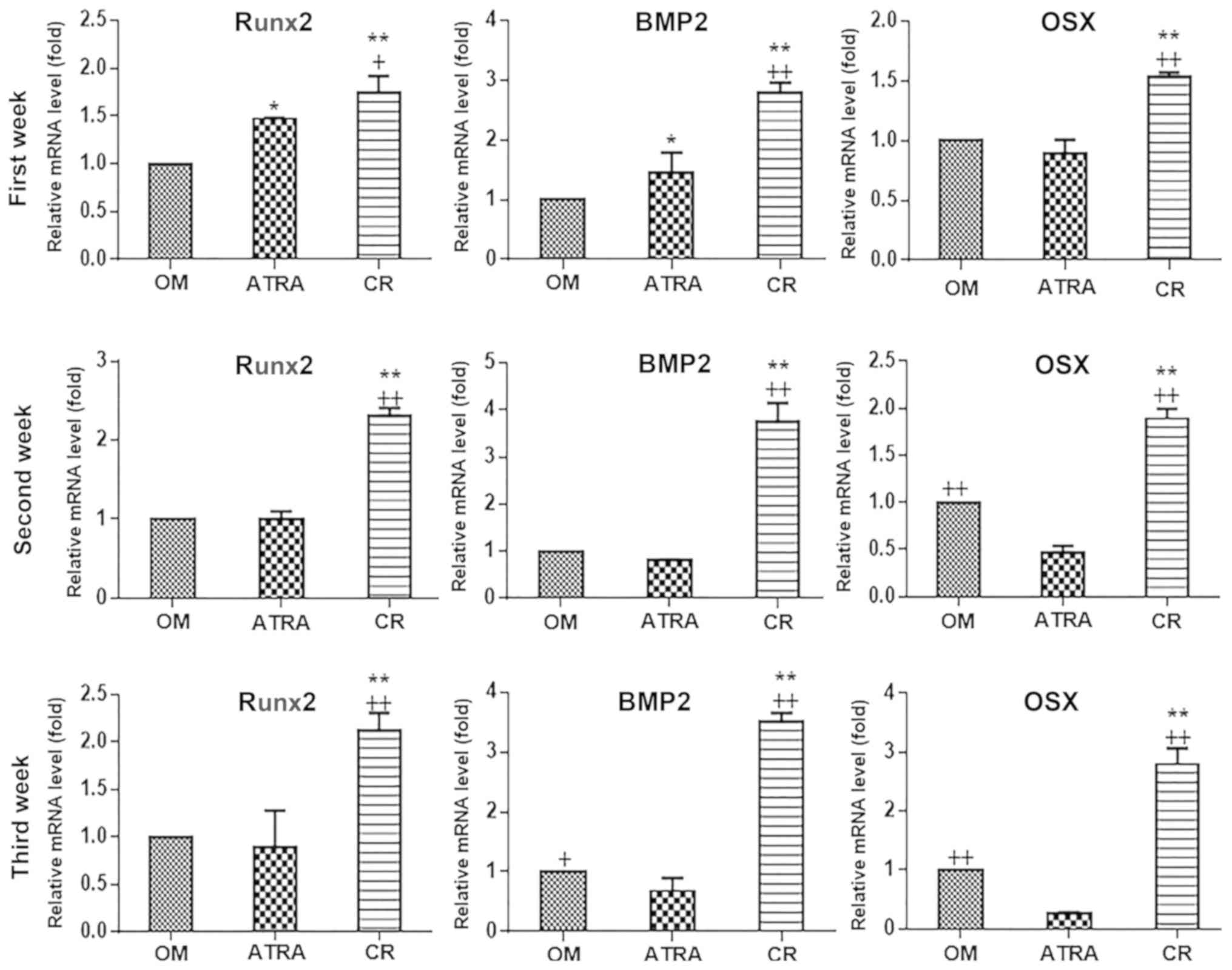
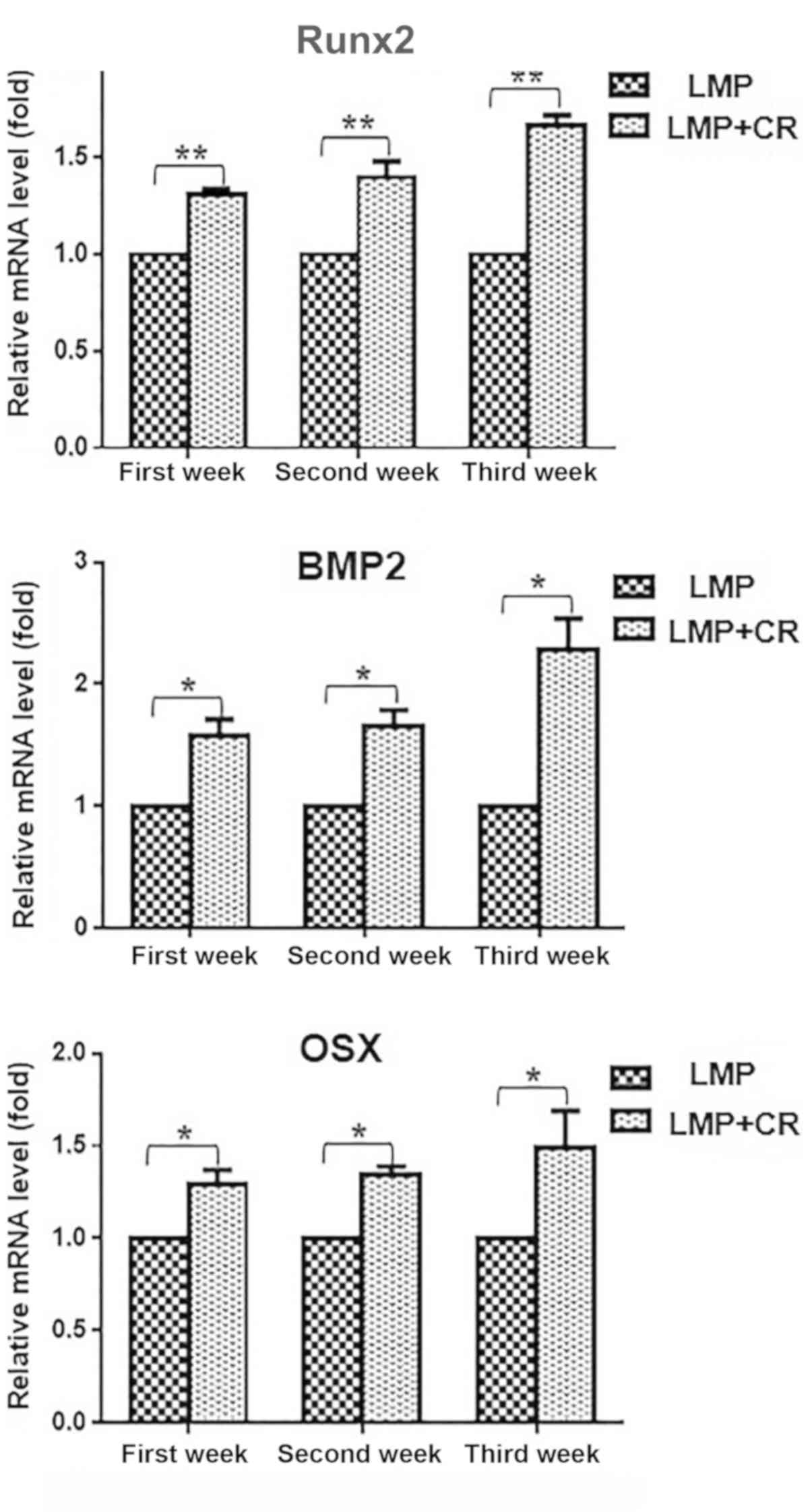
Effects of curcumin and ATRA on the
expression levels of bone-associated gene markers during BMSC
osteogenic differentiation
In order to evaluate the molecular changes following
the supplementation of the OM with curcumin and ATRA, the present
study examined the expression levels of the main genes associated
with osteoblastic differentiation, namely runt-related
transcription factor 2 (Runx2), osterix and BMP2. These markers are
well known for their roles in numerous osteoblast differentiation
pathways. The expression levels of these bone markers were
quantified at 7, 14 and 21 days (Fig.
4). The results revealed that curcumin-supplemented OM induced
a significant upregulation of osteo-specific markers when compared
with the ATRA and OM groups, and this upregulation appeared to be
time-dependent. After 7 days, the CR group exhibited induced
expression of osteo-specific markers when compared with the OM and
ATRA groups. After 14 days, the CR group had significantly higher
expression levels of the three tested markers when compared with
the OM and ATRA groups (P<0.01). The expression levels of the
markers had begun to decline in the ATRA group, and were not
significantly different when compared with the OM group for Runx2
and BMP2. Notably, the OM group exhibited significantly higher
(P<0.01) osterix expression when compared with the ATRA group.
These results were consistent with the results of the
mineralization assay, which revealed the inhibitory effect of ATRA
during the differentiation process. Finally, after 21 days, the
results revealed that the CR group had upregulated levels of the
three markers when compared with the OM and ATRA groups
(P<0.01). In addition, the expression levels of osteo-specific
markers in the ATRA group appeared to decline further compared with
those in the OM group. These RT-qPCR results demonstrated the
positive effect of curcumin-supplemented medium on the osteogenic
differentiation of BMSCs. By contrast, ATRA supplementation
exhibited an inhibitory effect.
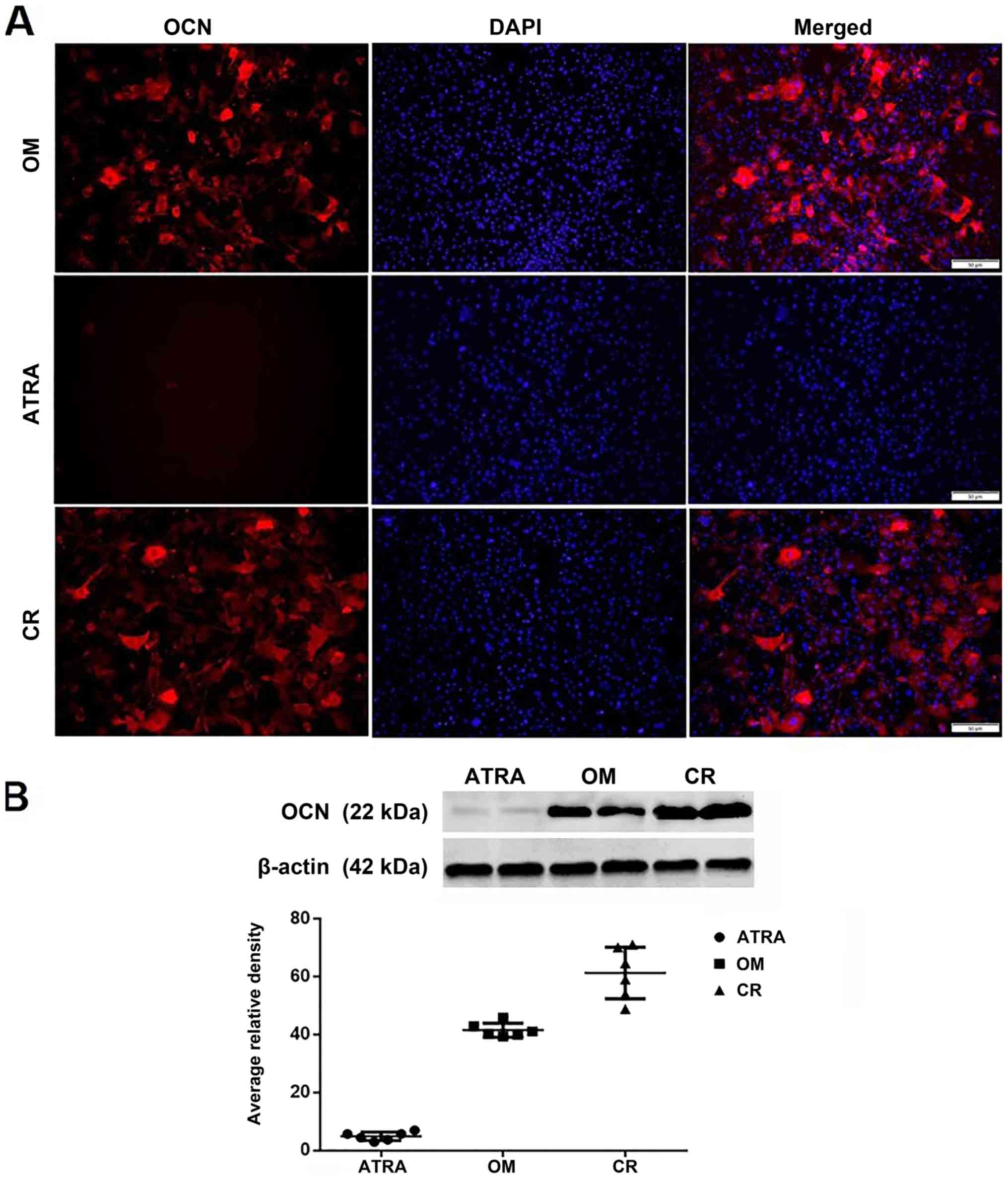
Expression of OCN during the
osteogenic differentiation process
OCN, a marker of mature osteoblasts, was evaluated
during the differentiation process using immunostaining and the
protein levels of OCN were determined by western blot analysis. In
line with the previous results, the CR group exhibited a distinct
increase in the expression level of OCN when compared with the OM
and ATRA groups. Furthermore, the addition of ATRA inhibited the
induction of OCN expression (Fig.
5).
Effect of curcumin-supplemented OM on
the osteogenic differentiation of lentivirus-transduced MEFs with
hLMP-3
The aforementioned results reveal the positive role
of curcumin-supplemented medium on the osteogenic differentiation
of BMSCs. Therefore, the present study investigated whether this
positive role could be applied in other cell types. In a previous
study, the present research team studied the reprogramming of MEFs
to osteoblast cells using hLMP-3 (86). The results revealed that the
transduction of MEFs with a lentiviral vector expressing the
osteogenic factor hLMP-3 induced osteoblast cell formation in
vitro (86). The present study
examined the effect of curcumin enrichment on the osteogenic
differentiation of MEFs reprogrammed with the osteogenic factor
hLMP-3. MEFs were successfully transduced with pGMLVPE1-hLMP-3, as
previously reported (86). At 2 days
post-transduction, the culture medium was changed to OM
supplemented with curcumin. The effect of curcumin-supplemented
medium was compared with that of the non-supplemented medium using
RT-qPCR to detect the difference in the expression of bone markers
between the two groups. The RT-qPCR results revealed that the
addition of curcumin to the OM upregulated the expression of the
bone markers Runx2, BMP and osterix at 7, 14 and 21 days
post-transduction (Fig. 6).
Discussion
In the present study, the effects of two natural
compounds on the in vitro osteogenic differentiation of
BMSCs were investigated. BMSCs were isolated from the bone marrow
of BALB/c mice, and the osteogenic differentiation of the BMSCs to
osteoblasts was induced using a standard protocol. In certain
treatment groups, the OM was supplemented with 15 µM curcumin (the
CR group) or 1 µM ATRA (the ATRA group). Cells were examined for
osteogenic differentiation to determine the effect of curcumin or
ATRA. Curcumin supplementation was revealed to increase the
osteogenic differentiation capacity of BMSCs, as detected by the
mineralization assay and RT-qPCR analysis of bone markers and OCN
expression. By contrast, ATRA downregulated the osteogenic
differentiation of BMSCs. To determine if the positive effect of
curcumin also occurred in other cell types, curcumin was added to
the culture medium during MEF reprogramming to osteoblasts using
the osteogenic factor hLMP-3. Again, an elevated expression of all
the bone markers was observed when compared with hLMP-3 transduced
cells cultured in OM without curcumin supplementation.
The effect of ATRA on osteogenic differentiation is
currently unclear in the literature, and whether its effect is
pro-osteogenic, involved in delaying osteogenesis, or even
anti-osteogenic is unknown (12,14,16,20,87). In
the present study, ATRA increased ALP expression during the
mineralization assay in the first week. Later, during the
differentiation process, a decreased expression of the bone markers
was observed, as well as the inability to develop matrix
mineralization. All these findings indicate that ATRA served a
negative role during the osteogenic differentiation of BMSCs. These
results are consistent with those of previous studies (27,82),
which suggested that this inhibitory effect of ATRA is mediated via
BMP signaling. They reported that ATRA upregulated the expression
of BMP-receptor IA which is responsible for adipogenic
differentiation, and reduced the expression of BMP-receptor IB,
which is responsible for the osteogenic differentiation of BMSCs.
Another explanation posed by Green et al (88) is that retinoic acid receptor (RAR)
agonists, such as ATRA, impair osteogenesis through RARα and RARγ,
which may explain why high intake and serum levels of retinol are
associated with fracture risk. By contrast, several studies have
demonstrated the positive role of ATRA during the osteogenic
differentiation of cell types other than BMSCs (18,89,90). In
the present study, BMSCs treated with ATRA did not express OCN,
which is the main non-collagenous protein of bone. This may be
explained by interactions between ATRA and the OCN promotor
(91).
In the present study, curcumin upregulated the
osteogenic differentiation of BMSCs. Several studies have examined
the role of curcumin in the orthopedic field (35–38). The
present results were consistent with those of Gu et al
(53), who reported that curcumin
increased the osteoblast differentiation of rat MSCs with a
reduction in adipocytes. The authors considered that this increase
occurred due to an increase in HO-1 expression induced by curcumin,
which in turn promoted osteoblast differentiation. In the same
context, Son et al (92)
reported that curcumin increased the expression of genes such as
Dlx5, Runx2, ALP and OCN, which subsequently induced osteoblast
differentiation in C3H10T1/2 cells. These findings were interpreted
through a new hypothesis, which is that curcumin induced mild ER
stress, similar to BMP2 functioning, in osteoblast cells.
Furthermore, Son et al (92)
reported that curcumin is similar to BMP2 as it induces the
phosphorylation of Smad 1/5/9. By contrast, another study revealed
that the administration of curcumin did not efficiently improve
bone mineralization in ovariectomized rats, and indicated that
curcumin affected the level of osteogenesis commitment, not
osteoblast maturation (36). It has
also been revealed that curcumin reduces the expression of RANKL
and inhibits osteoclastogenesis by acting on BMSCs (61). This could be explained by the
activity of curcumin as a scavenger of reactive oxygen species.
Upregulation of RANKL induced by estrogen deficiency in humans
results in increased bone resorption (93). In addition to RANKL inhibition,
curcumin also inhibits NF-κB (47),
which is associated with impaired bone formation in osteoporosis,
and inhibits the differentiation and mineralization of mature
osteoblasts. The inhibition of NF-κB results in stimulation of the
differentiation and mineralization of primary murine BMSCs and
pre-osteoblasts (94,95). Thus, curcumin may enhance
osteogenesis through its interactions with NF-κB. Curcumin has also
been demonstrated to affect other signaling pathways associated
with bone remodeling, including the Wnt (96) and transforming growth factor-β
signaling pathways (97).
In conclusion, the results of the present study
highlight the potential use of some natural osteogenic inducers in
the orthopedic field. The positive role of curcumin during the
osteogenic differentiation of BMSCs was demonstrated, and curcumin
was revealed to be capable of enriching the osteogenic
differentiation of other cell types, namely, MEFs reprogrammed with
hLMP-3. By contrast, the use of ATRA inhibited the osteogenic
differentiation process of BMSCs rather, which is contrary to its
important role in the osteogenic induction of other cell types.
Acknowledgements
The authors would like to thank the members of the
Key Laboratory of Animal Breeding, Reproduction and Molecular
Design for Jiangsu Province, College of Animal Science and
Technology, Yangzhou University, China for their help with the
isolation of BMSCs and MEFs, and also for taking care of the
laboratory animals during the study period.
Funding
The present study was funded by National Natural
Science Foundation of China (grant nos. 31472087 and 31572390),
College Students' Innovation and Entrepreneurship Training Program,
Yangzhou University (grant no. X20170702), the Project Funded by
the Priority Academic Program Development of Jiangsu Higher
Education Institutions, the Key Research and Development Program
(grant no. 2017YFE0108000) and the High Level Talents Q7 Support
Program of Yangzhou University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MFA and AKE have performed the experiments,
contributed to data analysis and wrote the manuscript. HC
substantially contributed to the interpretation of data and
revision of the manuscript. RZ and QZ contributed to the study
conception, and analysis and interpretation of data. MSY, YZ and BL
conceived and designed the study, and approved the final version to
be published.
Ethics approval and consent to
participate
All animal experiments were reviewed and approved by
the Institutional Animal Care and Use Committee of School of Animal
Science and Technology, Yangzhou University (Yangzhou, China;
approval no. YZUDWSY2017-0029).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wrobel E, Leszczynska J and Brzoska E: The
characteristics of human bone-derived cells (HBDCS) during
osteogenesis in vitro. Cell Mol Biol Lett. 21:262016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: Nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kon E, Muraglia A, Corsi A, Bianco P,
Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M,
et al: Autologous bone marrow stromal cells loaded onto porous
hydroxyapatite ceramic accelerate bone repair in critical-size
defects of sheep long bones. J Biomed Mater Res. 49:328–337. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petite H, Viateau V, Bensaïd W, Meunier A,
de Pollak C, Bourguignon M, Oudina K, Sedel L and Guillemin G:
Tissue-engineered bone regeneration. Nat Biotechnol. 18:959–963.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quarto R, Mastrogiacomo M, Cancedda R,
Kutepov SM, Mukhachev V, Lavroukov A, Kon E and Marcacci M: Repair
of large bone defects with the use of autologous bone marrow
stromal cells. N Engl J Med. 344:385–386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beresford JN, Bennett JH, Devlin C, Leboy
PS and Owen ME: Evidence for an inverse relationship between the
differentiation of adipocytic and osteogenic cells in rat marrow
stromal cell cultures. J Cell Sci. 102:341–351. 1992.PubMed/NCBI
|
|
7
|
Caplan AI: Adult mesenchymal stem cells
for tissue engineering versus regenerative medicine. J Cell
Physiol. 213:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaiswal N, Haynesworth SE, Caplan AI and
Bruder SP: Osteogenic differentiation of purified, culture-expanded
human mesenchymal stem cells in vitro. J Cell Biochem. 64:295–312.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu L, Chaudhary SC, Atigadda VR, Belyaeva
OV, Harville SR, Elmets CA, Muccio DD, Athar M and Kedishvili NY:
Retinoid X receptor agonists upregulate genes responsible for the
biosynthesis of all-trans-retinoic acid in human epidermis. PLoS
One. 11:e01535562016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacobson A, Johansson S, Branting M and
Melhus H: Vitamin A differentially regulates RANKL and OPG
expression in human osteoblasts. Biochem Biophys Res Commun.
322:162–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michaëlsson K, Lithell H, Vessby B and
Melhus H: Serum retinol levels and the risk of fracture. N Engl J
Med. 348:287–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skillington J, Choy L and Derynck R: Bone
morphogenetic protein and retinoic acid signaling cooperate to
induce osteoblast differentiation of preadipocytes. J Cell Biol.
159:135–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hisada K, Hata K, Ichida F, Matsubara T,
Orimo H, Nakano T, Yatani H, Nishimura R and Yoneda T: Retinoic
acid regulates commitment of undifferentiated mesenchymal stem
cells into osteoblasts and adipocytes. J Bone Miner Metab.
31:53–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malladi P, Xu Y, Yang GP and Longaker MT:
Functions of vitamin D, retinoic acid, and dexamethasone in mouse
adipose-derived mesenchymal cells. Tissue Eng. 12:2031–2040. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mirsaidi A, Kleinhans KN, Rimann M, Tiaden
AN, Stauber M, Rudolph KL and Richards PJ: Telomere length,
telomerase activity and osteogenic differentiation are maintained
in adipose-derived stromal cells from senile osteoporotic SAMP6
mice. J Tissue Eng Regen Med. 6:378–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song HM, Nacamuli RP, Xia W, Bari AS, Shi
YY, Fang TD and Longaker MT: High-dose retinoic acid modulates rat
calvarial osteoblast biology. J Cell Physiol. 202:255–262. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tiaden AN, Breiden M, Mirsaidi A, Weber
FA, Bahrenberg G, Glanz S, Cinelli P, Ehrmann M and Richards PJ:
Human serine protease HTRA1 positively regulates osteogenesis of
human bone marrow-derived mesenchymal stem cells and mineralization
of differentiating bone-forming cells through the modulation of
extracellular matrix protein. Stem Cells. 30:2271–2282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan DC, Shi YY, Nacamuli RP, Quarto N,
Lyons KM and Longaker MT: Osteogenic differentiation of mouse
adipose-derived adult stromal cells requires retinoic acid and bone
morphogenetic protein receptor type IB signaling. Proc Natl Acad
Sci USA. 103:12335–12340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan DC, Siedhoff MT, Kwan MD, Nacamuli RP,
Wu BM and Longaker MT: Refining retinoic acid stimulation for
osteogenic differentiation of murine adipose-derived adult stromal
cells. Tissue Eng. 13:1623–1631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choong PF, Martin TJ and Ng KW: Effects of
ascorbic acid, calcitriol, and retinoic acid on the differentiation
of preosteoblasts. J Orthop Res. 11:638–647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Descalzi Cancedda F, Gentili C, Manduca P
and Cancedda R: Hypertrophic chondrocytes undergo further
differentiation in culture. J Cell Biol. 117:427–435. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leboy PS, Beresford JN, Devlin C and Owen
ME: Dexamethasone induction of osteoblast mRNAs in rat marrow
stromal cell cultures. J Cell Physiol. 146:370–378. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iba K, Chiba H, Yamashita T, Ishii S and
Sawada N: Phase-independent inhibition by retinoic acid of
mineralization correlated with loss of tetranectin expression in a
human osteoblastic cell line. Cell Struct Funct. 26:227–233. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lind T, Sundqvist A, Hu L, Pejler G,
Andersson G, Jacobson A and Melhus H: Vitamin a is a negative
regulator of osteoblast mineralization. PLoS One. 8:e823882013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohishi K, Nishikawa S, Nagata T, Yamauchi
N, Shinohara H, Kido J and Ishida H: Physiological concentrations
of retinoic acid suppress the osteoblastic differentiation of fetal
rat calvaria cells in vitro. Eur J Endocrinol. 133:335–341. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nallamshetty S, Wang H, Rhee EJ, Kiefer
FW, Brown JD, Lotinun S, Le P, Baron R, Rosen CJ and Plutzky J:
Deficiency of retinaldehyde dehydrogenase 1 induces BMP2 and
increases bone mass in vivo. PLoS One. 8:e713072013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen M, Huang HZ, Wang M and Wang AX:
Retinoic acid inhibits osteogenic differentiation of mouse
embryonic palate mesenchymal cells. Birth Defects Res A Clin Mol
Teratol. 88:965–970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xin M, Yang Y, Zhang D, Wang J, Chen S and
Zhou D: Attenuation of hind-limb suspension-induced bone loss by
curcumin is associated with reduced oxidative stress and increased
vitamin D receptor expression. Osteoporos Int. 26:2665–2676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aggarwal BB, Sundaram C, Malani N and
Ichikawa H: Curcumin: The Indian solid gold. Adv Exp Med Biol.
595:1–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shishodia S, Sethi G and Aggarwal BB:
Curcumin: Getting back to the roots. Ann N Y Acad Sci.
1056:206–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riva A, Togni S, Giacomelli L, Franceschi
F, Eggenhoffner R, Feragalli B, Belcaro G, Cacchio M, Shu H and
Dugall M: Effects of a curcumin-based supplementation in
asymptomatic subjects with low bone density: A preliminary 24-week
supplement study. Eur Rev Med Pharmacol Sci. 21:1684–1689.
2017.PubMed/NCBI
|
|
32
|
Lone J, Choi JH, Kim SW and Yun JW:
Curcumin induces brown fat-like phenotype in 3T3-L1 and primary
white adipocytes. J Nutr Biochem. 27:193–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rohanizadeh R, Deng Y and Verron E:
Therapeutic actions of curcumin in bone disorders. Bonekey Rep.
5:7932016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yun JW: Possible anti-obesity therapeutics
from nature-a review. Phytochemistry. 71:1625–1641. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho DC, Jung HS, Kim KT, Jeon Y, Sung JK
and Hwang JH: Therapeutic advantages of treatment of high-dose
curcumin in the ovariectomized rat. J Korean Neurosurg Soc.
54:461–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Folwarczna J, Zych M and Trzeciak HI:
Effects of curcumin on the skeletal system in rats. Pharmacol Rep.
62:900–909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hussan F, Ibraheem NG, Kamarudin TA, Shuid
AN, Soelaiman IN and Othman F: Curcumin protects against
ovariectomy-induced bone changes in rat model. Evid Based
Complement Alternat Med. 2012:1749162012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim WK, Ke K, Sul OJ, Kim HJ, Kim SH, Lee
MH, Kim HJ, Kim SY, Chung HT and Choi HS: Curcumin protects against
ovariectomy-induced bone loss and decreases osteoclastogenesis. J
Cell Biochem. 112:3159–3166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang MW, Wang TH, Yan PP, Chu LW, Yu J,
Gao ZD, Li YZ and Guo BL: Curcumin improves bone microarchitecture
and enhances mineral density in APP/PS1 transgenic mice.
Phytomedicine. 18:205–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
French DL, Muir JM and Webber CE: The
ovariectomized, mature rat model of postmenopausal osteoporosis: An
assessment of the bone sparing effects of curcumin. Phytomedicine.
15:1069–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuncha M, Naidu VG, Sahu BD, Gadepalli SG
and Sistla R: Curcumin potentiates the anti-arthritic effect of
prednisolone in Freund's complete adjuvant-induced arthritic rats.
J Pharm Pharmacol. 66:133–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jain S, Meka SRK and Chatterjee K:
Curcumin eluting nanofibers augment osteogenesis toward
phytochemical based bone tissue engineering. Biomed Mater.
11:0550072016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23:363–398. 2003.PubMed/NCBI
|
|
44
|
Biswas S and Rahman I: Modulation of
steroid activity in chronic inflammation: A novel anti-inflammatory
role for curcumin. Mol Nutr Food Res. 52:987–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goel A, Jhurani S and Aggarwal BB:
Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food
Res. 52:1010–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
López-Lázaro M: Anticancer and
carcinogenic properties of curcumin: Considerations for its
clinical development as a cancer chemopreventive and
chemotherapeutic agent. Mol Nutr Food Res. 52 Suppl 1:S103–S127.
2008.PubMed/NCBI
|
|
47
|
Bharti AC, Takada Y and Aggarwal BB:
Curcumin (diferuloylmethane) inhibits receptor activator of
NF-kappa B ligand-induced NF-kappa B activation in osteoclast
precursors and suppresses osteoclastogenesis. J Immunol.
172:5940–5947. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chan WH, Wu HY and Chang WH: Dosage
effects of curcumin on cell death types in a human osteoblast cell
line. Food Chem Toxicol. 44:1362–1371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Notoya M, Nishimura H, Woo JT, Nagai K,
Ishihara Y and Hagiwara H: Curcumin inhibits the proliferation and
mineralization of cultured osteoblasts. Eur J Pharmacol. 534:55–62.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ozaki K, Kawata Y, Amano S and Hanazawa S:
Stimulatory effect of curcumin on osteoclast apoptosis. Biochem
Pharmacol. 59:1577–1581. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Von Metzler I, Krebbel H, Kuckelkorn U,
Heider U, Jakob C, Kaiser M, Fleissner C, Terpos E and Sezer O:
Curcumin diminishes human osteoclastogenesis by inhibition of the
signalosome-associated I kappaB kinase. J Cancer Res Clin Oncol.
135:173–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamaguchi M, Hamamoto R, Uchiyama S and
Ishiyama K: Effects of flavonoid on calcium content in femoral
tissue culture and parathyroid hormone-stimulated
osteoclastogenesis in bone marrow culture in vitro. Mol Cell
Biochem. 303:83–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gu Q, Cai Y, Huang C, Shi Q and Yang H:
Curcumin increases rat mesenchymal stem cell osteoblast
differentiation but inhibits adipocyte differentiation. Pharmacogn
Mag. 8:202–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hou M, Song Y, Li Z, Luo C, Ou JS, Yu H,
Yan J and Lu L: Curcumin attenuates osteogenic differentiation and
calcification of rat vascular smooth muscle cells. Mol Cell
Biochem. 420:151–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen F, Wang H, Xiang X, Yuan J, Chu W,
Xue X, Zhu H, Ge H, Zou M, Feng H and Lin J: Curcumin increased the
differentiation rate of neurons in neural stem cells via wnt
signaling in vitro study. J Surg Res. 192:298–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tiwari SK, Agarwal S, Seth B, Yadav A,
Nair S, Bhatnagar P, Karmakar M, Kumari M, Chauhan LK, Patel DK, et
al: Curcumin-loaded nanoparticles potently induce adult
neurogenesis and reverse cognitive deficits in Alzheimer's disease
model via canonical Wnt/β-catenin pathway. ACS Nano. 8:76–103.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tiwari SK, Agarwal S, Tripathi A and
Chaturvedi RK: Bisphenol-A mediated inhibition of hippocampal
neurogenesis attenuated by curcumin via canonical Wnt pathway. Mol
Neurobiol. 53:3010–3029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cui L, Jia X, Zhou Q, Zhai X, Zhou Y and
Zhu H: Curcumin affects β-catenin pathway in hepatic stellate cell
in vitro and in vivo. J Pharm Pharmacol. 66:1615–1622. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
He M, Li Y, Zhang L, Li L, Shen Y, Lin L,
Zheng W, Chen L, Bian X, Ng HK and Tang L: Curcumin suppresses cell
proliferation through inhibition of the Wnt/β-catenin signaling
pathway in medulloblastoma. Oncol Rep. 32:173–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Moran JM, Roncero-Martin R,
Rodriguez-Velasco FJ, Calderon-Garcia JF, Rey-Sanchez P, Vera V,
Canal-Macias ML and Pedrera-Zamorano JD: Effects of curcumin on the
proliferation and mineralization of human osteoblast-like cells:
Implications of nitric oxide. Int J Mol Sci. 13:16104–16118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Oh S, Kyung TW and Choi HS: Curcumin
inhibits osteoclastogenesis by decreasing receptor activator of
nuclear factor-kappaB ligand (RANKL) in bone marrow stromal cells.
Mol Cells. 26:486–489. 2008.PubMed/NCBI
|
|
62
|
Vierbuchen T, Ostermeier A, Pang ZP,
Kokubu Y, Südhof TC and Wernig M: Direct conversion of fibroblasts
to functional neurons by defined factors. Nature. 463:1035–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ieda M, Fu JD, Delgado-Olguin P, Vedantham
V, Hayashi Y, Bruneau BG and Srivastava D: Direct reprogramming of
fibroblasts into functional cardiomyocytes by defined factors.
Cell. 142:375–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sekiya S and Suzuki A: Direct conversion
of mouse fibroblasts to hepatocyte-like cells by defined factors.
Nature. 475:390–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Y, Wang Y, Yu J, Ma Z, Bai Q, Wu X, Bao
P, Li L, Ma D..Liu J, et al: Direct conversion of human fibroblasts
into osteoblasts and osteocytes with small molecules and a single
factor, Runx2. bioRxiv. 1274802017.
|
|
66
|
Wang Y, Wu MH, Cheung MPL, Sham MH,
Akiyama H, Chan D, Cheah KSE and Cheung M: Reprogramming of dermal
fibroblasts into osteo-chondrogenic cells with elevated osteogenic
potency by defined transcription factors. Stem Cell Reports.
8:1587–1599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nam YJ, Song K, Luo X, Daniel E, Lambeth
K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R and Olson
EN: Reprogramming of human fibroblasts toward a cardiac fate. Proc
Natl Acad Sci USA. 110:5588–5593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qian L, Huang Y, Spencer CI, Foley A,
Vedantham V, Liu L, Conway SJ, Fu JD and Srivastava D: In vivo
reprogramming of murine cardiac fibroblasts into induced
cardiomyocytes. Nature. 485:593–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Minamide A, Boden SD, Viggeswarapu M, Hair
GA, Oliver C and Titus L: Mechanism of bone formation with gene
transfer of the cDNA encoding for the intracellular protein LMP-1.
J Bone Joint Surg Am. 85-A:1030–1039. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Salgia R, Li JL, Lo SH, Brunkhorst B,
Kansas GS, Sobhany ES, Sun Y, Pisick E, Hallek M, Ernst T, et al:
Molecular cloning of human paxillin, a focal adhesion protein
phosphorylated by P210BCR/ABL. J Biol Chem. 270:5039–5047. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lattanzi W, Barba M, Novegno F, Massimi L,
Tesori V, Tamburrini G, Galgano S, Bernardini C, Caldarelli M,
Michetti F and Di Rocco C: Lim mineralization protein is involved
in the premature calvarial ossification in sporadic
craniosynostoses. Bone. 52:474–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lattanzi W, Parrilla C, Fetoni A,
Logroscino G, Straface G, Pecorini G, Stigliano E, Tampieri A,
Bedini R, Pecci R, et al: Ex vivo-transduced autologous skin
fibroblasts expressing human Lim mineralization protein-3
efficiently form new bone in animal models. Gene Ther.
15:1330–1343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yoon ST and Boden SD: Spine fusion by gene
therapy. Gene Ther. 11:360–367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Soleimani M and Nadri S: A protocol for
isolation and culture of mesenchymal stem cells from mouse bone
marrow. Nat Protoc. 4:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pola E, Gao W, Zhou Y, Pola R, Lattanzi W,
Sfeir C, Gambotto A and Robbins PD: Efficient bone formation by
gene transfer of human LIM mineralization protein-3. Gene Ther.
11:683–693. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang S, Xu L, Sun Y, Wu T, Wang K and Li
G: An improved protocol for isolation and culture of mesenchymal
stem cells from mouse bone marrow. J Orthop Translat. 3:26–33.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang T, Lee YW, Rui YF, Cheng TY, Jiang
XH and Li G: Bone marrow-derived mesenchymal stem cells promote
growth and angiogenesis of breast and prostate tumors. Stem Cell
Res Ther. 4:702013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chang R, Sun L and Webster TJ: Short
communication: Selective cytotoxicity of curcumin on osteosarcoma
cells compared to healthy osteoblasts. Int J Nanomedicine.
9:461–465. 2014.PubMed/NCBI
|
|
79
|
Wang N, Wang F, Gao Y, Yin P, Pan C, Liu
W, Zhou Z and Wang J: Curcumin protects human adipose-derived
mesenchymal stem cells against oxidative stress-induced inhibition
of osteogenesis. J Pharmacol Sci. 132:192–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bi W, Gu Z, Zheng Y, Wang L, Guo J and Wu
G: Antagonistic and synergistic effects of bone morphogenetic
protein 2/7 and all-trans retinoic acid on the osteogenic
differentiation of rat bone marrow stromal cells. Dev Growth
Differ. 55:744–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sheng N, Xie Z, Wang C, Bai G, Zhang K,
Zhu Q, Song J, Guillemot F, Chen YG, Lin A and Jing N: Retinoic
acid regulates bone morphogenic protein signal duration by
promoting the degradation of phosphorylated Smad1. Proc Natl Acad
Sci USA. 107:18886–18891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang A, Ding X, Sheng S and Yao Z:
Retinoic acid inhibits osteogenic differentiation of rat bone
marrow stromal cells. Biochem Biophys Res Commun. 375:435–439.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yamamoto K, Kishida T, Sato Y, Nishioka K,
Ejima A, Fujiwara H, Kubo T, Yamamoto T, Kanamura N and Mazda O:
Direct conversion of human fibroblasts into functional osteoblasts
by defined factors. Proc Natl Acad Sci USA. 112:6152–6157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ahmed MF, El-Sayed AK, Chen H, Zhao R, Jin
K, Zuo Q, Zhang Y and Li B: Direct conversion of mouse embryonic
fibroblast to osteoblast cells using hLMP-3 with Yamanaka factors.
Int J Biochem Cell Biol. 106:84–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
James AW, Levi B, Xu Y, Carre AL and
Longaker MT: Retinoic acid enhances osteogenesis in cranial
suture-derived mesenchymal cells: Potential mechanisms of
retinoid-induced craniosynostosis. Plast Reconstr Surg.
125:1352–1361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Green AC, Kocovski P, Jovic T, Walia MK,
Chandraratna RAS, Martin TJ, Baker EK and Purton LE: Retinoic acid
receptor signalling directly regulates osteoblast and adipocyte
differentiation from mesenchymal progenitor cells. Exp Cell Res.
350:284–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang S, Chen X, Hu Y, Wu J, Cao Q, Chen S
and Gao Y: All-trans retinoic acid modulates Wnt3A-induced
osteogenic differentiation of mesenchymal stem cells via activating
the PI3K/AKT/GSK3β signalling pathway. Mol Cell Endocrinol.
422:243–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ding J, Woo JT and Nagai K: The effects of
retinoic acid on reversing the adipocyte differentiation into an
osteoblastic tendency in ST2 cells, a murine bone marrow-derived
stromal cell line. Cytotechnology. 36:125–136. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cohen-Tanugi A and Forest N: Retinoic acid
suppresses the osteogenic differentiation capacity of murine
osteoblast-like 3/A/1D-1M cell cultures. Differentiation.
63:115–123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Son HE, Kim EJ and Jang WG: Curcumin
induces osteoblast differentiation through mild-endoplasmic
reticulum stress-mediated such as BMP2 on osteoblast cells. Life
Sci. 193:34–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Eghbali-Fatourechi G, Khosla S, Sanyal A,
Boyle WJ, Lacey DL and Riggs BL: Role of RANK ligand in mediating
increased bone resorption in early postmenopausal women. J Clin
Invest. 111:1221–1230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li Y, Li A, Strait K, Zhang H, Nanes MS
and Weitzmann MN: Endogenous TNFalpha lowers maximum peak bone mass
and inhibits osteoblastic Smad activation through NF-kappaB. J Bone
Miner Res. 22:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chang J, Wang Z, Tang E, Fan Z, McCauley
L, Franceschi R, Gaun K, Krebsbach PH and Wang CY: Inhibition of
osteoblast functions by IKK/NF-κB in osteoporosis. Nat Med.
15:682–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang X, Yin WK, Shi XD and Li Y: Curcumin
activates Wnt/β-catenin signaling pathway through inhibiting the
activity of GSK-3β in APPswe transfected SY5Y cells. Eur J Pharm
Sci. 42:540–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Thacker PC and Karunagaran D: Curcumin and
emodin down-regulate TGF-β signaling pathway in human cervical
cancer cells. PLoS One. 10:e01200452015. View Article : Google Scholar : PubMed/NCBI
|