Introduction
Age-associated macular degeneration (AMD) is one of
the most important causes of blindness in individuals aged >50
years worldwide. The incidence of AMD has been rapidly increasing
in recent years (1). The prevalent
lesion of AMD is an irreversible vision loss caused by
retrogression of retinal pigment epithelium (RPE) and neural retina
(2). AMD is classified into dry AMD
(geographic atrophy) and wet AMD (exudative). Dry AMD is
characterized by drusen accumulation around RPE and retrogression
of the RPE, while wet AMD is characterized by choroidal
neovascularization (CNV) and results in severe vision loss. As CNV
is a major cause of severe vision loss (3,4),
therapeutic strategies for AMD focus on reversing
neovascularization; they include photodymatic therapy and
anti-angiogenic drugs (5).
AMD-associated pathways and factors that stimulate
CNV remain to be fully elucidated. However, vascular endothelial
growth factor A (VEGF-A), a cytokine that promotes angiogenesis and
vascular permeability, is one of the most important factors that
promotes neovascularization (6).
Active forms of VEGF-A have been identified in CNV (7–9).
Anti-VEGF therapies are now becoming the focus of AMD treatment
(7). Aflibercept and ranibizumab,
two recombinant humanized monoclonal antibodies that inactivate
VEGF-A, are novel therapies that help numerous AMD patients gain a
sustainable vision (8). They were
respectively approved in 2006 and 2011 by the US Food and Drug
Administration for use in treating wet AMD (9,10). The
clinical implementation of such drugs, which directly inhibit VEGF
activity, may offer affected patients hope for improving their
vision.
Chronic inflammation may induce AMD by stimulating
the formation of an abnormal vessel structure. Hemamoeba was
discovered in choroiditis in the eyes of AMD patients (7). It was identified that auto-antibodies
to attack vitreous bodies, retinal pigment epithelium and retinal
tissue in AMD patients (11).
Lymphocytes and macrophages secrete an increased amount of
inflammatory factors in subjects with AMD (12). Certain inflammatory factors,
including transforming growth factor-β1 (TGF-β1), monocyte
chemoattractant protein 1 (MCP-1) and interleukin 6 (IL-6), were
reported to be closely associated with the angiogenesis occurring
as part of the pathogenesis of AMD (11,13).
Regarding drugs for AMD, investigating the correlation between
inflammatory factors and drug treatment effects in clinical studies
may help to further assess their efficiency.
The present study aimed to compare the treatment
outcome of aflibercept and ranibizumab in patients with wet AMD by
evaluating their visual acuity letter score (VAS) and measuring
central subfield thickness (CST). In addition, the possible
correlation between inflammatory factors and the treatment efficacy
of aflibercept and ranibizumab was investigated. The present study
provides a reference for the clinical treatment of wet AMD.
Materials and methods
Patients and aqueous humor
collection
A total of 80 patients with wet AMD (mean age, 57±10
years) who presented at Ningbo No. 6 Hospital (Ningbo, China)
between May 2016 and November 2017 were recruited for the present
study. The patients enrolled all had primary or recurrent CNV
associated with wet AMD. None of the patients included had received
any anti-VEGF treatment for one year prior to the study commencing.
Subjects with hyperlipidaemia, hypertension, diabetes mellitus,
heart failure and renal failure were excluded. The collection of
aqueous humor samples was in accordance with the procedures
approved by the institutional review board of the independent
ethics committee of Ningbo No. 6 Hospital (Ningbo, China) and
following a standard sterilization procedure. After topical
anesthesia with 0.4% oxybuprocaine hydrochloride eye drops (Eisai
Co., Ltd., Tokyo, Japan), 100 µl of aqueous humor was withdrawn
with a tuberculin syringe (30-gauge needle) at the corneal limbus
and was immediately stored at −80°C.
Treatment
A total of 40 patients with wet AMD were
intravitreously injected with aflibercept (Eylea; Regeneron
Pharmaceuticals, Eastview, NY, USA) at a dose of 2.0 mg, and the
other 40 patients with wet AMD were intravitreously injected with
0.3 mg ranibizumab in a dose of 0.5 mg (LUCENTIS™; Genentech Inc.,
San Francisco, CA, USA) every 4 weeks (±1 week) for 1 year. If two
eyes were available in one patient, the eye with the better visual
acuity was chosen to be treated, unless the clinician considered
the other eye more appropriate for certain medical reasons
(14).
Observations
The VAS (ranging from 0 to 100) and CST were
monitored every 4 weeks (±1 week) during the one-year treatment
period. The VAS was measured based on using the Electronic Early
Treatment of Diabetic Retinopathy Study Visual Acuity Test
(15). A VAS of <69 was
equivalent to 20/50 or worse according to a previous study
(16), and thus, 69 was selected as
the cut-off point for the initial VAS. The CST was measured using a
Cirrus™ SD-OCT (Zeiss AG, Oberkochen, Germany) with best-corrected
visual acuity. The higher VAS and lower CST correlated with better
visual acuities. An increase in the VAS by 5 or a decrease in CST
by 10% (~1 Snellen line) was considered to indicate an improvement
in visual acuity. After 6 months, the treatment would be terminated
if the VAS or CST was not improved or even deteriorated after 2
successive injections, or when the visual acuity was better than
20/20. All cases with adverse events were recorded and
monitored.
ELISA
The quantities of TGF-β1, IL-6 and MCP-1 in aqueous
humor samples collected from the patients at baseline and the
follow-up time-points were determined using ELISA kits (R&D
Systems, Minneapolis, MN, USA) following the manufacturer's
protocols, including Human TGF-beta 1 Quantikine ELISA kit (cat.
no. SB100B), Human IL-6 Quantikine ELISA kit (cat. no. S6050) and
Human CCL2/MCP-1 Quantikine ELISA kit (cat. no. SCP00). Finally,
the optical density values were read at 450 nm by using Multiskan
FC microplate photometer (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The quantities of the analytes were determined by using a
standard curve.
Statistical analysis
The mean changes of VAS and CST were calculated and
compared among the different treatment groups. All results are
expressed as the mean ± standard deviation. Statistical analysis
was performed using the SPSS 22.0 statistical package (IBM Corp.,
Armonk, NY, USA) and GraphPad Prism 6.0 (GraphPad Inc., La Jolla,
CA, USA). The Chi-squared test was used to compare categorical
variables. Spearman's correlation analysis was used to evaluate the
correlation among numerical data. One-way analysis of variance
followed by Dunnett's test was used to compare differences among
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients and treatments
The 80 patients with wet AMD were randomly assigned
into two groups that were respectively injected with aflibercept or
ranibizumab intravitreously. The clinical characteristics of the
patients are displayed in Table I.
At baseline, the characteristics in the two groups were similar.
When the initial VAS was ≥69, the median number of injections was
10 in each group (data not shown). However, when the initial VAS
was <69, the median number of injections was 11 in each group
(data not shown). These differences were not significant.
 | Table I.Characteristics of patients included
in the present study. |
Table I.
Characteristics of patients included
in the present study.
| Characteristic | Aflibercept
(n=40) | Ranibizumab
(n=40) | P-value |
|---|
| Sex |
|
|
|
| Male | 17 (42.5) | 19 (47.5) | >0.05 |
|
Female | 23 (57.5) | 21 (52.5) |
|
| Age (years) | 60.5±4.3 | 62.4±7.1 | 0.15 |
| Course of disease
(months) | 39.5±9.2 | 43.5±13.2 | 0.12 |
Effect of aflibercept or ranibizumab
on VAS
The mean VAS improved significantly during the
one-year treatment period, with an overall increase by 13.1 in the
aflibercept group and by 11.0 in the ranibizumab group. As
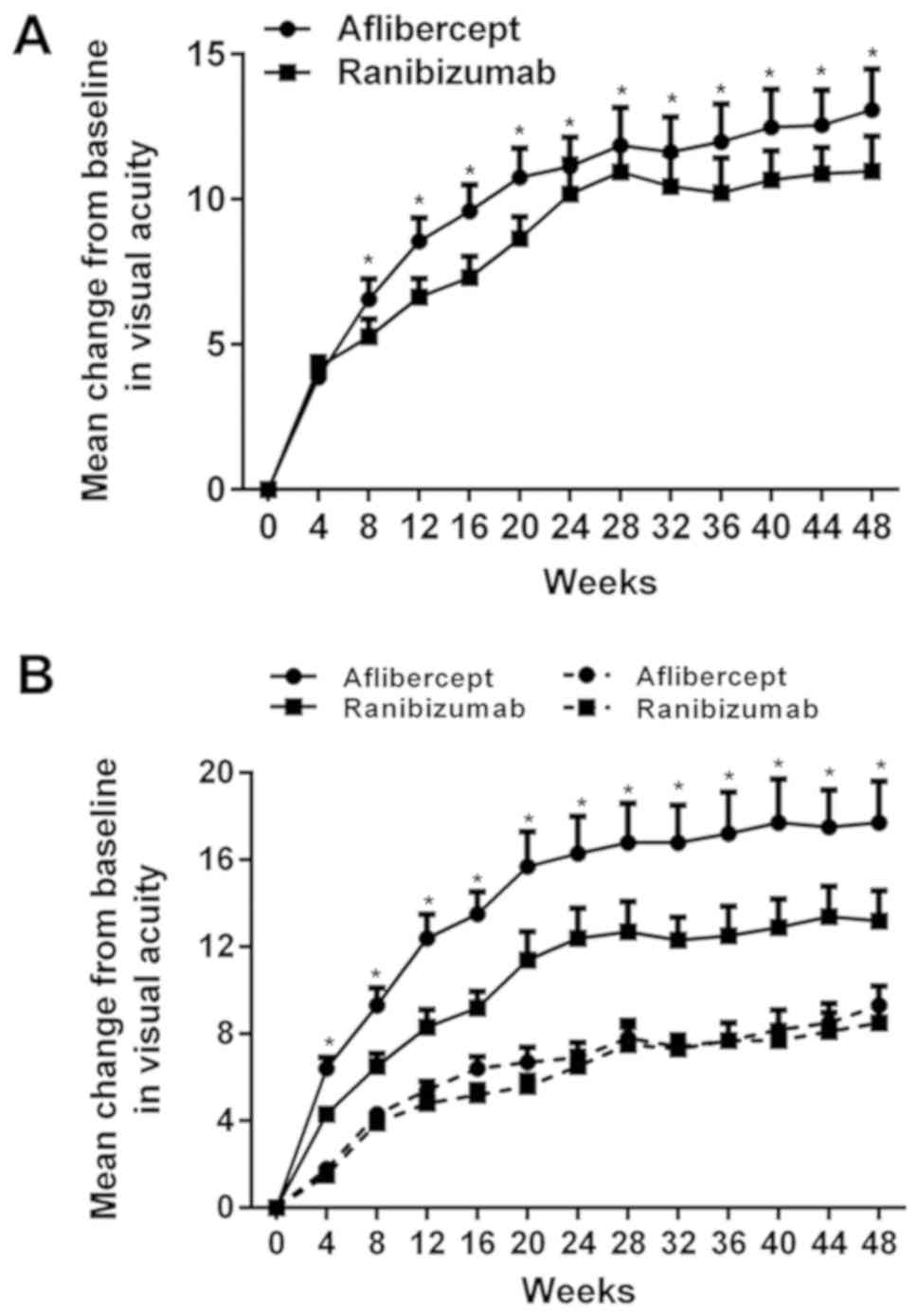
presented in Fig. 1A, the
improvement of VAS in the aflibercept group was significantly
higher than that in the ranibizumab group. The extent of
improvement of the VAS varied depending on the initial visual
acuity. When the initial VAS was <69 (Snellen equivalent,
20/50), the mean improvement in VAS was 17.7 in the aflibercept
group and 13.2 in the ranibizumab group (P<0.01; Table II), with a significant difference
between the groups. When the initial VAS was ≥69, the mean
improvement in VAS was 9.3 in the aflibercept group and 8.5 in the
ranibizumab group, with no significant difference between the
groups. As presented in Fig. 1B, the
mean improvement of VAS after drug injection was higher when the
initial VAS of the patients was <69 (Snellen equivalent, 20/50)
compared with that in the subgroups with an initial VAS of ≥69. In
addition, when the initial VAS was <69 (18 eyes for those who
were treated with aflibercept and 21 eyes for those who were
treated with ranibizumab), the number of eyes with a VAS
improvement of ≥15 was 61.1% (11/18) for patients who received
aflibercept and 28.5% (6/21) for patients who received ranibizumab,
and a significant difference was identified (P<0.05).
Furthermore, the number of eyes with a VAS improvement of 10–15 was
27.8% (5/18) for patients who received aflibercept and 52.4%
(11/21) for patients who received ranibizumab, and no significant
difference was observed. When the initial VAS was ≥69 (22 eyes
treated with aflibercept and 19 eyes treated with ranibizumab), the
number of eyes with an improvement in the VAS by ≥15 was 31.8%
(7/22) for patients using aflibercept and 21.1% (4/19) for patients
using ranibizumab, and no significant difference was identified.
Furthermore, the number of eyes with an improvement in the VAS by
10–15 was 59.1% (13/22) for those using aflibercept and 63.2%
(12/19) for those using ranibizumab, and no significant difference
was observed.
 | Table II.Changes in VAS in different
groups. |
Table II.
Changes in VAS in different
groups.
| A, <69 |
|---|
|
|---|
| VAS | Aflibercept
(n=40) | Ranibizumab
(n=40) | P-value |
|---|
| Eyes (n) | 18 | 21 | – |
| Mean improvement | 17.7±5.2 | 13.2±4.9 | 0.009 |
| Change VAS |
|
|
|
| ≥15 | 11 (61.1) | 6 (28.5) | 0.044 |
|
10–15 | 5 (27.8) | 11 (52.4) | 0.124 |
| 0±10 | 1 (5.5) | 2 (9.5) | 0.647 |
|
−(10–15) | 1 (5.5) | 1 (4.7) | 0.355 |
|
−(≥15) | 0 (0.0) | 1 (4.7) | – |
|
| B, ≥69 |
|
| VAS | Aflibercept
(n=40) | Ranibizumab
(n=40) | P-value |
|
| Eyes (n) | 22 | 19 |
|
| Mean improvement | 9.3±3.7 | 8.5±4.2 | 0.52 |
| Change in VAS |
|
|
|
|
≥15 | 7 (31.8) | 4 (21.1) | 0.443 |
|
10–15 | 13 (59.1) | 12 (63.2) | 0.627 |
|
0±10 | 2 (9.1) | 2 (10.5) | 0.879 |
|
−(10–15) | 0 (0.0) | 1 (5.3) | 0.282 |
|
−(≥15) | 0 (0.0) | 0 (0.0) | 1.000 |
Effect of aflibercept or ranibizumab
on CST
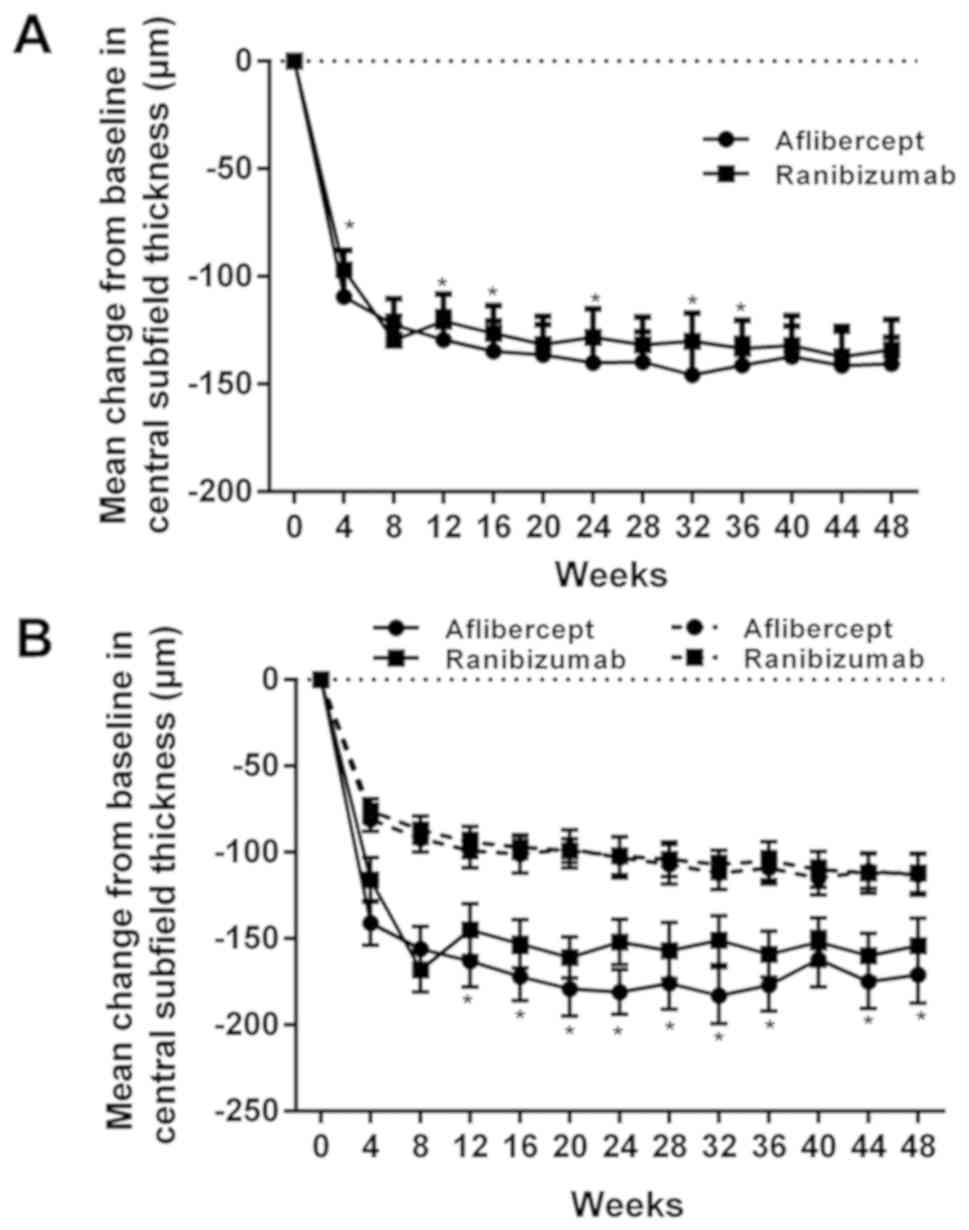
The mean CST decreased significantly within one year
of treatment. In the aflibercept group, the CST was decreased by
140 µm and in the ranibizumab group by 134 µm. As presented in
Fig. 2A, the decrease in CST in the
aflibercept group was larger than that in the ranibizumab group.
The reduction in CST was dependent on the initial VAS, as presented
in Fig. 2B. When the initial VAS was
<69, the mean decline was 171 µm in the aflibercept group and
154 µm in the ranibizumab group, and a significant difference was
identified. When the initial VAS was ≥69, the decline in the CST
was 113 µm in the aflibercept group and 112 µm in the ranibizumab
group, with no significant inter-group difference (Table III). The mean decline in CST after
drug injection was obvious when the initial VAS of the patients was
<69 in comparison with that in the subgroup with an initial VAS
of ≥69 (Fig. 2B). When the initial
VAS was <69 (18 eyes in the aflibercept subgroup and 21 in the
ranibizumab subgroup), the number of eyes with a CST of <250 µm
after 1 year was 61.1% (11/18) for those treated with aflibercept
and 38.1% (8/21) for those treated with ranibizumab. When the
initial VAS was ≥69 (22 eyes in the aflibercept subgroup and 19 in
the ranibizumab subgroup), the number of eyes with a CST of <250
µm after 1 year was 54.5% (12/22) for those treated with
aflibercept and 42.1% (8/19) for those who received ranibizumab,
and no significant difference was identified.
 | Table III.CST changes in the different
groups. |
Table III.
CST changes in the different
groups.
| A, <69 |
|---|
|
|---|
| Visual acuity
letter score | Aflibercept
(n=40) | Ranibizumab
(n=40) | P-value |
|---|
| Eyes (n) | 18 | 21 |
|
| Mean change in CST
from baseline (µm) | −171±48.5 | −154±43.6 | 0.127 |
| CST <250 µm at 1
year | 11 (61.1) | 8 (38.1) | 0.152 |
|
| B, ≥69 |
|
| Visual acuity
letter score | Aflibercept
(n=40) | Ranibizumab
(n=40) | P-value |
|
| Eyes (n) | 22 | 19 |
|
| Mean change in CST
from baseline (µm) | −113±32.7 | −112±30.8 | 0.923 |
| CST <250 µm at 1
year | 12 (54.5%) | 8 (38.1) | 0.427 |
Safety evaluation
The occurrence of adverse events is listed in
Table IV. No death or
endophthalmitis induced by injection occurred during the study
period. One case of inflammation other than endophthalmitis was
encountered in each group treated with aflibercept or ranibizumab.
The rate of patients with serious adverse events was identical
(25%, 10 in 40 eyes) in the two treatment groups. The adverse
events that occurred at higher rates, e.g., gastrointestinal (9 for
aflibercept and 7 for ranibizumab) or renal events (6 for
aflibercept and 5 for ranibizumab), were similar among the two
treatment groups, and no significant difference was identified. The
rates of vascular events (determined according to the Anti-platelet
Trialists' Collaboration definition), including non-fatal
myocardial infarction and non-fatal stroke were reported in a
previous study (16), were similar
between the two treatment groups, and no significant difference was
identified.
 | Table IV.Serious adverse events within 1 year
of recruitment. |
Table IV.
Serious adverse events within 1 year
of recruitment.
| Events | Aflibercept
(n=40) | Ranibizumab
(n=40) | P-value |
|---|
|
Endophthalmitis | 0 (0.0) | 0 (0.0) | – |
| Ocular
inflammation | 1 (2.5) | 1 (2.5) | 1.000 |
| Retinal detachment
or tear | 0 (0.0) | 1 (2.5) | 0.314 |
| Vitreous
hemorrhage | 1 (2.5) | 2 (5.0) | 0.556 |
|
Injection-associated cataract | 1 (2.5) | 1 (2.5) | 1.000 |
| Elevation of
intraocular pressure | 6
(15.0) | 5
(12.5) | 0.745 |
| Non-fatal
myocardial infarction | 1 (2.5) | 0 (0.0) | 0.314 |
| Non-fatal
stroke | 0 (0.0) | 1 (2.5) | 0.314 |
| Death from any
cause | 0 (0.0) | 0 (0.0) | – |
| Gastrointestinal
events | 9
(22.5) | 7
(17.5) | 0.576 |
| Renal events | 6
(15.0) | 5
(12.5) | 0.745 |
| Hypertension | 5
(12.5) | 5
(12.5) | 1.000 |
Effects of aflibercept or ranibizumab
on inflammatory factors in aqueous humor of patients with wet
AMD
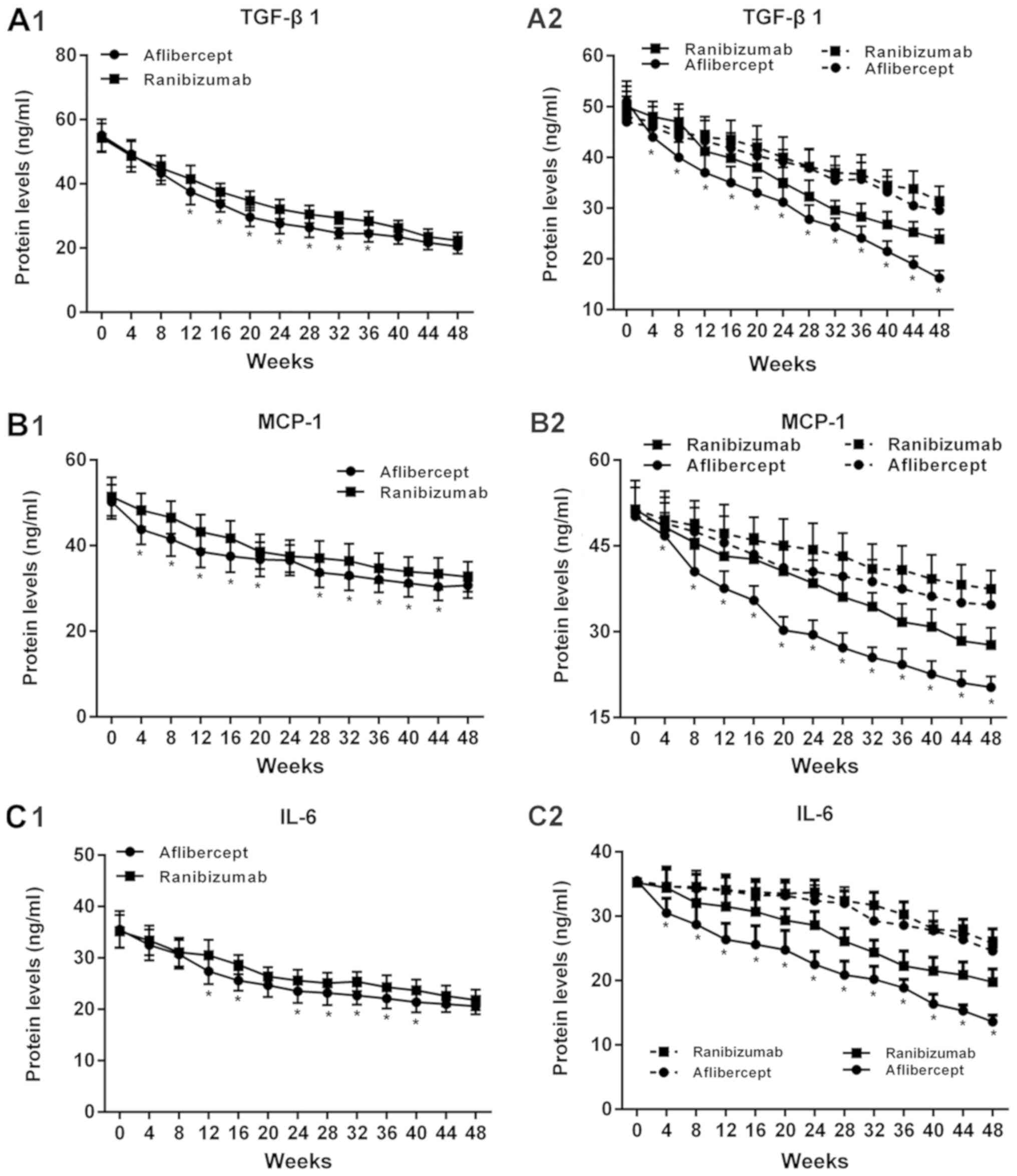
The concentrations of TGF-β1, MCP-1 and IL-6 in
aqueous humor samples from patients with wet AMD treated with
aflibercept or ranibizumab were identified by using ELISA (Fig. 3). The mean concentrations of TGF-β1,
MCP-1 and IL-6 all decreased significantly in the two treatment
groups over the 1-year period (P<0.05; Fig. 3A1-C1). TGF-β1 decreased by 62.7% in
the aflibercept group and by 58.7% in the ranibizumab group. MCP-1
was decreased by 38.8% in the aflibercept group and by 36.4% in the
ranibizumab group. Furthermore, IL-6 was decreased by 42.0% in the
aflibercept group and by 38.1% in the ranibizumab group. The
decline of TGF-β1, MCP-1 and IL-6 levels after drug injection was
more noticeable when the initial VAS of the patients was <69
compared with that in the subgroup with a VAS of ≥69 (Fig. 3A2-C2).
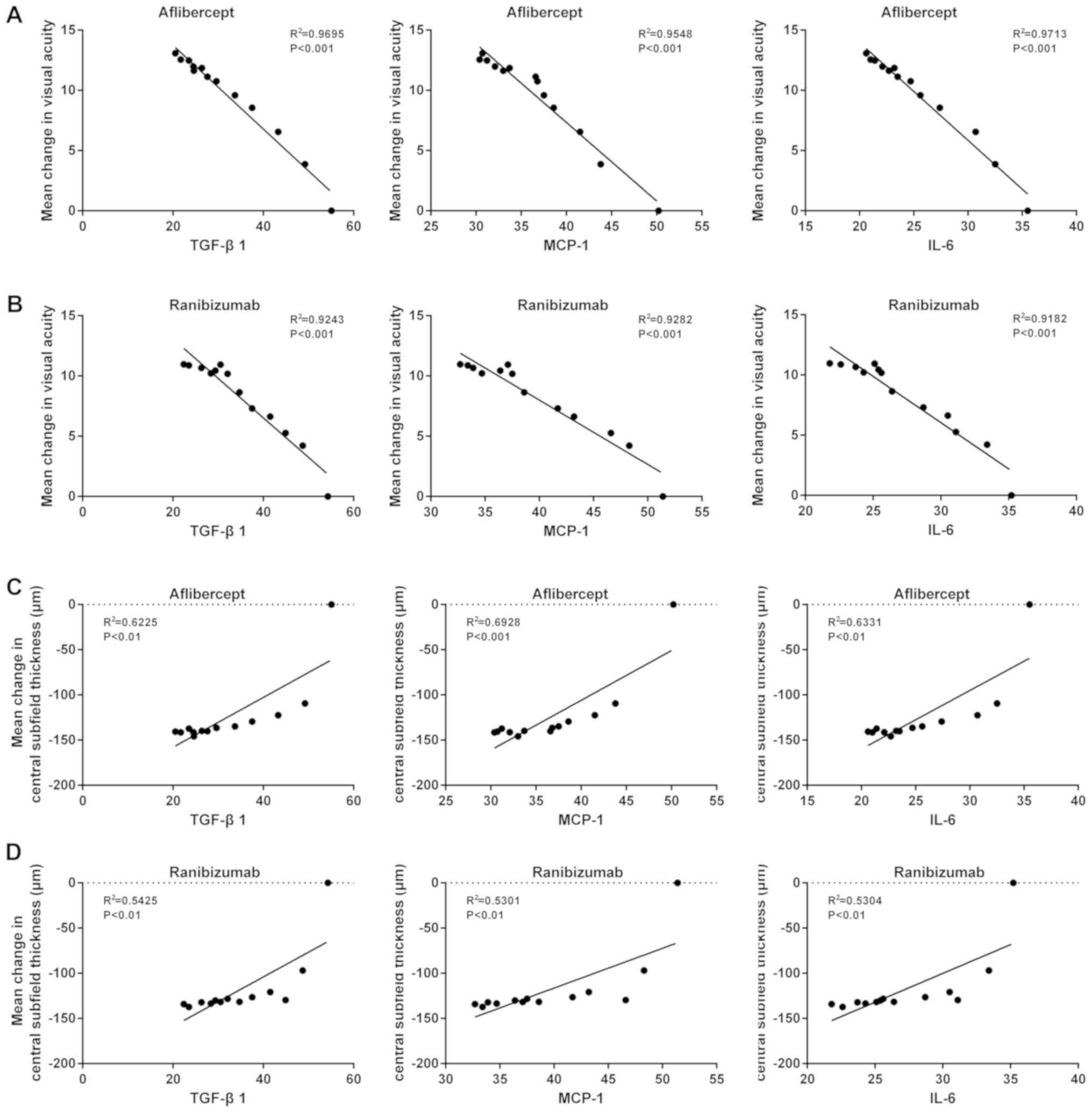
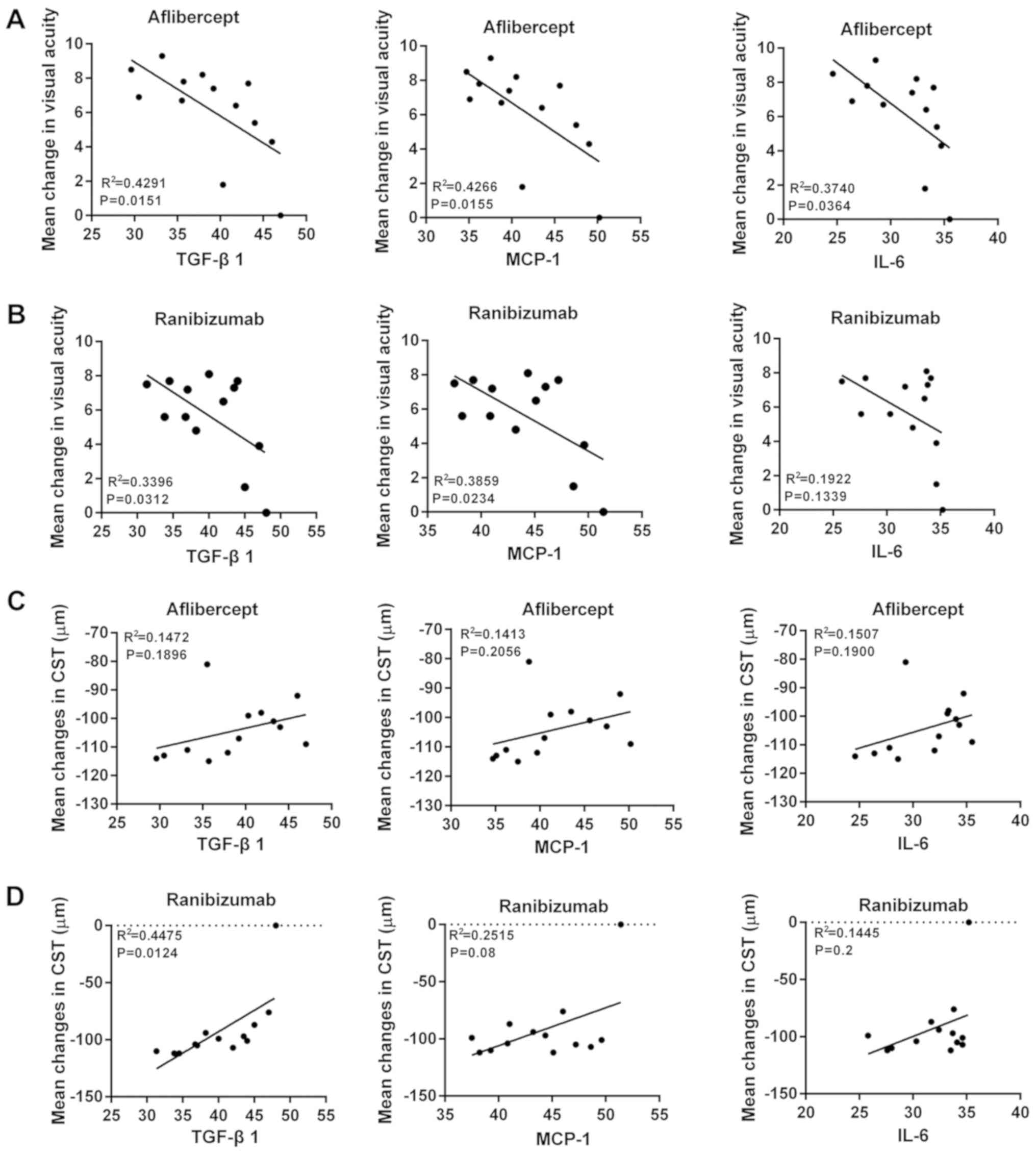
A correlation analysis was then performed to
determine the correlation between inflammatory factors and the mean
change of VAS and CST in patients with wet AMD treated with
aflibercept or ranibizumab. Negative correlations were identified
between the levels of TGF-β1, MCP-1 or IL-6 and the mean change of
VAS if the initial VAS was <69 (Fig.
4A and B). Furthermore, a positive correlation between the
levels of TGF-β1, MCP-1 and IL-6 and the mean change of CST was
observed when the initial VAS of the patients was <69 (Fig. 4C and D). However, while the levels of
TGF-β1, MCP-1 and IL-6 were negatively correlated with the mean
change of VAS when the initial VAS of the patients was ≥69, the
degree of the correlation was relatively low in comparison with
that for the group of patients with an initial VAS of <69
(Fig. 5A and B). In addition, the
levels of TGF-β1, MCP-1 and IL-6 had no significant correlation
with the mean change of CST when the initial VAS of the patients
was ≥69 (Fig. 5C and D).
Discussion
AMD, a retinal eye disease that affects aged
individuals, is characterized by retrogression of RPE and the
neural retina. The therapeutic strategies for wet AMD, including
aflibercept or ranibizumab treatment (as recombinant humanized
monoclonal antibodies inhibiting VEGF), which is a major regulator
of normal and pathological angiogenesis, are focusing on reversing
neovascularization (17).
In the present study, the treatment effects of
aflibercept and ranibizumab on 80 patients with wet AMD patients,
as evaluated via the VAS and the CST, as well as the correlation
between these effects and the decrease of inflammatory factors,
were assessed. At baseline, the VAS and CST were equal among the
groups. When the initial VAS was ≥69, the median injection number
was 10 in each group. During the one-year treatment period,
aflibercept was more effective in treating wet AMD than ranibizumab
based on the improvement in VAS and CST. When the initial VAS was
<69, the effect of aflibercept on the improvement of VAS and the
decrease of CST was more significant than that of ranibizumab. The
visual acuity improvement was mostly in the scope of ≥15 letter
scores in aflibercept-treated patients with wet AMD, while the
improvement was mostly in the range of 10–15 letter scores in the
ranibizumab group. While changes in VAS and CST were achieved by
each of the two treatments, aflibercept was more effective than
ranibizumab when the VAS at baseline was <69. By contrast, when
the initial VAS was ≥69, no difference was identified between the
effects of aflibercept and ranibizumab on VAS and CST. However, the
average changes in VAS and CST were not significantly different
between the aflibercept and ranibizumab treatment groups. Hence, in
patients with a VAS of <69, aflibercept should be
prescribed.
The safety of the two drugs aflibercept and
ranibizumab was monitored during the present clinical study. Apart
from the common medical history inquiry, blood routine examination
was performed in the present study in order to exclude recent
infections and the possibility that general infection affects the
results. Mortalities and endophthalmitis did not occur in the
present study. The incidence of adverse events, including serious
adverse events, e.g., gastrointestinal, renal or vascular events,
was similar between the aflibercept and ranibizumab groups. It may
be concluded that aflibercept and ranibizumab are safe and
effective reagents for improving VAS and decreasing CST in patients
with wet AMD. When the initial VAS was low (<96), the effect of
aflibercept on improving the VAS was slightly better than that of
ranibizumab. By contrast, when the initial VAS was high (≥96), the
effect of aflibercept and ranibizumab was similar.
Previous studies have indicated that complement
cascades and immunological mechanisms mediating inflammatory
reactions are critical elements in the initiation and development
of wet AMD (18,19). Wet AMD is a type of continuous low
chronic inflammatory disease, in which the blood-retinal barrier
breakdown and release of inflammatory factors are induced by
regional tissue damage (20). To
further elucidate the mechanisms by which aflibercept and
ranibizumab restore eyesight in patients with wet AMD, particularly
in terms of changes of inflammatory factors, the present study
determined the concentrations of characteristic inflammatory
cytokines.
The pathophysiology of AMD involves systemic and
ocular inflammation (21).
Aflibercept and ranibizumab are recombinant humanized monoclonal
antibodies that inactivate VEGF (22). VEGF is a potent angiogenic factor,
the overexpression of which is known to deteriorate AMD (23). TGF-β1, a critical regulator in
various physiological and pathological processes, stimulates
endothelial cells to synthesize as well as secrete VEGF. CNV is the
imbalance of angiogenic and anti-angiogenic factors within/among
the choroid, RPE and retina. TGF-β1 induces VEGF secretion in
choroid cells, and it may have a key role in CNV development in AMD
(24). Secreted by mononuclear
cells, macrophages, lymphocytes and endothelial cells, MCP-1 is an
important inflammatory factor that induces mononuclear cell
migration and differentiation to macrophages in tissues (25). VEGF may cause increases in the mRNA
expression of MCP-1, which in turn participates in the development
and infiltration of neovasculature. MCP-1 is known as a key factor
to promote neovascular development, and it has important roles in
regulating the migration and infiltration of mononuclear cells. The
levels of MCP-1 in wet AMD are dependent on the degree of macular
edema (26). IL-6 is a
multifunctional cytokine secreted by mononuclear cells, macrophages
and lymphocytes, and it is a major inflammation-inducing factor in
infection or the acute-phase response to injury. IL-6 is able to
activate the production of antibodies, promote the generation of
fibrinogens, and induce the expression of proteins as well as the
accumulation of T lymphocytes in the acute phase of inflammation
(27). Hence, IL-6 may induce
disorders of immune mechanisms and the autoimmune response.
Furthermore, IL-6 may stimulate transformation factor 3 and promote
CNV generation (28). The present
study indicated that the expression levels of TGF-β1, MCP-1 and
IL-6 decreased significantly during one year of treatment with
aflibercept or with ranibizumab (P<0.05). No significant
difference between the 2 groups was identified. A correlation
analysis for inflammatory factors (TGF-β1, MCP-1 or IL-6) and the
improvement of VAS or the decrease of CST was performed. The
results revealed a negative correlation between the levels of the
inflammatory factors and the effect of aflibercept or ranibizumab
treatment when the initial VAS of the patients was <69,
suggesting that the relative treatment effect on AMD varied
depending on the initial VAS. If the initial VAS was ≥69, there was
no difference, and it may be recommended that, if the initial VAS
is <69, aflibercept should be prescribed. In addition, this
correlation was higher in the aflibercept group than that in the
ranibizumab group. This suggested that aflibercept and ranibizumab
alleviate wet AMD by inhibiting inflammatory factors, including
TGF-β1, MCP-1 and IL-6, to improve the VAS and decrease the CST.
The mechanism behind the actions of aflibercept and ranibizumab may
need further corroborative studies. Taken together, the increase in
VAS, reduction of CST and inhibition of inflammatory factors were
more noticeable in the aflibercept treatment group than those in
the ranibizumab treatment group. Therefore, the inhibition of
inflammation may be a secondary effect of the treatment effect
produced by aflibercept and ranibizumab, the primary effect should
be assessed in future studies.
In addition, in the previous SCORE2 trial, the
effect of bevacizumab and aflibercept in treating macula edema was
compared (29). After 6 months of
treatment, it was indicated that intravitreal bevacizumab was not
inferior to aflibercept with regard to its ability to improve the
VAS. However, the present study compared the effect of aflibercept
and ranibizumab and in addition, the association between
pro-inflammatory cytokines, and the VAS and CST of patients with
AMD was assessed. The results indicated that the treatment effect
on AMD of aflibercept was better than that of ranibizumab. Taken
together, the present study may provide references for deciding on
the treatment strategy for AMD.
In conclusion, the present study suggested that
aflibercept and ranibizumab improved the VAS and decreased the CST
of patients with wet AMD. The drug treatment outcome was dependent
on the patients' initial VAS. Aflibercept was only better than that
of ranibizumab if the initial VAS was <69, while the effect was
similar for VAS ≥69. Therefore, the initial VAS can be used to
guide the treatment decision between aflibercept and ranibizumab,
which appears to be a novel finding of the current study.
Aflibercept and ranibizumab alleviated wet AMD by inhibiting the
expression of TGF-β1, MCP-1 and IL-6. The inhibition of
inflammation may be a secondary effect produced by aflibercept and
ranibizumab, the primary effect should be assessed in future
studies. Thus, the present study provides evidence for the effect
of aflibercept and ranibizumab in treating wet AMD.
Acknowledgements
Not applicable.
Funding
The present study was funded by the General Plan of
Medical and Health Research of the Health and Family Planning
Commission of Zhejiang Province (grant no. 2013 KYA186).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY designed the study, collected and analyzed the
data, and wrote the manuscript.
Ethical approval and consent to
participate
Informed consent was obtained from each patient
prior to enrolment. The present study (Chinese Clinical Trial
Registry no. 1800017782) was approved by the ethics committee of
Ningbo No. 6 Hospital (Ningbo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neely DC, Bray KJ, Huisingh CE, Clark ME,
McGwin G Jr and Owsley C: Prevalence of undiagnosed age-related
macular degeneration in primary eye care. JAMA Ophthalmol.
135:570–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bastawrous A, Mathenge W, Peto T, Shah N,
Wing K, Rono H, Weiss HA, Macleod D, Foster A, Burton M and Kuper
H: Six-year incidence and progression of age-related macular
degeneration in Kenya: Nakuru eye disease cohort study. JAMA
Ophthalmol. 135:631–638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang Z, Zhang Y, Wang Y, Zhang D, Shen B,
Luo M and Gu P: Progress of stem/progenitor cell-based therapy for
retinal degeneration. J Transl Med. 15:992017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ho AC, Chang TS, Samuel M, Williamson P,
Willenbucher RF and Malone T: Experience with a subretinal
cell-based therapy in patients with geographic atrophy secondary to
age-related macular degeneration. Am J Ophthalmol. 179:67–80. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Wiesmann C, Fuh G, Li B,
Christinger HW, McKay P, de Vos AM and Lowman HB: Selection and
analysis of an optimized anti-VEGF antibody: Crystal structure of
an affinity-matured Fab in complex with antigen. J Mol Biol.
293:865–881. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyamoto N, Mandai M, Kojima H, Kameda T,
Shimozono M, Nishida A and Kurimoto Y: Response of eyes with
age-related macular degeneration to anti-VEGF drugs and
implications for therapy planning. Clin Ophthalmol. 11:809–816.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Lin Z, Liu CH, Gong Y, Liegl R,
Fredrick TW, Meng SS, Burnim SB, Wang Z, Akula JD, et al:
Inflammatory signals from photoreceptor modulate pathological
retinal angiogenesis via c-Fos. J Exp Med. 214:1753–1767. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Oliveira Dias JR, Costa de Andrade G,
Kniggendorf VF, Novais EA, Takahashi VKL, Maia A, Meyer C, Watanabe
SES, Farah ME and Rodrigues EB: Intravitreal Ziv-Aflibercept for
neovascular age-related macular degeneration: 52-week results.
Retina. Dec 11–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reich O, Schmid MK, Rapold R, Bachmann LM
and Blozik E: Injections frequency and health care costs in
patients treated with aflibercept compared to ranibizumab: New
real-life evidence from Switzerland. BMC Ophthalmol. 17:2342017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilke RGH, Finger RP and Sachs HG: Time
course of changes in visual acuity after a single injection of
aflibercept or ranibizumab in neovascular age-related macular
degeneration-analysis of aggregated real life data. Klin Monbl
Augenheilkd. 234:1508–1514. 2017.(In German). PubMed/NCBI
|
|
11
|
Wu KH, Tan AG, Rochtchina E, Favaloro EJ,
Williams A, Mitchell P and Wang JJ: Circulating inflammatory
markers and hemostatic factors in age-related maculopathy: A
population-based case-control study. Invest Ophthalmol Vis Sci.
48:1983–1988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuse Y, Tsuruma K, Kanno Y, Shimazawa M
and Hara H: CCR3 is associated with the death of a photoreceptor
cell-line induced by light exposure. Front Pharmacol. 8:2072017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein R, Klein BE, Knudtson MD, Wong TY,
Shankar A and Tsai MY: Systemic markers of inflammation,
endothelial dysfunction, and age-related maculopathy. Am J
Ophthalmol. 140:35–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fouda SM and Bahgat AM: Intravitreal
aflibercept versus intravitreal ranibizumab for the treatment of
diabetic macular edema. Clin Ophthalmol. 11:567–571. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beck RW, Moke PS, Turpin AH, Ferris FL
III, SanGiovanni JP, Johnson CA, Birch EE, Chandler DL, Cox TA,
Blair RC and Kraker RT: A computerized method of visual acuity
testing: Adaptation of the early treatment of diabetic retinopathy
study testing protocol. Am J Ophthalmol. 135:194–205. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells JA, Glassman AR, Ayala AR, Jampol
LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR,
Jhaveri C, et al: Aflibercept, bevacizumab, or ranibizumab for
diabetic macular edema: Two-year results from a comparative
effectiveness randomized clinical trial. Ophthalmology.
123:1351–1359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Giet M, Henkel C, Schuchardt M and
Tolle M: Anti-VEGF drugs in eye diseases: Local therapy with
potential systemic effects. Curr Pharm Des. 21:3548–3556. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anderson DH, Mullins RF, Hageman GS and
Johnson LV: A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol. 134:411–431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin T, Walker GB, Kurji K, Fang E, Law G,
Prasad SS, Kojic L, Cao S, White V, Cui JZ and Matsubara JA:
Parainflammation associated with advanced glycation endproduct
stimulation of RPE in vitro: Implications for age-related
degenerative diseases of the eye. Cytokine. 62:369–381. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Chen M and Forrester JV:
Para-inflammation in the aging retina. Prog Retin Eye Res.
28:348–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato T, Takeuchi M, Karasawa Y, Enoki T
and Ito M: Intraocular inflammatory cytokines in patients with
neovascular age-related macular degeneration before and after
initiation of intravitreal injection of anti-VEGF inhibitor. Sci
Rep. 8:10982018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demirel S, Bilici S, Batioglu F and Ozmert
E: Is there any difference between ranibizumab and aflibercept
injections in terms of inflammation measured with anterior chamber
flare levels in age-related macular degeneration patients: A
comparative study. Ophthalmic Res. 56:35–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tosi GM, Caldi E, Neri G, Nuti E,
Marigliani D, Baiocchi S, Traversi C, Cevenini G, Tarantello A,
Fusco F, et al: HTRA1 and TGF-β1 concentrations in the aqueous
humor of patients with neovascular age-related macular
degeneration. Invest Ophthalmol Vis Sci. 58:162–167. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fisichella V, Giurdanella G, Platania CB,
Romano GL, Leggio GM, Salomone S, Drago F, Caraci F and Bucolo C:
TGF-β1 prevents rat retinal insult induced by amyloid-β (1–42)
oligomers. Eur J Pharmacol. 787:72–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kramer M, Hasanreisoglu M, Feldman A,
Axer-Siegel R, Sonis P, Maharshak I, Monselise Y, Gurevich M and
Weinberger D: Monocyte chemoattractant protein-1 in the aqueous
humour of patients with age-related macular degeneration. Clin Exp
Ophthalmol. 40:617–625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jonas JB, Tao Y, Neumaier M and Findeisen
P: Monocyte chemoattractant protein 1, intercellular adhesion
molecule 1, and vascular cell adhesion molecule 1 in exudative
age-related macular degeneration. Arch Ophthalmol. 128:1281–1286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stewart MW: Clinical and differential
utility of VEGF inhibitors in wet age-related macular degeneration:
Focus on aflibercept. Clin Ophthalmol. 6:1175–1186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lazzeri S, Orlandi P, Piaggi P, Sartini
MS, Casini G, Guidi G, Figus M, Fioravanti A, Di Desidero T,
Ripandelli G, et al: IL-8 and VEGFR-2 polymorphisms modulate
long-term functional response to intravitreal ranibizumab in
exudative age-related macular degeneration. Pharmacogenomics.
17:35–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scott IU, Vanveldhuisen PC, Ip MS, Blodi
BA, Oden NL, Awh CC, Kunimoto DY, Marcus DM, Wroblewski JJ and King
J; SCORE2 Investigator Group, : Effect of bevacizumab vs.
aflibercept on visual acuity among patients with macular edema due
to central retinal vein occlusion: The SCORE2 randomized clinical
trial. JAMA. 317:2072–2087. 2017. View Article : Google Scholar : PubMed/NCBI
|