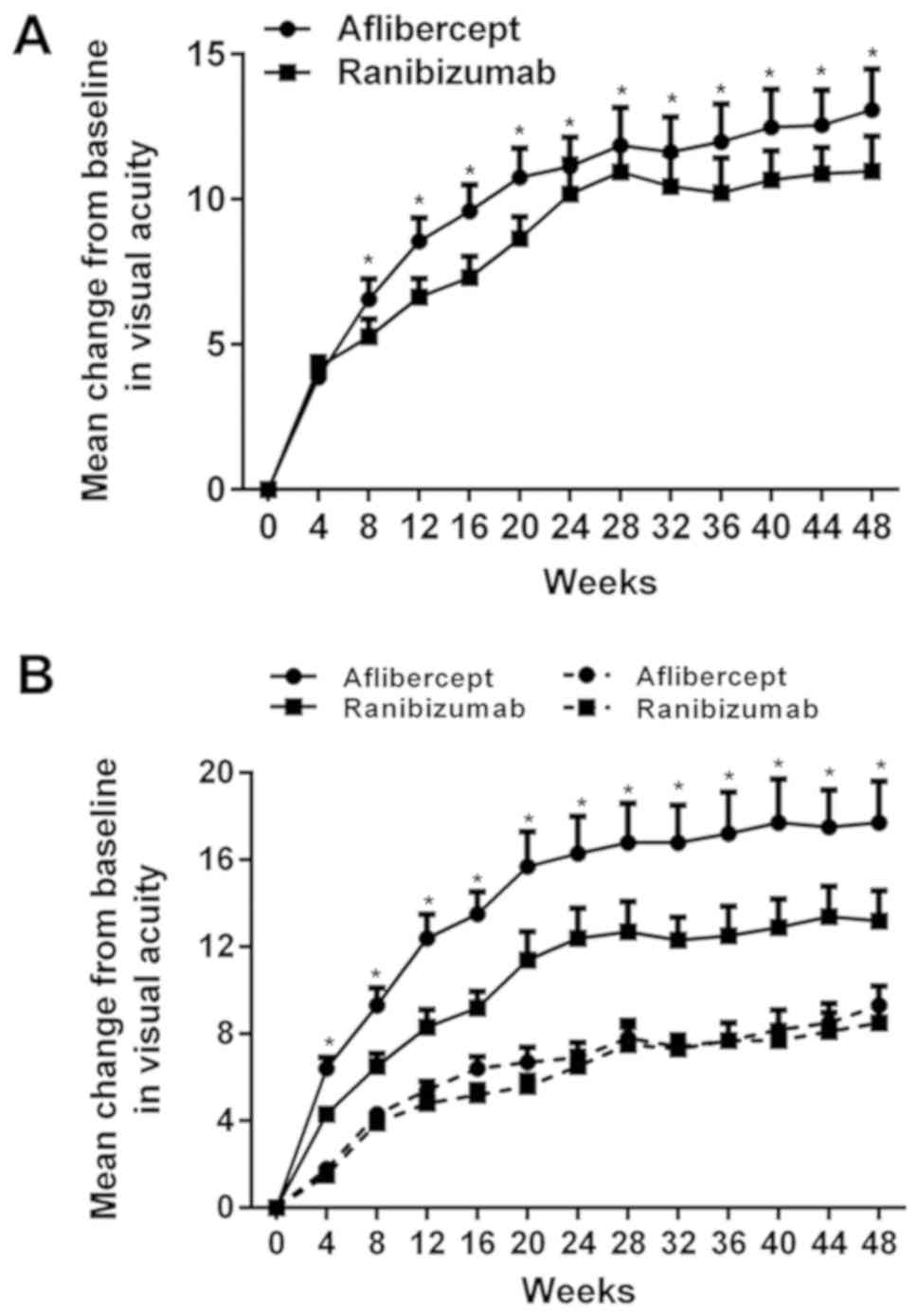
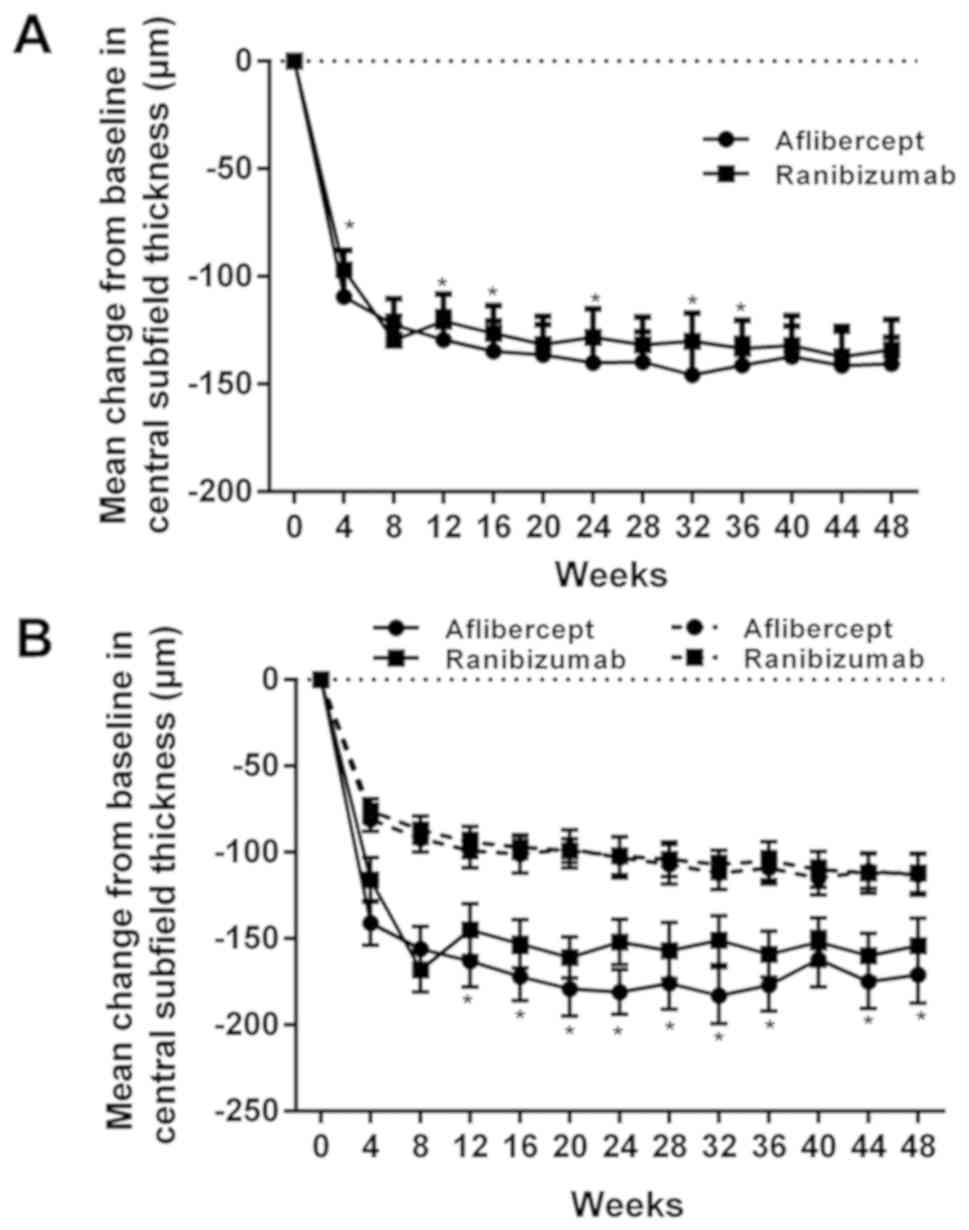
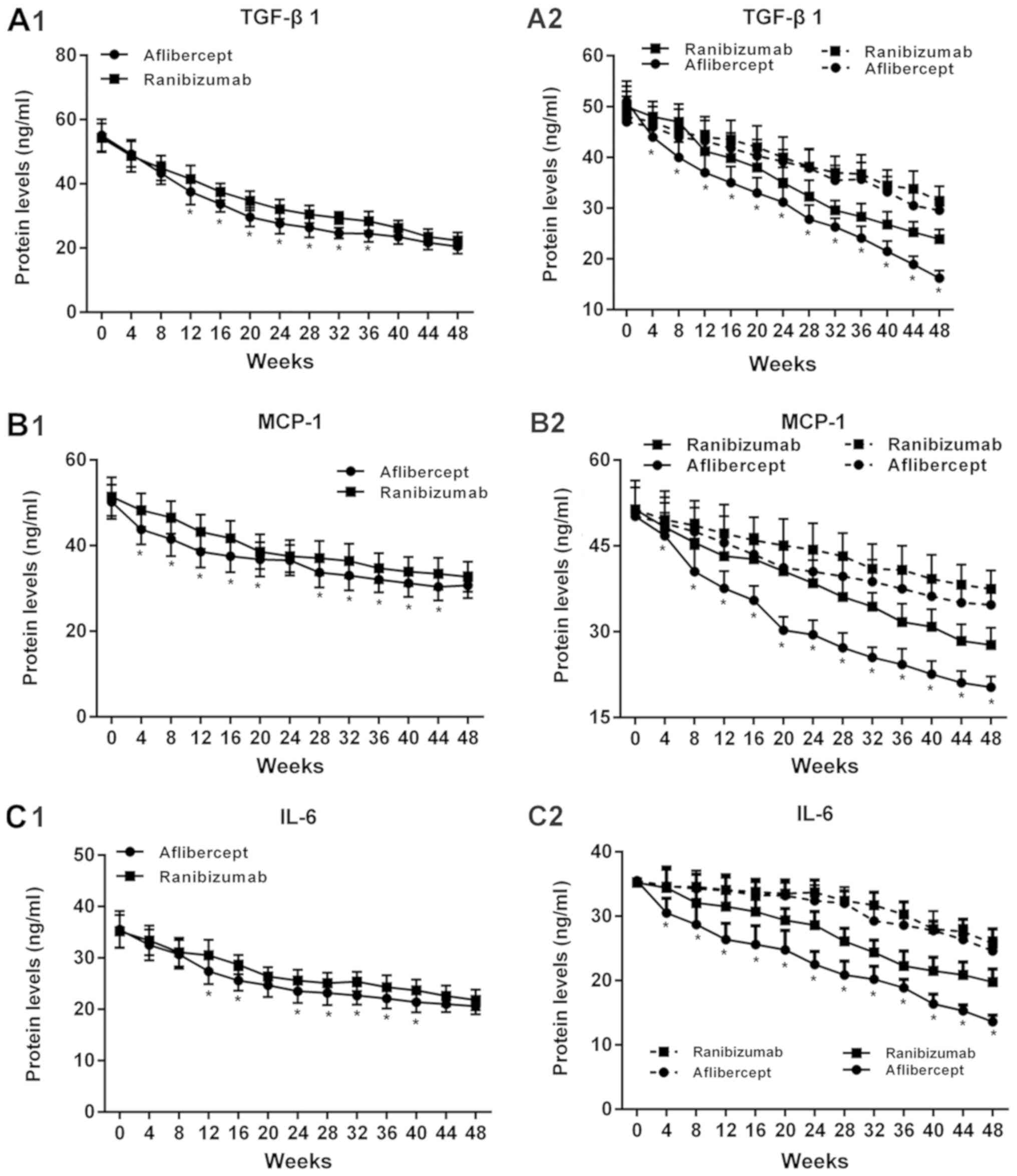
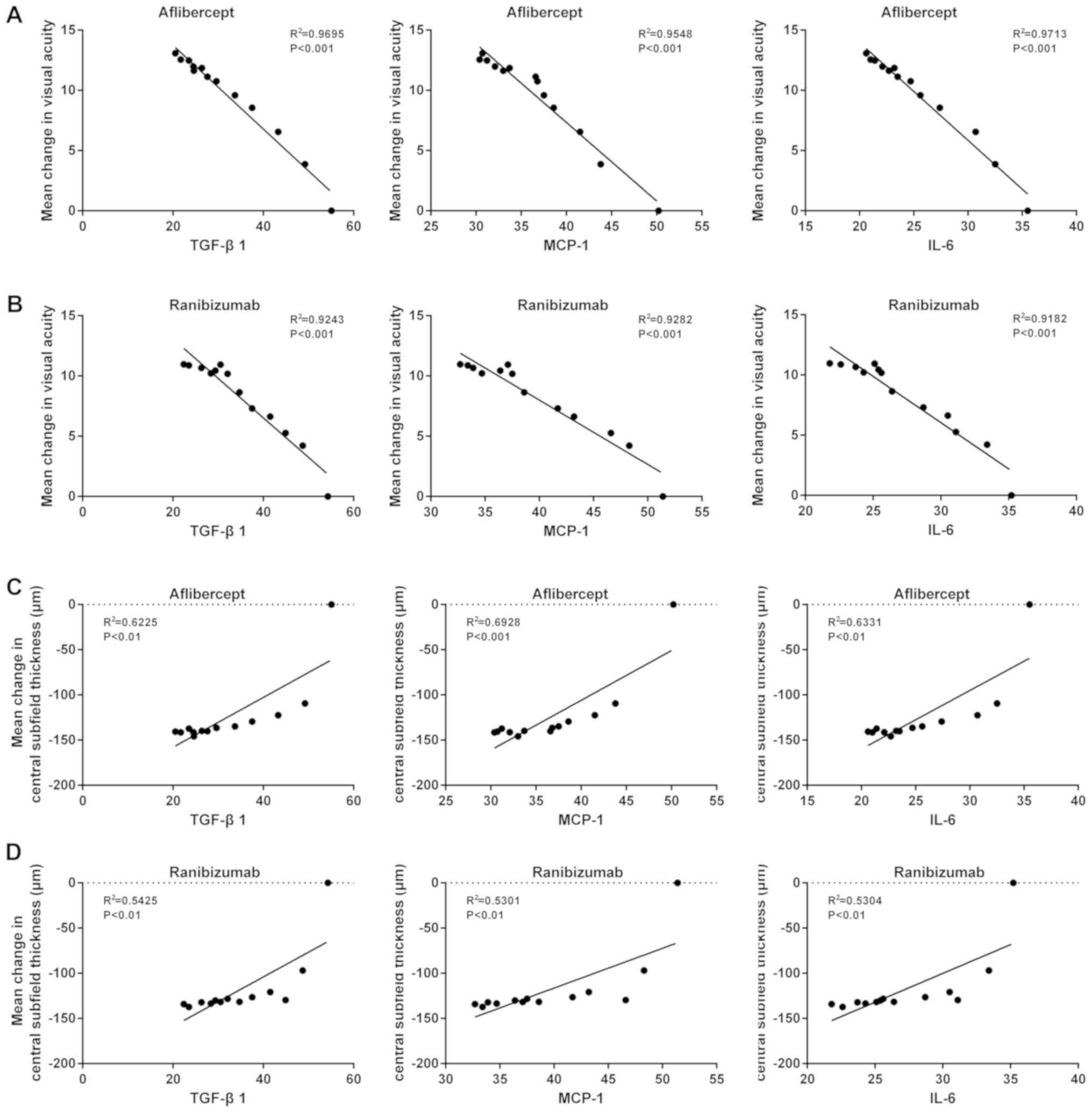
|
1
|
Neely DC, Bray KJ, Huisingh CE, Clark ME,
McGwin G Jr and Owsley C: Prevalence of undiagnosed age-related
macular degeneration in primary eye care. JAMA Ophthalmol.
135:570–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bastawrous A, Mathenge W, Peto T, Shah N,
Wing K, Rono H, Weiss HA, Macleod D, Foster A, Burton M and Kuper
H: Six-year incidence and progression of age-related macular
degeneration in Kenya: Nakuru eye disease cohort study. JAMA
Ophthalmol. 135:631–638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang Z, Zhang Y, Wang Y, Zhang D, Shen B,
Luo M and Gu P: Progress of stem/progenitor cell-based therapy for
retinal degeneration. J Transl Med. 15:992017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ho AC, Chang TS, Samuel M, Williamson P,
Willenbucher RF and Malone T: Experience with a subretinal
cell-based therapy in patients with geographic atrophy secondary to
age-related macular degeneration. Am J Ophthalmol. 179:67–80. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Wiesmann C, Fuh G, Li B,
Christinger HW, McKay P, de Vos AM and Lowman HB: Selection and
analysis of an optimized anti-VEGF antibody: Crystal structure of
an affinity-matured Fab in complex with antigen. J Mol Biol.
293:865–881. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyamoto N, Mandai M, Kojima H, Kameda T,
Shimozono M, Nishida A and Kurimoto Y: Response of eyes with
age-related macular degeneration to anti-VEGF drugs and
implications for therapy planning. Clin Ophthalmol. 11:809–816.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Lin Z, Liu CH, Gong Y, Liegl R,
Fredrick TW, Meng SS, Burnim SB, Wang Z, Akula JD, et al:
Inflammatory signals from photoreceptor modulate pathological
retinal angiogenesis via c-Fos. J Exp Med. 214:1753–1767. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Oliveira Dias JR, Costa de Andrade G,
Kniggendorf VF, Novais EA, Takahashi VKL, Maia A, Meyer C, Watanabe
SES, Farah ME and Rodrigues EB: Intravitreal Ziv-Aflibercept for
neovascular age-related macular degeneration: 52-week results.
Retina. Dec 11–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reich O, Schmid MK, Rapold R, Bachmann LM
and Blozik E: Injections frequency and health care costs in
patients treated with aflibercept compared to ranibizumab: New
real-life evidence from Switzerland. BMC Ophthalmol. 17:2342017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilke RGH, Finger RP and Sachs HG: Time
course of changes in visual acuity after a single injection of
aflibercept or ranibizumab in neovascular age-related macular
degeneration-analysis of aggregated real life data. Klin Monbl
Augenheilkd. 234:1508–1514. 2017.(In German). PubMed/NCBI
|
|
11
|
Wu KH, Tan AG, Rochtchina E, Favaloro EJ,
Williams A, Mitchell P and Wang JJ: Circulating inflammatory
markers and hemostatic factors in age-related maculopathy: A
population-based case-control study. Invest Ophthalmol Vis Sci.
48:1983–1988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuse Y, Tsuruma K, Kanno Y, Shimazawa M
and Hara H: CCR3 is associated with the death of a photoreceptor
cell-line induced by light exposure. Front Pharmacol. 8:2072017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein R, Klein BE, Knudtson MD, Wong TY,
Shankar A and Tsai MY: Systemic markers of inflammation,
endothelial dysfunction, and age-related maculopathy. Am J
Ophthalmol. 140:35–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fouda SM and Bahgat AM: Intravitreal
aflibercept versus intravitreal ranibizumab for the treatment of
diabetic macular edema. Clin Ophthalmol. 11:567–571. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beck RW, Moke PS, Turpin AH, Ferris FL
III, SanGiovanni JP, Johnson CA, Birch EE, Chandler DL, Cox TA,
Blair RC and Kraker RT: A computerized method of visual acuity
testing: Adaptation of the early treatment of diabetic retinopathy
study testing protocol. Am J Ophthalmol. 135:194–205. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells JA, Glassman AR, Ayala AR, Jampol
LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR,
Jhaveri C, et al: Aflibercept, bevacizumab, or ranibizumab for
diabetic macular edema: Two-year results from a comparative
effectiveness randomized clinical trial. Ophthalmology.
123:1351–1359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Giet M, Henkel C, Schuchardt M and
Tolle M: Anti-VEGF drugs in eye diseases: Local therapy with
potential systemic effects. Curr Pharm Des. 21:3548–3556. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anderson DH, Mullins RF, Hageman GS and
Johnson LV: A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol. 134:411–431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin T, Walker GB, Kurji K, Fang E, Law G,
Prasad SS, Kojic L, Cao S, White V, Cui JZ and Matsubara JA:
Parainflammation associated with advanced glycation endproduct
stimulation of RPE in vitro: Implications for age-related
degenerative diseases of the eye. Cytokine. 62:369–381. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Chen M and Forrester JV:
Para-inflammation in the aging retina. Prog Retin Eye Res.
28:348–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato T, Takeuchi M, Karasawa Y, Enoki T
and Ito M: Intraocular inflammatory cytokines in patients with
neovascular age-related macular degeneration before and after
initiation of intravitreal injection of anti-VEGF inhibitor. Sci
Rep. 8:10982018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demirel S, Bilici S, Batioglu F and Ozmert
E: Is there any difference between ranibizumab and aflibercept
injections in terms of inflammation measured with anterior chamber
flare levels in age-related macular degeneration patients: A
comparative study. Ophthalmic Res. 56:35–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tosi GM, Caldi E, Neri G, Nuti E,
Marigliani D, Baiocchi S, Traversi C, Cevenini G, Tarantello A,
Fusco F, et al: HTRA1 and TGF-β1 concentrations in the aqueous
humor of patients with neovascular age-related macular
degeneration. Invest Ophthalmol Vis Sci. 58:162–167. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fisichella V, Giurdanella G, Platania CB,
Romano GL, Leggio GM, Salomone S, Drago F, Caraci F and Bucolo C:
TGF-β1 prevents rat retinal insult induced by amyloid-β (1–42)
oligomers. Eur J Pharmacol. 787:72–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kramer M, Hasanreisoglu M, Feldman A,
Axer-Siegel R, Sonis P, Maharshak I, Monselise Y, Gurevich M and
Weinberger D: Monocyte chemoattractant protein-1 in the aqueous
humour of patients with age-related macular degeneration. Clin Exp
Ophthalmol. 40:617–625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jonas JB, Tao Y, Neumaier M and Findeisen
P: Monocyte chemoattractant protein 1, intercellular adhesion
molecule 1, and vascular cell adhesion molecule 1 in exudative
age-related macular degeneration. Arch Ophthalmol. 128:1281–1286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stewart MW: Clinical and differential
utility of VEGF inhibitors in wet age-related macular degeneration:
Focus on aflibercept. Clin Ophthalmol. 6:1175–1186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lazzeri S, Orlandi P, Piaggi P, Sartini
MS, Casini G, Guidi G, Figus M, Fioravanti A, Di Desidero T,
Ripandelli G, et al: IL-8 and VEGFR-2 polymorphisms modulate
long-term functional response to intravitreal ranibizumab in
exudative age-related macular degeneration. Pharmacogenomics.
17:35–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scott IU, Vanveldhuisen PC, Ip MS, Blodi
BA, Oden NL, Awh CC, Kunimoto DY, Marcus DM, Wroblewski JJ and King
J; SCORE2 Investigator Group, : Effect of bevacizumab vs.
aflibercept on visual acuity among patients with macular edema due
to central retinal vein occlusion: The SCORE2 randomized clinical
trial. JAMA. 317:2072–2087. 2017. View Article : Google Scholar : PubMed/NCBI
|