Introduction
Acute lymphoblastic leukemia (ALL) is the most
common malignancy in childhood, accounting for 25–30% of all
childhood malignancies (1,2). Pediatric patients with ALL are
classified into standard-, intermediate- or high-risk categories
based on age, white blood cell count, existing central nervous
system leukemia or testicular leukemia, immunophenotype,
cytogenetic and molecular characteristics, prednisone response,
morphological remission at the end of induction therapy, and the
different expression of minimal residual disease (MRD) (3,4). Newly
diagnosed patients with ALL require a rigorous, standardized and
long-course chemotherapy treatment strategy, which includes
induction, consolidation, intensification and maintenance therapy
(5). Additionally, stratification of
treatment intensity is based on the risk stratification of leukemic
blasts identified in ALL (4). The
disease-free survival and the curative rate have greatly improved
with improved diagnosis and treatment regimens (6). However, treatment interruption or
discontinuation due to hematopoietic toxicity is a common adverse
event and results in a higher risk of relapse (7).
As an anticancer and immunosuppressive agent,
6-mercaptopurine (6-MP) is commonly used as part of the treatment
strategy in patients with ALL, including consolidation,
intensification and maintenance therapy (8). 6-MP is specifically important for
maintenance therapy, the longest phase lasting ~2–3 years (9). 6-MP-induced life-threatening
myelotoxicity is commonly associated with polymorphisms in genes
encoding thiopurine methyltransferase (TPMT), inosine
triphosphatase (ITPA) and nudix hydrolase 15 (NUDT15) (8–12). Given
the low prevalence of polymorphisms in TPMT in the Asian
population, polymorphisms in ITPA and NUDT15 are considered to be
the main causes of severe myelotoxicity in the Asian population
(13). Previous studies determined
that adult patients with inflammatory bowel disease and pediatric
patients with ALL homozygous for the NUDT15 variant were extremely
sensitive to 6-MP (14,15), which suggests that the NUDT15 variant
may be a potential factor associated with 6-MP-induced
myelotoxicity.
To the best of our knowledge, the present report is
the first to present the case of a Chinese pediatric patient with
ALL who experienced 6-MP-induced life-threatening myelotoxicity and
agranulocytosis due to the homozygous mutant (TT genotype) for
rs116855232 (NUDT15).
Case report
A 5-year-old male presenting with intermittent
fever, anorexia, increasing fatigue and a cough that had persisted
for 15 days was hospitalized in January 2017 at the Department of
Hematology of The First Hospital of Lanzhou University. The patient
had a normal medical history with no known drug allergies, no
travel to epidemic areas and no known exposure to toxins. There was
no family history of malignancy. On physical examination, the
skin-mucous membrane of the patient was pale and no petechiae or
edema was identified on either of the lower limbs. There was no
involvement of the superficial lymph nodes and rales were not
detected. The patient had a normal cardiac rhythm without murmurs.
In addition, the patient's abdomen was soft and the liver was not
palpable. No splenic tenderness or costovertebral angle tenderness
was observed. The patient's white blood cell (WBC) count was
97.72×109/l (normal range, 4–10×109/l), with
a platelet count of 13×109/l (normal range,
100–300×109/l), and a hemoglobin count of 48 g/l (normal
range, 110–150 g/l). Bone marrow aspiration revealed bone marrow
characteristics typically observed in ALL. Immunophenotypic
analysis of the blast population revealed that CD34, HLA-DR, CD10
and CD19 were expressed on all cells, while CD33 was partially
expressed. The patient demonstrated an absence of blasts in the
cerebrospinal fluid. Cytogenetic analysis of bone marrow cells from
this patient demonstrated 46, XY of the 20 metaphases examined.
Detection of fusion genes identified no fusion gene expression in
the patient. In addition, molecular studies did not identify any
genetic mutations. These findings were consistent with a diagnosis
of acute B-cell lymphoblastic leukemia with immediate-risk. The
patient was tested for prednisone sensitivity and treated according
to the CCLG-2008 protocol (16)
developed at Beijing Children's Hospital.
The patient received induction chemotherapy,
achieved morphological remission and was negative for MRD four
weeks post-therapy. However, during early consolidation therapy
with cyclophosphamide (1 g/m2 on day 1), cytarabine (75
mg/m2 on days 1–3 and 10–13) and 6-MP (60
mg/m2 for 14 days), the patient was admitted to the
pediatric intensive care unit with a severe lung infection due to
long-term and serious myelosuppression and agranulocytosis.
Subsequently, the patient developed respiratory failure and
required ventilator support for several days. In addition, clear
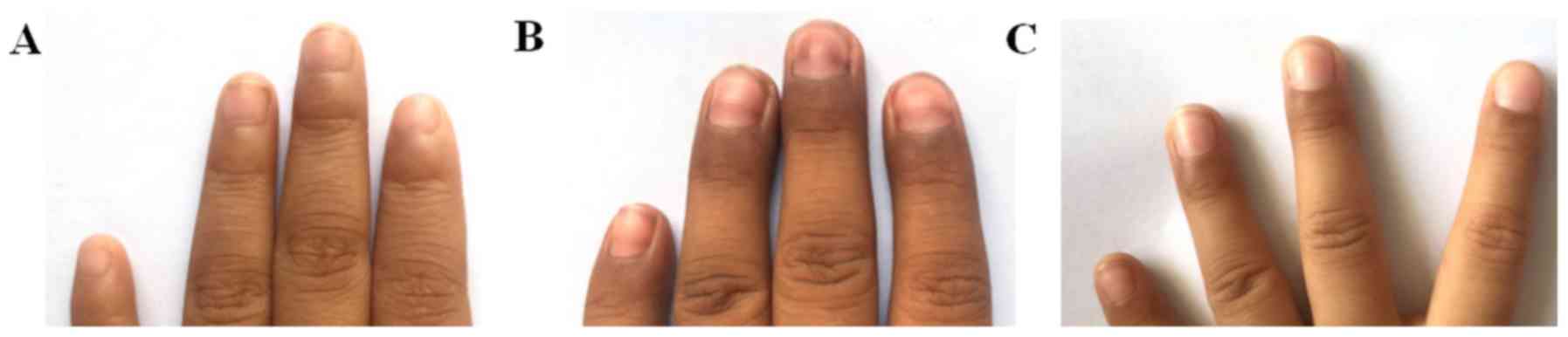
hyperpigmentation appeared on the patient's hands and feet, which
was pronounced at the interphalangeal joints and in the subungual
region (Fig. 1). The dosage of 6-MP
was not decreased as the patient's TPMT enzyme activity was within
the normal range, and no genetic mutations were previously
identified. However, consolidation therapy could not be performed
according to the schedule outlined in the protocol (due to
myelosuppression and agranulocytosis). Three months after the
patient's initial presentation, examination of the patient's bone
morrow cells confirmed an increased proportion of lymphoblast-like
cells, although it had not increased sufficiently to be classified
as a relapse. Due to the severe myelotoxicity and significant
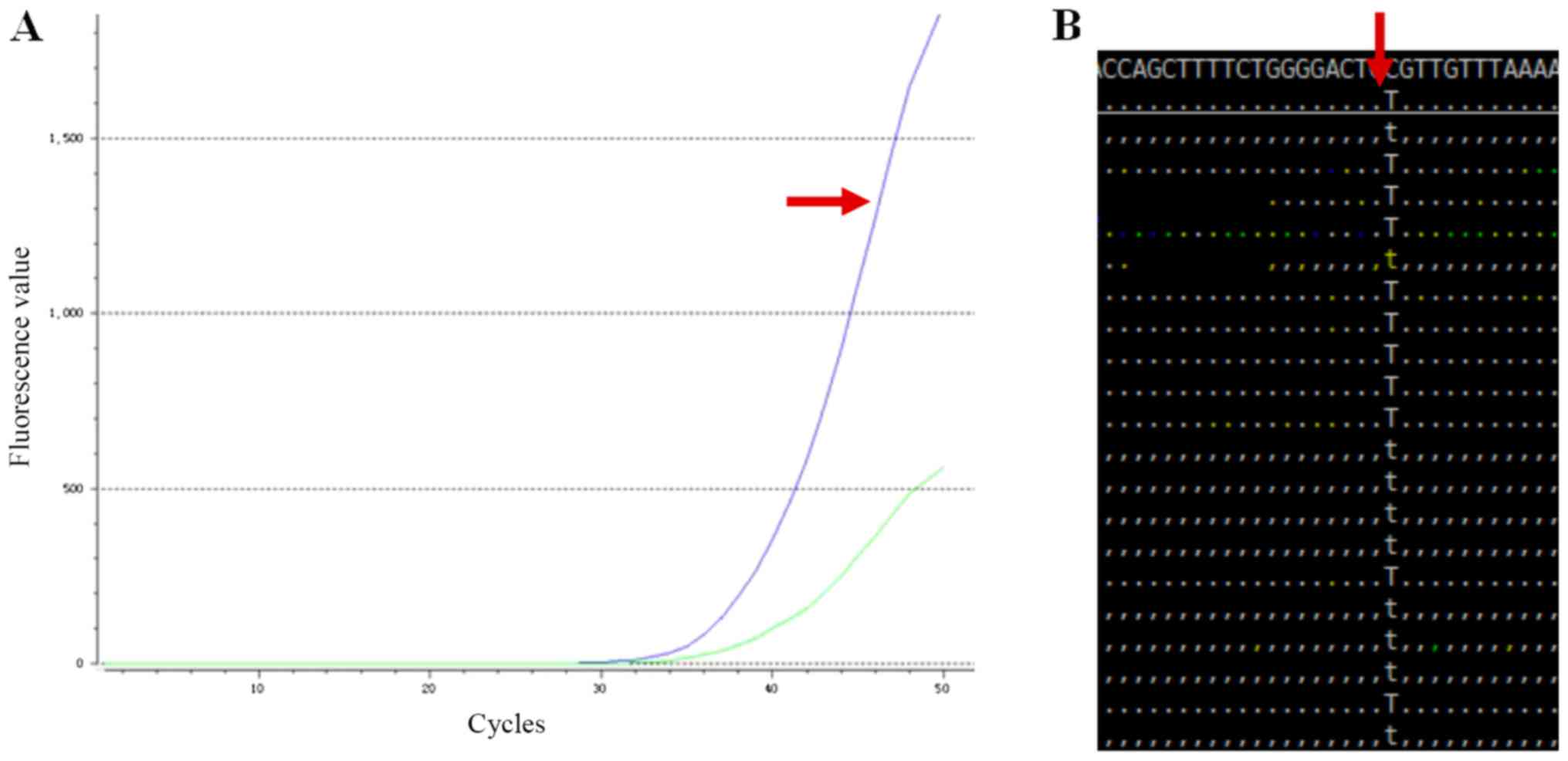
hyperpigmentation observed, NUDT15 testing including fluorescence
in situ hybridization and whole exome sequencing was
performed. The results demonstrated that the patient was a
homozygous carrier (415C>T, TT) for rs116855232 (NUDT15)
(Fig. 2). During follow-up
consolidation treatment, as the 6-thioguanine nucleotide plasma
concentration could not be detected, the dose of 6-MP was adjusted
from 30 to 10% of the total recommended dose after examining
previous hematological toxicity records. The dosage adjustment of
6-MP alleviated the clinical symptoms of myelotoxicity and
hyperpigmentation observed in the patient. Four months after the
patient's initial presentation, the patient is having regular
follow-ups and is continuing maintenance therapy with 8% of the
recommended dose of 6-MP.
Discussion
A major component of the standardized chemotherapy
treatment strategy used to treat patients with childhood ALL,
involves daily exposure to 6-MP for 2–3 years (17). As a prodrug, 6-MP is enzymatically
converted into TGTP, through multiple sequential reactions, and
TGTP is further reduced into deoxy-thioguanosine triphosphate
(TdGTP). TdGTP can be incorporated into double-strand DNA during
cell replication, to trigger ineffective mismatch repair and
eventually apoptosis, causing cell cytotoxicity (18). Myelosuppression is a serious
complication, which can occur during treatment of ALL, and is
associated with 6-MP therapy (19).
Myelosuppression results in treatment disruption leading to an
increased risk of relapse, an increased risk of life-threatening
infections and the need for more extensive treatment (19,20).
6-MP-induced myelosuppression is largely associated with
polymorphisms in TPMT and ITPA genes (21). The four major mutant alleles of TPMT,
TPMT*2 (238G>C), *3A (460G>A, 719A>G), *3B (460G>A) and
*3C (719A>G), account for the majority of TPMT deficiency;
deletions of exons and copy number variations can also account for
the variability in TPMT efficacy (22). The TPMT*3A and *3C variants are
significantly associated with dose intensity (19). There is a high prevalence of all
major TPMT polymorphisms in African and European populations,
however there is a low prevalence in the East Asian population
(13).
ITPA genetic polymorphisms exhibited no significant
differences between genotypes, and 6-MP-induced myelosuppression
can occur at any time point during maintenance therapy (12). ITPA-associated risks of
myelosuppression induced by 6-MP have been less convincingly
demonstrated, therefore testing for those variants may not be
clinical warranted (15). A
meta-analysis study demonstrated that genetic polymorphisms in
NUDT15 were strongly associated with adverse drug reaction of
thiopurines, and therefore NUDT15 may be considered as a highly
credible pharmacogenetic indicator for the use of thiopurines,
especially in the Asian population (23). In 2015, a genome-wide association
study of childhood ALL revealed a significant association between a
germline NUDT15 variant (rs116855232) and 6-MP dose intensity
(13). Out of 657 patients, 31
patients were heterozygous (CT) and only 2 were homozygous (TT) for
the NUDT15 variant. Patients with the TT genotype were sensitive to
6-MP and required an average dose intensity of 8.3% of the planned
dose (13). The NUDT15 variant was
most frequent in East Asians and Hispanics, while rare in Europeans
and not observed in Africans, thereby contributing to
ancestry-associated differences in 6-MP tolerance (13,24). In
a study of 404 Taiwan Chinese patients with ALL, 5 patients were
homozygous (TT) for the NUDT15 variant and the maximal tolerable
daily dose of 6-MP in this group was 9.4±3.7 mg/m2/day
(24). A previous study demonstrated
that 5 out of 182 Korean pediatric patients with ALL were
homozygous for the NUDT15 variant and the lowest dose of 6-MP
administered was 7.5 mg/m2/day (25). Previous studies suggest that a
comprehensive pharmacogenetic model incorporating the specific
genetic variation of NUDT15 may allow for further personalized
thiopurine therapy (13,23–26).
Although genetic testing prior to treatment revealed that the
patient possessed the TPMT wild-type allele, the patient suffered
from severe myelosuppression, in particular, neutropenia and
hyperpigmentation, all of which led to a lung infection and
treatment delay. The patient's blood sample was subsequently sent
for genetic testing for the NUDT15 and ITPA variant, and it was
revealed that the patient possessed the ITPA wild-type allele, but
was homozygous (TT) for rs116855232 (NUDT15), which resulted in the
observed adverse reaction to 6-MP.
In conclusion, to the best of our knowledge, the
present report is the first to present the case of a Chinese
pediatric patient with ALL who experienced 6-MP-induced
life-threatening myelosuppression due to the homozygous mutant (TT
genotype) for rs116855232 (NUDT15). The present report demonstrates
the importance of NUDT15 pharmacogenetics and early detection of
genetic polymorphisms in children with ALL prior to treatment with
6-MP. Further studies are required to better understand the
implications associated with NUDT15 variants in the treatment of
childhood ALL, in order to determine the association between the
necessary dosage of and length of treatment with 6-MP needed to
achieve desirable therapeutic effects and avoid
myelosuppression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC contributed to conception of the study,
interpretation of data and preparation of the manuscript. HZ
performed the data analysis and contributed to the discussion. HZM
and JL were responsible for data acquisition. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient's parents prior to participation in the present report.
Patient consent for publication
The patient's parents provided their consent for the
publication of the associated data of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pui CH, Carroll WL, Meshinchi S and Arceci
RJ: Biology, risk stratification, and therapy of pediatric acute
leukemias: An update. J Clin Oncol. 29:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopez-Lopez E, Gutierrez-Camino A,
Bilbao-Aldaiturriaga N, Pombar-Gomez M, Martin-Guerrero I and
Garcia-Orad A: Pharmacogenetics of childhood acute lymphoblastic
leukemia. Pharmacogenomics. 15:1383–1398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silverman LB, Declerck L, Gelber RD,
Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A,
Samson Y, et al: Results of Dana-farber cancer institute consortium
protocols for children with newly diagnosed acute lymphoblastic
leukemia (1981–1995). Leukemia. 14:2247–2256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rudin S, Marable M and Huang RS: The
promise of pharmacogenomics in reducing toxicity during acute
lymphoblastic leukemia maintenance treatment. Genomics Proteomics
Bioinformatics. 15:82–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper SL and Brown PA: Treatment of
pediatric acute lymphoblastic leukemia. Pediatr Clin North Am.
62:61–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunger SP and Mullighan CG: Acute
lymphoblastic leukemia in children. N Engl J Med. 373:1541–1552.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arya LS, Kotikanyadanam SP, Bhargava M,
Saxena R, Sazawal S, Bakhshi S, Khattar A, Kulkarni KP, Adde M,
Vats TS and Magrath I: Pattern of relapse in childhood all:
Challenges and lessons from a uniform treatment protocol. J Pediatr
Hemato Oncol. 32:370–375. 2010. View Article : Google Scholar
|
|
8
|
Mei L, Ontiveros EP, Griffiths EA,
Thompson JE, Wang ES and Wetzler M: Pharmacogenetics predictive of
response and toxicity in acute lymphoblastic leukemia therapy.
Blood Rev. 29:243–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang DS, Yu CH, Chou WC, Chang YH, Lin CY,
Lin KH, Jou ST, Lu MY, Chang HH, Lin SW, et al: Adjusting dosage of
mercaptopurine By NUDT15 polymorphisms in childhood acute
lymphoblastic leukemia. Blood. 130:25662017.
|
|
10
|
Farfan MJ, Salas C, Canales C, Silva F,
Villarroel M, Kopp K, Torres JP, Santolaya ME and Morales J:
Prevalence of TPMT and ITPA gene polymorphisms and effect on
mercaptopurine dosage in Chilean children with acute lymphoblastic
leukemia. BMC Cancer. 14:2992014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishii R, Moriyama T, Janke LJ, Yang W,
Suiter CC, Lin TN, Li L, Kihira K, Toyoda H, Hofmann U, et al:
Preclinical evaluation of NUDT15-guided thiopurine therapy and its
effects on toxicity and anti-leukemic efficacy. Blood.
131:2466–2474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiengthong K, Ittiwut C, Muensri S,
Sophonphan J, Sosothikul D, Seksan P, Suppipat K, Suphapeetiporn K
and Shotelersuk V: NUDT15 c.415C>T increases risk of
6-mercaptopurine induced myelosuppression during maintenance
therapy in children with acute lymphoblastic leukemia.
Haematologica. 101:e24–e26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JJ, Landier W, Yang W, Liu C, Hageman
L, Cheng C, Pei D, Chen Y, Crews KR, Kornegay N, et al: Inherited
NUDT15 variant is a genetic determinant of mercaptopurine
intolerance in children with acute lymphoblastic leukemia. J Clin
Oncol. 33:1235–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang SK, Hong M, Baek J, Choi H, Zhao W,
Jung Y, Haritunians T, Ye BD, Kim KJ, Park SH, et al: A common
missensevariant in NUDT15 confers susceptibility to
thiopurine-induced leukopenia. Nat Genet. 46:1017–1020. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh M, Bhatia P, Khera S and Trehan A:
Emerging role of NUDT15 polymorphisms in 6-mercaptopurine
metabolism and dose related toxicity in acute lymphoblastic
leukaemia. Leuk Res. 62:17–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui L, Li ZG, Chai YH, Yu J, Gao J, Zhu
XF, Jin RM, Shi XD, Zhang LP, Gao YJ, et al: Outcome of children
with newly diagnosed acute lymphoblastic leukemia treated with
CCLG-ALL 2008: The first nation-wide prospective multicenter study
in China. Am J Hematol. 93:913–920. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmiegelow K, Nielsen SN, Frandsen TL and
Nersting J: Mercaptopurine/Methotrexate maintenance therapy of
childhood acute lymphoblastic leukemia: Clinical facts and fiction.
J Pediatr Hematol Oncol. 36:503–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ebbesen MS, Nersting J, Jacobsen JH,
Frandsen TL, Vettenranta K, Abramsson J, Wesenberg F and
Schmiegelow K: Incorporation of 6-thioguanine nucleotides into DNA
during maintenance therapy of childhood acute lymphoblastic
leukemia-the influence of thiopurine methyltransferase genotypes. J
Clin Pharmacol. 53:670–674. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldberg R and Irving PM: Toxicity and
response to thiopurines in patients with inflammatory bowel
disease. Expert Rev Gastroenterol Hepatol. 9:891–900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lennard L, Cartwright CS, Wade R and Vora
A: Thiopurine dose intensity and treatment outcome in childhood
lymphoblastic leukaemia: The influence of thiopurine
methyltransferase pharmacogenetics. Br J Haematol. 169:228–240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong FC, Leung AW, Kwok JS, Chan MH, Li CK
and Yuen YP: NUDT15 variant and thiopurine-induced leukopenia in
Hong Kong. Hong Kong Med J. 22:185–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azimi F, Jafariyan M, Khatami S, Mortazavi
Y and Azad M: Assessment of Thiopurine-based drugs according to
Thiopurine S-methyltransferase genotype in patients with acute
lymphoblastic leukemia. Iran J Ped Hematol Oncol. 4:32–38.
2014.PubMed/NCBI
|
|
23
|
Yin D, Xia X, Zhang J, Zhang S, Liao F,
Zhang G, Zhang Y, Hou Q, Yang X, Wang H, et al: Impact of NUDT15
polymorphisms on thiopurines-induced myelotoxicity and thiopurines
tolerance dose. Oncotarget. 8:13575–13585. 2017.PubMed/NCBI
|
|
24
|
Liang DC, Yang CP, Liu HC, Jaing TH, Chen
SH, Hung IJ, Yeh TC, Lin TH, Lai CL, Lai CY and Shih LY: NUDT15
gene polymorphism related to mercaptopurine intolerance in Taiwan
Chinese children with acute lymphoblastic leukemia.
Pharmacogenomics J. 16:536–539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yi ES, Choi YB, Choi R, Lee NH, Lee JW,
Yoo KH, Sung KW, Lee SY and Koo HH: NUDT15 variants cause
hematopoietic toxicity with low 6-TGN levels in children with acute
lymphoblastic leukemia. Cancer Res Treat. 50:872–882. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moriyama T, Nishii R, Perez-Andreu V, Yang
W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira
K, et al: NUDT15 polymorphisms alter thiopurine metabolism and
hematopoietic toxicity. Nat Genet. 48:367–373. 2016. View Article : Google Scholar : PubMed/NCBI
|