Introduction
Colon cancer is a type of cancer that causes severe
damage to human health, and its pathogenesis to date remains
unclear. Colon cancer is a leading cause of mortality in developed
Western countries, second only to lung cancer (1). In China, in addition to the development
of social economy and the improvement in the standard of living,
the dietary structure and way of life has also changed for decades;
for example, the typical dietary structure of residents in China
has transitioned from consisting predominantly of grain,
vegetables, sugar and high-fiber, to meat, eggs, milk, fat,
high-protein and low-fiber (2,3). Changes
in the dietary structure have been indicated as a notable causes of
colon cancer (4). Along with an
increase in the aging population in China, the incidence of colon
cancer and fatalities have continued to rise (5). According to the World Health
Organization, the incidence of colon cancer and mortality in
malignant tumors ranked third and fourth respectively for Chinese
residents (4). Despite the fact that
treatment with surgery, chemotherapy, radiotherapy and
comprehensive biological treatment has progressed, the 5-year
survival rate of advanced colon cancer has not improved (6). Colon cancer with early-onset symptoms
is not typical and the primary causes of the poor prognosis in
patients with colon cancer include the strong tendency of invasion
and metastasis (7,8). In light of these factors,
identification of novel tumor markers and research towards colon
cancer prevention and control has vital practical importance.
The occurrence and development of colon cancer is a
multi-factorial, multi-phase, complicated multi-step process. As
the understanding of tumor molecular biology has increased, an
increasing number of genes have been identified to participate in
the complex process of colon cancer development (9). microRNAs (miRNAs or miRs) are small
non-coding RNAs that exist widely in eukaryotic cells, are 19–24
nucleotides in length and inhibit the expression of target genes at
transcriptional or translational levels (10,11). In
recent years, research has revealed that miR-193b is a typical
multi-function gene that is mediated by its downstream genes, and
is associated with various physiological and pathological
processes, including inflammation, immunodeficiency, and the
occurrence and development of tumors (12,13). The
miR-193b gene is located on human chromosome 16p13.12 and has an
abnormal expression in prostate cancer, hepatocellular carcinoma
and breast cancer (14–16). However, the specific biological
functions of its invasion and metastasis in colon cancer have not
yet been elucidated.
To explore the influences and mechanisms associated
with miR-193b, mature miR-193b sequences (mimics) were synthesized
and transfected into SW480 colon cancer cells. The present study
aimed to detect cell migration, cell invasion and metastasis
ability changes following miR-193b transfection. RAB22A, the
predicted target gene regulated by miR-193b, may have an effect on
invasion and metastasis of colon cancer, although its mechanism
requires further study. The present research provides a novel
theoretical basis for the treatment of colon cancer.
Materials and methods
Patient tissue samples
A total of 62 tumor tissues and adjacent
counterparts (<2 cm from the tumor site) were extracted from
patients (42 male, 20 female; age, 28–79 years; median age, 66
years; T1-T4 stage; no distant metastasis or preoperative
chemotherapy) with colon cancer who received tumor resections
between January 2010 and December 2012 at the Department of Anus
and Intestine Surgery, Weifang People's Hospital (Weifang, China).
Patients' basic information was recorded, including
Tumor-Node-Metastasis (TNM) stage, the degree of differentiation,
lymph node metastasis and distant metastasis, according to the 2002
International Cancer Alliance TNM staging criteria (17). All tissues were rapidly placed in
liquid nitrogen within half an hour after tumor resection. The
Ethics Committee of Weifang People's Hospital approved the present
study and informed consent from all patients was received upon
admission. The clinical features of all cases are indicated in
Table I.
 | Table I.Association between miR-193b
distribution and clinicopathological characteristics of patients
with colon cancer. |
Table I.
Association between miR-193b
distribution and clinicopathological characteristics of patients
with colon cancer.
|
|
| miR-193b expression
(n) |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Patients (n) | Low | High | χ2 | P-value |
|---|
| Total cases | 62 | 40 | 22 |
|
|
| Age (years) |
|
|
| 0.015 | 0.903 |
|
≥60 | 36 | 23 | 13 |
|
|
|
<60 | 26 | 17 | 9 |
|
|
| Sex |
|
|
| 1.974 | 0.160 |
|
Male | 30 | 22 | 8 |
|
|
|
Female | 32 | 18 | 14 |
|
|
| Tumor size |
|
|
| 0.282 | 0.596 |
| ≥5
cm | 31 | 21 | 10 |
|
|
| <5
cm | 31 | 19 | 12 |
|
|
| TNM stage |
|
|
| 4.700 | 0.030 |
|
I+II | 28 | 14 | 14 |
|
|
|
III+IV | 34 | 26 | 8 |
|
|
| Lymph node
metastasis status |
|
|
| 7.297 | 0.007 |
|
Positive | 34 | 27 | 7 |
|
|
|
Negative | 28 | 13 | 15 |
|
|
| Tumor
differentiation |
|
|
| 0.448 | 0.799 |
|
Well-differentiated | 20 | 12 | 8 |
|
|
|
Moderately-differentiated | 20 | 14 | 6 |
|
|
|
Poorly-differentiated | 22 | 14 | 8 |
|
|
| Tumors (n) |
|
|
| 0.518 | 0.472 |
| 1 | 30 | 18 | 12 |
|
|
|
>1 | 32 | 22 | 10 |
|
|
Cell transfection and cell invasion
activity detection
The human colorectal cancer cell lines SW480, HT-29,
LOVO, HCT116 and CACO-2 were purchased from the Chinese Academy of
Sciences (Shanghai, China). All cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cells were cultured at 37°C in
an atmosphere containing 5% CO2 until they reached a
60–80% confluence for subsequent experiments.
miR-193b was transfected in 6-well plates for 24 h
at 37°C with 6×105 SW480 cell culture cells (70–80%
confluence). An miR-193b mimic (transfected with miR-193b mimic;
all obtained from Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China), negative-scramble control (transfected with the negative
mimic, NC) and blank control (untransfected cells, BC) were used
for transfection. The sequences of the miRNAs used were as follows:
miR-193b mimic, sense 5′-AACUGGCCCUCAAAGUCCCGCU-3′ and antisense
5′-AGCGGGACUUUGAGGGCCAGUU-3′; and miR-193b SC, sense
5′-UUCUCGGAACCUGUCCAGUTT-3′ and antisense
5′-ACGUCAGACGUUGCGACAATT-3′. The transfection procedure was
performed using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The following mixtures were prepared: A) 10 µl
miR-193b mimic (50 pmol/µl) + 250 µl opti-MEM, as provided by the
kit; and B) 10 µl Lipofectamine® 2000 + 250 µl opti-MEM.
The A+B liquid was mixed together and plated for 20 min at room
temperature. Subsequently, 500 µl cell culture was added drop-wise
into the liquid media and 1.5 ml opti-MEM was added in each 6-well
plate. The transfected cells were incubated at 37°C for 6 h and
were left to culture with 2 ml RPMI-1640 medium (Invitrogen; Thermo
Fisher Scientific, Inc.) containing 10% FBS for 36 h at 37°C prior
to further experiments. Each group was established in three
replicate wells.
Transwell migration assay
Using the above-described transfection method,
1×105 cells/well were counted manually using a
hemocytometer at 2 days following transfection. Transfected SW480
cells, which exhibited the lowest relative expression of miR-193b,
were seeded at 8×103 cells/well in a 6-well plate in
serum-free RPMI-1640 medium for 12 h at 37°C in the upper chamber
of Transwell plates (Costar; Corning Incorporated, Corning, NY,
USA). The lower chamber contained 500 µl 20% fetal bovine
Dulbecco's modified Eagle's medium. Following incubation for 36 h
at 37°C the cells in the upper chamber were wiped out, fixed with
0.1% ethanol at room temperature for 20 min, stained with crystal
violet at room temperature for 20 min. All migrated cells from the
upper chamber were observed under an optical microscope
(magnification, ×400) with crystal violet staining. Cells were
counted in 5–6 randomly visual fields. Experiments were performed
in triplicate.
Wound healing assay
Following transfection, transfected cells were
seeded in 12-well plates (4×105 cells/well) and cultured
at 37°C. On reaching 100% confluence, a wound was created by
scratching the cell surface with a 1 ml pipette tip. Cells were
then washed three times with PBS and incubated in RPMI-1640 with 2%
FBS. Wounds were observed under a light microscope (magnification,
×400) at 0 and 48 h. Cell migration was determined by subtracting
the final wound width (µm) from the initial wound width (µm).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted from tissues and all cell lines
using TRIzol reagent extract (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA RT-qPCR TaqMan
method was used to detect miR-193b expression level. Specific steps
were followed using the TaqMan miRNA reverse transcription kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The following conditions were applied to conduct the
RT reaction: 16°C for 30 min, 42°C for 30 min and 85°C for 5 min.
The PCR product was preserved at 4°C. qPCR reaction system
operation steps were performed according to the TaqMan Small RNA
Assay instructions (Takara Bio, Inc., Otsu, Japan). The following
thermocycling conditions were applied for qPCR: 95°C for 10 min;
95°C for 15 sec and 60°C for 60 sec for 42 cycles; and 72°C for 10
min using a Stratagene Mx3005P PCR system. The results were
calculated using the 2−ΔΔCq method (18). The miR-193b and U6 Ct value in each
sample was revised via U6 internal calibration, with
2−ΔΔCt relative quantitative method to compare the
miR-193b expressed in colon cancer and matched normal tissues:
ΔΔCq=Cqcarcinoma miR-193b-Cqcarcinoma U6-(Cqadjacent
miR-193b-Cqadjacent U6). The relative levels of gene expression
were represented as ΔCq-Cq gene-Cq reference, and the fold change
of the gene expression was calculated by the 2-ΔΔCt method.
Experiments were repeated in triplicate. The following primers were
used: RAB22A, forward 5′-TTGTAGTTGCCATTGCAGGA-3′ and reverse
5′-AGGCTGTCTTCGGAGTTTGA-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Western blot analysis
Total SW480 cell protein was extracted using
radioimmunoprecipitation assay lysis buffer (Pierce; Thermo Fisher
Scientific, Inc.), centrifuged at 12,000 × g for 15 min at 4°C and
quantified using the Bradford method (19). Protein (50 µg) was separated using
10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA), which were then blocked for 1
h with 5% skimmed milk in Tris-buffered saline with Tween-20 at
room temperature. Primary antibodies against RAB22A (cat. no.
ab137093; 1:5,000, Abcam, Cambridge, UK), extracellular
signal-related kinase (ERK; cat. no. AF1576; 1:800; Novus
Biologicals Canada ULC; Oakville, ON, Canada), GAPDH (cat. no.
sc-25778; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), Ras (cat. no. sc-68743; 1:1,000; Santa Cruz Biotechnology,
Inc.) and phosphorylated (p-)ERK l/2 (cat. no. sc-101761; 1:800;
Santa Cruz Biotechnology, Inc.) were added and incubated at 4°C
overnight. Following elution, mouse anti-rabbit horseradish
peroxidase conjugated Immunoglobulin G (cat. no. sc-2357; 1:5,000;
Santa Cruz Biotechnology, Inc.) was added and incubated for 2 h at
room temperature. GAPDH was used as the reference protein. Enhanced
chemiluminescent color (EMD Millipore) was added, and the blots
were developed. The experiment was repeated in triplicate.
Ras separation and detection was performed using the
Active Ras Pull-Down and Detection kit (cat. no. 16117; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. A total of 2×106 SW480 cells were seeded
in 6-well plates and transfected with miR-193b mimic for 24 h at
37°C. Subsequently, cells were treated with 500 µl pyrolysis liquid
with protease inhibitors for 5 min to lyse the cells, prior to
incubation with 1 mg sample and 80 µg Glutathione
S-transferase-Rafl-RBD (part of the detection kit) for 1 h at 4°C.
Protein centrifugation (6,000 × g for 30 sec at 4°C) was performed
followed by protein concentration determination (20). Following electrophoresis and transfer
to the polyvinylidene fluoride membrane, the blot was incubated at
4°C overnight with primary antibody (anti-Ras antibody; 1:200),
which was included in the Ras Pull-Down and Detection kit.
miR-193b target gene prediction and
dual-luciferase assay
Targetscan (http://www.targetscan.org/), Pictar (http://pictar.mdc-berlin.de/) and miRanda (http://www.microrna.org/microrna/home.do) were used to
predict the downstream target gene of miR-193b, through
bioinformatics analysis. The RAB22A 3′ untranslated region (UTR)
was identified at miR-193b binding sites, and wild-type and mutant
RAB22A 3′UTR luciferase reporter gene plasmids (Shanghai GenePharma
Co., Ltd., Shanghai, China) were constructed for further study.
Luc-RAB22A-3′UTR wild-type or mutant-type 3′pGL3 vector were
constructed by Shanghai GeneChem Co., Ltd. (Shanghai, China).
HEK293T cells were plated onto 96-well plates at density of
2×104 cells/ml for each well 24 h prior to transfection.
The Luc-RAB22A-3′ UTR wild-type or mutant-type 3′ pGL3 vector and
miR-193b mimics were co-transfected into 293T cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At
the time of transfection, 50 µl RPMI-1640 medium, 50 µl Dual Glo
Luciferase Reagent (premixed; Promega Corporation, Madison, WI,
USA), and 100 µl Glo Stop & Glo Reagent were added to each well
of a LockWell MaxiSorp test plate. After 48 h of transfection, the
Firefly and Renilla luciferase fluorescence values were
detected using a Dual-Luciferase® Reporter Assay System
(Promega Corporation). Results were normalized to that of
Renilla luciferase and the experiment was repeated in
triplicate.
Flow cytometric analysis
SW480 Cells were harvested by trypsinization, washed
with PBS and fixed in cold 70% EtOH at room temperature for 1 h.
Then SW480 cells were washed with PBS. Staining was performed with
propidium iodide (cat. no. 81845; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 15 min and cell cycle
profiles were analyzed using a FACS Aria II flow cytometer (BD
FACSAria II; BD Biosciences, San Jose, CA, USA). Data were acquired
and analyzed using the FACS DiVa software program (BD
Biosciences).
Statistical analysis
GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA) and SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) were
used for systemic analyses and plotting data. One-way Analysis of
variance was used to determine significant differences between the
test conditions. ImageJ 1.51p 22 software (National Institutes of
Health, Bethesda, MD, USA) was used to analyze the grey ratios of
western blotting bands. The association between miR-193b and
clinicopathological parameters was compared using a χ2
test. Data are presented as the mean ± or + standard error of the
mean as indicated. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-193b in clinical
tissue samples and cell lines
RT-qPCR was used to measure the expression levels of
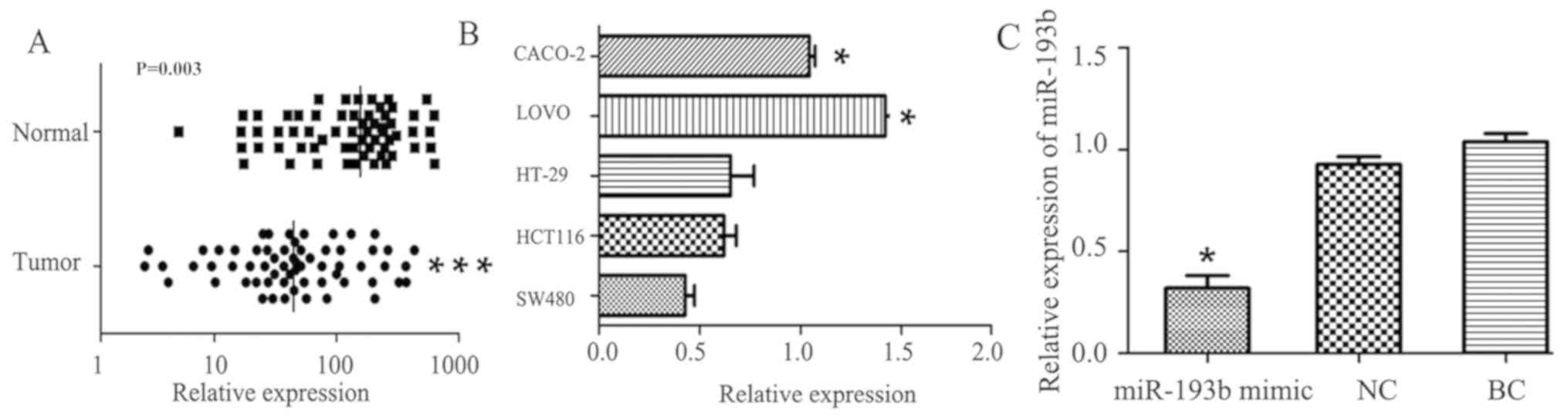
miR-193 in 62 colon cancer tissues and adjacent normal tissue. Of
the 62 patients, 40 (64.5%) exhibited a significantly decreased
level of miR-193b in colon cancer tissue specimens compared with
adjacent normal tissue specimens (P=0.003; Fig. 1A), The median expression value in
colon cancer tissues (64.32, normalized by the internal U6 gene)
was significantly decreased compared with the median value (163.7,
normalized to internal U6 gene) in the corresponding normal tissues
(Fig. 1A). miR-193b was expressed at
the lowest level (0.43±0.08) in the SW480 cell line compared with
the other four cancer cell lines (HCT116, HT-29, LOVO and CACO-2)
as indicated in Fig. 1B. The SW480
cell line was therefore selected for further study. The relative
expression of miR-193b in the miR-193b mimic group was
significantly downregulated compared with the NC group (P<0.05),
with no significant differences between the NC and BC groups
(Fig. 1C).
Association between miR-193b
expression and colon cancer clinical pathology parameters
miR-193b expression levels were significantly
altered in accordance with TNM stage and lymph node metastasis
status (Table I). Results indicated
the miR-193b expression levels in patients with stage III+IV were
significantly lower compared with those at stage I+II (P=0.030).
Furthermore, patients with lymph node metastasis exhibited
significantly decreased miR-193b expression levels compared with
those with no lymph node metastasis (P=0.007). However, no
significant differences were observed relating to age, histological
type or tumor size (Table I).
miR-193b function in SW480 cancer
cells following transfection
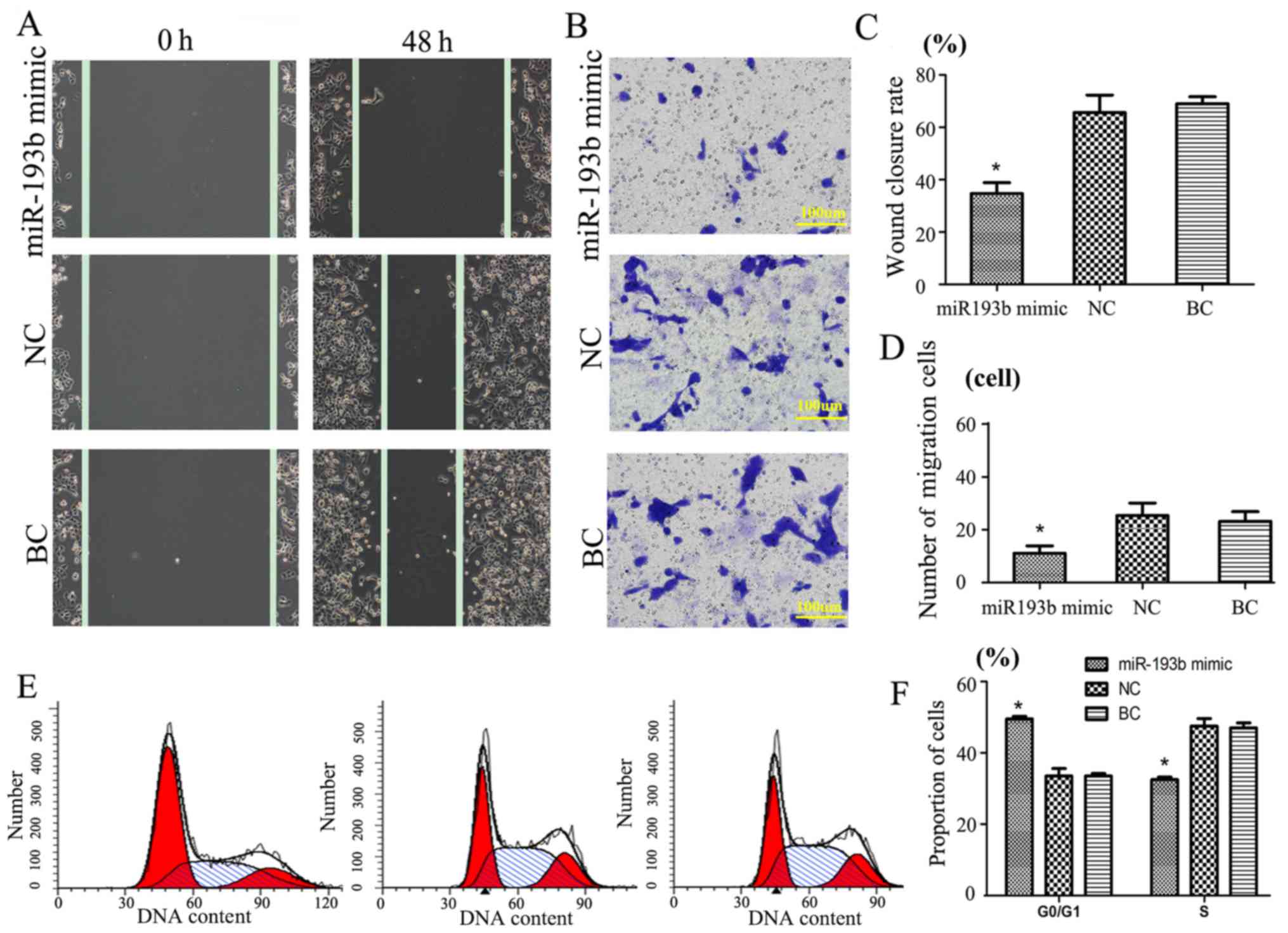
A Transwell migration assay at 48 h was performed to
evaluate the function of miR-193b in SW480 cancer cells (Fig. 2). Results revealed that, following
transfection of miR-193b mimic in SW480 cells group, the wound
closure rate was 38.3±4.4%, whereas the wound closure rate of the
NC and BC groups (compared with miR-193b mimic; P<0.05) were
significantly increased in comparison (64.1±6.7 and 66.9±5.2%,
respectively; Fig. 2A and C).
Additionally, Transwell assay results indicated that
miR-193b may have a negative correlation with invasion. The results
suggested that, compared with the NC (25±4) and BC groups (22±3),
the miR-193b mimic group cell migration number (11±2) was
significantly decreased (P<0.05, Fig.
2B and D). No significant difference was detected between the
NC and BC groups. These results suggest that exogenous miR-193b may
significantly inhibit SW480 cell migration.
SW480 cycle cell cycle results indicated that
transfection with miR-193b mimic compared with the other groups was
significantly different (P<0.05); the proportion of cells in the
G0/G1 phase of the miR-193b mimic group was significantly
increased, whereas the proportion of cells in the S phase was
significantly reduced compared with the NC and BC groups,
respectively (P<0.05; Fig. 2E and
F). There was no significant difference between the groups in
the G2/M phase (data not shown).
miR-193b biological function and
pathway research in SW480 cells
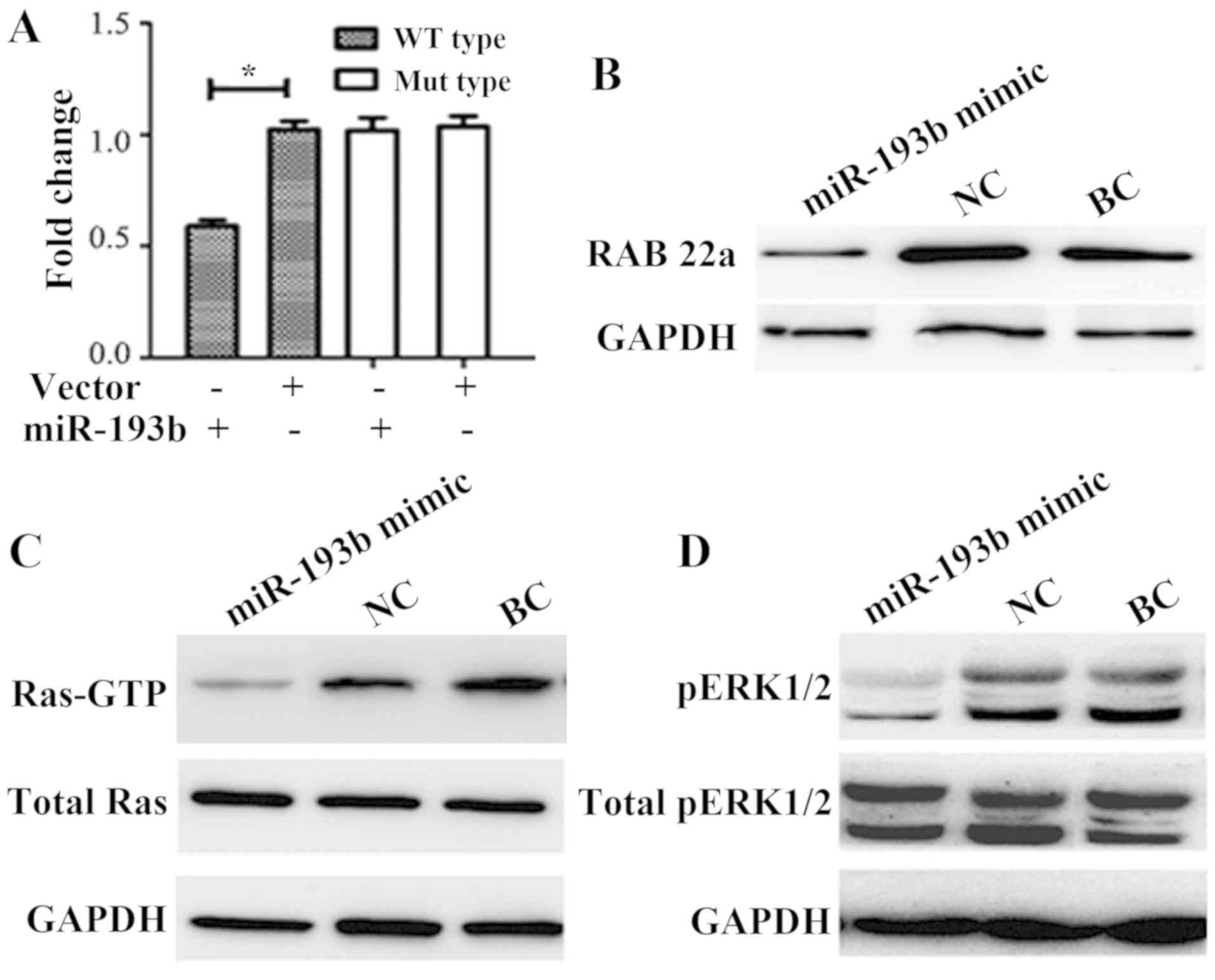
miR-193b mimic and pMIR-REPORT luciferase vector
containing the RAB22A 3′UTR binding site fragment were
co-transfected for 24 h. Results indicated that the luciferase
activity compared with the NC or BC group, the enzyme activity was
significantly reduced by ~40% in the mimic co-transfection group
(P<0.05; Fig. 3A). The luciferase
assay directly demonstrated that RAB22A may be a target of miR-193b
(Fig. 3A).
The primary downstream target gene of miR-193b was
predicted to be RAB22A according to bioinformatics analysis.
Following transfection with miR-193b mimic into SW480 cells, RAB22A
expression was significantly decreased compared with that of the NC
group (0.35±0.11 vs. 1.11±0.19; P<0.01; BC group densitometry
value=1) via western blot analysis. No marked differences were
detected between the BC and NC groups (Fig. 3B).
The association of miR-193b and RAB22A was further
analyzed by exploring the role of the Ras signaling pathway.
Results indicated that the miR-193b mimic markedly reduced the
protein expression levels of Ras-GTP compared with the NC and BC
groups (Fig. 3C). Furthermore, ERK
protein is a key Ras signaling factor (21), and the phosphorylation of ERK1/2 was
evaluated by western blot analysis. In SW480 cells transfected with
miR-193b, the protein expression levels of p-ERK l/2 were markedly
decreased compared with the NC and BC groups (Fig. 3D).
Discussion
A number of previous studies have demonstrated that
miR is associated with the occurrence of colon cancer (22,23).
miRNA is important in the field of gene expression regulation and
control, and is associated with apoptosis, cell cycle, cell
differentiation and development (24,25). For
example, in B-cell lymphoma, the miR-17–92 family is located on
chromosome 13 q31 and the amplification of miR-17-92 may increase
the degree of the malignant tumor formation (26). Currently, investigation into novel
miR targets for cancer treatment is actively being conducted; some
results have revealed the potential clinical significance of miRNA
as therapeutic targets. For example, in bile duct cancer cells,
miR-21, miR-141 and miR-200b were expressed at high levels, and
inhibition of miR-21 and miR-200b was indicated to increase the
sensitivity of the bile duct cancer cells to gemcitabine, and
inhibition of miR-141 reduced cell proliferation (22,23,27). To
facilitate gemcitabine treatment in transplanted tumors, a variety
of changes in miR expression are required (28). Additionally, miR-21 may activate
phosphatidylinositol 3 kinase signaling pathways to regulate cell
apoptosis induced by gemcitabine (29). Tazawa et al (30) previously indicated that miR-34a
downregulated E2F and upregulated the p53 signaling pathway,
inducing an HCT116 and phenotypic RKO colon cancer cell
senescence-like phenotype, which subsequently inhibited cell
proliferation. These findings indicate that miR-34a has a critical
role in the development of colon cancer (30). Additionally, Akao et al
(31) identified that let-7
expression was reduced in colon tumors, and pre-miR let-7-1a
transfected in the DLD colon cancer cell line significantly
inhibited cancer cell growth.
RAB22A is a member of the RAS oncogene family that
is amplified or expressed in specific tumors, including lung
cancer, melanoma and osteosarcoma (32–34).
Rabs serves as master regulator of transport between membrane
compartments within cells, recruiting multiple effector molecules
that recognize cargo, promote membrane fission and fusion, alter
lipid composition, mediate transport along cytoskeletal elements
and regulate other Rabs. (35,36).
RAB22A is highly expressed in hepatocellular carcinoma (37). Furthermore, overexpression of RAB22A
causes a prominent morphological enlargement of early and late
endosomes, but hampers the transport between endosomes and the
Golgi apparatus (38,39). RAB22A is associated with the
co-localization of auto phagosomes, which is associated with
another mechanism that leads to excessive RAB22A activation in
tumor cells, including the interaction with early endosome antigen
1 and membrane transport (40).
Previous research has indicated that RAB22A depletion may activate
the RAF-mitogen-activated protein kinase-ERK signaling pathway,
eventually leading to the occurrence of tumor development (41). The present study indicated that
miR-193b targeted RAB22A through the Ras signaling pathway,
resulting in the dephosphorylation of ERK1/2, repression of
downstream factors and the inhibition of cell proliferation,
migration, and other biological functions.
Through the study of molecular biology on tumor
occurrence and development, the research suggests that the role of
oncogenes and tumor suppressor genes are associated with the cell
cycle mechanism by directly or indirectly regulating the cell
cycle, which leads to disorder of cell cycle and uncontrolled
growth even into cancerous (42).
miR-193b abnormal expression is associated with human malignant
tumor development (12). Xu et
al (43) demonstrated that
upregulated miR-193b expression targeted cyclin D1 (CCND1) and
ETS1, resulting in blocking the cell cycle and suppression of liver
cancer cell migration and invasion. Chen et al (44) suggested that increased expression of
miR-193b inhibited malignant melanoma cell proliferation and
suppressed CCND1 expression. CCND1, which is a type of oncogene
that encodes protein, is the key factor in the G1 proliferation
phase that has been identified to have an important role in the
development of malignant tumors, and is therefore associated with
tumor occurrence, metastasis and prognosis (45). A previous study has indicated that
overexpression of CCND1 may shorten the G1 phase and promote cells
to enter S phase, leading to uncontrolled cell proliferation and
resulting in hyperplastic lesions or canceration (46). Therefore, it may be speculated that
miR-193b regulates the cell cycle, not only by RAB22A-Ras signaling
as indicated in the present study, but also by targeting the CCND1
gene.
The present study demonstrated that miR-193b
expressed in colon cancer tissues was significantly decreased
compared with that in the adjacent normal tissues and reduced
expression was also observed in the SW480 colon cancer cell line.
miR-193b mimic transfection into SW480 cells resulted in a
reduction in migration, indicating the potential role that miR-193b
has as a tumor suppressor gene in colon cancer. Furthermore,
results indicated that miR-193b combined with RAB22A in the 3′UTR
region, and that the specific binding of RAB22A is an miR-193b
downstream target. Western blot analysis revealed that miR-193b may
inhibit RAB22A protein expression levels in SW480 colon cancer
cells.
These findings suggest that miR-193b has potential
applications in the diagnosis and treatment of colon cancer and
that it may have an important role in colon cancer development.
Further studies into the function of miR-193b on the occurrence and
development of colon cancer and further elucidation into the
underlying molecular mechanism are required. In conclusion, these
findings suggest the theoretical basis of miR-193b for the
diagnosis and treatment of colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors’ contributions
ZMF and SCL performed the experiments and wrote the
manuscript. CRL contributed significantly to the data analysis. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research and ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committee of Weifang People's Hospital
(Weifang, China) approved the present study and informed consent
from all patients was received upon admission.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Janakiram NB and Rao CV: The role of
inflammation in colon cancer. Adv Exp Med Biol. 816:25–52. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng M, Lan Y and Luo S: Quality of life
estimate in stomach, colon, and rectal cancer patients in a
hospital in China. Tumour Biol. 34:2809–2815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fengju S, Guanglin W and Kexin C:
Incidence of colon cancer in Tianjin, China, 1981–2000. Asia Pac J
Public Health. 17:22–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou L, Ji BT, Blair A, Dai Q, Gao YT and
Chow WH: Commuting physical activity and risk of colon cancer in
Shanghai, China. Am J Epidemiol. 160:860–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiu BC, Ji BT, Dai Q, Gridley G,
McLaughlin JK, Gao YT, Fraumeni JF Jr and Chow WH: Dietary factors
and risk of colon cancer in Shanghai, China. Cancer Epidemiol
Biomarkers Prev. 12:201–208. 2003.PubMed/NCBI
|
|
6
|
Diao D, Wang L, Wan J, Chen Z, Peng J, Liu
H, Chen X, Wang W and Zou L: MEK5 overexpression is associated with
the occurrence and development of colorectal cancer. BMC Cancer.
16:3022016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lassmann S, Kreutz C, Schoepflin A, Hopt
U, Timmer J and Werner M: A novel approach for reliable microarray
analysis of microdissected tumor cells from formalin-fixed and
paraffin-embedded colorectal cancer resection specimens. J Mol Med
(Berl). 87:211–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu J, Chen Y, Fu X, Zhou X, Peng Y, Shi L,
Chen T and Wu Y: Invasive Fusobacterium nucleatum may play a role
in the carcinogenesis of proximal colon cancer through the serrated
neoplasia pathway. Int J Cancer. 139:1318–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Li X, Wu M, Lin C, Guo Y and Tian
B: Knockdown of long noncoding RNA GHET1 inhibits cell
proliferation and invasion of colorectal cancer. Oncol Res.
23:303–309. 2016. View Article : Google Scholar
|
|
10
|
Ren W, Shen S, Sun Z, Shu P, Shen X, Bu C,
Ai F, Zhang X, Tang A, Tian L, et al: Jak-STAT3 pathway triggers
DICER1 for proteasomal degradation by ubiquitin ligase complex of
CUL4A(DCAF1) to promote colon cancer development. Cancer Lett.
375:209–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mullany LE, Wolff RK, Herrick JS, Buas MF
and Slattery ML: SNP regulation of microRNA expression and
subsequent colon cancer risk. PLoS One. 10:e01438942015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Xu Y, Qiu W, Zhao D and Zhang Y:
Tissue miR-193b as a novel biomarker for patients with ovarian
cancer. Med Sci Monit. 21:3929–3934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feuermann Y, Kang K, Gavrilova O,
Haetscher N, Jang SJ, Yoo KH, Jiang C, Gonzalez FJ, Robinson GW and
Hennighausen L: MiR-193b and miR-365-1 are not required for the
development and function of brown fat in the mouse. RNA Biol.
10:1807–1814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Kong F, Wu K, Song K, He J and Sun
W: miR-193b directly targets STMN1 and uPA genes and suppresses
tumor growth and metastasis in pancreatic cancer. Mol Med Rep.
10:2613–2620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XF, Yan PJ and Shao ZM: Downregulation
of miR-193b contributes to enhance urokinase-type plasminogen
activator (uPA) expression and tumor progression and invasion in
human breast cancer. Oncogene. 28:3937–3948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao K, Zhang J, He C, Xu K, Liu J, Sun J,
Wu G, Tan C, Zeng Y, Wang J and Xiao Z: Restoration of miR-193b
sensitizes Hepatitis B virus-associated hepatocellular carcinoma to
sorafenib. Cancer Lett. 352:245–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu H, Krasinskas A and Willis J:
Perspectives on current tumor-node-metastasis (TNM) staging of
cancers of the colon and rectum. Semin Oncol. 38:500–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiu VK, Bivona T, Hach A, Sajous JB,
Silletti J, Wiener H, Johnson RL II, Cox AD and Philips MR: Ras
signaling on the endoplasmic reticulum and the Golgi. Nat Cell
Biol. 4:343–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Gao J, Du Z, Zhang X, Yang F and
Gao W: Expression of factors and key components associated with the
PI3K signaling pathway in colon cancer. Oncol Lett. 15:5465–5472.
2018.PubMed/NCBI
|
|
22
|
Wang YN, Chen ZH and Chen WC: Novel
circulating microRNAs expression profile in colon cancer: A pilot
study. Eur J Med Res. 22:512017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Du Y, Liu X, Cho WC and Yang Y:
MicroRNAs as regulator of signaling networks in metastatic colon
cancer. Biomed Res Int. 2015:8236202015.PubMed/NCBI
|
|
24
|
Li X, Qin B and Liu BO: Delineating the
effect of demethylating agent 5-aza-2′-deoxycytidine on human
Caco-2 colonic carcinoma cells. Oncol Lett. 12:139–143. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pagliara V, Saide A, Mitidieri E,
d'Emmanuele di Villa Bianca R, Sorrentino R, Russo G and Russo A:
5-FU targets rpL3 to induce mitochondrial apoptosis via
cystathionine-beta-synthase in colon cancer cells lacking p53.
Oncotarget. 7:50333–50348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schneider B, Nagel S, Ehrentraut S,
Kaufmann M, Meyer C, Geffers R, Drexler HG and MacLeod RA:
Neoplastic MiR-17~92 deregulation at a DNA fragility motif (SIDD).
Genes Chromosomes Cancer. 51:219–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knudsen KN, Lindebjerg J, Nielsen BS,
Hansen TF and Sørensen FB: MicroRNA-200b is downregulated in colon
cancer budding cells. PLoS One. 12:e01785642017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng
L, Shi Y, Wang H, Yin B, Xia J and Wang Z: Down-regulation of
miR-223 reverses epithelial-mesenchymal transition in
gemcitabine-resistant pancreatic cancer cells. Oncotarget.
6:1740–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Chen J, Liu X, Guo Z, Sun X and
Zhang J: The real-time dynamic monitoring of microRNA function in
cholangiocarcinoma. PLoS One. 9:e994312014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:15472–15477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akao Y, Nakagawa Y and Naoe T: Let-7
microRNA functions as a potential growth suppressor in human colon
cancer cells. Biol Pharm Bull. 29:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Wu B, Li JH, Nan G, Jiang JL and
Chen ZN: Rab22a enhances CD147 recycling and is required for lung
cancer cell migration and invasion. Exp Cell Res. 357:9–16. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su F, Chen Y, Zhu S, Li F, Zhao S, Wu L,
Chen X and Su J: RAB22A overexpression promotes the tumor growth of
melanoma. Oncotarget. 7:71744–71753. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang D, Liu G and Wang K: miR-203 acts as
a tumor suppressor gene in osteosarcoma by regulating RAB22A. PLoS
One. 10:e01322252015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Opdam FJ, Kamps G, Croes H, van Bokhoven
H, Ginsel LA and Fransen JA: Expression of Rab small GTPases in
epithelial Caco-2 cells: Rab21 is an apically located GTP-binding
protein in polarised intestinal epithelial cells. Eur J Cell Biol.
79:308–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ngalame NN, Tokar EJ, Person RJ, Xu Y and
Waalkes MP: Aberrant microRNA expression likely controls RAS
oncogene activation during malignant transformation of human
prostate epithelial and stem cells by arsenic. Toxicol Sci.
138:268–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He H, Dai F, Yu L, She X, Zhao Y, Jiang J,
Chen X and Zhao S: Identification and characterization of nine
novel human small GTPases showing variable expressions in liver
cancer tissues. Gene Expr. 10:231–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mesa R, Salomón C, Roggero M, Stahl PD and
Mayorga LS: Rab22a affects the morphology and function of the
endocytic pathway. J Cell Sci. 114:4041–4049. 2001.PubMed/NCBI
|
|
39
|
Mesa R, Magadán J, Barbieri A, López C,
Stahl PD and Mayorga LS: Overexpression of Rab22a hampers the
transport between endosomes and the Golgi apparatus. Exp Cell Res.
304:339–353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kauppi M, Simonsen A, Bremnes B, Vieira A,
Callaghan J, Stenmark H and Olkkonen VM: The small GTPase Rab22
interacts with EEA1 and controls endosomal membrane trafficking. J
Cell Sci. 115:899–911. 2002.PubMed/NCBI
|
|
41
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slattery ML, Herrick JS, Mullany LE,
Samowitz WS, Sevens JR, Sakoda L and Wolff RK: The co-regulatory
networks of tumor suppressor genes, oncogenes, and miRNAs in
colorectal cancer. Genes Chromosomes Cancer. 56:769–787. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu C, Liu S, Fu H, Li S, Tie Y, Zhu J,
Xing R, Jin Y, Sun Z and Zheng X: MicroRNA-193b regulates
proliferation, migration and invasion in human hepatocellular
carcinoma cells. Eur J Cancer. 46:2828–2836. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen J, Feilotter HE, Paré GC, Zhang X,
Pemberton JG, Garady C, Lai D, Yang X and Tron VA: MicroRNA-193b
represses cell proliferation and regulates cyclin D1 in melanoma.
Am J Pathol. 176:2520–2529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fusté NP, Castelblanco E, Felip I,
Santacana M, Fernández-Hernández R, Gatius S, Pedraza N, Pallarés
J, Cemeli T, Valls J, et al: Characterization of cytoplasmic cyclin
D1 as a marker of invasiveness in cancer. Oncotarget.
7:26979–26991. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|