Introduction
Traumatic optic neuropathy (TON) is an acute injury
to the optic nerve due to trauma. Optic nerve axons may be affected
directly or indirectly and cause partial or complete vision loss.
Indirect trauma can occur after head trauma with intraneural edema,
shearing of the optic nerve fibers, and altered cerebrospinal fluid
circulation, which indirectly disrupts the function and anatomy of
the optic nerve. The most common site of indirect TON is the part
of the optic nerve that lies within the optic canal. The mechanism
of TON can be mechanical shearing of optic nerve axons, ischemia,
or apoptosis of adjacent neurons.
Experimental unilateral transection of the optic
nerve also causes acute glial activation of the retina on the
contralateral side (1–3). These experiments suggest that the
processes of bilateral glial activation actually may also occur on
the level of the retinal ganglion cells in the visual tract in
humans. The current literature contains no mention of this topic,
and we decided to present the case report below.
Materials and methods
A young man (born in 1981) suffered an injury to the
right orbit upon hitting a concrete pole in the summer of 2013
while riding his bicycle. A severe vision disorder persisted after
neurosurgical treatment with plastic surgery and reconstruction of
the right orbit. The patient underwent his first complex assessment
at our department six months after the injury. Standard
ophthalmological tests and visual field testing (Medmont M700;
Medmont International Pty Ltd., Nunawading, Australia) were
performed on this patient after TON. The retinal nerve fibre layers
(RNFL) and the ganglion cell complex (GCC) were tested using
spectral domain optical coherence tomography (SD-OCT) RTVue-100.
The pattern electroretinogram (PERG) and pattern visual evoked
potential (PVEP; Roland Consult, Brandenburg an der Have, Germany)
were performed using the ISCEV methodology. The stimulating board
size was 30×38 cm for both the PERG and PVEP. Square size at
PERG-48 min, PVEP 60 min. The distance of the stimulating board
from the eye was 30 cm, and the eye was set for this distance if
necessary. Reversal rate was 4.02 Hz for PERG and 1.827 Hz for
PVEP. The PERG voltage was detected using corneal DTL electrodes.
Dish-shaped skin electrodes were placed 1 cm laterally from the
outer corner of the eye. We evaluated the P50 and N95 latencies
(ms) and the P50-N95 amplitudes (uV). In the PVEP (Fz-Oz
electrodes), we evaluated the P100 latency and the N70-P100
amplitudes (uV).
Magnetic resonance imaging was used for structural
and functional brain imaging. Magnetic resonance imaging (MRI)
examinations were performed using the Philips Achieva 3T TX MR
system (Philips Healthcare, Eindhoven, The Netherlands), with a
magnetic field strength of 3 Tesla. Functional MRI (fMRI) uses
blood oxygen level-dependent (BOLD) contrast. A standard 32-channel
head coil was used, and each measurement was performed with a
gradient-echo echo-planar imaging sequence (TR/TE=3000/30 ms,
spatial resolution of 2 × 2 × 2 mm3). Optical
stimulation was performed using a black/white checkerboard
alternated with its negative image at a frequency of 2 Hz. The
visual size of the black and white checkerboard was 25.8×6.2
degrees. Measurements consisted of a sequence of five 30-second
active phase periods and five rest periods of the same length (for
each of the ten dynamic scans). During the resting phase, a static
crosshair situated in the centre of the visible field was projected
for view fixation. Each measurement included 100 dynamic scans and
took 5 min. Each eye was examined using the means of separate fMRI
measurements (LE and RE), and one control measurement was performed
via stimulation of both eyes together (LE+RE).
Structural magnetic resonance imaging was performed
in the basic planes using the following sequences: T1 TFE SAG 3D
1×1×1 mm3, TR=8.1 ms, TE=3.7 ms, NSA 1, 170 slices; T2
mDIXON COR 2.5 mm, TR=3,000 ms, TE=80 ms, 36 slices; T2 4 mm TRA,
TR=3,000 ms, TE=80 ms, NSA 1, 30 slices; FLAIR 4 mm, TR=11,000 ms,
TE=125 ms; VenBold, TR=15 ms, TE=21 ms, NSA 2, 3DFFE 1×1×1
mm3, 290 slices; T1 IR COR 5 mm, TR=3873 ms, TE=15 ms,
NSA 1, 28 slices, gap 1; and DWI 4 mm, TR=3,443 ms, TE=76 ms,
b-factor 0–800, 30 slices. RNFL and GCC were examined using SD-OCT
RTvue 100 with navigation on the right and fixation of a light
point 37 and 50 months from the first ophthalmological testing on
the left. PERG and PVEP were assessed on the first day of testing
in February 2014.
Functional magnetic resonance imaging was performed
nine months after the first ophthalmological testing.
Results
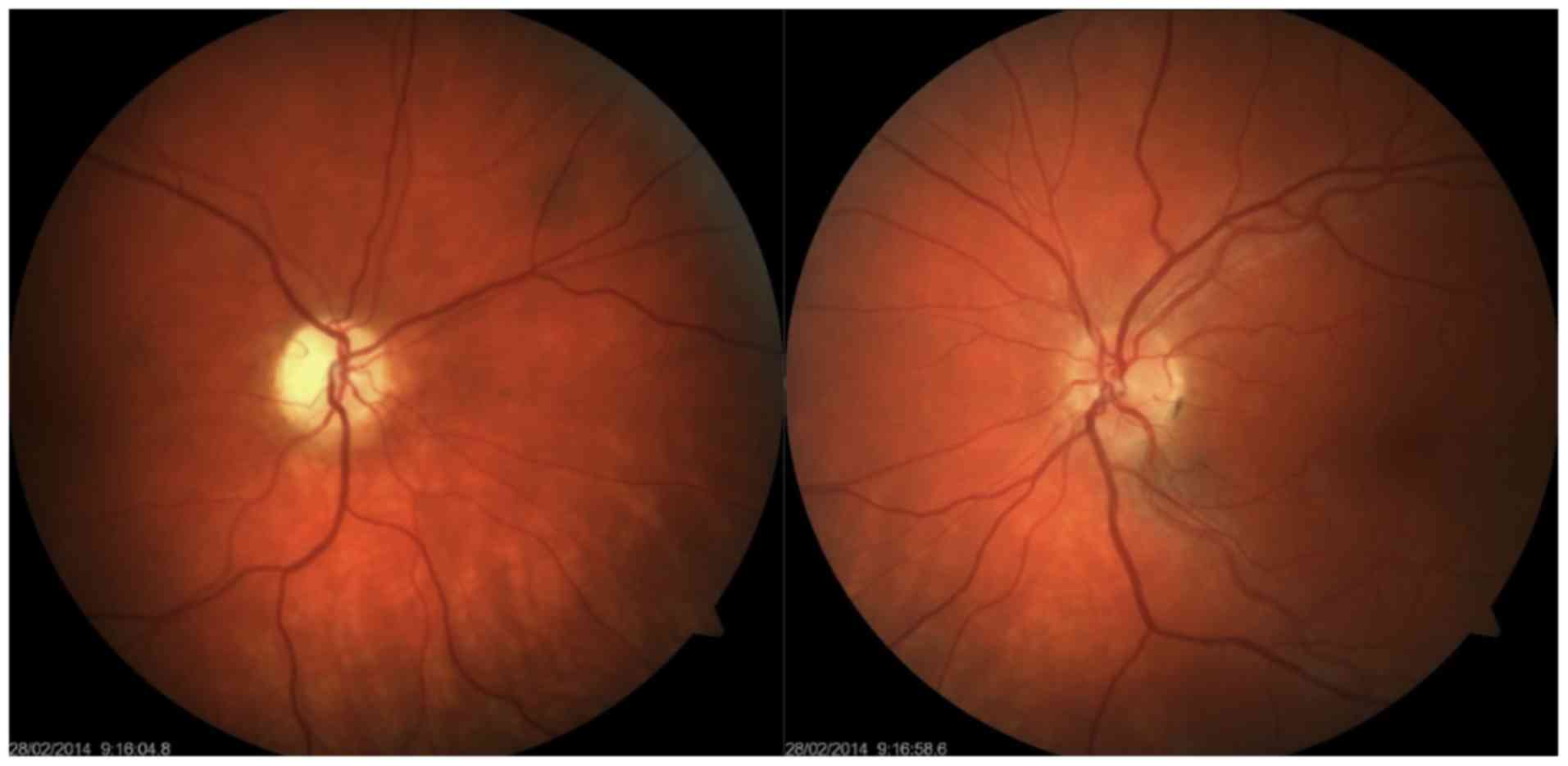
Enophthalmos was detected on the right, with the
bulb in the 5-degree divergent position with free motility.
Right-sided anisocoria was found with an amaurotic pupil, which
responded to an indirect stimulus. Simple atrophy of the optic disc
was also noted, but other ophthalmological findings were normal.
This finding was normal on the left, and the pupil responded only
to a direct light stimulus. Visual acuity was 0 and 1.0 (natural)
on the right and left, respectively. Intraocular pressure (IOP) was
9/9 mmHg (Fig. 1). Colour perception
was unimpaired on the left. The visual field exhibited no decrease
in sensitivity on the left.
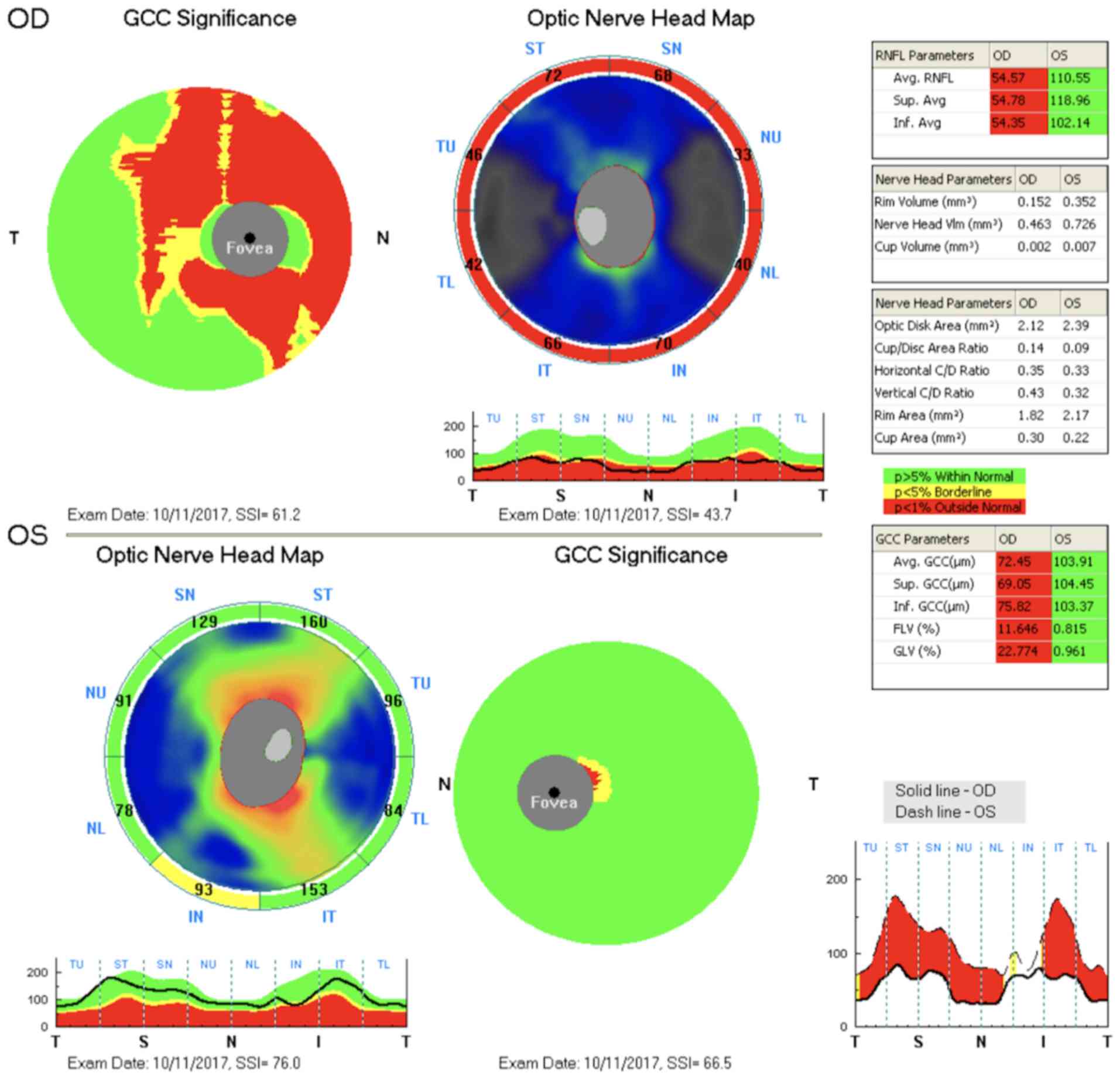
The findings were identical at the given time
points. A considerable decrease in RNFL and GCC thickness was
observed on the right, and both of these findings were normal on
the left (Fig. 2).
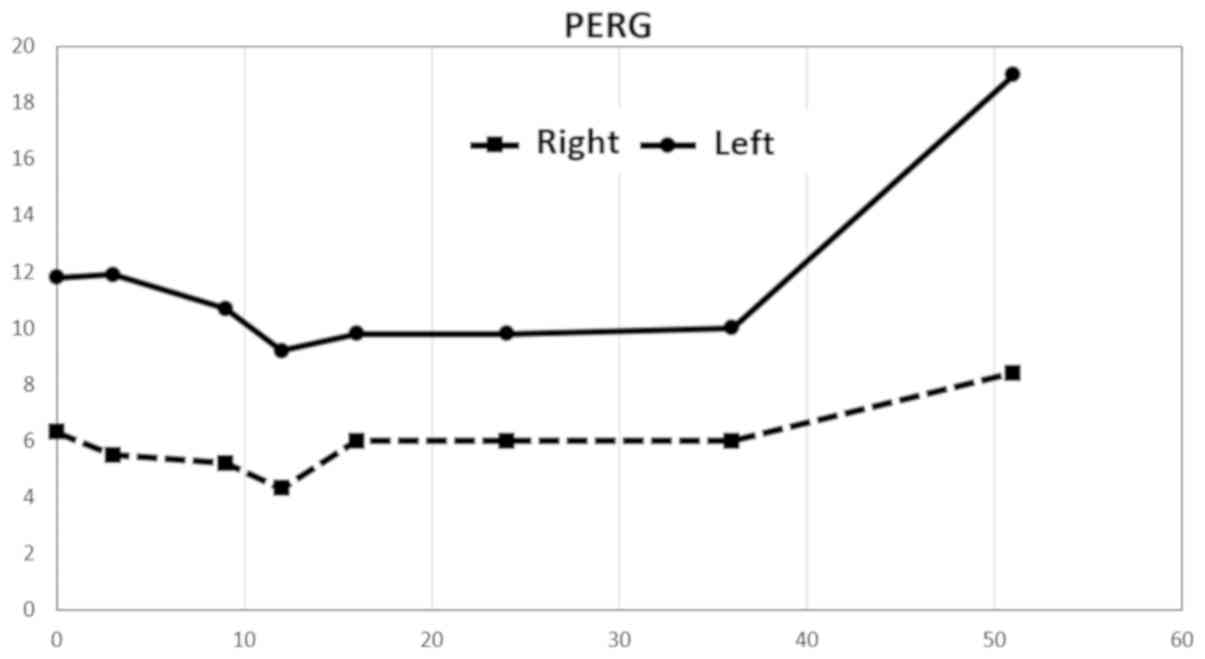
A considerable decrease in amplitudes in PERG was
observed after stimulation of the right eye, up to 50% compared to
the left eye. Values on the right were at approximately the lower
limit of normal. The repeated examination approximately eight
months later revealed a decrease in amplitude in the left eye, and
this finding remained virtually unchanged until 37 months from the
first examination. The amplitude values increased at this time. The
measured amplitude values remained virtually unchanged on the right
throughout the follow-up period. The last examination was completed
52 months after the first examination and revealed a considerable
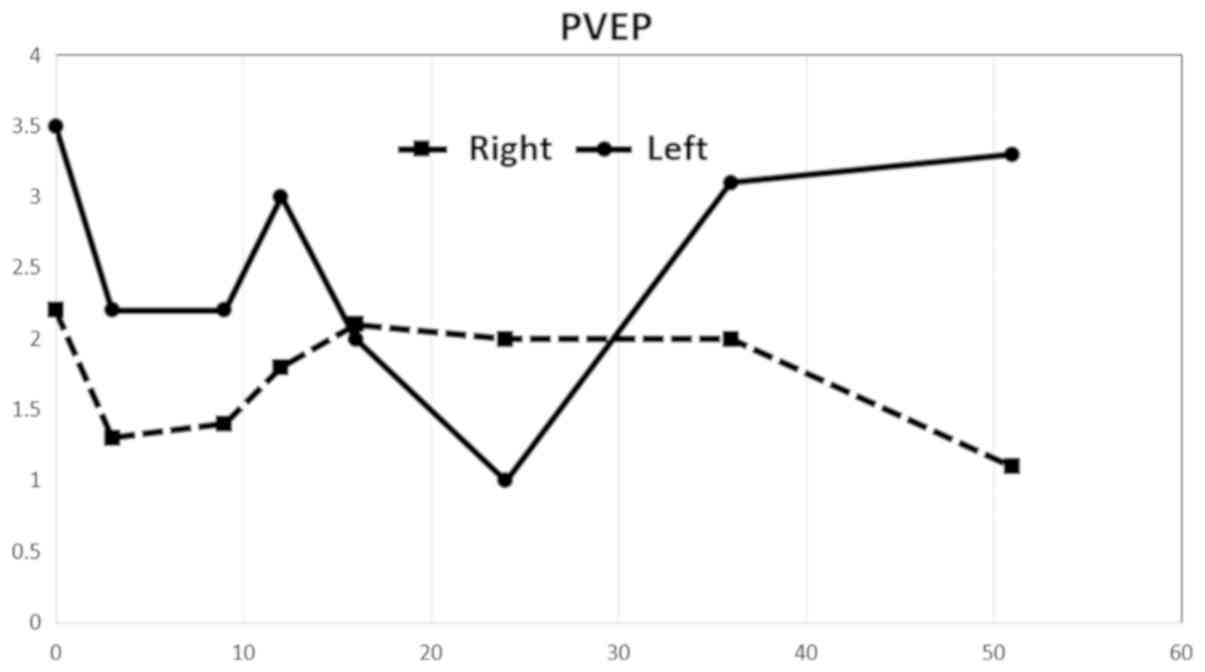
improvement in amplitudes in the left eye (Fig. 3). The amplitude values of the PVEP
were markedly pathological on both sides from the first examination
and exhibited a more profound decrease after stimulation of the
right eye. An increase in PVEP amplitudes was observed after
stimulation of the left eye starting from the 37-month time point.
The values were considerably abnormal despite this increase
(Fig. 4).
Normal PERG amplitude values P50-N95: 14.80±2.51 uV.
Normal PVEP amplitude values N70-P100: 12.22±3.22 uV (4).
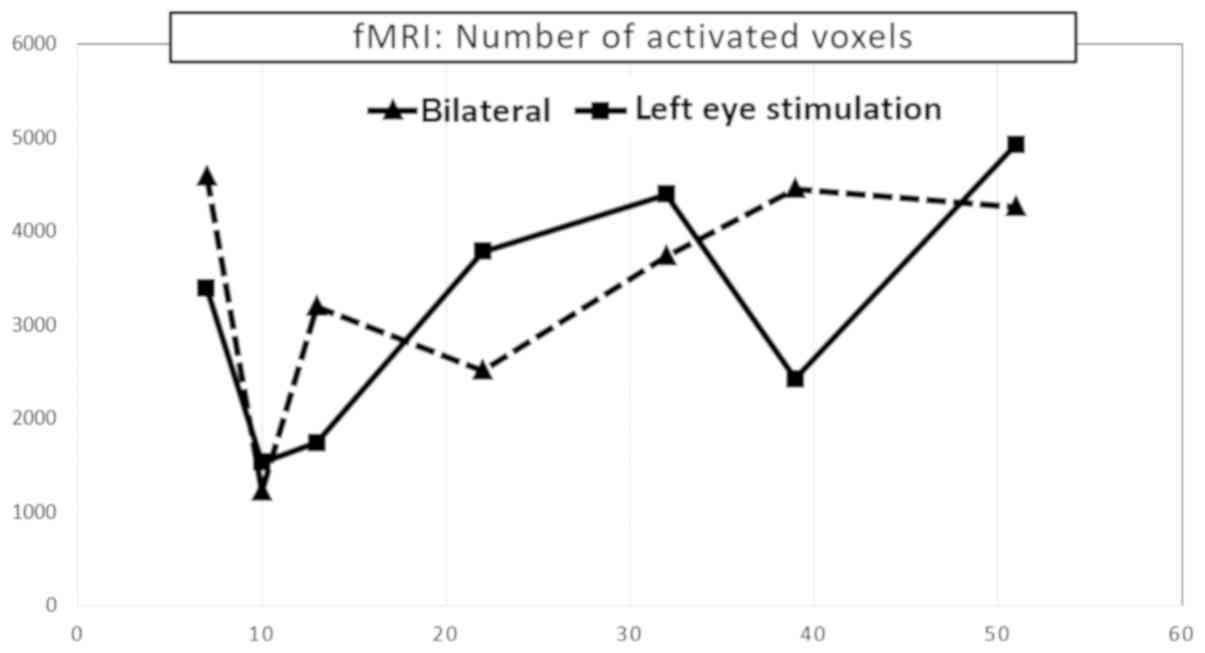
Functional magnetic resonance imaging was performed
nine months after the first ophthalmological testing. fMRI
exhibited a considerable decrease in visual cortex activity after
stimulation of the left eye. A further decrease was observed ten
months after the first ophthalmological testing, followed by
culmination with a gradual increase in voxel activity, similarly as
the PVEP. Values remained below the normal range despite this
increase. fMRI activity of the right eye alone was not examined
because its fixation to a relatively small stimulation area could
not be ensured. The following mean values were observed in a
healthy population using our methodology: fMRI response was
compared with normal control database which is 9,200 ± 2,700
monocular voxels for the corresponding age group (Fig. 5).
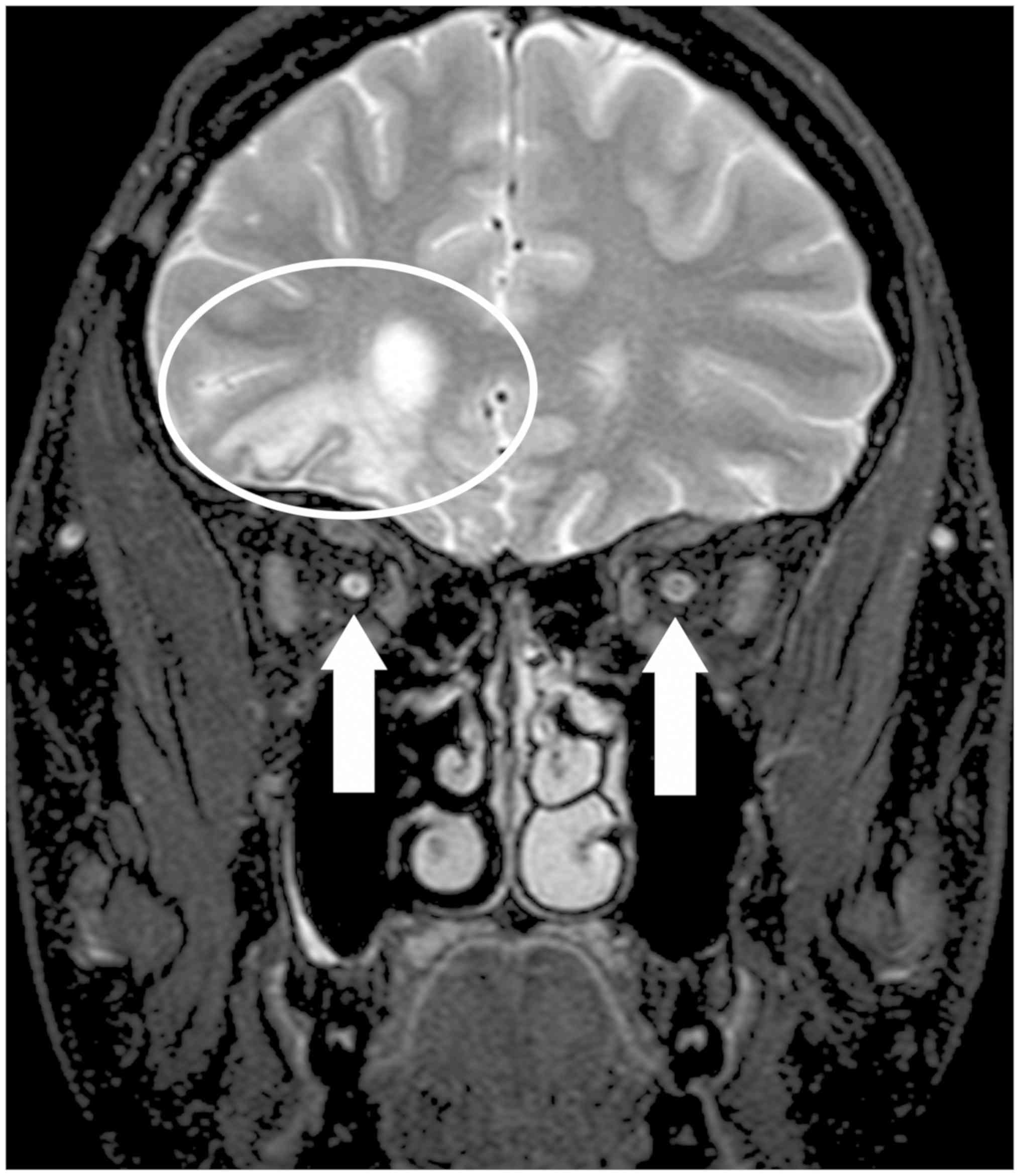
Structural MRI revealed post-contusion alternations
of the brain in the frontal-basal and frontal-polar regions on the
right, with minor haemosiderin deposits. No other foci were
observed, and no acute cerebral ischaemia was demonstrated.
Periventricular atrophy of the frontal horn of the right lateral
ventricle and an atrophying process of the entire optic nerve on
the right and the right half of the optic chiasm were found
(Fig. 6).
Discussion
Glial activation in the retina on the ipsilateral
and contralateral sides was demonstrated in experimental models in
the literature (1–3). These experimental conclusions suggest
that the processes of bilateral glial activation may also occur on
the level of the retinal ganglion cells themselves and the visual
tract in humans. This hypothesis was supported by our
electrophysiological testing of other patients after TON. The
normal visual field and colour perception of our patients revealed
no abnormalities on the contralateral side. This result was likely
because an up to 25–30% loss of retinal ganglion cells is needed to
detect the first perimetric changes using static automatic
perimetry. Colour perception tends to be affected similarly.
RNFL and GCC examinations detected a loss only on
the ipsilateral side. These alternations may also be found on the
contralateral side, but they cannot be captured using current
methods. OCT only scans GCC thickness, which may be affected by
glial proliferation.
The problem of damage to the contralateral optic
nerve, or more precisely the contralateral visual tract, after TON
in human medicine, is not known from the literature.
Liu et al (5)
discovered PERG changes in the ipsilateral and contralateral eyes
after unilateral damage to the optic nerve in a murine model. These
findings preceded morphological alterations in the layer of retinal
ganglion cells (5). The PERG
findings obtained in our patient confirm this finding. However, we
cannot explain the increased amplitude on the damaged side and the
contralateral side 43 months after the injury. PVEP amplitudes also
increased, approximately from 30 months after the injury. Ganglion
cells in the retina pass through the pathological process of
shrinkage before cell death. This corresponds to a decrease in
their action potential, and this change can be diagnosed with PERG,
even when OCT findings of the ganglion cell complex are normal.
We also considered the possibility of influencing
PVEP associated with brain contusions and contralateral side optic
tract changes. The authors have their own experience with
contralateral side optic tract injuries related to TON lesions of
the optic nerve caused by tumor compression.
No PVEP findings are available in the literature for
human TON, and no fMRI examinations are found. Our results indicate
damage to the entire visual tract that was not limited to the
damaged side but also included the contralateral side. We are
convinced that the ganglion cells of the retina respond initially
to glial activation. These changes are, in our view, followed by
changes in the visual pathway.
Potential therapy should be briefly mentioned. In
Czech literature, the first solid opinion on this topic was adopted
by Otradovec (6), who summarized his
TON results in ten points: i) Injury to one optic nerve is the most
common visual tract disorder after a blunt injury of the head; ii)
the vision disorder develops at the moment of the injury; iii) the
condition is characterized by a remarkable capability of
spontaneous restitution of the visual functions, which may be
expected in approximately one-half of injured persons. The first
signs of improvement occur 3 to 4 days after injury and reach peak
intensity in the first 3–4 weeks; iv) altitudinal scotoma is a
typical perimetric defect; v) the bulb is generally not damaged,
and pupil function disorder is the only objective sign of the
sensory disorder; vi) cranial bone fractures are common, but not
obligatory, finding; vii) surgical revisions of 17 optic nerves in
16 patients with post-traumatic amaurosis never revealed any
changes that could be rectified by the procedure; viii) sagittal
displacement of the brain inside the skull at the moment of impact
is likely involved; ix) indications for surgical revision of the
canalicular region in cases of post-traumatic amaurosis are
exceptional, and generally, they are extraocular, such as
liquorrhoea, pneumocephalus, and similar reasons; x) the first four
days after the injury are a contraindication for the surgical
procedure, i.e., the period when spontaneous restitution of visual
functions may begin.
We have previous experience with this type of
problem. We previously described the case of a 22-year-old male
with a car accident injury and 0 visual acuity on the left. The
optic canal was decompressed on the second day after injury, and
the patient's visual acuity began to improve as early as the second
postoperative day, as seen by his ability to recognize light from
dark. The patient's visual acuity improved to 0.1 one month after
injury. These visual function results are consistent with the
above-mentioned evaluations of Otradovec (7).
Review papers in recent years have not adopted a
uniform position for this problem. No evidence of TON therapy
exists. Therefore, there is insufficient consensus among clinicians
for the suitability of TON therapy. Our overview of recent
literature revealed no reliable evidence that suggests that TON
therapy using corticosteroids, surgical decompression of the optic
canal, or both provides any benefit compared to mere observation.
Furthermore, these interventions pose an additional risk that may
not warrant their routine use (8,9).
Our study demonstrated that unilateral damage to the
optic nerve in TON was also associated with considerable
alterations of the contralateral visual tract, including functional
alterations of the retina.
Acknowledgements
Not applicable.
Funding
The present study was supported by MH CZ-DRO, Motol
University Hospital, Prague, Czech Republic (grant no. 00064203).
Progres Q35. The present study was supported by the Charles
University research program PROGRES Q35. The present study was
supported by the EU Structural Funds OPP competitiveness (grant no.
CZ.2.16/3.1.00/21532).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
All the authors were involved in conceiving and
designing the study. KM and LJ drafted and wrote the manuscript.
KM, LJ and TJ were responsible for the collection and analysis of
the patient data. HP critically revised the manuscript for
important intellectual content. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the local Ethics
Committee of JL Clinic, Faculty of Biomedical Engineering, Czech
Technical University in Prague, and the study was performed in
accordance with Good Clinical Practice and the Declaration of
Helsinki. The patient provided written informed consent.
Patient consent for publication
The patient provided written informed consent;
however, the authors made efforts to remove the identifying
information to protect the privacy of the patient.
Disclosure of interest
The authors declare that they have no competing
interests.
References
|
1
|
Bodeutsch N, Siebert H, Dermon C and
Thanos S: Unilateral injury to the adult rat optic nerve causes
multiple cellular responses in the contralateral site. J Neurobiol.
38:116–128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panagis L, Thanos S, Fischer D and Dermon
CR: Unilateral optic nerve crush induces bilateral retinal glial
cell proliferation. Eur J Neurosci. 21:2305–2309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cen LP, Han M, Zhou L, Tan L, Liang JJ,
Pang CP and Zhang M: Bilateral retinal microglial response to
unilateral optic nerve transection in rats. Neuroscience.
311:56–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lestak J, Nutterova E, Pitrova S, Krejcova
H, Bartosova L and Forgacova V: High tension versus normal tension
glaucoma. A comparison of structural and functional examinations. J
Clin Exp Ophthalmol. 3:1–4. 2012.
|
|
5
|
Liu Y, McDowell CM, Zhang Z, Tebow HE,
Wordinger RJ and Clark AF: Monitoring retinal morphologic and
functional changes in mice following optic nerve crush. Invest
Ophthalmol Vis Sci. 55:3766–3774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Otradovec J: Klinická Neurooftalmologie.
Prague Grada Publishing. 2003.
|
|
7
|
Haninec P, Klener J, Houst'ava L and
Lesták J: Restoration of vision after surgical treatment of
traumatic optic neuropathy of an uncommon type. Case report and
literature review. Cesk Slov Oftalmol. 52:249–252. 1996.(In Czech).
PubMed/NCBI
|
|
8
|
Kumaran AM, Sundar G and Chye LT:
Traumatic optic neuropathy: A review. Craniomaxillofac Trauma
Reconstr. 8:31–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaon BC and Lee MS: Is there treatment
for traumatic optic neuropathy? Curr Opin Ophthalmol. 26:445–449.
2015. View Article : Google Scholar : PubMed/NCBI
|