Introduction
Liver resection is considered to be the most
effective therapy for patients with hepatocellular carcinoma (HCC)
to date. However, due to the high incidence of tumor recurrence and
metastasis, the overall prognosis of HCC remains unsatisfactory
(1). It has been reported that
>70% of patients with HCC develop recurrence within 5 years
after surgery (2). The mechanism
underlying recurrence and metastasis of HCC is complex and remains
unclear. Therefore, novel molecular prognostic biomarkers of HCC
are required to improve the survival of patients with HCC (3). Complement factor H-related protein 1
(CFHL1), a member of the complement factor H (CFH) family, was
demonstrated to inhibit the activity of C5 convertase, as well as
the assembly and membrane insertion of the terminal complement
components (4,5). It was also reported that human CFHL1,
had a function and structure similar to human CFH (6). Several studies reported that CFHR is
associated with bladder cancer and may be used as a quantitative
urinary tumor marker in selected patients (7,8). In
2011, Yang et al (9) revealed
that CFH exhibited decreased mRNA expression and increased CpG site
methylation in surgically resected HCC tissues; however, no further
studies verified this result. Therefore, the aim of the present
study was to investigate the potential prognostic value of CFHL1 in
HCC. First, immunohistochemistry (IHC) and western blot analysis
were used to evaluate CFHL1 protein levels in HCC and paired
peritumoral tissues. Subsequently, the association between CFHL1
expression and overall survival (OS), pathological characteristics
of HCC and time-to-recurrence (TTR) was analyzed and the prognostic
value of CFHL1 for postoperative patients with HCC was determined
via receiver operating characteristic (ROC) curves.
Materials and methods
Patients and specimens
Eight pairs of fresh frozen tissue samples selected
for western blotting (collected in January 2017) and a total of 354
formalin-fixed, paraffin-embedded (FFPE) pathological specimens
(collected between December 2005 and December 2008) were obtained
at the Eastern Hepatobiliary Surgery Hospital (Shanghai, China).
The inclusion criteria were as follows: i) No preoperative
anticancer treatment; ii) no extrahepatic metastases prior to
surgery; and iii) pathological diagnosis of HCC based on the
histological diagnostic criteria of the World Health Organization
(10). The present study was
approved by the Institutional Review Board of the Eastern
Hepatobiliary Surgery Hospital and each patient provided written
informed consent. FFPE specimens were divided into two groups,
namely 76 specimens with paired peritumoral liver tissues and 278
specimens with prognostic data. All 278 cases with prognostic data
were followed up after surgery every 3 months for the first year
and every 6 months thereafter until December 2013. Serum
α-fetoprotein (AFP) examination, abdominal ultrasonography and
chest X-ray were performed monthly within the first year after
surgery and every 3–6 months thereafter. Computed tomography
scanning (CT) or magnetic resonance imaging (MRI) of the abdomen
was performed every 6 months or immediately upon suspicion of
recurrence. The criteria for recurrence were the same as the
preoperative diagnostic criteria (11). TTR was defined as the time between
tumor resection and tumor recurrence, and OS was defined as the
time between surgery and the date of the last follow-up or the date
of mortality from any cause. Each FFPE tissue specimen was stained
by hematoxylin and eosin and two experienced liver pathologists
reviewed the stained sections. Sections (4-µm thick) were placed on
slides coated with 3-aminopropyltriethoxysilane. Subsequently,
samples were deparaffinized, washed twice with xylene (each 10 min)
and rehydrated twice with 100% alcohol for 5 min each, once with
95% alcohol for 2 min and once with 70% alcohol for 2 min. Samples
were washed with distilled water and stained with Harris
hematoxylin solution for 6 min. Sections were washed under tap
water for 5 min, differentiated in 1% acid alcohol for 10 sec,
washed under tap water for 1 min and counterstained with
eosin-phloxine solution for 30 sec. Subsequently, samples were
dehydrated with 95 and 100% alcohol (each, 5 min), and washed twice
with xylene (each, 5 min). Xylene based Neutral balsam was used to
mount samples. All staining steps were performed at room
temperature.
Immunohistochemistry, scoring and
tissue microarray (TMA)
The method described by Kononen et al
(12) was used for the TMAs.
Briefly, two experienced pathologists reviewed HE-stained sections
and then pre-marked the representative cores in the paraffin
blocks. A 1.5-mm diameter tissue cylinder, which was subsequently
incorporated into a recipient paraffin block, was punched from a
marked area of each block. Subsequently, 4-mm sections were placed
on slides coated with 3-aminopropyltriethoxysilane. The paraffin
sections were deparaffinized in xylene and rehydrated through
decreasing concentrations of ethanol (100, 95 and 85%; 5 min each).
Antigens were retrieved by microwave irradiation for 5 min in
citrate buffer (pH 6.0) and cooled at room temperature for 120 min,
according to the protocol reported by Jin et al (13) with minor modifications (microwave
irradiation was applied for 5 min in the current study, compared
with 3 min in the previous study). The slides were incubated in 3%
H2O2/phosphate-buffered saline to block
endogenous peroxidase activity and non-specific binding sites were
blocked with goat serum. Samples were treated with Rabbit
polyclonal primary antibodies specific to CFHL1 (cat. no. ab103162;
1:50 dilution; cytoplasmic staining; Abcam, Cambridge, MA, USA;) at
4°C overnight. Tissue antigens were visualized with an EnVision
Detection kit (cat. no. GK500705: Gene Tech Co., Ltd., Hong Kong,
China), which included the ChemMate™ EnVision™/HRP and Rabbit/Mouse
(ENV) reagent (a peroxidase-conjugated polymer). Counterstaining
with hematoxylin was performed for 5 min. Negative control slides
were created for all assays by omission of the primary antibodies.
As previously reported (14), the
integrated optical density (IOD) of CFHL1 was considered to reflect
the expression level. A Leica CCD camera DFC420 connected to a
Leica DM IRE2 microscope (Leica Microsystems, Ltd., Milton Keynes,
UK) was used for imaging. High-power magnification (×200) with
Leica QWin Plus software (version 3; Leica Microsystems, Ltd.) was
used to capture images of representative fields. The IOD of each
image was counted and measured using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc., Bethesda, MD, USA).
Western blot analysis
Another eight pairs of fresh frozen tissue samples
were selected for western blotting which was performed as
previously described (15).
Characteristics of patients the samples were collected from are
summarized in Table I. Briefly,
tissue samples were homogenized in a radioimmunoprecipitation assay
buffer (Qiagen China Co., Ltd., Shanghai, China) with a cocktail of
proteinase inhibitors (Roche Applied Science, Basel, Switzerland)
and a cocktail of phosphatase inhibitors (Roche Applied Science).
The protein concentrations were determined using the bicinchoninic
acid kit (Pierce; Thermo Fisher Scientific Inc., Waltham, MA, USA).
Total protein (20 µg/lane) was separated by 10% SDS-PAGE and
transferred to nitrocellulose membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were incubated with primary
antibodies against CFHL1 (1:500 dilution; cat. no. ab103162; Abcam)
overnight at 4°C and subsequently incubated with Goat anti rabbit
IgG-HRP (1:3,000; cat. no. Sc-2004; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 60 min at room temperature. In addition, for
detection of loading control protein, anti-actin-HRP conjugated
antibody was incubated for 60 min at room temperature (1:4,000;
cat. no. HRP-60008; Proteintech Group, Inc.). After washing the
membrane, CFHL1 or actin were visualized using a Pierce ECL Western
Blotting Substrate (cat. no. 32106; Pierce; Thermo Fisher
Scientific, Inc.) Enhanced Chemiluminescence development solution.
The visualized bands were quantified using Quantity One software
(Bio-Rad Laboratories, Inc.).
 | Table I.Characteristics of patients the
samples for western blotting were collected from. |
Table I.
Characteristics of patients the
samples for western blotting were collected from.
| Variable | Number of
patients |
|---|
| Sex |
|
| Male | 6 |
|
Female | 2 |
| Age (years) |
|
| ≤50 | 4 |
|
>50 | 4 |
| HBsAg |
|
|
Negative | 1 |
|
Positive | 7 |
| Serum AFP |
|
| ≤20
ng/ml | 5 |
| >20
ng/ml | 3 |
| Liver cirrhosis |
|
| No | 5 |
| Yes | 3 |
| TNM |
|
| I | 3 |
| II | 2 |
|
III–IV | 1 |
| Child-pugh score |
|
| A | 8 |
| B | 0 |
| Tumor size |
|
| ≤5
cm | 3 |
| >5
cm | 5 |
| Tumor number |
|
|
Single | 5 |
|
Multiple | 3 |
| Tumor
differentiation |
|
| Well | 0 |
|
Moderate | 8 |
| Poor | 0 |
| Vascular
invasion |
|
| No | 6 |
| Yes | 2 |
Validation of the association between
CFHL1 mRNA expression levels and prognosis
To validate the association between CFHL1 mRNA
expression and the prognosis of patients with HCC, the current
study analyzed data from the Human Protein Atlas dataset (HPA,
www.proteinatlas.org). According to the
expected number of fragments per kilobase of transcript sequence
per millions base pairs sequenced (FPKM) of CFHL1, patients
included in the HPA were classified into low expression CFHL1 and
high CFHL1 expression groups. CFHL1 with a median expression FPKM
<1 were excluded. Kaplan-Meier survival and log-rank analysis
were used in the data from HPA website. Genes with log rank
P<0.001 were considered to be prognostic genes. (http://www.proteinatlas.org/ENSG00000244414-CFHR1/pathology/tissue/liver+cancer).
Statistical analysis
Optimization of the cut-point values was based on
outcome and the assessment of CFHL1 expression (expressed as IOD)
based on X-tile plots (16). Paired
t-test was used to analyze mean differences between two sets of
observations. A standard log-rank method was used to analyze the
cut-off scores, which were derived from 278 cases. The association
between CFHL1 expression and patient survival was analyzed via the
Mantel Cox log-rank test and Kaplan-Meier analysis. A χ2
test was used to analyze the association between
clinicopathological characteristics and CFHL1 expression. The SPSS
statistical software package (version 13.0; SPSS, Inc., Chicago,
IL, USA) was used for data analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
CFHL1 is downregulated in HCC tissues
at the protein level
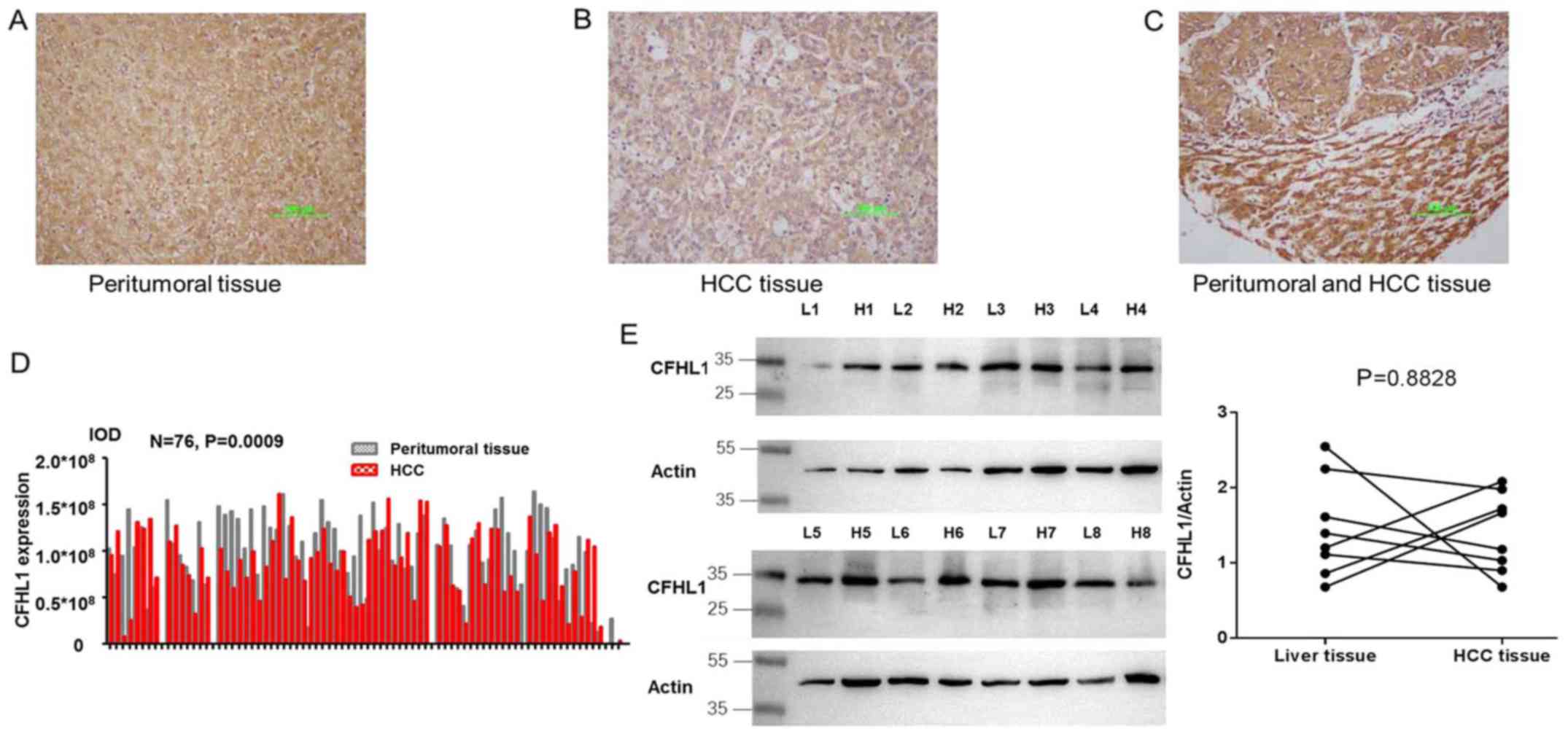
Cytoplasmic expression of CFHL1 was detected in both
HCC and peritumoral tissues (Fig.
1A-C). The results demonstrated that CFHL1 expression was
downregulated in 51 and upregulated in 25 HCC tissue samples,
compared with the peritumoral tissue samples (P=0.0009; Fig. 1D). Additionally, the expression level
of CFHL1 was analyzed in 8 paired HCC and peritumoral tissue
samples by western blotting. The expression level of CHFL1 was
lower in HCC tissues compared with peritumoral samples; however,
the difference was not statistically significant (Fig. 1).
Association between
clinicopathological characteristics and CFHL1 expression
In order to determine the association between CFHL1
expression and clinicopathologcal variables in patients with HCC,
data from the 354 patients with HCC were collected and evaluated by
χ2 test. The results demonstrated that low CFHL1
expression was associated with vascular invasion (P=0.024) and
serum AFP levels (P=0.039); however, no significant associations
were observed with other clinicopathological parameters, including
liver cirrhosis, sex, TNM stage, age, HBsAg, tumor size, Child-Pugh
class, tumor differentiation or tumor number (all P>0.05;
Table II).
 | Table II.CFHL1 expression level associations
with pathological characteristics of HCC. |
Table II.
CFHL1 expression level associations
with pathological characteristics of HCC.
|
| CFHL1 |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Sex |
|
| 0.554 |
| Male | 233 | 78 |
|
|
Female | 34 | 9 |
|
| Age (years) |
|
| 0.426 |
| ≤51 | 142 | 42 |
|
|
>51 | 125 | 45 |
|
| HBsAga |
|
| 0.196 |
|
Negative | 50 | 11 |
|
|
Positive | 215 | 75 |
|
| Serum
AFPa |
|
| 0.039 |
| ≤20
ng/ml | 88 | 39 |
|
| 20
ng/ml | 178 | 47 |
|
| Liver cirrhosis |
|
| 0.463 |
| No | 72 | 27 |
|
| Yes | 195 | 60 |
|
| TNM |
|
| 0.182 |
| I | 79 | 35 |
|
| II | 142 | 3 |
|
|
III–IV | 46 | 13 |
|
| Child-pugh
scorea |
|
| 0.953 |
| A | 243 | 78 |
|
| B | 24 | 8 |
|
| Tumor size |
|
| 0.260 |
| ≤5
cm | 78 | 20 |
|
| >5
cm | 189 | 67 |
|
| Tumor number |
|
| 0.324 |
|
Single | 210 | 64 |
|
|
Multiple | 57 | 23 |
|
| Tumor
differentiation |
|
| 0.881 |
|
Well | 22 | 8 |
|
|
Moderate | 226 | 74 |
|
|
Poor | 19 | 5 |
|
| Vascular
invasion |
|
| 0.024 |
| No | 90 | 41 |
|
|
Yes | 177 | 46 |
|
Association of low CFHL1 expression
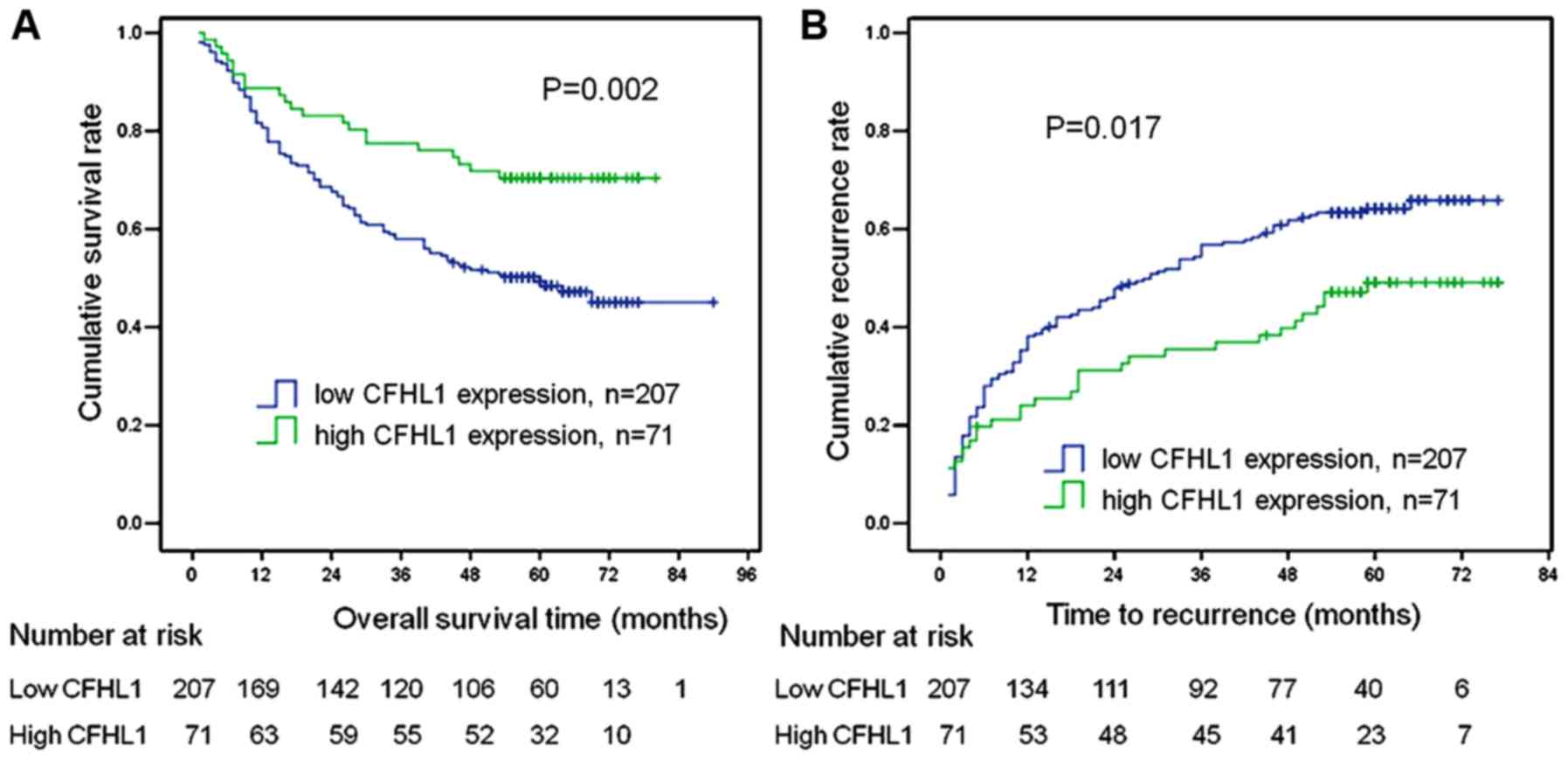
with OS and TTR in patients with HCC
The association of low CFHL1 expression with OS and
TTR was further assessed in 278 cases of HCC via univariate and
multivariate analyses (Table III).
The results demonstrated that tumor size, liver cirrhosis, TNM
stage, tumor number, CFHL1 expression and vascular invasion were
significant prognostic factors for OS and TTR on univariate
analysis (all P<0.05). With regard to tumor differentiation in
the univariate analysis, there was a statistically significant
difference for TTR (P=0.039), while there was no difference for OS
(P=0.271). In the multivariate analysis, liver cirrhosis, tumor
size, low CFHL1 expression and tumor number were independent
prognostic factors for OS and TTR (all P<0.05). Furthermore, as
presented in Fig. 2, both OS and TTR
were significantly worse in the low CFHL1 expression group (number
of patients, 207) compared with those in the high CFHL1 expression
group (n=71; all P<0.05).
 | Table III.Univariate and multivariate analyses
of clinico-pathological factors associated with OS and TTR. |
Table III.
Univariate and multivariate analyses
of clinico-pathological factors associated with OS and TTR.
|
| OS | TTR |
|---|
|
|
|
|
|---|
|
|
| Multivariate |
| Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factor | Univariate
P-value | HR | 95% Cl | P-value | Univariate
P-value | HR | 95% Cl | P-value |
|---|
| Sex, male vs.
female | 0.637 |
|
|
| 0.946 |
|
|
|
| Age, ≤51 vs.
>51 | 0.678 |
|
|
| 0.395 |
|
|
|
| HBsAg, positive vs.
negative | 0.174 |
|
|
| 0.173 |
|
|
|
| Serum AFP (ng/ml),
≤20 vs. >20 | 0.162 |
|
|
| 0.248 |
|
|
|
| Liver cirrhosis,
yes vs. no | 0.005 | 1.933 | 1.283–2.911 | 0.002 | <0.0001 | 2.176 | 1.476–2.913 | <0.0001 |
| TNM, I vs. II vs.
III–IV | <0.0001 |
|
|
| <0.0001 |
|
|
|
| Child-pugh score, A
vs. B | 0.379 |
|
|
| 0.126 |
|
|
|
| Tumor size, ≤5 vs.
>5 | 0.025 | 1.736 | 1.104–2.731 | 0.017 | 0.006 | 1.937 | 1.307–2.871 | 0.001 |
| Tumor number,
single vs. multiple | <0.0001 | 2.142 | 1.465–3.132 | <0.0001 | <0.0001 | 2.082 | 1.480–2.929 | <0.0001 |
| Tumor
differentiation, well vs. moderate vs. poor | 0.271 |
|
|
| 0.039 |
|
|
|
| Vascular invasion,
no vs. yes | 0.015 |
|
|
| 0.025 |
|
|
|
| CFHL1, low vs.
high | 0.003 | 0.470 | 0.294–0.751 | 0.002 | 0.020 | 0.630 | 0.431–0.920 | 0.017 |
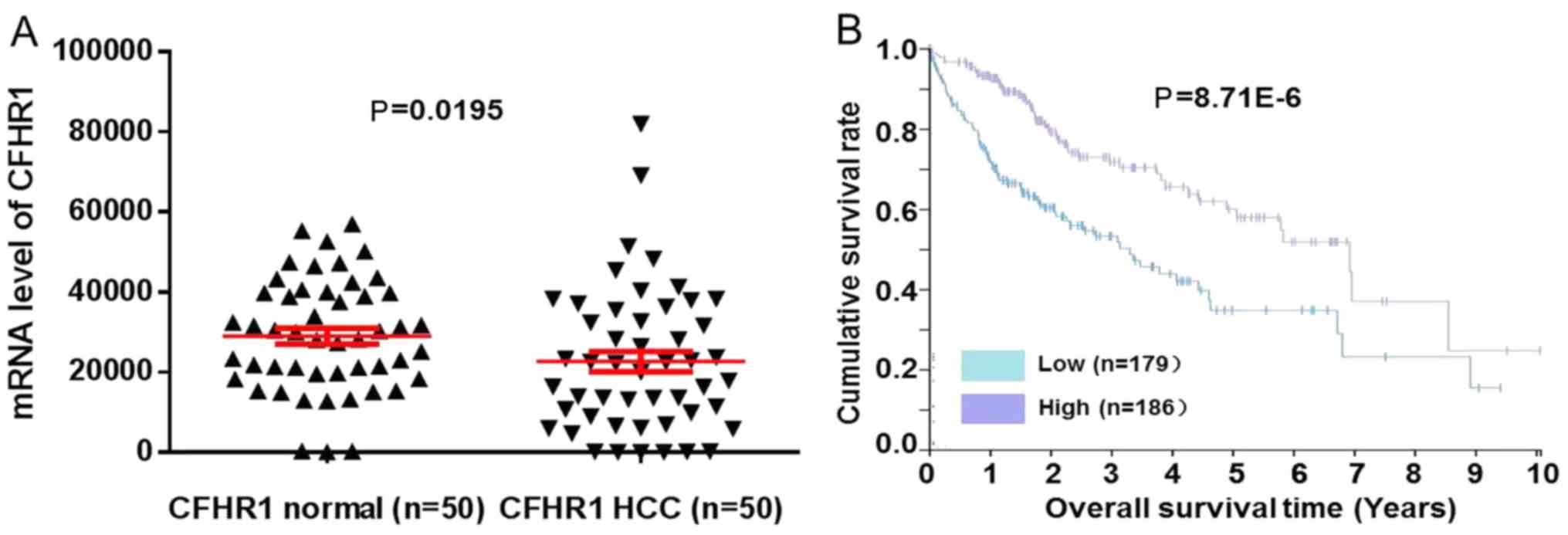
Further validation in TCGA and The
Human Protein Atlas datasets
To validate the expression of CFHL1, CFHL1 mRNA
expression data in 50 pairs of HCC and normal tissue samples were
obtained from the database of The Cancer Genome Atlas (TCGA;
cancergenome.nih.gov). Fig. 3A revealed that CFHL1 mRNA expression
levels in 50 pairs of HCC were statistically lower compared with
normal tissue samples (P=0.0195). CFHL1 mRNA expression level of
365 patients in The Human Protein Atlas dataset (www.proteinatlas.org) was used for prognosis
validation. Results revealed that low CFHL1 mRNA level was
associated with worse prognosis compared with the high-expression
group (P=8.71×10−6; Fig.
3B).
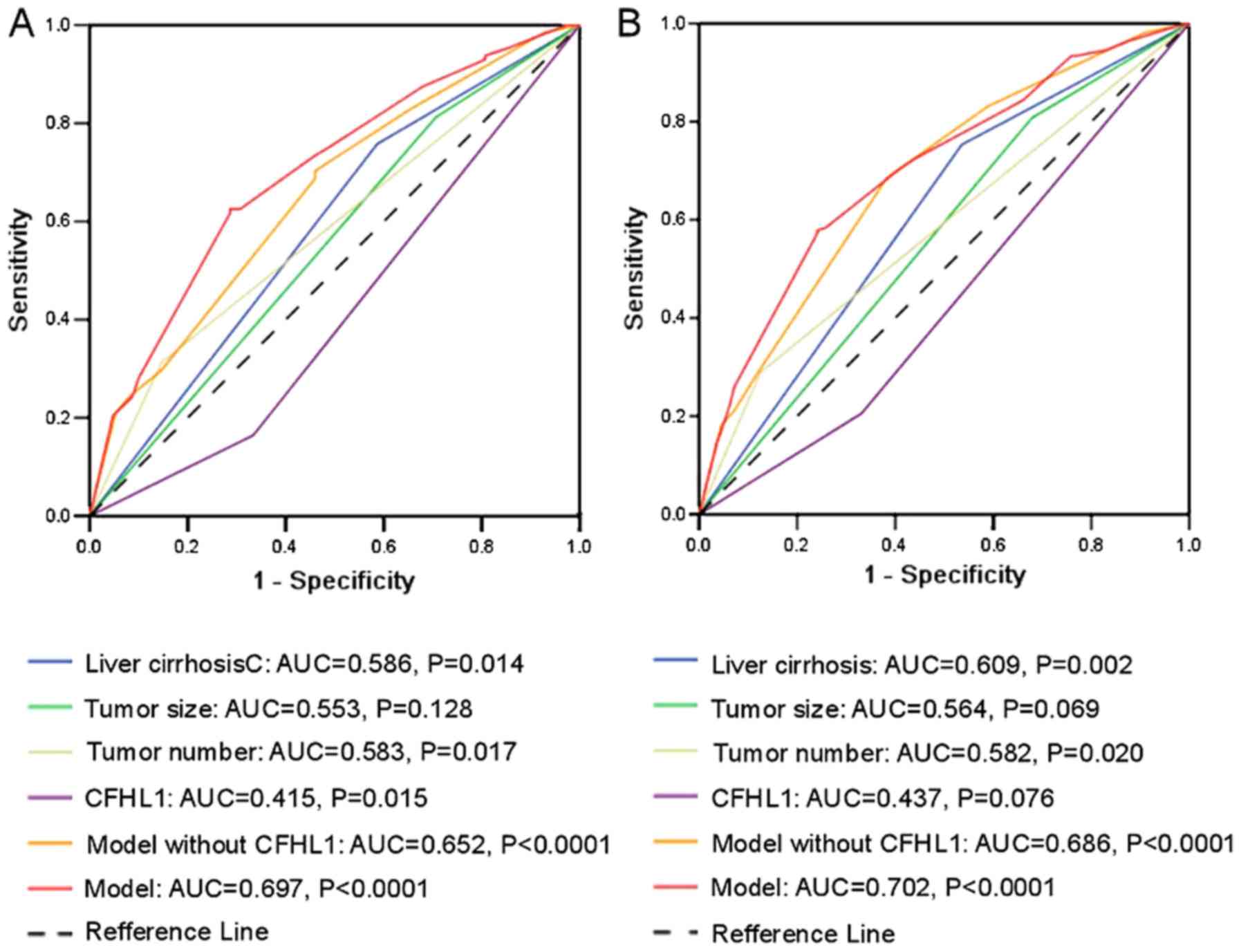
Prognostic model including tumor size,
tumor number, liver cirrhosis and CFHL1
Based on the results presented in Table III, a new prognostic model was
proposed that included liver cirrhosis, tumor size, tumor number
and CFHL1 for both OS and TTR. As presented in Fig. 4, the ROC curve analysis demonstrated
that the predictive accuracy of the new prognostic model [area
under the curve (AUC) 0.697 for OS and 0.702 for TTR] was higher
compared with that for any other factor and the prognostic model
without CFHL1 (liver cirrhosis/tumor number/tumor size
combination). The AUC values for OS were 0.553, 0.586, 0.583, 0.652
and 0.415 for tumor size, liver cirrhosis, tumor number, model
without CFHL1 and CFHL1, respectively, whereas the respective AUC
values for TTR were 0.564, 0.609, 0.582, 0.686 and 0.437.
Discussion
The present study demonstrated that CFHL1 expression
was decreased in HCC tissues, and patients with low CFHL1
expression exhibited worse OS and TTR. Furthermore, after proposing
a new prognostic model, the AUC of this new model was higher
compared with that of the liver cirrhosis/tumor size/tumor number
combination. Collectively, these data indicated that decreased
CFHL1 expression was associated with poor outcome in patients with
HCC.
To the best of our knowledge, there are no studies
in the literature on the association between CFHL1 and cancer,
including HCC. In the current study, the expression of CFHL1 was
examined in HCC and paired peritumoral tissues by western blot
analysis and immunohistochemical examination. The results revealed
that CFHL1 was downregulated in HCC tissues, with a significant
difference between HCC and peritumoral tissues, indicating that
CFHL1 may be a risk factor for HCC. In addition, based on the
Kaplan-Meier analysis, an association was observed between CFHL1
protein expression and clinical outcome in patients with HCC after
surgery. Patients with low CFHL1 expression had worse OS and
shorter TTR compared with cases with high CFHL1 expression. The
multivariate analysis indicated that CFHL1 was an independent
postoperative predictor of recurrence and OS. Furthermore, in order
to validate the prognostic value of decreased CFHL1 expression in
postoperative patients with HCC, ROC curve analysis and
bioinformatics analysis were performed. The results revealed that
the new prognostic model was superior to that without CFHL1 (liver
cirrhosis/tumor size/tumor number combination) and low CFHL1 mRNA
level had worse prognosis, indicating that CFHL1 may be a reliable
prognostic factor for postoperative patients with HCC.
To the best of our knowledge, the present study was
the first to investigate the association between CFHL1 expression
and the prognosis of postoperative patients with HCC, however,
there were several limitations. First, similar studies on the role
of CFHL1 in other types of cancer, which could expand the
prognostic value of CFHL1, are required. Second, more in
vivo and in vitro studies should be performed in the
future to elucidate why the downregulated expression of CFHL1 is
associated with unfavorable prognosis in postoperative HCC
patients. Previous studies have demonstrated an association of
CFHL1 with human neutrophil granulocytes (17) and the human immune system (18).
In conclusion, the current study was the first to
demonstrate that CFHL1 may be of high prognostic value in
postoperative patients with HCC and it may be used as a new
prognostic biomarker in such individuals. However, further studies,
particularly on the association between CFHL1 and the tumor
microenvironment, should be conducted in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the youth
projects of the general logistics department of Chinese people's
liberation army (grant no. 13QNP101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYY and YH were responsible for the study conception
and design. Experiments were performed by HF and FF. HF, FF, LY and
MX were responsible for patient follow-up. HF and FF performed data
analysis. HF and FF were responsible for the drafting of the
manuscript. XYY and YH performed the review and editing of the
manuscript. All authors have read and approved the final version of
this manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of the Eastern Hepatobiliary Surgery Hospital and all the
patients signed written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li F, Guo Z and Wang H: Influencing
elements and treatment strategies associated with the relapse of
hepatocellular carcinoma after surgery. Hepatogastroenterology.
60:1148–1155. 2013.PubMed/NCBI
|
|
2
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases, :
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Umeda S, Kanda M and Kodera Y: Emerging
evidence of molecular biomarkers in hepatocellular carcinoma.
Histol Histopathol. 33:343–355. 2018.PubMed/NCBI
|
|
4
|
Skerka C, Timmann C, Horstmann RD and
Zipfel PF: Two additional human serum proteins structurally related
to complement factor H. Evidence for a family of factor H-related
genes. J Immunol. 148:3313–3318. 1992.PubMed/NCBI
|
|
5
|
Siegel C, Hallström T, Skerka C, Eberhardt
H, Uzonyi B, Beckhaus T, Karas M, Wallich R, Stevenson B, Zipfel PF
and Kraiczy P: Complement factor H-related proteins CFHR2 and CFHR5
represent novel ligands for the infection-associated CRASP proteins
of Borrelia burgdorferi. PLoS One. 5:e135192010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinders R, Jones T, Root R, Bruce C,
Murchison H, Corey M, Williams L, Enfield D and Hass GM: Complement
factor H or a related protein is a marker for transitional cell
cancer of the bladder. Clin Cancer Res. 4:2511–2520.
1998.PubMed/NCBI
|
|
7
|
Heicappell R, Wettig IC, Schostak M,
Müller M, Steiner U, Sauter T and Miller K: Quantitative detection
of human complement factor H-related protein in transitional cell
carcinoma of the urinary bladder. Eur Urol. 35:81–87. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malkowicz SB: The application of human
complement factor H-related protein (BTA TRAK) in monitoring
patients with bladder cancer. Urol Clin North Am. 2763–73.
(ix)2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang JD, Seol SY, Leem SH, Kim YH, Sun Z,
Lee JS, Thorgeirsson SS, Chu IS, Roberts LR and Kang KJ: Genes
associated with recurrence of hepatocellular carcinoma: Integrated
analysis by gene expression and methylation profiling. J Korean Med
Sci. 26:1428–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin GZ, Yu WL, Dong H, Zhou WP, Gu YJ, Yu
H, Yu H, Lu XY, Xian ZH, Liu YK, et al: SUOX is a promising
diagnostic and prognostic biomarker for hepatocellular carcinoma. J
Hepatol. 59:510–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan N, Liu Q, Liu X, Gong Z, Zeng Y, Pan
G, Xu Q and He S: Low expression of B-cell-associated protein 31 in
human primary hepatocellular carcinoma correlates with poor
prognosis. Histopathology. 68:221–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin GZ, Li Y, Cong WM, Yu H, Dong H, Shu
H, Liu XH, Yan GQ, Zhang L, Zhang Y, et al: iTRAQ-2DLC-ESI-MS/MS
based identification of a new set of immunohistochemical biomarkers
for classification of dysplastic nodules and small hepatocellular
carcinoma. J Proteome Res. 10:3418–3428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin H, Wang C, Jin G, Ruan H, Gu D, Wei L,
Wang H, Wang N, Arunachalam E, Zhang Y, et al: Regulator of
calcineurin 1 gene isoform 4, down-regulated in hepatocellular
carcinoma, prevents proliferation, migration, and invasive activity
of cancer cells and metastasis of orthotopic tumors by inhibiting
nuclear translocation of NFAT1. Gastroenterology. 153:799–811.e33.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Losse J, Zipfel PF and Józsi M: Factor H
and factor H-related protein 1 bind to human neutrophils via
complement receptor 3, mediate attachment to Candida albicans, and
enhance neutrophil antimicrobial activity. J Immunol. 184:912–921.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel C, Schreiber J, Haupt K, Skerka C,
Brade V, Simon MM, Stevenson B, Wallich R, Zipfel PF and Kraiczy P:
Deciphering the ligand-binding sites in the Borrelia
burgdorferi complement regulator-acquiring surface protein 2
required for interactions with the human immune regulators factor H
and factor H-like protein 1. J Biol Chem. 283:34855–34863. 2008.
View Article : Google Scholar : PubMed/NCBI
|