Introduction
Air pollution has become one of the tremendous
problems of developing cities in China. Less than 13% of China's
cities meet the national ambient air quality standards (1). Air pollution has been listed as one of
the environmental risk factors that pose a threat to human health.
WHO estimates that air pollution causes 37,000 premature
deaths/year, mainly related to fine particulate matter (PM2.5)
exposure (2).
The frequent occurrence of foggy haze is due to the
excessive production of suspended particles in the atmosphere:
PM2.5 of diameter <2.5 µm, containing polycyclic aromatic
hydrocarbons, inorganic substances, especially transition metals,
and acidic oxides (3,4). The smaller the particle size, the more
severe the damage. PM2.5 levels affect health more than large
particulate matter because PM2.5 can penetrate into the lungs,
alveoli and lung sacs and can be dissolved into the bloodstream
(5). PM2.5 has strong toxicity to
cardiovascular and cerebral vascular (6), lung cancer (7,8) and
nervous system (9). Duan et
al established a PM2.5 exposure model for zebrafish and found
that PM2.5 predominantly affects organs and causes multiple organ
toxicological injuries, including cardiovascular injuries,
hepatotoxicity and neurotoxicity (10).
Thymidylate synthase (TS) is a key rate-limiting
enzyme in the folate metabolism which catalyzes the conversion of
deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate
(dTMP). This conversion essentially influences DNA repair,
methylation and synthesis through the production of nucleotides
(11). It also functions by
catalyzing the methylation of deoxyuridylate to deoxythymidylate
with a cofactor 5,10-methylenetetrahydrofolate (methylene THF).
This function is essential for the dTMP (thymidine-5-prime
monophosphate) pool. It affects DNA replication and repair and thus
has been considered to be a target for cancer chemotherapeutic
agents, such as 5-fluorouracil, 5-fluoro-2-prime-deoxyuridine and
some folate analogs (3,4).
Mammalian target of rapamycin (mTOR) is an
evolutionarily conserved serine, threonine protein kinase
discovered in recent years and is also an important signal
transduction molecule that can be integrated to energy, hormones,
amino acids, cellular oxidative stress and other signals. mTOR
signaling pathway is involved in many biological processes, such as
cell growth, proliferation, apoptosis and autophagy (12,13).
P70S6K1 acts as a downstream substrate of mTOR protein. p-mTOR is
activated, which regulates the activation of P70S6K1 in cells,
thereby controlling cell growth from G1 to S phase (14,15).
This study found that PM2.5-induced cell cycle
arrest might be due to the downregulation of mTOR/P70S6K1 signaling
pathway, and thus inhibits the expression of TS protein.
Materials and methods
Collection and preparation of
PM2.5
Sampling sites comprised the streets of Shenyang and
samples were collected over 48 h. Filters were cut into 2 cm × 2 cm
size and placed into beakers containing a certain amount of saline.
Ultrasonic shock was performed 3 times, 10 min each. The filtrates
were centrifuged at 1,000 × g, 4°C, for 5 min and the supernatants
were discarded to collect the underlying particles. Then, the
particles were freeze-dried in vacuum and preserved at −80°C
(16–18). Ma et al have carried out a
composition analysis and have revealed that toxic heavy metals and
polycyclic aromatic hydrocarbon substances are the main elements of
PM2.5 (19).
Animal model
Forty SPF Wistar rats, aged 6–8 weeks with a body
weight of 150±10 g, were selected. All rats were males, purchased
from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[production license no. SCXK (Beijing) 2012–0001; Beijing, China].
The rats were housed at a temperature of 20–22°C, relative humidity
of 50–60%, and 12/12 h light/dark cycle. During the experiment, the
rats were allowed to eat and drink water freely. The rats were
randomly divided into 4 groups: PM2.5 exposure group and saline
control group, at 2 weeks and 4 weeks, respectively, with 10 rats
in each group. Using a mouth-nose exposure system (Beijing
Huironghe Technology Co., Ltd., Beijing, China), the experimental
group animals were exposed to concentrated PM2.5 at 10 ambient
concentrations of 750 µg/cm3 for 4 h/day, 5 days/week
for a total of 4 weeks, and the control group animals were treated
with physiological saline under similar conditions. The study was
approved by the Ethics Committee of Shenyang Medical College
(Shenyang, China).
Cell culture and PM2.5 treatment
H1299 and H292 cells, obtained from the American
Type Culture Collection (cat. nos. CRL-5803 and CRL-1848; ATCC,
Manassas, VA, USA), were seeded in RPMI-1640 medium (10% fetal
bovine serum, penicillin, streptomycin 100 kU/l) and subcultured at
37°C, 5% CO2 at 3 days fluid was changed and passaged
once, using 0.25% trypsin-EDTA digestion. After 2–3 passages, the
cells in logarithmic growth phase were equally divided into 4
groups and inoculated into complete RPMI-1640 medium. After 24 h,
the cells were treated with different concentrations of PM2.5
suspension (0, 50, 100 and 200 µg/ml) for 48 h. Cells were defined
as PM2.5 suspension-treated groups and control group, with an equal
amount of complete RPMI-1640 medium.
Western blotting
Total protein was extracted with Total Protein
Extraction kit (Nanjing Kaiji Biotechnology Development Co., Ltd.,
Nanjing, China) from the lung tissues of rats and the H1299 and
H292 cells, and the protein concentration was determined by the BCA
method. A total of 20 µg protein/lane were separated via 8%
SDS-PAGE and were subsequently transferred onto a PVDF transfer
membrane and blocked with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 20°C for 1 h. The membranes
were incubated with a primary rabbit anti-human thymidylate
synthase, cyclin D1, p-mTOR, p70, β-actin monoclonal antibodies
(dilution, 1:1,000; cat. nos. 5449, 2978, 5536, 2708, and 8457)
overnight at 4°C. Then, the membranes were incubated with secondary
goat anti-rabbit IgG polyclonal antibody (dilution, 1:1,000; cat.
no. 7074) at room temperature for 1 h. Both primary and secondary
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). ECL chemiluminescence (SuperSignal Western Pico
Chemiluminescent Substrate; Pierce Biotechnology, Inc.; Thermo
Fisher Scientific, Inc., Dallas, TX, USA) was used to determine the
changes in the expression of various factors in the cell lysates.
ImageJ software (version 1.48u; National Institutes of Health,
Bethesda, MD, USA) was used for densitometry.
RT-qPCR analysis
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) was used to extract total RNA
of lung tissue and H1299 and H292 cells. RT kit was purchased from
Qiagen GmbH (Hilden, Germany). After the synthesis of the first
strand cDNA, qPCR was performed using SYBR-Green and GAPDH as the
internal control. The reaction system (20 µl) contained 10 µl of 1X
SYBR-Green Supermix reagent (Promega Corp., Madison, WI, USA), 0.8
µl of upper and lower primers (Sangon Biotech Co., Ltd., Shanghai,
China), 2 µl of cDNA (10 ng), and 6.4 µl of ddH2O. qPCR
amplification parameters were: annealing at 25°C for 5 min,
extension at 42°C for 60 min; inactivation at 70°C for 15 min,
after extension of the detection of fluorescence signals, a total
of 40 cycles. Three replicate wells were set up for each sample,
and the relative expression of mRNA was calculated according to the
2−ΔΔCq method (20).
Primer sequences are shown in Table
I.
 | Table I.Primer sequences of qPCR. |
Table I.
Primer sequences of qPCR.
| Gene | Primer sequence |
|---|
| Human-TYMS | U:
5′-TTGAGGAATTTTGCATCAATG-3′ |
|
| D:
5′-CCAAAAAGAGTCCAGGACCA-3′ |
| Human-GAPDH | U:
5′-TCTCTTAGATTTGGTCGTATT-3′ |
|
| D:
5′-CATATTGGAACATGTAAACCT-3′ |
| Rat-TYMS | U:
5′-GTGGATGAAGTAGCCGTGGT-3′ |
|
| D:
5′-GGCCATTTTACCAAAAGCAA-3′ |
| Rat-GAPDH | U:
5′-GACATGCCGCCTGGAGAAAC-3′ |
|
| D:
5′-AGCCCAGGATGCCCTTTAGT-3′ |
Transient transfection
Cells were seeded into 6-well plates without the
addition of antibiotics. After 24 h, the cells were transfected
with siRNA inhibitor (Shanghai GenePharma Co., Ltd., Shanghai,
China). Approximately 800 µl of 150 mM sterilized NaCl solution
were diluted and mixed and placed on the bench for 5 min.
Lipofectamine transfection reagent (~50 µl; Shanghai GenePharma
Co., Ltd.) was added to the 5 µg DNA dilution solution. The final
volume of the transfection solution was 1 ml and was let to stand
for 20 min. The transfection solution was added dropwise to the
cell culture medium. After 48–72 h of transfection, gene expression
was detected (21).
Flow cytometry analysis
Cells were treated with PM2.5 suspension for 48 h,
centrifuged at 13,000 × g for 3 min at 4°C and washed twice with
PBS. Cells were resuspended with PBS, and 2 volumes of 5 ml ethanol
were slowly added and mixed, stationary set was at 4°C. The cells
were washed twice with PBS, resuspended in 1 ml of PI solution and
incubated at room temperature for 30 min. The cell cycle was
detected by flow cytometry (Muse™ flow cytometer; Merck Millipore,
Burlington, MA, USA) and the data were analyzed with ModFit 2.0
software (Verity Software House, Inc., Berlin, Germany).
Statistical analysis
SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used for
the statistical analysis. Results are presented as the mean ±
standard deviation from three independent experiments. Statistical
significance was determined with the unpaired Student's t-test and
ANOVA. Dunnett's test was the post hoc test used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Exposure to PM2.5 induces cell cycle
arrest and downregulation of the expression of cyclin D1
protein
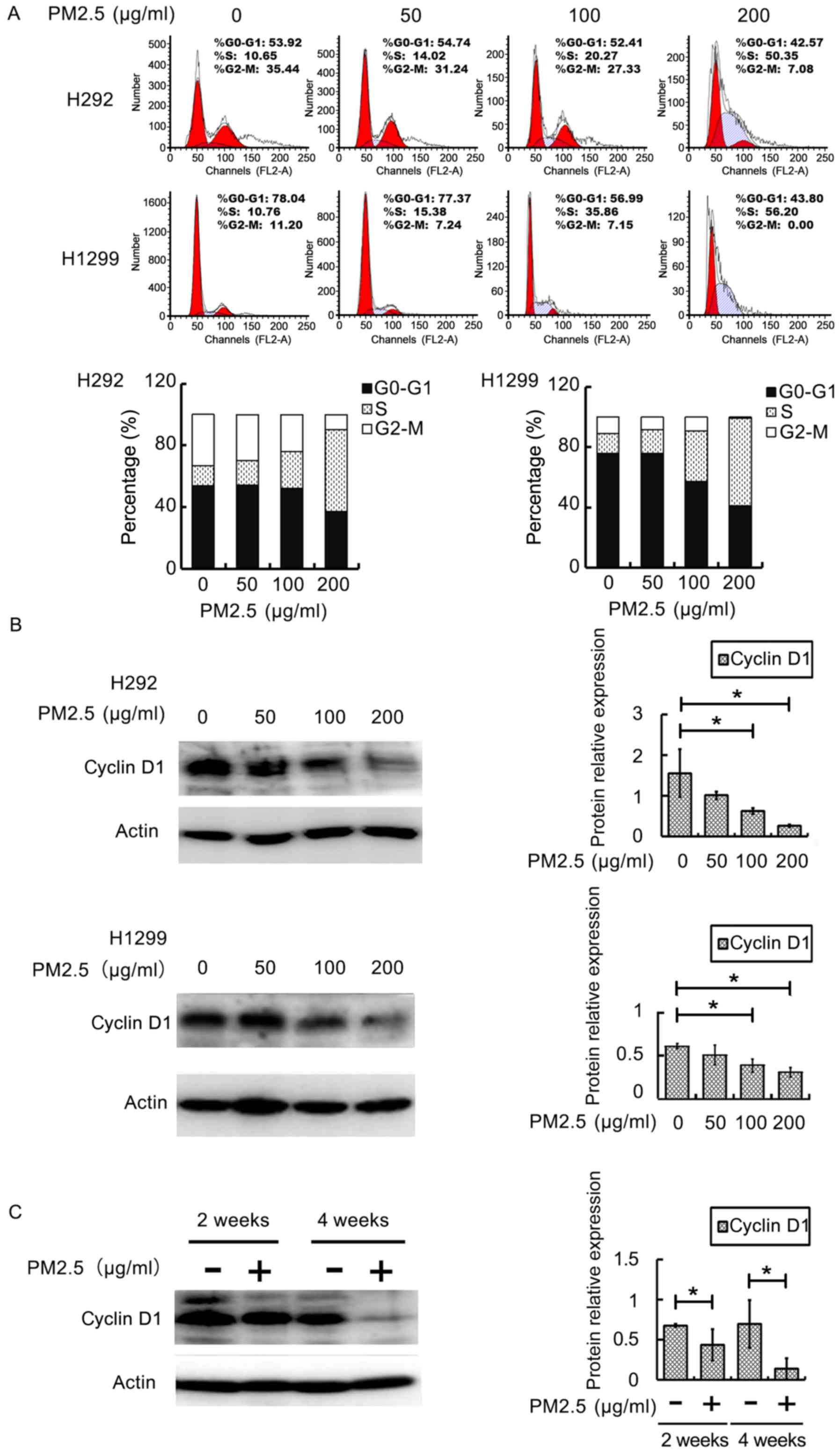
H292 and H1299 cells were exposed to PM2.5 for 48 h.
The results showed that with the increase of PM2.5 concentration,
the cell cycles of H292 and H1299 cells were mostly blocked at the
S phase. When the concentration of PM2.5 was 200 µg/ml, the
percentage of H292 and H1299 cells in S phase reached 50.35 and
56.20%, respectively (Fig. 1A).
Also, with the increase of PM2.5 concentration, the expression of
cyclin D1 at 100 and 200 µg/ml PM2.5 was downregulated, compared
with the control group (P<0.05) (Fig.
1B).
To analyze PM2.5-induced lung toxicity after injury,
an in vivo exposure method was used. It was found that
cyclin D1 protein was significantly downregulated (P<0.05) as
the exposure time increased at 2 and 4 weeks, compared with the
control group, as seen in Fig.
1C.
Conclusively, our obtained data showed that exposure
to PM2.5 for a few weeks can lead to the downregulation of the
expression of cyclin D1 protein.
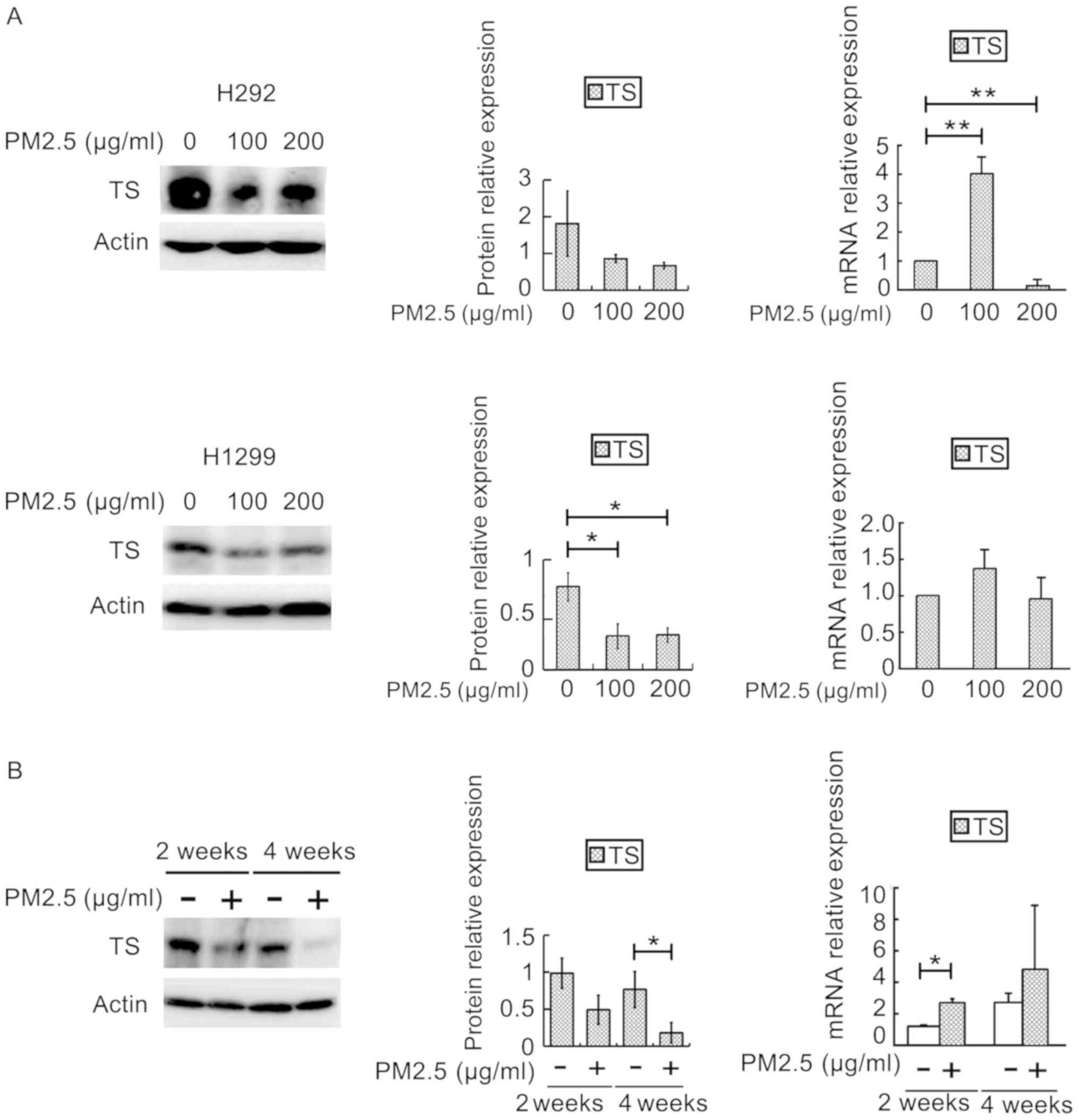
The protein expression of TS enzyme is
downregulated and the mRNA expression of TS is upregulated after
PM2.5 exposure
As the cell cycle was arrested and the expression of
cyclin D1 was downregulated, we hypothesized that DNA synthesis was
inhibited after PM2.5 exposure. Therefore, we studied the effect of
PM2.5 exposure on the protein and mRNA expression of TS, a key
enzyme in DNA synthesis. With the increase of PM2.5 concentration,
the expression of TS protein in H1299 cells was downregulated
(P<0.05), the expression of mRNA in H292 cells was more
upregulated (P<0.01) at 100 µg/ml PM2.5, and the expression of
mRNA in H1299 cells was increased or expressed similarly
(P>0.05) at 100 and 200 µg/ml PM2.5, respectively, compared with
the control group (Fig. 2A). The
protein expression of TS was downregulated at 4 weeks (P<0.05)
in vivo, and the expression of mRNA of lung tissues was
upregulated at 2 weeks (P<0.05) in PM2.5-exposed group (Fig. 2B).
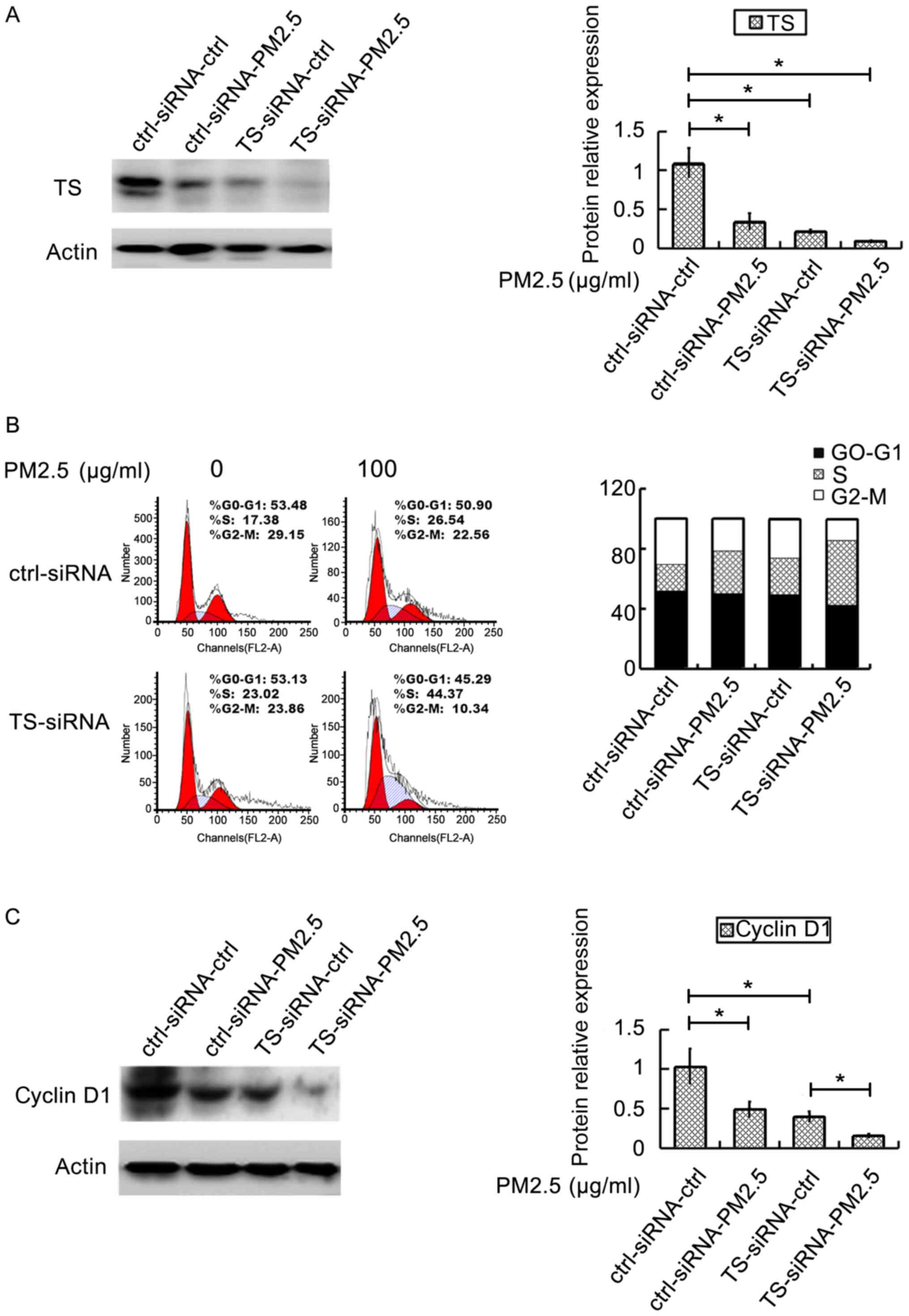
Knockout of the TS gene promotes the
cell cycle arrest and the downregulation of the expression of
cyclin D1 protein after PM2.5 exposure
H292 cells were transfected with TS for 48 h before
exposed to PM2.5. Compared with ctrl-siRNA-ctrl (control group),
the expression of TS enzyme protein in the ctrl-siRNA-PM2.5 (with
PM2.5 exposure), TS-siRNA-ctrl (with TS enzyme inhibitor) and
TS-siRNA-PM2.5 (with PM2.5 exposure and TS enzyme inhibitors)
groups was downregulated (P<0.05) (Fig. 3A). The results also showed that the
percentage of S phase cells in TS-siRNA groups was higher
(P<0.05) (Fig. 3B). Compared with
ctrl-siRNA-ctrl, the expression of cyclin D1 after PM2.5 exposure
(ctrl-siRNA-PM2.5), was downregulated (P<0.05), and the
expression of cyclin D1 in TS-siRNA-ctrl group (inhibitor of TS
enzyme protein) was downregulated (P<0.05). Compared with
TS-siRNA-ctrl, the expression of cyclin D1 protein in the
TS-siRNA-PM2.5 group, was downregulated after PM2.5 exposure
(P<0.05) (Fig. 3C).
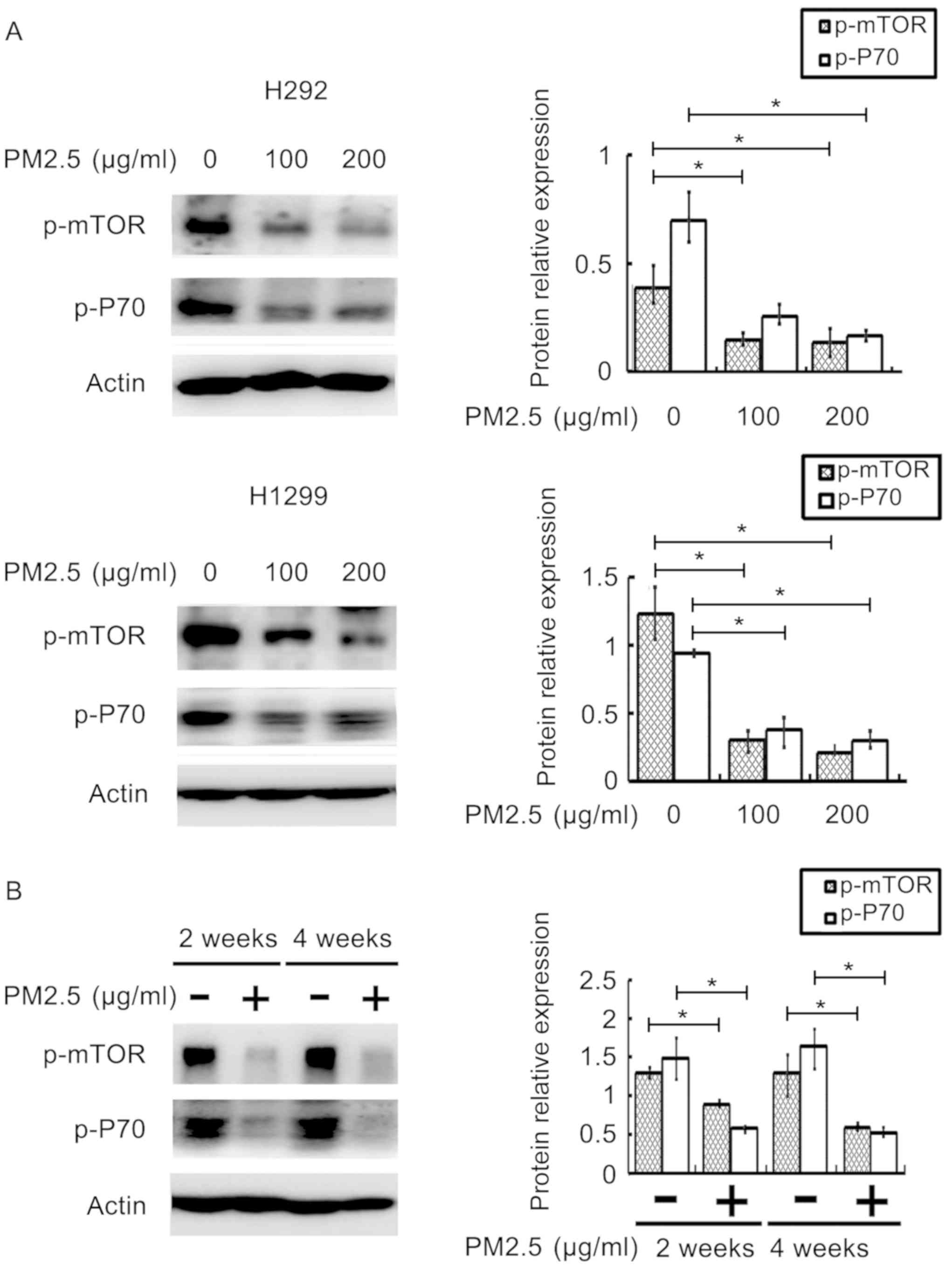
PM2.5 exposure downregulates the
expression of TS and cyclin D1 partially through the downregulation
of the mTOR/P70S6K1 signaling pathway
We have found that PM2.5 exposure induced cell cycle
arrest in H292 and H1299 cells, and both cyclin D1 and TS proteins
were downregulated after PM2.5 exposure. To investigate the
downregulation of cyclin D1 and TS enzyme after PM2.5 exposure
through the mTOR signaling pathway, we examined the expression of
p-mTOR and its downstream molecule p-P70S6K1 in both H292 and H1299
cells. The results showed that the expression levels of p-mTOR and
p-P70S6K1 were downregulated (P<0.05) in H1299 and H292 cells
with increasing PM2.5 concentration at 200 µg/ml (Fig. 4A). In vivo, the expression
levels of p-mTOR and p-P70S6K1 were significantly more
downregulated (P<0.05) in the 2- and 4-weeks PM2.5 exposure
groups compared with the 2- and 4-weeks control groups (Fig. 4B).
Discussion
Air pollution is one of the important risk factors
affecting public health. In recent years, severe hazy weather
appears in many parts of the world, and the occurrence of hazy
weather is closely related to the PM2.5 concentration of
environmental fine particles. PM2.5 has small particle size, large
specific surface area, and presents adsorption of toxic and harmful
substances, leading to respiratory and cardiovascular diseases
(22,23). In this study, H292 and H1299 cells,
as well as and Wistar rats were exposed to PM2.5 suspension to
simulate the in vivo and in vitro exposure
experiments, respectively, with saline and culture fluid exposure
used as control, in order to reduce the experimental error. The
results showed that after exposure, the expression of cyclin D1 is
downregulated and the cell cycle appears at S phase, in lung tissue
and H292 and H1299 cells. Also, this experiment demonstrated that
the expression levels of TS enzyme and cyclin D1 proteins are
inhibited by PM2.5 due to downregulation of mTOR and P70S6K1
signaling pathway.
Gualtieri et al have collected airborne
particulates during winter and summer in Milan, Italy, and have
applied them respectively to A549 cells (24). It was found that PM2.5 collected in
winter could induce the production of reactive oxygen species and
DNA damage by changing the expression of cellular genes. Billet
et al have found that volatile organic compounds and
polycyclic aromatic hydrocarbons in PM2.5 play an important role in
the toxicity of lung cancer cells (25). Some organic components carried by
PM2.5, such as PAH metabolites in human body, can bind covalently
to the amino terminal guanine ring of DNA nucleophilic sites to
form BPDE-DNA, cause DNA damage, induce gene mutation and
carcinogenesis (26,27). Cyclin is the basic process to ensure
the cells to carry out life activities. Cyclin D1 is a protein that
is closely related to cell growth, development, and tumorigenesis.
The cyclin D1 protein plays an important role in regulating the
G1/S phase transition of the cell cycle (28). In this experiment, TS enzyme was
selected as the research target, and the expression of TS protein
was found to be downregulated after exposure to PM2.5, which
resulted in the damage of DNA synthesis, thereby affecting the
expression of cyclin D1 and the cell cycle arrest.
In this study, we designed interference plasmids for
TS genes. After introducing liposome into H292 cells, we found that
the expression of TS protein is decreased, suggesting that the
expression of TS gene is disturbed by silencing. After knocking
down TS, the effects of PM2.5 were enhanced. Also, we found that
siRNA targeting silencing of TS gene could result in cell cycle
arrest at S phase in H292 cells exposed to PM2.5, and the
expression of cyclin D1 protein decreases more significantly. The
results were statistically significant (P<0.05).
It has been found that many tumors are associated
with an abnormal mTOR signaling pathway in which overexpression of
mTOR affects the translational expression of oncogenes such as
cyclin D1 (29). The phenomenon of
amplification or overexpression of downstream factor P70S6K1 in
mTOR also occurs in many tumors. The multiple phosphorylation sites
in P70S6K1 are directly regulated by mTOR (30). Phosphorylation is an activated form
of the mTOR/P70S6K1 pathway. This study found that exposure of
PM2.5 inhibits the activity of mTOR/P70S6K1 signaling pathway
leading to downregulated cyclin D1 and TS. PM2.5 occured at least
partially through the inhibition of the activity of mTOR/P70S6K1
signaling pathway.
In conclusion, in this study, we systematically
studied the toxic effects of PM2.5. The results revealed cell cycle
arrest at S phage and showed that cyclin D1 and TS enzyme protein
expressions are inhibited by the downregulation of mTOR and P70S6K1
signaling pathway. Knockdown of TS enhances the cell cycle arrest
by PM2.5 exposure that inhibits the expression of cyclin D1. TS
enzyme may be the target of PM2.5 exposure to regulate cell cycle
progression.
Acknowledgements
The authors wish to thank the following for their
support and encouragement: Professor Chunling Xiao, Professor of
Shenyang Medical College; Mrs. Dan Yang, Professor Mingyue Ma, Mr.
Yang Biao, Miss Guo Jie and Miss Li Bingyu. Thank you to Mr. Aasi's
for his support of Afghan foreign students in our College.
Funding
The study was supported by the Shenyang Medical
College Graduate Innovation Fund Project (no. Y20170610), the
Liaoning Provincial Department of Science and Technology Project
(2017225076), the Shenyang Science and Technology Bureau ‘Major
Technology R&D Project’ (18–400409), and the Liaoning Province
Natural Science Foundation (no. 20170520037).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and CX assisted with the construction of the
animal model. YZ and DY were responsible for the cell culture and
PM2.5 treatment, and were involved in the writing of the
manuscript. BY and BL performed western blotting. JG contributed to
RT-qPCR. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shenyang Medical College (Shenyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang YL and Cao F: Fine particulate
matter (PM2.5) in China at a city level. Sci Rep. 5:148842015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
WHO Regional Office for Europe, . Review
of evidence on health aspects of air pollution - REVIHAAP Project:
Technical Report. WHO Regional Office for Europe. (Copenhagen).
2013.
|
|
3
|
Wu S, Deng F, Wei H, Huang J, Wang H,
Shima M, Wang X, Qin Y, Zheng C, Hao Y, et al: Chemical
constituents of ambient particulate air pollution and biomarkers of
inflammation, coagulation and homocysteine in healthy adults: A
prospective panel study. Part Fibre Toxicol. 9:492012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahman I, Biswas SK and Kode A: Oxidant
and antioxidant balance in the airways and airway diseases. Eur J
Pharmacol. 533:222–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franklin BA, Brook R and Arden Pope C III:
Air pollution and cardiovascular disease. Curr Probl Cardiol.
40:207–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogino K, Zhang R, Takahashi H, Takemoto K,
Kubo M, Murakami I, Wang DH and Fujikura Y: Allergic airway
inflammation by nasal inoculation of particulate matter (PM2.5) in
NC/Nga mice. PLoS One. 9:e927102014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai L, Zanobetti A, Koutrakis P and
Schwartz JD: Associations of fine particulate matter species with
mortality in the United States: A multicity time-series analysis.
Environ Health Perspect. 122:837–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu J, Jiang D, Lin G, Liu K and Wang Q: An
ecological analysis of PM2.5 concentrations and lung cancer
mortality rates in China. BMJ Open. 5:e0094522015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng L, Lau WKW, Fung TKH, Lau BWM, Chau
BKH, Liang Y, Wang Z, So KF, Wang T, Chan CCH, et al: PM2.5
exposure suppresses dendritic maturation in subgranular zone in
aged rats. Neurotox Res. 32:50–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan J, Hu H, Zhang Y, Feng L, Shi Y,
Miller MR and Sun Z: Multi-organ toxicity induced by fine
particulate matter PM2.5 in zebrafish (Danio rerio) model.
Chemosphere. 180:24–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henríquez-Hernández LA, Murias-Rosales A,
Hernández- González A, Cabrera De León A, Díaz-Chico BN, Mori De
Santiago M and Fernández Pérez L: Gene polymorphisms in TYMS,
MTHFR, p53 and MDR1 as risk factors for breast cancer: A
case-control study. Oncol Rep. 22:1425–1433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N, Sui J, Liu H, Zhong M, Zhang M, Wang
Y and Hao F: Expression of phosphorylated Akt/mTOR and clinical
significance in human ameloblastoma. Int J Clin Exp Med.
8:5236–5244. 2015.PubMed/NCBI
|
|
14
|
Peponi E, Drakos E, Reyes G, Leventaki V,
Rassidakis GZ and Medeiros LJ: Activation of mammalian target of
rapamycin signaling promotes cell cycle progression and protects
cells from apoptosis in mantle cell lymphoma. Am J Pathol.
169:2171–2180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X and Proud CG: The mTOR pathway in
the control of protein synthesis. Physiology (Bethesda).
21:362–369. 2006.PubMed/NCBI
|
|
16
|
Yang B, Li X, Chen D and Xiao C: Effects
of fine air particulates on gene expression in non-small-cell lung
cancer. Adv Med Sci. 62:295–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Yang B, Xu J, Chen DM and Xiao CL:
PM2.5-induced alterations of cell cycle associated gene expression
in lung cancer cells and rat lung tissues. Environ Toxicol
Pharmacol. 52:77–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao C, Li S, Zhou W, Shang D, Zhao S, Zhu
X, Chen K and Wang R: The effect of air pollutants on the
microecology of the respiratory tract of rats. Environ Toxicol
Pharmacol. 36:588–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma M, Li S, Jin H, Zhang Y, Xu J, Chen D,
Kuimin C, Yuan Z and Xiao C: Characteristics and oxidative stress
on rats and traffic policemen of ambient fine particulate matter
from Shenyang. Sci Total Environ. 526:110–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li R: Transient transfection of CHO cells
using linear polyethylenimine is a simple and effective means of
producing rainbow trout recombinant IFN-γ protein. Cytotechnology.
67:987–993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kroll A, Gietl JK, Wiesmüller GA, Günsel
A, Wohlleben W, Schnekenburger J and Klemm O: In vitro toxicology
of ambient particulate matter: Correlation of cellular effects with
particle size and components. Environ Toxicol. 28:76–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samoli E, Stafoggia M, Rodopoulou S, Ostro
B, Declercq C, Alessandrini E, Díaz J, Karanasiou A, Kelessis AG,
Le Tertre A, et al MED PARTICLES Study Group, : Associations
between fine and coarse particles and mortality in Mediterranean
cities: Results from the MED-PARTICLES project. Environ Health
Perspect. 121:932–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gualtieri M, Longhin E, Mattioli M,
Mantecca P, Tinaglia V, Mangano E, Proverbio MC, Bestetti G,
Camatini M and Battaglia C: Gene expression profiling of A549 cells
exposed to Milan PM2.5. Toxicol Lett. 209:136–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Billet S, Garçon G, Dagher Z, Verdin A,
Ledoux F, Cazier F, Courcot D, Aboukais A and Shirali P: Ambient
particulate matter (PM2.5): Physicochemical characterization and
metabolic activation of the organic fraction in human lung
epithelial cells (A549). Environ Res. 105:212–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Topinka J, Rossner P Jr, Milcova A,
Schmuczerova J, Svecova V and Sram RJ: DNA adducts and oxidative
DNA damage induced by organic extracts from PM2.5 in an acellular
assay. Toxicol Lett. 202:186–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vodicka P, Koskinen M, Arand M, Oesch F
and Hemminki K: Spectrum of styrene-induced DNA adducts: The
relationship to other biomarkers and prospects in human
biomonitoring. Mutat Res. 511:239–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shirali S, Aghaei M, Shabani M, Fathi M,
Sohrabi M and Moeinifard M: Adenosine induces cell cycle arrest and
apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian
cancer cell line OVCAR-3. Tumour Biol. 34:1085–1095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gera JF, Mellinghoff IK, Shi Y, Rettig MB,
Tran C, Hsu JH, Sawyers CL and Lichtenstein AK: AKT activity
determines sensitivity to mammalian target of rapamycin (mTOR)
inhibitors by regulating cyclin D1 and c-myc expression. J Biol
Chem. 279:2737–2746. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bjornsti MA and Houghton PJ: The TOR
pathway: A target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|