Introduction
Breast cancer is the most commonly diagnosed cancer
and the leading cause of cancer-associated mortality among women
worldwide (1,2). The prognosis of patients with advanced
breast cancer is poor despite having a number of treatment options
available, which include surgical resection, chemotherapy and
radiotherapy (1,2). Therefore, an understanding of the
regulatory mechanisms underlying breast cancer progression is
needed for the development of novel and effective treatment
strategies.
MicroRNAs (miRNAs) are small non-coding RNAs 18–25
nucleotides in length (3,4). miRNAs can function as regulators of
gene expression by pairing with complementary binding sites in the
3′-untranslated region (UTR) of target mRNAs, resulting in mRNA
degradation or inhibition of protein translation (3,4).
Recently, several miRNAs were revealed to be involved in the
development and progression of breast cancer, these include
miRNA-148 (5), miRNA-181 (6) and miRNA-200 (7). The role of miRNA-153 (miR-153) in
breast cancer has been investigated in several studies (8–11). Wu
et al (8) demonstrated that
miR-153 induced apoptosis in breast cancer cells by inhibiting the
expression of HECT domain E3 ubiquitin ligase 3. In addition, Li
et al (9) revealed that
miR-153 demonstrated suppressive effects on epithelial-mesenchymal
transition (EMT) in human breast cancer cells by inhibiting the
expression of metadherin. Furthermore, miR-153 was demonstrated to
suppress the expression of the oncogene BRCA1 in breast cancer MCF7
cells (10). Together, these results
suggest that miR-153 may serve a tumor suppressive role in breast
cancer. However, Anaya et al (11) demonstrated that miR-153 knockdown
induced apoptosis in MDA-MB-231 breast cancer cells. In addition,
Wang et al (12) revealed
that miR-153 could decrease apoptosis and increase colony formation
in breast epithelial cells, and following treatment with E2,
miR-153 was upregulated in human breast cell lines. Therefore, the
exact role of miR-153 in breast cancer growth and metastasis, as
well as the underlying molecular mechanism of miR-153 in breast
cancer should be further investigated.
Runt-related transcription factor 2 (RUNX2) is an
important member of the RUNX family of transcription factors
(13–15). It acts as a scaffold for nucleic
acids and regulatory factors involved in osteoblastic
differentiation and skeletal morphogenesis (13–15). It
was recently revealed that RUNX2 can promote breast cancer cell
survival under metabolic stress, as well as bone metastases
(16,17). Furthermore, the targeting of RUNX2 by
miR-135 and miR-203 impairs breast cancer progression and distant
metastasis (18). However, whether
other miRNAs regulate RUNX2 expression in breast cancer remains
unclear.
The present study aimed to investigate the
underlying molecular mechanism of miR-153 and RUNX2 in breast
cancer growth and metastasis.
Materials and methods
Sample collection
The present study analyzed tissue samples obtained
from 67 patients (age range, 31–69 years; mean age, 52.5 years)
diagnosed with breast cancer in the Second Xiangya Hospital of
Central South University (Changsha, China) from September 2010 and
March 2012. Primary breast cancer tissue and adjacent healthy
tissue were collected and stored at −80°C until further use,
following histopathological evaluation. The follow-up period was 5
years. The current study was conducted with the approval from the
Ethics Committee at Second Xiangya Hospital of Central South
University (Changsha, China). Written informed consent was obtained
from all patients.
Cell culture and transfection
Human breast cancer cell lines BT-549, MCF-7,
MDA-MB-453, MDA-MB-231 and SK-BR-3, and a normal human breast
epithelial cell line MCF-10A were purchased from Shanghai Institute
of Biochemistry and Cell Biology (SIBCB; Shanghai, China). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc.) and maintained at 37°C in a 5%
CO2-humidified incubator. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for cell
transfection according to the manufacturer's protocol. SK-BR-3 and
BT-549 cells were transfected with miR-NC (100 nM; 4464058; Thermo
Fisher Scientific, Inc.), miR-153 mimic (100 nM; 4464066; Thermo
Fisher Scientific, Inc.), NC inhibitor (100 nM; 4464076; Thermo
Fisher Scientific, Inc.) or miR-153 inhibitor (100 nM; 4464084;
Thermo Fisher Scientific, Inc.), or co-transfected with miR-153
mimic and empty pc-DNA3.1 (blank) vector or miR-153 mimic and
pc-DNA3.1-RUNX2 plasmid (100 nM; Yearthbio, Changsha, China),
respectively. Cells were used for subsequent experimentation 48 h
post-transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues or cell lines
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Total
RNA (1 µg) was reverse transcribed into cDNA using the miScript
Reverse Transcription kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. qPCR was subsequently
performed on an ABI 7500 PCR machine (Thermo Fisher Scientific,
Inc.) using the miScript SYBR Green PCR kit (Qiagen, Inc.),
according to the manufacturer's protocol. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 5 min; 40 cycles of 95°C for 10 sec, 60°C
for 30 sec and 72°C for 15 sec. The mRNA levels were quantified
using the 2−∆∆Cq method (19). The primers used were as follows:
miR-153, forward 5′-TTGCATAGTCACAAAAGTGAT-3′ and reverse
5′-CAGTGCGTGTCGTGGAGT-3′; U6, forward 5′-CTCGCTTCGGCAGCACATATACT-3′
and reverse 5′-ACGCTTCACGAATTTGCGTGTC-3′; RUNX2, forward
5′-TGGTTACTGTCATGGCGGGTA-3′ and reverse
5′-TCTCAGATCGTTGAACCTTGCTA-3′; and GAPDH, forward
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Western blot analysis
Total protein was extracted from tissues or cells
using cold radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.). Total protein was quantified using a
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.) and
50 µg protein/lane was separated via SDS-PAGE on a 12% gel. The
separated proteins were transferred onto polyvinylidene difluoride
membranes and blocked with 5% non-fat milk in Tris-buffered saline
with Tween® 20 (Thermo Fisher Scientific, Inc.) for 3 h
at room temperature. Membranes were washed with PBS for 10 min,
followed by incubation with rabbit anti-human E-cadherin primary
antibody (1:200; ab15148; Abcam, Cambridge, MA, USA), rabbit
anti-human N-cadherin primary antibody (1:200; ab18203; Abcam),
rabbit anti-human vimentin primary antibody (1:100; ab8978; Abcam)
or rabbit anti-human GAPDH primary antibody (1:200; ab9485; Abcam)
for 3 h at room temperature. Following primary incubation,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:5,000; ab6721; Abcam) for 1
h at room temperature. Protein bands were visualized using an ECL
Western Blotting kit (Thermo Fisher Scientific, Inc.), and protein
expression was quantified using Image-Pro Plus software (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Cell proliferation assay
Cell Counting kit-8 (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) was used to study cell proliferation.
SK-BR-3 and BT-549 cells were seeded into 96-well plates at a
density of 5×103 cells/well and cultured for 0, 24, 48
and 72 h. The absorbance was measured at a wavelength of 450 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Wound healing assay
Transfected SK-BR-3 and BT-549 cells were cultured
until confluence reached >90%. Cell layers were scratched with a
plastic scriber (~1 mm width) and cells were washed twice with PBS,
and incubated at 37°C for 24 h. The wound was observed and images
were captured at 0 and 24 h using an inverted microscope
(magnification, ×40; Nikon Corporation, Tokyo, Japan).
Cell invasion assay
The cell suspension of transfected SK-BR-3 or BT-549
cells (50,000 cells) in DMEM was added to the upper chamber of 8-mM
Transwell inserts which was pre-coated with Matrigel®
(BD Biosciences, Franklin Lakes, NJ, USA), and DMEM supplemented
with 10% FBS was added to the lower chamber. After incubation for
24 h at 37°C, SK-BR-3 and BT-549 transfected cells remaining in the
upper chamber of the inserts were removed using a cotton-tipped
swab. SK-BR-3 and BT-549 transfected cells that had migrated to the
lower chamber were stained with gentian violet (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at room temperature for 5 min, and
the number of migrated cells was calculated using a light inverted
microscope (magnification, ×200).
Bioinformatics analysis and luciferase
reporter gene assay
TargetScan 7.2 software (www.targetscan.org) was used to predict the putative
target genes of miR-153. The psi-CHECK2 luciferase reporter
plasmids containing the wild-type (WT) 3′UTR or the mutant type
(MT) 3′UTR of RUNX2 were obtained from Yearthbio. SK-BR-3 and
BT-549 cells were co-transfected with miR-153 mimic (or miR-NC) and
WT-RUNX2-3′UTR plasmid (or MT-RUNX2-3′UTR plasmid) using
Lipofectamine® 2000, according to the manufacturer's
protocol. Following incubation for 48 h, cells were collected and
the luciferase activity was detected using a Dual Luciferase
Reporter Assay system (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. The method of
normalization was comparison with Renilla luciferase activity.
Statistical analysis
Data are presented as the mean ± standard error. All
statistical analyses were performed using SPSS software (version
20.0; IBM Corp., Armonk, NY, USA). Student's t-test was performed
for two-group comparisons. One-way analysis of variance followed by
Tukey's post hoc test was performed for multiple-group comparisons.
Chi-square test was conducted to analyze the association between
gene expression and the clinical characteristics of patients with
breast cancer. The Kaplan-Meier method and the log-rank rest were
used for survival analysis. The Pearson correlation test was used
to measure the association between miR-153 and RUNX2 expression in
breast cancer tissue samples. P<0.05 was considered to indicate
a statistically significant difference.
Results
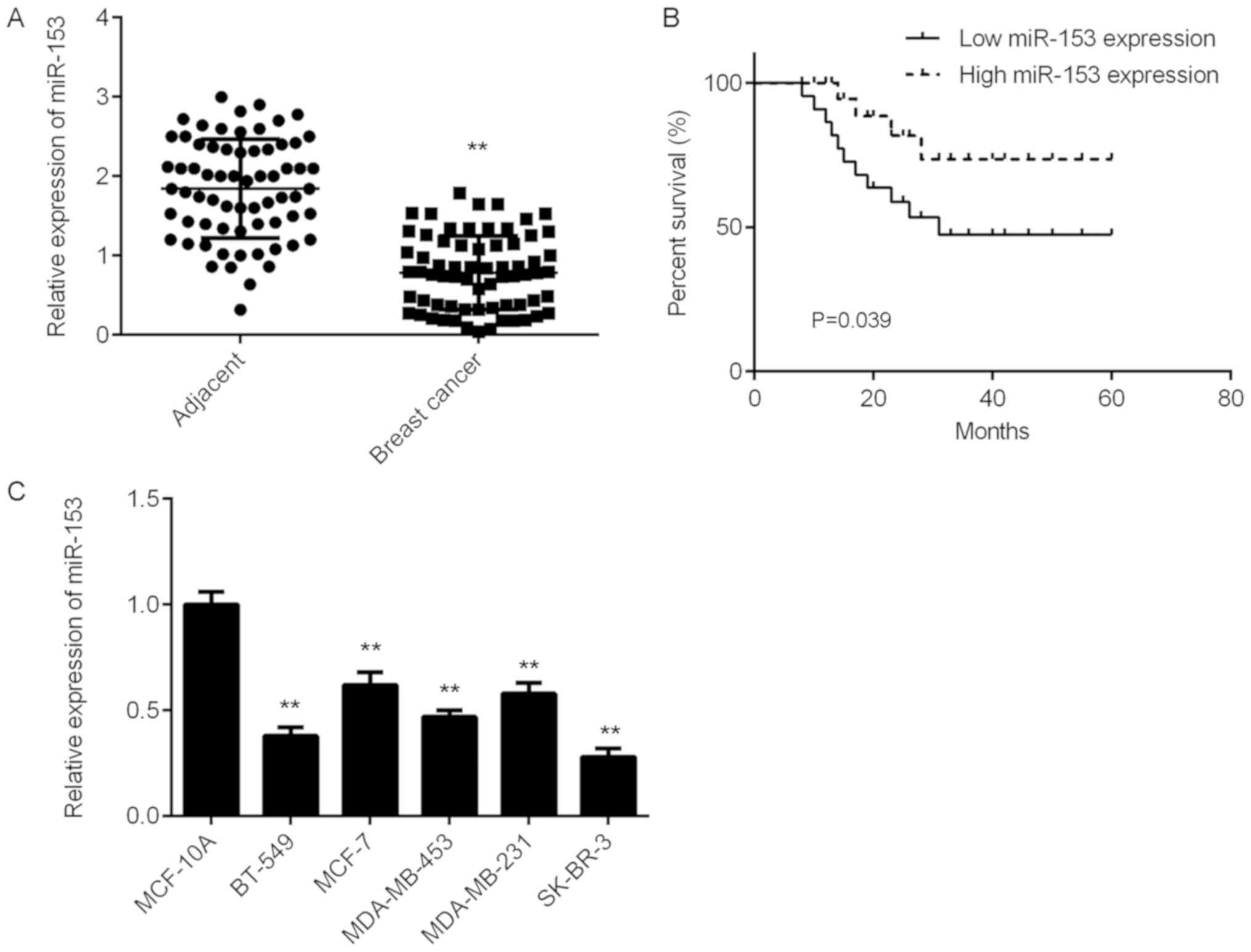
miR-153 is downregulated in breast
cancer
The expression level of miR-153 was significantly
decreased in breast cancer tissue samples compared with matched
adjacent healthy tissue samples from patients with breast cancer
(Fig. 1A). The clinical significance
of miR-153 expression in breast cancer was examined. According to
the mean miR-153 expression value (0.784), patients with breast
cancer were divided into two groups: high miR-153 expression and
low miR-153 expression. The low miR-153 expression group was
associated with advanced clinical staging as well as metastasis in
patients with breast cancer, however no association with age,
subtype, or differentiation was identified (Table I). Patients with breast cancer with
low miR-153 expression demonstrated a poor prognosis (Fig. 1B). In addition, the mRNA expression
levels of miR-153 significantly decreased in several breast cancer
cell lines compared with normal human breast cell line MCF-10A
(Fig. 1C).
 | Table I.Association between miR-153 expression
and clinicopathological characteristics of patients with breast
cancer. |
Table I.
Association between miR-153 expression
and clinicopathological characteristics of patients with breast
cancer.
| Variables | No. of patients
(n=67) | Low expression
(n=34) | High expression
(n=33) | P-value |
|---|
| Age, years |
|
|
| 1.00 |
| ≤50 | 30 | 18 | 12 |
|
|
>50 | 37 | 16 | 11 |
|
| Subtype |
|
|
| 0.814 |
| Lunimal A
type | 33 | 15 | 18 |
|
| Lunimal B
type | 8 | 4 | 4 |
|
| HER2
positive | 11 | 6 | 5 |
|
| TNBC | 15 | 9 | 6 |
|
| Differentiation |
|
|
| 0.136 |
| Well and
moderately | 40 | 17 | 23 |
|
| Poor | 27 | 17 | 10 |
|
| Lymph node
metastasis |
|
|
| 0.039 |
|
Present | 45 | 27 | 18 |
|
|
Absent | 22 | 7 | 15 |
|
| Distant
metastasis |
|
|
| 0.011 |
|
Present | 7 | 7 | 0 |
|
|
Absent | 60 | 27 | 33 |
|
| TNM stage |
|
|
| 0.010 |
|
I–II | 44 | 17 | 27 |
|
|
III–IV | 23 | 17 | 6 |
|
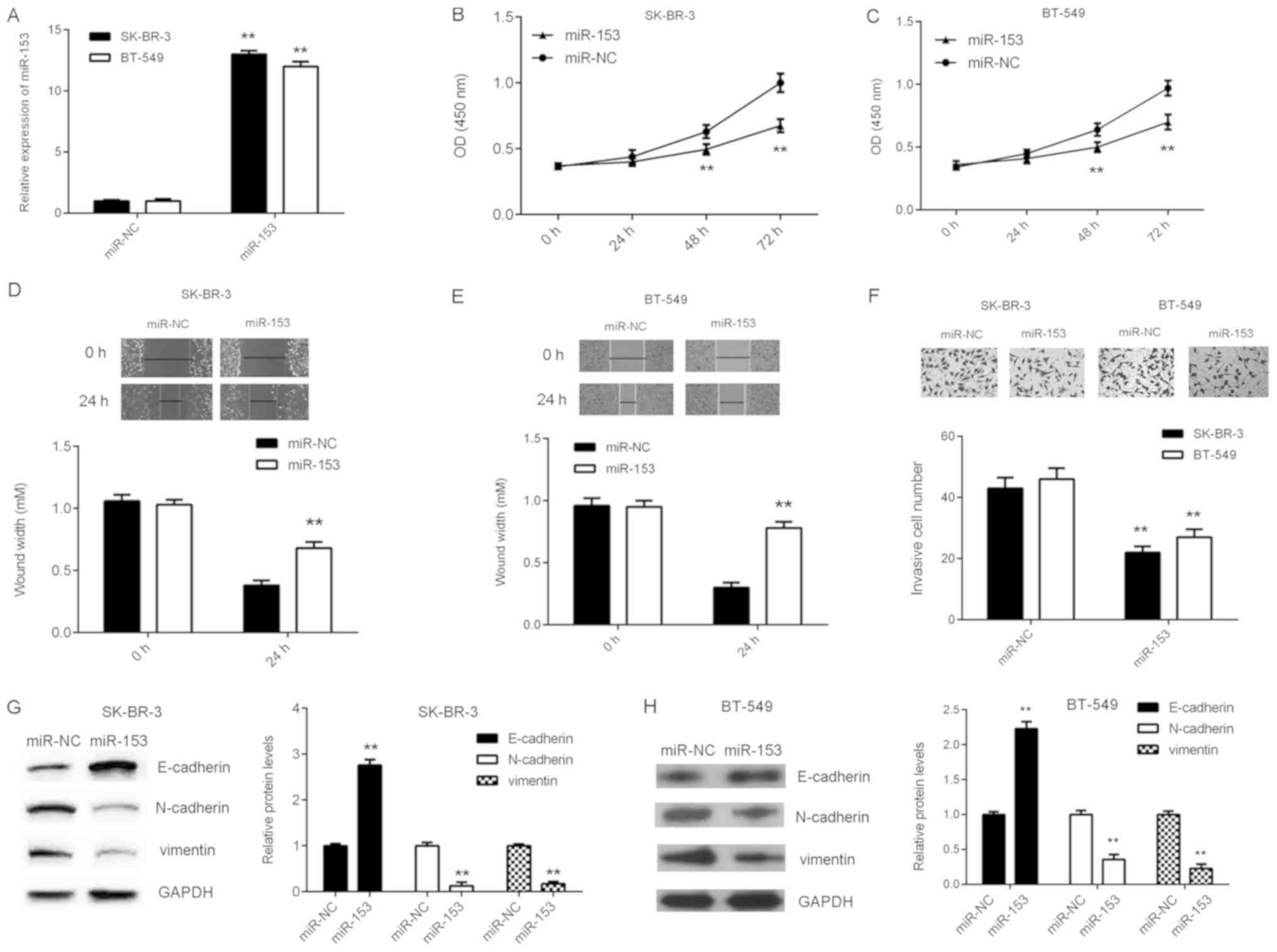
Overexpression of miR-153 inhibits the
malignant phenotype in breast cancer cell lines
To investigate the function of miR-153 in breast
cancer, cellular proliferation, migration and invasion were
analyzed in breast cancer cell lines SK-BR-3 and BT-549 following
transfection with either miR-153 mimic or miR-NC. The mRNA
expression level of miR-153 significantly increased in breast
cancer cells transfected with miR-153 mimic compared with miR-NC
(Fig. 2A). In addition, SK-BR-3 and
BT-549 cell proliferation, migration and invasion significantly
decreased following miR-153 overexpression (Fig. 2B-F). The effect of miR-153 expression
on EMT in SK-BR-3 and BT-549 cells was subsequently examined. The
protein expression level of E-cadherin was significantly increased,
whilst the protein expression levels of N-cadherin and vimentin
were significantly reduced following overexpression of miR-153
compared with the miR-NC group (Fig. 2G
and H). These results indicated that miR-153 may downregulate
EMT in breast cancer cell lines.
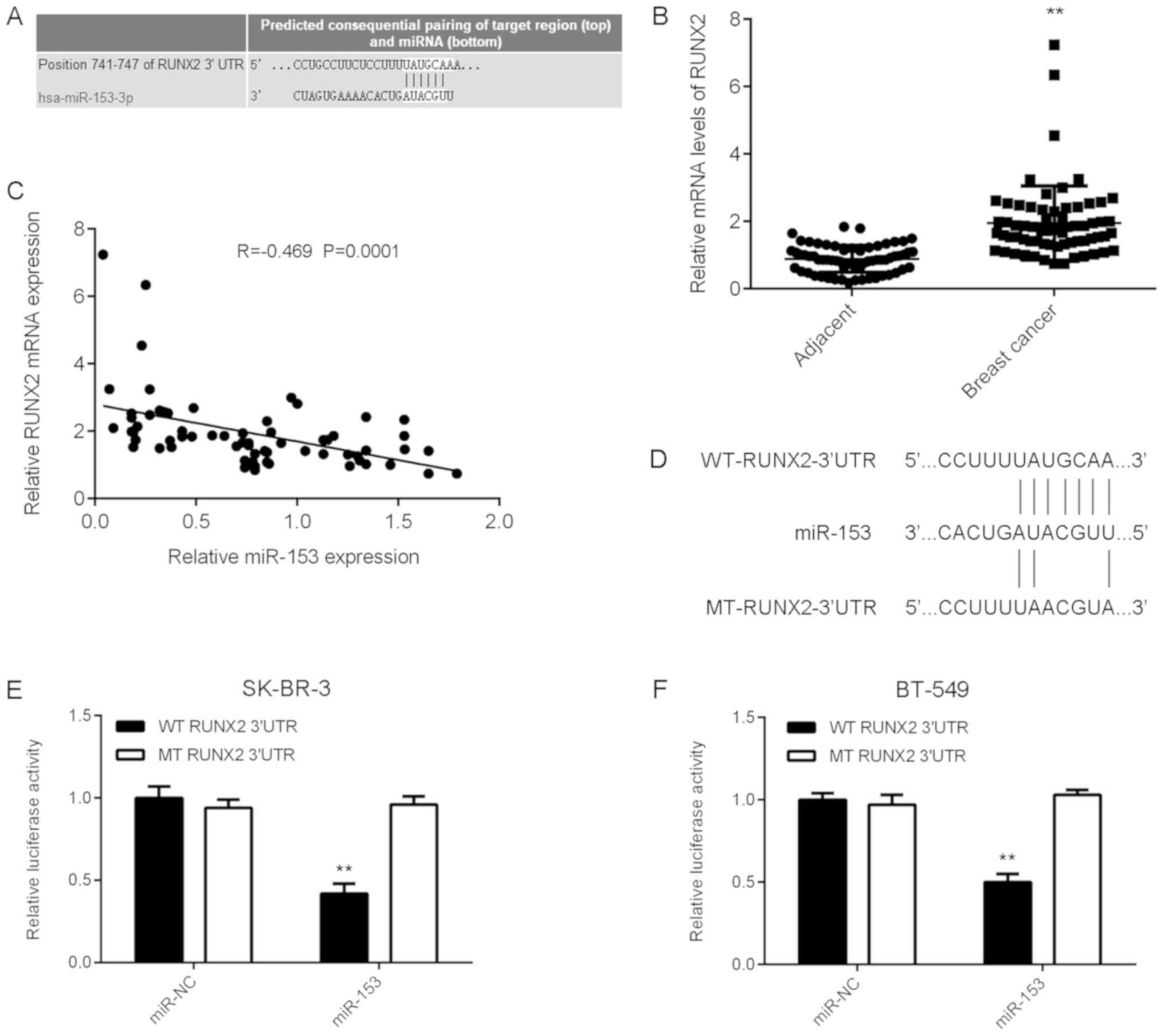
RUNX2, upregulated in breast cancer,
is a novel target of miR-153
To investigate miR-153 further, the potential
targets of miR-153 in breast cancer were examined. TargetScan
software was used to identify RUNX2 as a putative target gene of
miR-153 in breast cancer (Fig. 3A).
To further elucidate the potential association between RUNX2 and
miR-153 in breast cancer, RUNX2 expression was examined in tissue
samples from patients with breast cancer. The mRNA expression level
of RUNX2 was significantly increased in breast cancer tissue
samples compared with matched adjacent healthy tissue samples
(Fig. 3B). Furthermore, an inverse
correlation was observed between miR-153 and RUNX2 expression in
breast cancer tissue (Fig. 3C).
According to the mean RUNX2 expression value, which was used as the
cut-off, patients with breast cancer involved in the current study
were divided into two groups: High RUNX2 expression and low RUNX2
expression. The high RUNX2 expression group was associated with
advanced clinical staging and metastasis in patients with breast
cancer, however no association with age, subtype, or
differentiation was identified (Table
II). These results suggest that RUNX2 upregulation may
contribute to the process of malignant progression in human breast
cancer. Luciferase reporter plasmids containing the WT-RUNX2-3′UTR
or MT-RUNX2-3′UTR were generated (Fig.
3D) and used in the luciferase reporter gene assays which were
conducted in SK-BR-3 and BT-549 cells. Luciferase activity
indicated that miR-153 significantly reduced WT RUNX2 expression
compared with MT RUNX2, which demonstrated no effect on the
luciferase activity, in both breast cancer cell lines (Fig. 3E and F). These results confirm RUNX2
as a novel target gene of miR-153 in SK-BR-3 and BT-549 breast
cancer cell lines.
 | Table II.Association between RUNX2 expression
and clinicopathological characteristics of patients with breast
cancer. |
Table II.
Association between RUNX2 expression
and clinicopathological characteristics of patients with breast
cancer.
| Variables | No. of patients
(n=67) | Low expression
(n=43) | High expression
(n=24) | P-value |
|---|
| Age, years |
|
|
| 0.800 |
|
≤50 | 30 | 20 | 10 |
|
|
>50 | 37 | 23 | 14 |
|
| Subtype |
|
|
| 0.175 |
| Lunimal
A type | 33 | 25 | 8 |
|
| Lunimal
B type | 8 | 3 | 5 |
|
| HER2
positive | 11 | 6 | 5 |
|
|
TNBC | 15 | 9 | 6 |
|
|
Differentiation |
|
|
| 0.120 |
| Well
and moderately | 40 | 29 | 11 |
|
|
Poor | 27 | 14 | 13 |
|
| Lymph node
metastasis |
|
|
| 0.014 |
|
Present | 45 | 24 | 21 |
|
|
Absent | 22 | 19 | 3 |
|
| Distant
metastasis |
|
|
| 0.0004 |
|
Present | 7 | 0 | 7 |
|
|
Absent | 60 | 43 | 17 |
|
| TNM stage |
|
|
| 0.0005 |
|
I–II | 44 | 35 | 9 |
|
|
III–IV | 23 | 8 | 15 |
|
miR-153 negatively regulates RUNX2
expression in breast cancer cells
The effect of miR-153 on RUNX2 expression was
examined in breast cancer cell lines SK-BR-3 and BT-549 following
transfection with either miR-153 mimic or miR-NC. Overexpression of
miR-153 significantly decreased the mRNA and protein expression
levels of RUNX2 (Fig. 4A and B). To
further understand the effect of miR-153, SK-BR-3 and BT-549 cells
were transfected with either miR-153 inhibitor or NC inhibitor. The
mRNA expression level of miR-153 significantly decreased in breast
cancer cells transfected with miR-153 inhibitor compared with NC
inhibitor (Fig. 4C). Knockdown of
miR-153 significantly increased the mRNA and protein expression
levels of RUNX2 (Fig. 4D and E),
suggesting that miR-153 negatively regulates RUNX2 expression in
breast cancer cells.
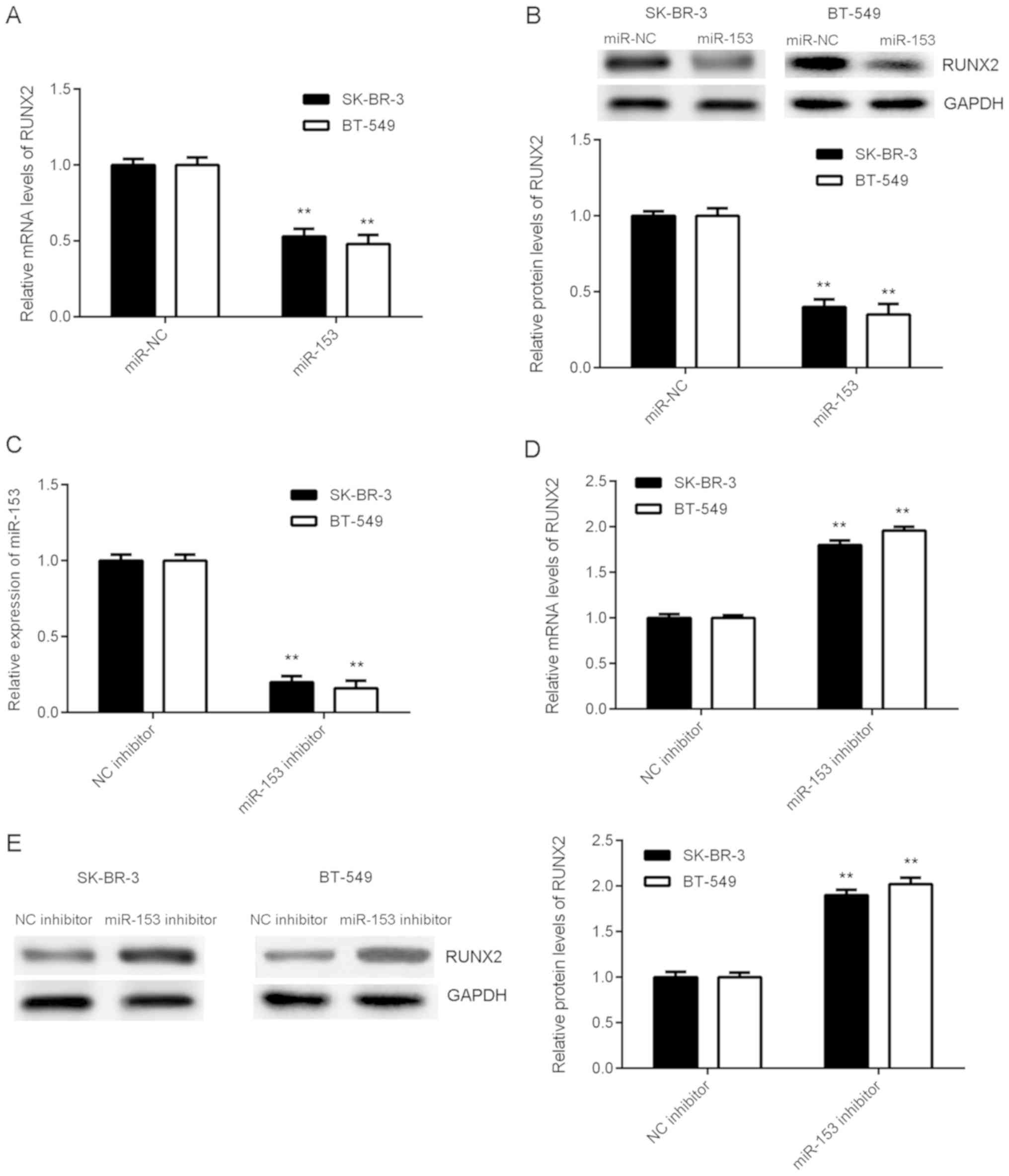 | Figure 4.miR-153 negatively regulates RUNX2
expression in SK-BR-3 and BT-549 cells. miR-153 mimic and scramble
miR mimic were transiently transfected into SK-BR-3 and BT-549
cells, respectively. (A) The mRNA expression level of RUNX2 in
SK-BR-3 and BT-549 cells was detected by RT-qPCR. (B) The protein
expression level of RUNX2 in SK-BR-3 and BT-549 cells was
determined by western blotting. **P<0.01 vs. miR-NC. miR-153
inhibitor and NC inhibitor were transiently transfected into
SK-BR-3 and BT-549 cells, respectively. The mRNA expression level
of (C) miR-153 and (D) RUNX2 in SK-BR-3 and BT-549 cells was
detected by RT-qPCR. (E) The protein expression level of RUNX2 in
SK-BR-3 and BT-549 cells was determined by western blotting.
**P<0.01 vs. NC inhibitor. RUNX2, runt-related transcription
factor 2; SK-BR-3 and BT-549, human breast cancer cell lines; miR,
microRNA; miR-NC, breast cancer cell lines SK-BR-3 and BT-549
transfected with scramble miR; miR-153, breast cancer cell lines
SK-BR-3 and BT-549 transfected with miR-153 mimic; NC inhibitor,
breast cancer cell lines SK-BR-3 and BT-549 transfected with miRNA
NC inhibitor; miR-153 inhibitor, breast cancer cell lines SK-BR-3
and BT-549 transfected with miR-153 inhibitor; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
RUNX2 reduces the suppressive effects
of miR-153 in breast cancer cells
RUNX2 was identified in the present study as
putative target gene of miR-153 in breast cancer cells. To
determine whether RUNX2 was involved in miR-153-mediated breast
cancer, SK-BR-3 and BT-549 cells overexpressing miR-153 were
transfected with pcDNA3.1-RUNX2 plasmid to increase RUNX2
expression.
Following transfection, the mRNA and protein
expression of levels of RUNX2 were significantly increased in both
breast cancer cell lines (Fig. 5A and
B). In addition, SK-BR-3 and BT-549 cell proliferation,
migration and invasion were significantly increased following
transfection with miR-153 + RUNX2 compared with miR-153 + blank
(Fig. 5C-G). The effect on EMT in
SK-BR-3 and BT-549 cells was subsequently examined. The protein
expression level of E-cadherin was significantly reduced, whilst
the protein expression levels of N-cadherin and vimentin were
significantly increased following transfection with miR-153 + RUNX2
compared with miR-153 + blank (Fig. 5H
and I), suggesting that RUNX2 overexpression increased EMT in
breast cancer cell lines. Taken together, these results suggested
that RUNX2 overexpression impaired the suppressive effects of
miR-153 on the malignant phenotype in SK-BR-3 and BT-549 breast
cancer cell lines.
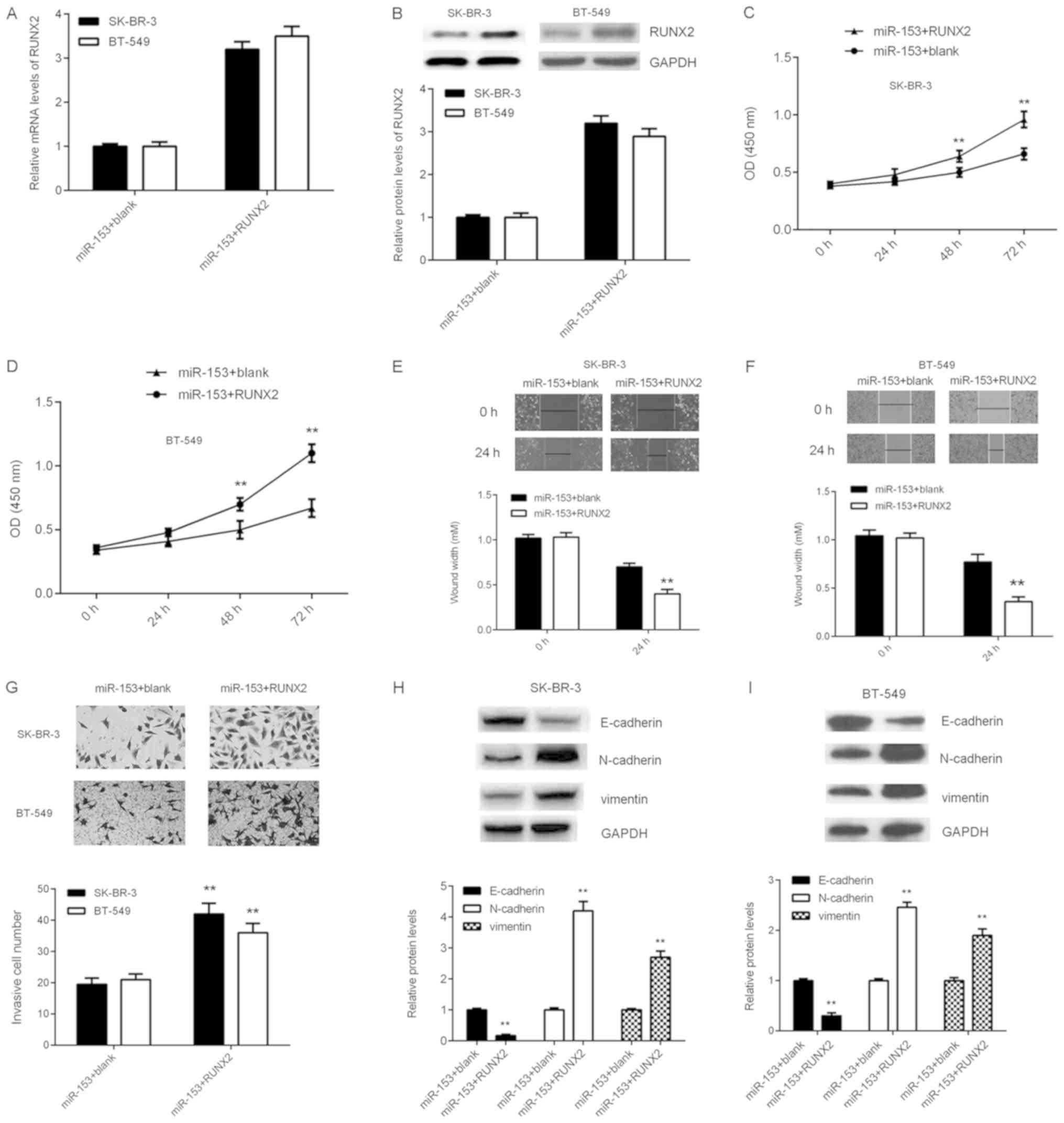 | Figure 5.RUNX2 overexpression reduces the
suppressive effects of miR-153 on SK-BR-3 and BT-549 cells. SK-BR-3
and BT-549 cells were co-transfected with miR-153 mimic, and
pcDNA3.1-RUNX2 plasmid or blank pcDNA3.1 vector, respectively. The
(A) mRNA and (B) protein expression levels of RUNX2 were detected
by reverse transcription-quantitative polymerase chain reaction and
western blotting, respectively. The effect on (C) SK-BR-3 and (D)
BT-549 cell growth was analyzed by cell counting kit-8 assay. The
effect on (E) SK-BR-3 and (F) BT-549 cell migration was analyzed
using the wound healing assay (magnification, ×40). (G) The effect
on SK-BR-3 and BT-549 cell invasion was analyzed using the
transwell invasion assay (magnification, ×200). The protein
expression levels of E-cadherin, N-cadherin and vimentin in (H)
SK-BR-3 and (I) BT-549 cells were determined by western blotting.
**P<0.01 vs. miR-153 + blank. SK-BR-3 and BT-549, human breast
cancer cell lines; miR, microRNA; miR-153 + blank, breast cancer
cell lines SK-BR-3 and BT-549 co-transfected with miR-153 mimic and
blank pc-DNA3.1 vector; miR-153+RUNX2, breast cancer cell lines
SK-BR-3 and BT-549 co-transfected with miR-153 mimic and
pc-DNA3.1-RUNX2 plasmid. |
Discussion
The underlying regulatory mechanism of miR-153 in
breast cancer progression remains unclear. The present study
demonstrated that miR-153 expression levels were significantly
reduced in breast cancer tissue samples and cell lines, compared
with adjacent healthy tissue samples and normal human breast cell
line MCF-10A. Furthermore, low miR-153 expression was associated
with advanced clinical staging and metastasis in patients with
breast cancer, however not association with age, subtype or
differentiation was identified. Furthermore, patients with breast
cancer with low miR-153 expression had poor prognosis, compared
with patients with breast cancer with high miR-153 expression.
Overexpression of miR-153 reduced the proliferation, migration,
invasion and EMT in breast cancer SK-BR-3 and BT-549 cells. RUNX2,
significantly upregulated in breast cancer, was confirmed to be a
novel target gene of miR-153 in SK-BR-3 and BT-549 cells by
luciferase reporter gene assay. High RUNX2 expression was
associated with advanced clinical staging as well as distant and
lymph node metastasis in patients with breast cancer; however no
association with age, subtype or differentiation was identified. In
addition, an inverse correlation between miR-153 and RUNX2 mRNA
expression levels was observed in breast cancer tissues. RUNX2
overexpression reduced the suppressive effects of miR-153
upregulation on the proliferation, migration, invasion and EMT of
SK-BR-3 and BT-549 cells.
In different types of human cancer, miR-153 appears
to be involved in either promoting or suppressing tumor growth and
progression (20–22). Wu et al (20) revealed that miR-153 promoted prostate
cancer cell proliferation by targeting PTEN. By contrast, miR-153
inhibited the proliferation and invasion of human laryngeal
squamous cell carcinoma cells by inhibiting the expression of
kruppel-like factor 5 (21). miR-153
may inhibit cell migration and invasion of gastric cancer cells by
directly targeting Snail (22). A
tumor suppressor function of miR-153 was revealed in glioblastoma
(23). The different roles
associated with miR-153 in different types of cancer may be as a
result of differences in tumor microenvironment and target genes.
In the current study, miR-153 expression was significantly reduced
in breast cancer tissues and cell lines. Downregulation of miR-153
expression was associated with tumor progression and metastasis as
well as poor prognosis in patients with breast cancer. A previous
study demonstrated that miR-153 was downregulated in breast cancer
(9). Downregulation of miR-153
expression may therefore serve a role during breast cancer
progression. In the current study, overexpression of miR-153 led to
a significant reduction in breast cancer cell proliferation,
migration and invasion. Epithelial cancer cells acquire molecular
alternations that facilitate the loss of epithelial features and
gain of mesenchymal phenotype during EMT, promoting tumor cell
migration and invasion (24). The
present study demonstrated that overexpression of miR-153 inhibited
EMT in breast cancer cells, which may have contributed to a
reduction in tumor cell migration and invasion. Therefore, miR-153
may induce suppressive effects on the growth and metastasis of
breast cancer.
The current study investigated the regulatory
mechanism of miR-153-induced malignant phenotype in breast cancer
cell lines. This study identified RUNX2 to be a novel target gene
of miR-153 in breast cancer cells. RUNX2 is involved in
osteogenesis and breast cancer bone metastases (16). The skeleton is one of the most common
metastatic sites in breast cancer (16). The present study demonstrated that
RUNX2 expression levels were significantly increased in breast
cancer tissues and cell lines. High expression level of RUNX2 was
associated with breast cancer progression including distant and
lymph node metastasis. Similarly, Chang et al (25) demonstrated that higher expression of
RUNX2 was associated with adverse outcomes in patients with breast
cancer, including poor prognosis and recurrence (25). RUNX2 acts as an oncogene in breast
cancer, activating the PI3K/AKT signaling, Indian Hedgehog
signaling and a downstream bone metastatic pathway in breast cancer
cells (26,27). The present study demonstrated that
miR-153 may negatively regulate RUNX2 expression in breast cancer
cells. An inverse correlation was identified between RUNX2 and
miR-153 expression levels in breast cancer tissue samples. In
addition, overexpression of RUNX2 impaired the suppressive effects
of miR-153 on the malignant phenotype in breast cancer cells.
Therefore, it was hypothesized that RUNX2 may be involved in the
miR-153-induced malignant phenotype in breast cancer cell
lines.
In conclusion, miR-153 inhibits cell proliferation,
migration, invasion and EMT in breast cancer through direct
targeting of RUNX2. miR-153 and RUNX2 may be potential molecular
targets in the treatment of breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG designed the experiments. FY and YG performed the
experiments. ZL analyzed the data. ZZ and JH prepared the
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Second Xiangya Hospital of Central South University
(Changsha, China). Written informed consent was obtained from
patients involved in this study.
Patient consent for publication
Patient consent for publication has been
obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu X, Zhang Y, Jasper J, Lykken E,
Alexander PB, Markowitz GJ, McDonnell DP, Li QJ and Wang XF:
MiR-148a functions to suppress metastasis and serves as a
prognostic indicator in triple-negative breast cancer. Oncotarget.
7:20381–20394. 2016.PubMed/NCBI
|
|
6
|
Zheng Y, Lv X, Wang X, Wang B, Shao X,
Huang Y, Shi L, Chen Z, Huang J and Huang P: MiR-181b promotes
chemoresistance in breast cancer by regulating Bim expression.
Oncol Rep. 35:683–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao Y, Hu J, Shen Z, Yao R, Liu S, Li Y,
Cong H, Wang X, Qiu W and Yue L: MiR-200b expression in breast
cancer: A prognostic marker and act on cell proliferation and
apoptosis by targeting Sp1. J Cell Mol Med. 19:760–769. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Li L, Li Y and Liu Z: MiR-153
promotes breast cancer cell apoptosis by targeting HECTD3. Am J
Cancer Res. 6:1563–1571. 2016.PubMed/NCBI
|
|
9
|
Li W, Zhai L, Zhao C and Lv S: MiR-153
inhibits epithelial-mesenchymal transition by targeting metadherin
in human breast cancer. Breast Cancer Res Treat. 150:501–509. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fkih M'hamed I, Privat M, Ponelle F,
Penault-Llorca F, Kenani A and Bignon YJ: Identification of
miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative
breast cancer biomarkers. Cell Oncol (Dordr). 38:433–442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anaya-Ruiz M, Cebada J, Delgado-López G,
Sánchez-Vázquez ML and Pérez-Santos JL: miR-153 silencing induces
apoptosis in the MDA-MB-231 breast cancer cell line. Asian Pac J
Cancer Prev. 14:2983–2986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Teng Y and Liu Q: MicroRNA-153
regulates NRF2 expression and is associated with breast
carcinogenesis. Clin Lab. 62:39–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rashid H, Ma C, Chen H, Wang H, Hassan MQ,
Sinha K, de Crombrugghe B and Javed A: Sp7 and Runx2 molecular
complex synergistically regulate expression of target genes.
Connect Tissue Res. 55 (Suppl 1):S83–S87. 2014. View Article : Google Scholar
|
|
14
|
McGee-Lawrence ME, Carpio LR, Bradley EW,
Dudakovic A, Lian JB, van Wijnen AJ, Kakar S, Hsu W and Westendorf
JJ: Runx2 is required for early stages of endochondral bone
formation but delays final stages of bone repair in Axin2-deficient
mice. Bone. 66:277–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Ghori-Javed FY, Rashid H, Adhami
MD, Serra R, Gutierrez SE and Javed A: Runx2 regulates endochondral
ossification through control of chondrocyte proliferation and
differentiation. J Bone Miner Res. 29:2653–2665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vishal M, Swetha R, Thejaswini G, Arumugam
B and Selvamurugan N: Role of Runx2 in breast cancer-mediated bone
metastasis. Int J Biol Macromol. 99:608–614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tandon M, Othman AH, Ashok V, Stein GS and
Pratap J: The role of Runx2 in facilitating autophagy in metastatic
breast cancer cells. J Cell Physiol. 233:559–571. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taipaleenmäki H, Browne G, Akech J, Zustin
J, van Wijnen AJ, Stein JL, Hesse E, Stein GS and Lian JB:
Targeting of Runx2 by miR-135 and miR-203 impairs progression of
breast cancer and metastatic bone disease. Cancer Res.
75:1433–1444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Z, He B, He J and Mao X: Upregulation
of miR-153 promotes cell proliferation via downregulation of the
PTEN tumor suppressor gene in human prostate cancer. Prostate.
73:596–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu JY, Lu JB and Xu Y: MicroRNA-153
inhibits the proliferation and invasion of human laryngeal squamous
cell carcinoma by targeting KLF5. Exp Ther Med. 11:2503–2508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z and Liu C: MiR-153 regulates
metastases of gastric cancer through Snail. Tumour Biol. 2015.
|
|
23
|
Ghasemi A, Fallah S and Ansari M: MiR-153
as a tumor suppressor in glioblastoma multiforme is downregulated
by DNA methylation. Clin Lab. 62:573–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Sarkissyan M and Vadgama JV:
Epithelial-mesenchymal transition and breast cancer. J Clin Med.
5:E132016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang CH, Fan TC, Yu JC, Liao GS, Lin YC,
Shih AC, Li WH and Yu AL: The prognostic significance of RUNX2 and
miR-10a/10b and their inter-relationship in breast cancer. J Transl
Med. 12:2572014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pratap J, Wixted JJ, Gaur T, Zaidi SK,
Dobson J, Gokul KD, Hussain S, van Wijnen AJ, Stein JL, Stein GS
and Lian JB: Runx2 transcriptional activation of Indian Hedgehog
and a downstream bone metastatic pathway in breast cancer cells.
Cancer Res. 68:7795–7802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tandon M, Chen Z and Pratap J: Runx2
activates PI3K/Akt signaling via mTORC2 regulation in invasive
breast cancer cells. Breast Cancer Res. 16:R162014. View Article : Google Scholar : PubMed/NCBI
|